Secretory Products in Petals of Centaurea cyanus L. Flowers: A Histochemistry, Ultrastructure, and Phytochemical Study of Volatile Compounds
Abstract
:1. Introduction
2. Results
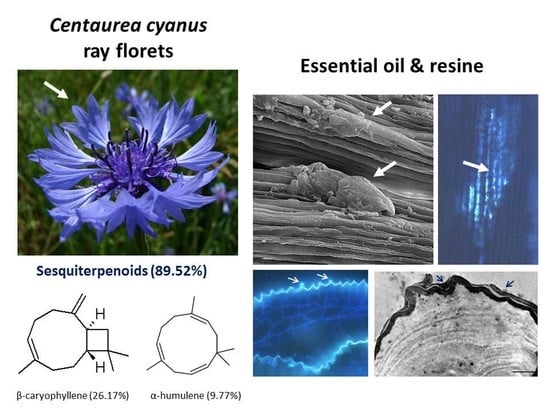
2.1. Micromorphology of Ray Florets
2.2. Secretory Activity of the Epidermis and Parenchyma
2.3. Ultrastructure of Secretory Cells
2.4. Volatile Compounds
3. Discussion
3.1. Secretory Activity of Corolla Tissues
3.2. Volatile Compounds of Petals
4. Materials and Methods
4.1. Plant Material
4.2. Light Microscopy
4.3. Fluorescence Microscopy
4.4. Transmission Electron Microscopy (TEM)
4.5. Scanning Electron Microscopy
4.6. Determination of Volatile Compounds in the Flowers
4.6.1. SPME Extraction of Floral Volatile Components
4.6.2. GC-MS determination
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mofikoya, A.O.; Bui, T.N.T.; ·Kivimäenpää, M.; Holopainen, J.K.; Himanen, S.J.; Blande, J.D. Foliar behaviour of biogenic semi-volatiles: Potential applications in sustainable pest management. Arthropod-Plant Interact. 2019, 13, 193–212. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Muhlemann, J.K.; Klempien, A.; Dudareva, N. Floral volatiles: From biosynthesis to function. Plant Cell Environ. 2014, 37, 1936–1949. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, H.A.; El-Ghorab, A.H.; Shibamoto, T. Bioactivity of essential oils and their volatile aroma components: Review. J. Essent. Oil Res. 2012, 24, 203–212. [Google Scholar] [CrossRef]
- Rahmani, R.; Andersson, F.; Andersson, M.N.; Yuvaraj, J.K.; Anderbrant, O.; Hedenström, E. Identification of sesquisabinene B in carrot (Daucus carota L.) leaves as a compound electrophysiologically active to the carrot psyllid (Trioza apicalis Förster). Chemoecology 2019, 29, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- González-Mas, M.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile compounds in Citrus essential oils: A comprehensive review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef]
- Chizzola, R. Regular monoterpenes and sesquiterpenes (essential oils). In Natural Products; Ramawat, K.G., Mérillon, J.M., Eds.; Springer: Berlin, Germany, 2013; pp. 2973–3008. [Google Scholar]
- Wiart, C. Terpenes in Lead Compounds from Medicinal Plants for the Treatment of Cancer; Chapter 2, Terpenes; Academic Press: London, UK; Elsevier: London, UK, 2013; pp. 97–265. [Google Scholar] [CrossRef]
- Caissard, J.-C.; Joly, C.; Bergougnox, V.; Hugueney, P.; Mauriat, M.; Baudino, S. Secretion mechanisms of volatile organic compounds inspecialized cells of aromatic plants. Rec. Res. Develop. Cell Biol. 2004, 2, 1–15. [Google Scholar]
- Rehman, R.; Hanif, M.A.; Mushtaq, Z.; Mochona, B.; Qi, X. Biosynthetic factories of essential oils: The aromatic plants. Nat. Prod. Chem. Res. 2016, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Metcalfe, C.R.; Chalk, L. Anatomy of the Dicotyledons; Oxford Press: London, UK, 1972; Volume 2. [Google Scholar]
- Rusak, G.; Pleše, N.; Kuštrak, D. Anatomical investigations of endemic species Centaurea rupestris L. and C. fritschii Hayek (Asteraceae). Acta Bot. Croat. 1992, 51, 27–36. [Google Scholar]
- Pagni, A.M.; Orlando, R.; Masini, A.; Ciccarelli, D. Secretory structures of Santolina ligustica Arrigoni (Asteraceae), an Italian endemic species. Isr. J. Plant Sci. 2003, 51, 185–192. [Google Scholar] [CrossRef]
- Andreucci, A.C.; Ciccarelli, D.; Desideri, I.; Pagni, A.M. Glandular hairs and secretory ducts of Matricaria chamomilla (Asteraceae): Morphology and histochemistry. Ann. Bot. Fennici. 2008, 45, 11–18. [Google Scholar] [CrossRef]
- Lersten, N.R.; Curtis, J.D. Polyacetylene reservoir (duct) development in Ambrosia trifida (Asteraceae) staminate flowers. Amer. J. Bot. 1989, 76, 1000–1005. [Google Scholar] [CrossRef]
- Poli, F.; Sacchetti, G.; Bruni, B. Distribution of internal secretory structures in Tagetes patula (Asteraceae). Nord. J. Bot. 1995, 15, 197–205. [Google Scholar] [CrossRef]
- Chiru, T.; Calalb, T.; Nistreanu, A. Morphological and anatomical studies of Cyani herba. Mod. Phytomorphol. 2013, 4, 65–68. [Google Scholar]
- Haratym, W.; Weryszko-Chmielewska, E.; Konarska, A. Microstructural and histochemical analysis of aboveground organsof Centaurea cyanus used in herbal medicine. Protoplasma 2020, 257, 285–298. [Google Scholar] [CrossRef] [Green Version]
- Erel, S.B.; Demirci, B.; Demir, S.; Karaalp, C.; Baser, K.H.C. Composition of the essential oils of Centaurea aphrodisea, C. polyclada, C. athoa, C. hyalolepis, C. iberica. J. Essent. Oil Res. 2013, 25, 79–84. [Google Scholar] [CrossRef]
- Erdogan, T.; Sumer, B.; Ozcınar, O.; Cakilcioglu, U.; Demirci, B.; Husnu, K.; Baser, C.; Kivcak, B. Essential oil composition of three Centaurea species from Turkey: Centaurea aggregata Fisch & Mey. ex. DC. subsp. aggregata, C. balsamita Lam. and C. behen L. Rec. Nat. Prod. 2014, 11, 69–73. [Google Scholar]
- Polatoglu, K.; Sen, A.; Bulut, G.; Bitis, L.; Gören, N. Essential oil composition of Centaurea kilaea Boiss. and C. cuneifolia Sm. from Turkey. Nat. Volatiles Essent. Oils 2014, 1, 55–59. [Google Scholar]
- Polatoglu, K.; Sen, A.; Bulut, G.; Bitis, L.; Gören, N. Essential oil composition of Centaurea stenolepis Kerner. from Turkey. J. Essent. Oil Bear. Plants 2014, 17, 1268–1278. [Google Scholar] [CrossRef]
- Yaglioglu, A.S.; Demirtas, I. Comparative essential oil composition of flowers, leaves, and stems of Centaurea polypodiifolia var polypodiifolia. Chem. Nat. Comd. 2015, 51, 982–984. [Google Scholar] [CrossRef]
- Politeo, O.; Skocibusic, M.; Carev, I.; Burcul, F.; Jerkovic, I.; Sarolic, M.; Milos, M. Phytochemical profiles of volatile constituents from Centaurea ragusina leaves and flowers and their antimicrobial effects. Nat. Prod. Commun. 2012, 7, 1087–1090. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.; Milia, A.; Catinella, G.; Bancheva, S. Volatile components from aerial parts of Centaurea diffusa and C. micrantha ssp. melanosticta and their biocidal activity on microorganisms affecting historical art crafts. Nat. Prod. Commun. 2018, 13, 1179–1182. [Google Scholar] [CrossRef] [Green Version]
- Azadi, B.; Nouri, E. The essential oil composition of Centaurea intricate Boiss. flowering aerial parts. Asian J. Biomed. Pharm. 2014, 4, 25–27. [Google Scholar] [CrossRef]
- Reda, E.H.; Shakour, Z.T.A.; El-Halawany, A.M.; El-Kashoury, E.-S.A.; Shams, K.A.; Mohamed, T.A.; Saleh, I.; Elshamy, A.I.; Atia, M.A.M.; El-Beih, A.A.; et al. Comparative study on the essential oils from five wild Egyptian Centaurea species: Effective extraction techniques, antimicrobial activity and in-silico analyses. Antibiotics 2021, 10, 252. [Google Scholar] [CrossRef]
- Józefczyk, A.; Kowalska, J. Ocena składu i zastosowanie olejków eterycznych z rodzaju Centaurea L. Kosmos. Probl. Nauk. Przyr. 2018, 67, 319–334. [Google Scholar] [CrossRef] [Green Version]
- Karamenderes, C.; Demirci, B.; Baser, K.H.C. Composition of essential oils of ten Centaurea L. taxa from Turkey. J. Essent. Oil Res. 2008, 20, 342–349. [Google Scholar] [CrossRef]
- Ascensão, L.; da Silva, J.A.T.; Barroso, J.G.; Figueiredo, A.C.; Pedro, L.G. Glandular trichomes and essential oils of Helichrysum stoechas. Isr. J. Plant Sci. 2001, 49, 115–122. [Google Scholar] [CrossRef]
- Sulborska, A. Micromorphology of flowers, anatomy and ultrastructure of Chamomilla recutita (L.) Rausch. (Asteraceae) nectary. Acta Agrobot. 2011, 64, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Sulborska, A. Structure and distribution of glandular and nonglandular trichomes on above-ground organs in Inula helenium L. (Asteraceae). Acta Agrobot. 2013, 66, 25–34. [Google Scholar] [CrossRef]
- Delbón., N.; Cosa, M.T.; Bernardello, G. Reproductive biology, seed germination and regeneration of Flourensia DC. species endemic to Central Argentina (Asteraceae). Adansonia 2017, 39, 71–85. [Google Scholar] [CrossRef]
- Haratym, W.; Weryszko-Chmielewska, E. The ecological features of flowers and inflorescences of two species of the genus Petasites Miller (Asteraceae). Acta Agrobot. 2012, 65, 37–46. [Google Scholar] [CrossRef]
- Tölke, E.D.; Capelli, N.V.; Pastori, T.; Alencar, A.C.; Cole, T.C.H.; Demarco, D. Diversity of floral glands and their secretions in pollinator attraction. In Co-Evolution of Secondary Metabolites; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Hansen, H.V. Phylogenetic studies in Compositeae tribe Mutisieae. Opera Bot. 1991, 109, 1–50. [Google Scholar]
- Koch, K.; Bennemann, M.; Bohn, H.F.; Albach, D.C.; Barthlott, W. Surface microstructures of daisy florets (Asteraceae) and characterization of their anisotropic wetting. Bioinspiration Biomim. 2013, 8, 036005. [Google Scholar] [CrossRef] [PubMed]
- Płachno, B.J.; Świątek, P.; Szymczak, G. Can a stench be beautiful? Osmophores in stem-succulent stapeliads (Apocynaceae-Asclepiadoideae-Ceropegieae-Stapeliinae). Flora 2010, 205, 101–105. [Google Scholar] [CrossRef]
- Amela Garcia, M.T.; Galati, B.G.; Hoc, P.S. Ultrastructure of the corona of scented and scentless flowers of Passiflora spp. (Passifloraceae). Flora 2007, 202, 302–315. [Google Scholar] [CrossRef]
- Paiva, E.A.S. How do secretory products cross the plant cell wall to be released? A new hypothesis involving cyclic mechanical actions of the protoplast. Ann. Bot. 2016, 117, 533–540. [Google Scholar] [CrossRef] [Green Version]
- Riedener, M. Biology of the plant cuticle. In Biology of the Plant Cuticle. Annual Plant Reviews; Riederer, M., Müller, C., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2006; Volume 23. [Google Scholar]
- Antoń, S.; Kamińska, M.; Stpiczyńska, M. Comparative structure of the osmophores in the flower of Stanhopea graveolens Lindley and Cycnoches chlorochilon Klotzsch (Orchidaceae). Acta Agrobot. 2012, 65, 11–22. [Google Scholar] [CrossRef] [Green Version]
- de Melo, M.C.; Borba, E.L.; Paiva, E.A.S. Morphological and histological characterization of the osmophores and nectaries of four species of Acianthera (Orchidaceae: Pleurothallidinae). Plant Syst. Evol. 2010, 286, 141–151. [Google Scholar] [CrossRef]
- Vogel, S. The Role of Scent Glands in Pollinationa: On the Structure and Function of Ssmophores; Smithsonian Institution Libraries and National Science Foundation: Washington, DC, USA, 1990. [Google Scholar]
- Skubatz, H.; Kunkel, D.D.; Patt, J.M.; Howald, W.N.; Hartman, T.G.; Meeuse, B.J.D. Pathway of terpene excretion by the appendix of Sauromatum guttatum. Proc. Natl. Acad. Sci. USA 1995, 92, 10084–10088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry and Molecular Biology of Plants; Wiley-Blackwell: Chichester, UK; West Sussex, UK; Hoboken, NJ, USA; New York, NY, USA, 2015. [Google Scholar]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Stahl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1–120. [Google Scholar] [CrossRef]
- Simkin, A.J.; Underwood, B.A.; Auldridge, M.; Loucas, H.M.; Shibuya, K.; Schmelz, E.; Clark, D.G.; Klee, H.J. Circadian regulation of the PhCCD1 carotenoid cleavage dioxygenase controls emission of β-ionone, a fragrance volatile of petunia flowers. Plant Physiol. 2004, 136, 3504–3514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulido, P.; Perello, C.; Rodriguez-Concepcion, M. New insights into plant isoprenoid metabolism. Mol. Plant. 2012, 5, 964–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flamini, G.; Ertugrul, K.; Cioni, P.L.; Morelli, I.; Dural, H.; Bagci, Y. Volatile constituents of two endemic Centaurea species from Turkey: C. pseudoscabiosa subsp. pseudoscabiosa and C. hadimensis. Biochem. Syst. Ecol. 2002, 30, 953–959. [Google Scholar] [CrossRef]
- Ertugrul, K.; Dural, H.; Tugay, O.; Flamini, G.; Cioni, P.L.; Morelli, I. Essential oils from flowers of Centaurea kotschyi var. kotschyi and C. kotschyi var. decumbens from Turkey. Flavour Fragr. J. 2003, 18, 95–97. [Google Scholar] [CrossRef]
- Flamini, G.; Tebano, M.; Cioni, P.L.; Bagci, Y.; Dural, H.; Ertugrul, K.; Uysal, T.; Savran, A. A multivariate statistical approach to Centaurea classification using essential oil composition data of some species from Turkey. Plant Syst. Evol. 2006, 261, 217–228. [Google Scholar] [CrossRef]
- Yayli, N.; Yasar, A.; Yayli, N.; Albay, C.; Asamaz, Y.; Coþkunçelebi, K.; Karaoğlu, S. Chemical composition and antimicrobial activity of essential oils from Centaurea appendicigera and Centaurea helenioides. Pharm. Biol. 2009, 47, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Kohlmünzer, S. Farmakognozja, Podręcznik dla Studentów Farmacji; Wydanie V unowocześnione; PZWL: Warszawa, Poland, 2000. [Google Scholar]
- Sharonova, N.; Nikitin, E.; Terenzhev, D.; Lyubina, A.; Amerhanova, S.; Bushmeleva, K.; Rakhmaeva, A.; Fitsev, I.; Sinyashin, K. Comparative assessment of the phytochemical composition and biological activity of extracts of flowering plants of Centaurea cyanus L., Centaurea jacea L. and Centaurea scabiosa L. Plants 2021, 10, 1279. [Google Scholar] [CrossRef]
- Novakovic, J.; Raj, N.; Milanovici, S.; Marin, P.D.; Janakovic, P. Essential oil composition of Centaurea atropurpurea and Centaurea orientalis inflorescences from the Central Balkans—Ecological significance and taxonomic implication. Chem. Biodivers. 2016, 13, 1221–1229. [Google Scholar] [CrossRef]
- Špaldoňová, A.; Havelcová, M.; Machovič, V.; Lapčák, L. Molecular resin composition of two taxodium taxa growing indifferent climate condition: Chromatographic and spectroscopic study. Adv. Med. Plant Res. 2020, 8, 60–72. [Google Scholar] [CrossRef]
- Haziri, A.; Faiku, F.; Rudhani, I.; Mehmeti, M.; Motori, D. Antibacterial activity of different extracts of Centaurea cyanus (L.) growing wild in Kosovo. Orient. J. Chem. 2017, 33, 1636–1641. [Google Scholar] [CrossRef] [Green Version]
- Mariani, E.; Vicaş, L.G.; Tunde, J.; Mureşan, M.; Stan, R.L.; Sevastre, B.; Diaconeasa, Z.; Ionescu, C.; Hangan, A.C. A comparative study on the biological activity of Centaurea cyanus versus Calendula officinalis. Farmacia 2017, 65, 940–946. [Google Scholar]
- Spring, O. Chemataxonomy based on matabolities from glandular trichomes. Adv. Bot Res. 2000, 31, 153–174. [Google Scholar] [CrossRef]
- Heinrich, G.; Pfeifhofer, H.W.; Stabentheiner, E.; Sawidis, T. Glandular hairs Sigesbeckia jorullensis Kunth (Asteraceae): Morphology, histochemistry and composition of essential oil. Ann. Bot. 2002, 89, 459–469. [Google Scholar] [CrossRef] [Green Version]
- Parimal, L.; Khale, A.; Pramod, K. Resins from herbal origin and a focus on their applications. Int. J. Pharm. Sci. Res. 2011, 2, 1077–1085. [Google Scholar]
- Silva, T.M.; Borges, L.L.; de Sousa Fiuza, T.; Tresvenzol, L.M.F.; da Conceição, E.C.; Batista, C.R.; Matos, C.B.; da Veiga Júnior, V.F.; Mourão, R.H.V. Viscosity of the oil-resins and chemical composition of the essential oils from oils-resins of Copaifera multijuga Hayne growing in the National Forest Saracá-Taquera Brazil. J. Essent. Oil Bear. Plants. 2017, 20, 1226–1234. [Google Scholar] [CrossRef]
- Johansen, D.A. Plant Microtechnique, 1st ed.; McGraw Hill: New York, NY, USA; London, UK, 1940. [Google Scholar]
- O’Brien, T.P.; McCully, M.E. The Study of Plant Structure, Principles and Selected Methods; Termarcarphi Pty. Ltd.: Melbourne, Australia, 1981. [Google Scholar]
- Heslop-Harrison, Y. The pollen stigma interaction: Pollen tube penetration in Crocus. Ann. Bot. 1977, 41, 913–922. [Google Scholar] [CrossRef]
- Stpiczyńska, M.; Davies, K.L.; Pacek-Bieniek, A.; Kamińska, M. Comparative anatomy of the floral elaiophore in representatives of the newly re-circumscribed Gomesa and Oncidium clades (Orchidaceae: Oncidiinae). Ann. Bot. 2013, 112, 839–854. [Google Scholar] [CrossRef] [Green Version]
- Mass Spectral Library. NIST/EPA/NIH, USA, 2020. Available online: https://chemdata.nist.gov/dokuwiki/doku.php?id=chemdata:start (accessed on 26 May 2020).





| No | Compound | Retention Index * | Retention Time [min] | Percent [%] |
|---|---|---|---|---|
| Oxygenated monoterpenes | ||||
| 1 | Bornyl acetate | 1290 | 15.91 | 5.22 |
| Total percent | 5.22 | |||
| Sesquiterpenoids | ||||
| 1 | bicycloelemene | 1342 | 16.77 | 2.56 |
| 2 | α-cubebene | 1354 | 16.98 | 1.98 |
| 3 | α-ylangene | 1378 | 17.38 | 1.21 |
| 4 | α-copaene | 1383 | 17.45 | 4.98 |
| 5 | β-elemene | 1397 | 17.69 | 1.26 |
| 6 | cyperene | 1410 | 17.89 | 2.74 |
| 7 | α-cedrene | 1424 | 18.11 | 2.01 |
| 8 | β-caryophyllene | 1429 | 18.20 | 26.17 |
| 9 | β-cedrene | 1432 | 18.25 | 4.21 |
| 10 | β-copaene | 1438 | 18.34 | 3.74 |
| 11 | aromadendrene | 1450 | 18.52 | 0.71 |
| 12 | α-humulene | 1464 | 18.75 | 9.77 |
| 13 | cis-muurola-3,5-diene | 1455 | 18.60 | 1.79 |
| 14 | trans-cadina-1(6)-4-diene | 1482 | 19.02 | 0.70 |
| 15 | γ-muurolene | 1485 | 19.07 | 2.83 |
| 16 | α-muurolene | 1489 | 19.13 | 1.18 |
| 17 | α-selinene | 1497 | 19.27 | 2.51 |
| 18 | cadina-1,4-diene | 1503 | 19.35 | 1.98 |
| 19 | epi zonarene | 1506 | 19.40 | 4.45 |
| 20 | γ-cadinene | 1523 | 19.66 | 2.20 |
| 21 | δ-cadinene | 1532 | 19.79 | 4.05 |
| 22 | cis calamanane | 1533 | 19.80 | 5.25 |
| 23 | α-cadinene | 1548 | 20.02 | 0.79 |
| 24 | α-calacorene | 1556 | 20.14 | 0.45 |
| Total percent | 89.52 | |||
| Unknown | ||||
| 1 | 1332 | 16.61 | 0.55 | |
| 2 | 1392 | 17.61 | 0.71 | |
| 3 | 1402 | 17.77 | 0.62 | |
| 4 | 1460 | 18.68 | 0.99 | |
| 5 | 1473 | 18.89 | 1.88 | |
| 6 | 1542 | 19.94 | 0.51 | |
| Total percent | 5.26 | |||
| Oxygenated Monoterpenes | ||||||
 bornyl acetate | ||||||
| Sesquiterpenoids | ||||||
 |  |  |  |  |  |  |
| Bicycloelemene | β-elemene | α-cubebene | cyperene | α-cedrene | β-cedrene | β-caryophyllene |
 |  |  |  |  |  | |
| α-humulene | aromadendrene | α-ylangene | α-copaene | β-copaene | cis-muurola-3,5-diene | |
 |  |  |  |  |  | |
| α-muurolene | γ-muurolene | α-selinene | trans-cadina-1(6)-4-diene | cadina-1,4-diene | epi zonarene | |
 |  |  |  |  | ||
| α-cadinene | γ-cadinene | δ-cadinene | cis calamenene | α-calacorene | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulborska-Różycka, A.; Weryszko-Chmielewska, E.; Polak, B.; Stefańczyk, B.; Matysik-Woźniak, A.; Rejdak, R. Secretory Products in Petals of Centaurea cyanus L. Flowers: A Histochemistry, Ultrastructure, and Phytochemical Study of Volatile Compounds. Molecules 2022, 27, 1371. https://doi.org/10.3390/molecules27041371
Sulborska-Różycka A, Weryszko-Chmielewska E, Polak B, Stefańczyk B, Matysik-Woźniak A, Rejdak R. Secretory Products in Petals of Centaurea cyanus L. Flowers: A Histochemistry, Ultrastructure, and Phytochemical Study of Volatile Compounds. Molecules. 2022; 27(4):1371. https://doi.org/10.3390/molecules27041371
Chicago/Turabian StyleSulborska-Różycka, Aneta, Elżbieta Weryszko-Chmielewska, Beata Polak, Beata Stefańczyk, Anna Matysik-Woźniak, and Robert Rejdak. 2022. "Secretory Products in Petals of Centaurea cyanus L. Flowers: A Histochemistry, Ultrastructure, and Phytochemical Study of Volatile Compounds" Molecules 27, no. 4: 1371. https://doi.org/10.3390/molecules27041371
APA StyleSulborska-Różycka, A., Weryszko-Chmielewska, E., Polak, B., Stefańczyk, B., Matysik-Woźniak, A., & Rejdak, R. (2022). Secretory Products in Petals of Centaurea cyanus L. Flowers: A Histochemistry, Ultrastructure, and Phytochemical Study of Volatile Compounds. Molecules, 27(4), 1371. https://doi.org/10.3390/molecules27041371