Synthesis of Polyanionic Cellulose Carbamates by Homogeneous Aminolysis in an Ionic Liquid/DMF Medium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Synthesis
2.3.1. General Information
2.3.2. Synthesis of Cellulose bis-2,3-O-(3,5-Dimethylphenyl Carbamate) 10
2.3.3. Synthesis of Cellulose 6-O-(Phenyl Carbonate)-bis-2,3-O-(3,5-Dimethyl Phenyl Carbamates) 11a–c (Oxycarbonylation Reaction)
2.3.4. Synthesis of Cellulose 6-O-(Propynyl Carbamate)-bis-2,3-O-(3,5-Dimethylphenyl Carbamate) 12 (Aminolysis)
2.3.5. Synthesis of Cellulose Derivatives 13a–b (Aminolysis)
- Procedure A—Heterogeneous Reaction
- Procedure B—Homogenous Reaction with N,O-bis(Trimethylsilyl)Acetamide (BSA)
- Procedure C—Two-Step, One-Pot Homogenous Reaction in DMF/[EMIM]OAc
- Procedure D—Two-Step, One-Pot Homogenous Reaction in DMF and DMF/[EMIM]OAc
3. Results and Discussion
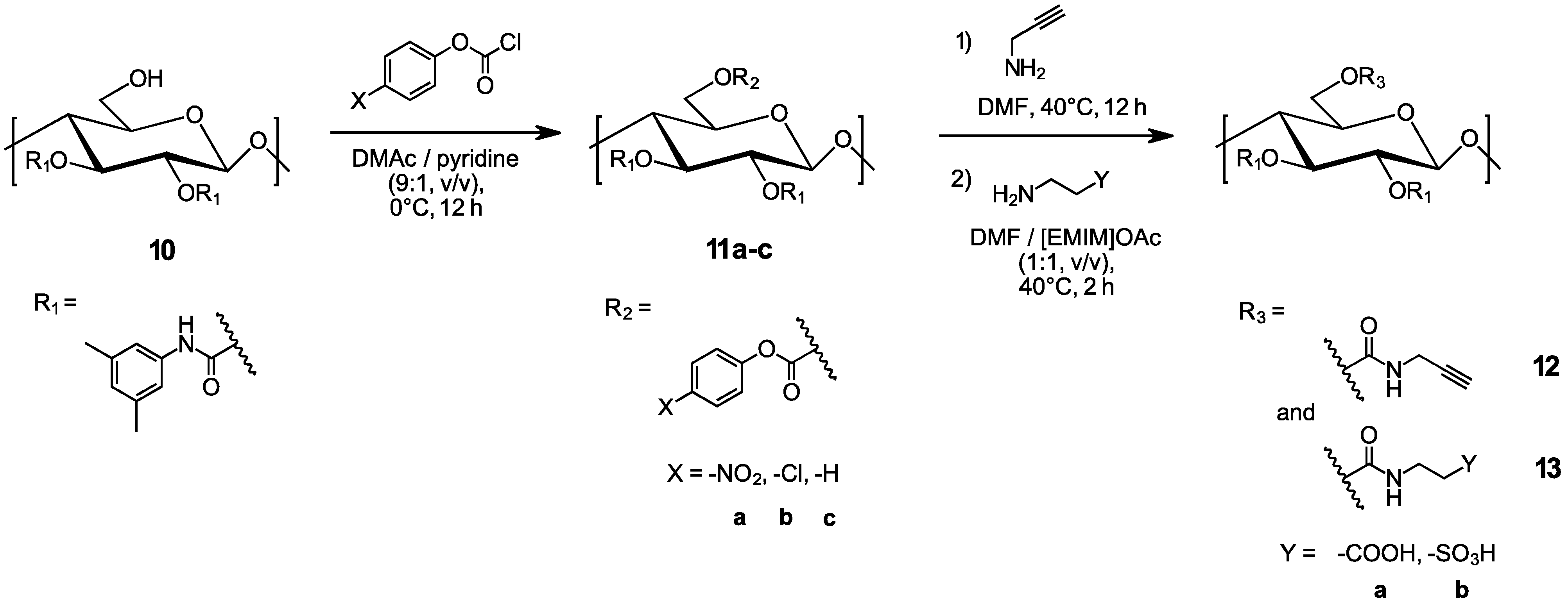
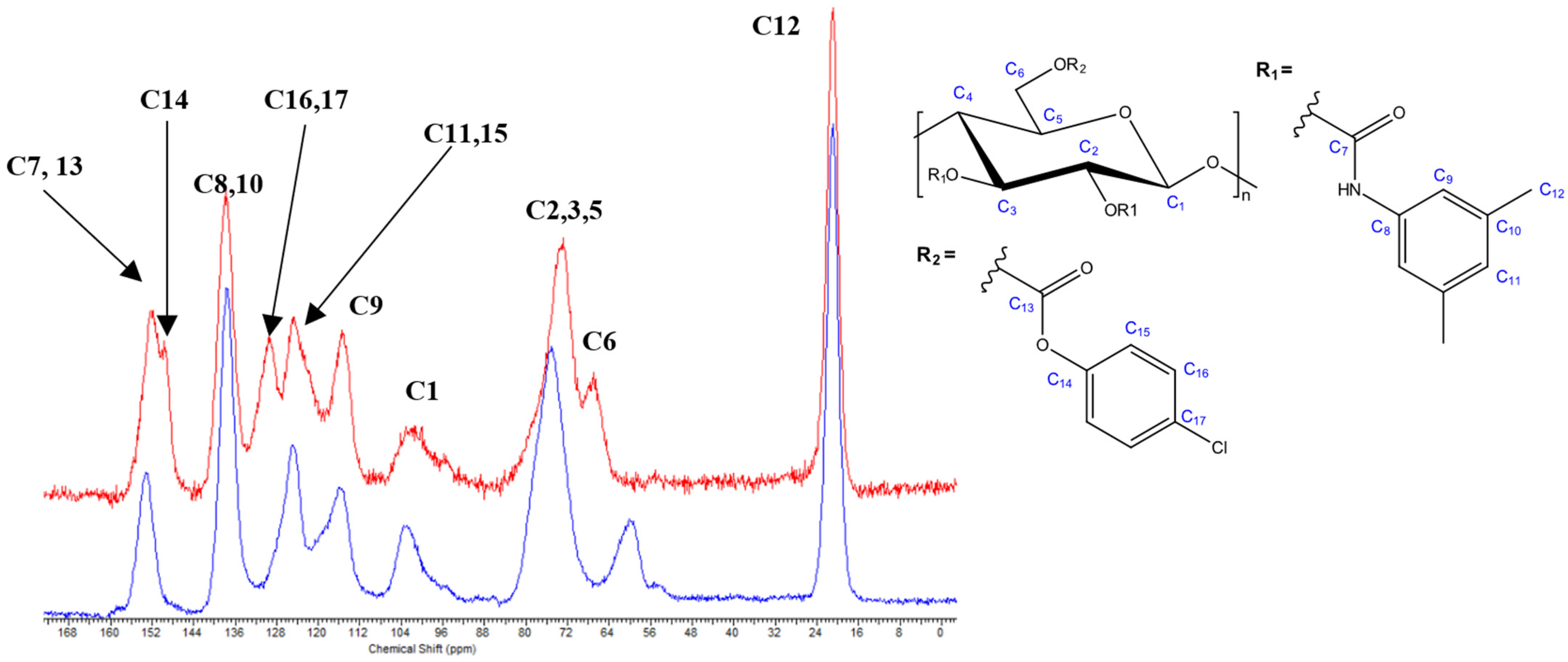
3.1. Evaluation of the Oxycarbonylation (Carbonate Formation) Reaction
3.2. Aminolysis Reaction with Propargyl Amine
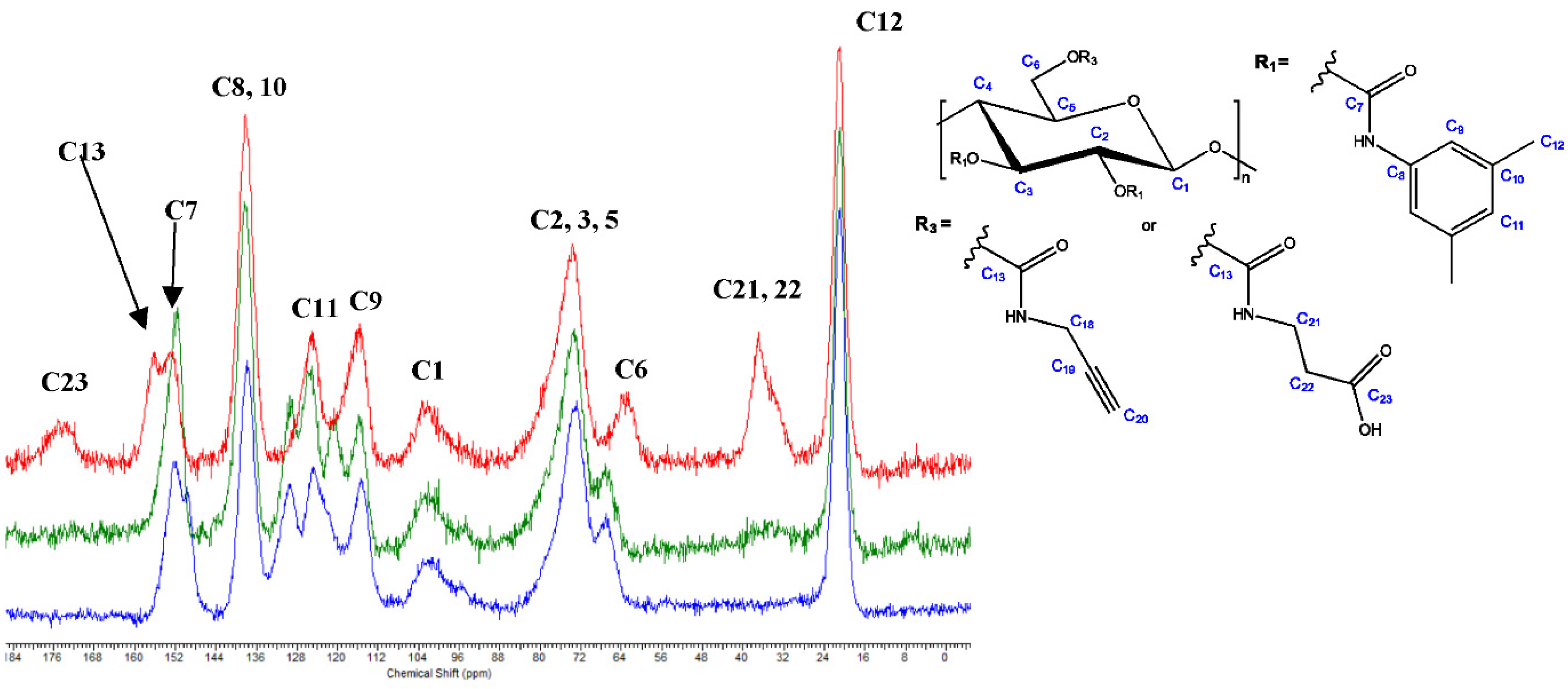
3.3. Aminolysis Procedures A–D
- Procedure A—Heterogeneous Comparison
- Procedure B—Silylation Followed by Aminolysis
- Procedure C—Two-Step, One-Pot Homogenous Reaction in DMF/[EMIM]OAc
- Procedure D—Two-Step, One-Pot Homogenous Reaction in DMF and DMF/[EMIM]OAc
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BSA | N,O-Bis-(trimethylsilyl)acetamide |
| CS | Chiral selector |
| DMAc | N,N-Dimethylacetamide |
| DMF | N,N-Dimethylformamide |
| DS | Degree of substitution |
| EA | Elemental analysis |
| [EMIM]OAc | 1-Ethyl-3-methylimidazolium acetate |
| EtOH | Ethanol |
| MeOH | Methanol |
| IL | Ionic liquid |
| RT | Room temperature |
| TBAF | tetra-n-Butylammonium fluoride hydrate |
References
- Heinze, T.; Liebert, T.; Koschella, A. Esterification of Polysaccharides; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Elschner, T.; Heinze, T. Cellulose carbonates: A platform for promising biopolymer derivatives with multifunctional capabilities. Macromol. Biosci. 2015, 15, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Hettegger, H.; Lindner, W.; Rosenau, T. Derivatized polysaccharides on silica and hybridized with silica in chromatography and separation—A mini review. In Recent Trends in Carbohydrate Chemistry: Synthesis, Structure and Function of Carbohydrates; Rauter, A.P., Christensen, B., Somsak, L., Kosma, P., Adamo, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Lüttringhaus, A.; Hess, U.; Rosenbaum, H.J.I. Mitt.: Optisch aktives 4.5. 6.7-Dibenzo-1.2-dithiacyclooctadien. Z. Für. Nat. B 1967, 22, 1296–1300. [Google Scholar] [CrossRef]
- Fanali, C.; D’Orazio, G.; Gentili, A.; Fanali, S. Analysis of Enantiomers in Products of Food Interest. Molecules 2019, 24, 1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minguillón, C.; Franco, P.; Oliveros, L.; López, P. Bonded cellulose-derived high-performance liquid chromatography chiral stationary phases I. Influence of the degree of fixation on selectivity. J. Chromatogr. A 1996, 728, 407–414. [Google Scholar] [CrossRef]
- Yin, C.; Chen, W.; Zhang, J.; Zhang, M.; Zhang, J. A facile and efficient method to fabricate high-resolution immobilized cellulose-based chiral stationary phases via thiol-ene click chemistry. Sep. Purif. Technol. 2019, 210, 175–181. [Google Scholar] [CrossRef]
- Ikai, T.; Okamoto, Y. Structure control of polysaccharide derivatives for efficient separation of enantiomers by chromatography. Chem. Rev. 2009, 109, 6077–6101. [Google Scholar] [CrossRef]
- Shen, J.; Okamoto, Y. Efficient separation of enantiomers using stereoregular chiral polymers. Chem. Rev. 2016, 116, 1094–1138. [Google Scholar] [CrossRef]
- Yin, C.; Zhang, J.; Chang, L.; Zhang, M.; Yang, T.; Zhang, X.; Zhang, J. Regioselectively substituted cellulose mixed esters synthesized by two-steps route to understand chiral recognition mechanism and fabricate high-performance chiral stationary phases. Anal. Chim. Acta 2019, 1073, 90–98. [Google Scholar] [CrossRef]
- Felix, G. Regioselectively modified polysaccharide derivatives as chiral stationary phases in high-performance liquid chromatography. J. Chromatogr. A 2001, 906, 171–184. [Google Scholar] [CrossRef]
- Acemoglu, M.; Küsters, E.; Baumann, J.; Hernandez, I.; Pong Mak, C. Synthesis of regioselectively substituted cellulose derivatives and applications in chiral chromatography. Chirality 1998, 10, 294–306. [Google Scholar] [CrossRef]
- Katoh, Y.; Tsujimoto, Y.; Yamamoto, C.; Ikai, T.; Kamigaito, M.; Okamoto, Y. Chiral recognition ability of cellulose derivatives bearing pyridyl and bipyridyl residues as chiral stationary phases for high-performance liquid chromatography. Polym. J. 2011, 43, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Chassaing, C.; Thienpont, A.; Félix, G. Regioselective carbamoylated and benzoylated cellulose for the separation of enantiomers in high-performance liquid chromatography. J. Chromatogr. A 1996, 738, 157–167. [Google Scholar] [CrossRef]
- Shen, J.; Wang, F.; Bi, W.; Liu, B.; Liu, S.; Okamoto, Y. Synthesis of cellulose carbamates bearing regioselective substituents at 2, 3-and 6-positions for efficient chromatographic enantioseparation. J. Chromatogr. A 2018, 1572, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Bragg, W.; Hou, J.; Lin, N.; Chandrasekaran, S.; Shamsi, S.A. Sulfated and sulfonated polysaccharide as chiral stationary phases for capillary electrochromatography and capillary electrochromatography–mass spectrometry. J. Chromatogr. A 2009, 1216, 857–872. [Google Scholar] [CrossRef] [PubMed]
- Ganske, K.; Heinze, T. Evaluation of the Synthesis of Soluble Aromatic Cellulose Carbonates of Low Degree of Substitution. Macromol. Chem. Phys. 2018, 219, 1800152. [Google Scholar] [CrossRef]
- Elschner, T.; Ganske, K.; Heinze, T. Synthesis and aminolysis of polysaccharide carbonates. Cellulose 2013, 20, 339–353. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Seidi, F.; Afjeh, S.S.; Nikoseresht, N.; Salimi, H.; Nemati, N. Synthesis of soluble N-functionalized polysaccharide derivatives using phenyl carbonate precursor and their application as catalysts. Starch-Stärke 2011, 63, 780–791. [Google Scholar] [CrossRef]
- Gericke, M.; Fardim, P.; Heinze, T. Ionic liquids—promising but challenging solvents for homogeneous derivatization of cellulose. Molecules 2012, 17, 7458–7502. [Google Scholar] [CrossRef] [Green Version]
- Schrems, M.; Brandt, A.; Welton, T.; Liebner, F.; Rosenau, T.; Potthast, A. Ionic liquids as media for biomass processing: Opportunities and restrictions. Holzforschung 2011, 65, 527–533. [Google Scholar] [CrossRef]
- Brandt, A.; Erickson, J.K.; Hallett, J.P.; Murphy, R.J.; Potthast, A.; Ray, M.J.; Rosenau, T.; Schrems, M.; Welton, T. Soaking of pine wood chips with ionic liquids for reduced energy input during grinding. Green Chem. 2012, 14, 1079–1085. [Google Scholar] [CrossRef] [Green Version]
- Stark, A. Ionic liquids in the biorefinery: A critical assessment of their potential. Energy Environ. Sci. 2011, 4, 19–32. [Google Scholar] [CrossRef]
- Mora-Pale, M.; Meli, L.; Doherty, T.V.; Linhardt, R.J.; Dordick, J.S. Room temperature ionic liquids as emerging solvents for the pretreatment of lignocellulosic biomass. Biotechnol. Bioeng. 2011, 108, 1229–1245. [Google Scholar] [CrossRef]
- Lopes, J.M.; Bermejo, M.D.; Martín, Á.; Cocero, M.J. Ionic liquid as reaction media for the production of cellulose-derived polymers from cellulosic biomass. ChemEngineering 2017, 1, 10. [Google Scholar] [CrossRef] [Green Version]
- Koide, M.; Wataoka, I.; Urakawa, H.; Kajiwara, K.; Henniges, U.; Rosenau, T. Intrinsic characteristics of cellulose dissolved in an ionic liquid: The shape of a single cellulose molecule in solution. Cellulose 2019, 26, 2233–2242. [Google Scholar] [CrossRef]
- Kosan, B.; Michels, C.; Meister, F. Dissolution and forming of cellulose with ionic liquids. Cellulose 2008, 15, 59–66. [Google Scholar] [CrossRef]
- Fukaya, Y.; Hayashi, K.; Wada, M.; Ohno, H. Cellulose dissolution with polar ionic liquids under mild conditions: Required factors for anions. Green Chem. 2008, 10, 44–46. [Google Scholar] [CrossRef]
- Pang, J.-H.; Liu, X.; Wu, M.; Wu, Y.-Y.; Zhang, X.-M.; Sun, R.-C. Fabrication and characterization of regenerated cellulose films using different ionic liquids. J. Spectrosc. 2014, 2014, 214057. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Li, H.; Zhang, Y.; Zhang, J.; He, J. Structure and properties of novel regenerated cellulose films prepared from cornhusk cellulose in room temperature ionic liquids. J. Appl. Polym. Sci. 2010, 116, 547–554. [Google Scholar] [CrossRef]
- Gericke, M.; Trygg, J.; Fardim, P. Functional cellulose beads: Preparation, characterization, and applications. Chem. Rev. 2013, 113, 4812–4836. [Google Scholar] [CrossRef]
- Stepan, A.M.; King, A.W.; Kakko, T.; Toriz, G.; Kilpeläinen, I.; Gatenholm, P. Fast and highly efficient acetylation of xylans in ionic liquid systems. Cellulose 2013, 20, 2813–2824. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Cao, Y.; Sang, S.; Zhang, J.; He, J. Synthesis of cellulose benzoates under homogeneous conditions in an ionic liquid. Cellulose 2009, 16, 299–308. [Google Scholar] [CrossRef]
- Peng, X.; Ren, J.; Zhong, L.; Sun, R. Homogeneous synthesis of hemicellulosic succinates with high degree of substitution in ionic liquid. Carbohydr. Polym. 2011, 86, 1768–1774. [Google Scholar] [CrossRef]
- Cao, Y.; Li, H.; Zhang, J. Homogeneous synthesis and characterization of cellulose acetate butyrate (CAB) in 1-allyl-3-methylimidazolium chloride (AmimCl) ionic liquid. Ind. Eng. Chem. Res. 2011, 50, 7808–7814. [Google Scholar] [CrossRef]
- Singh, R.K.; Gupta, P.; Sharma, O.P.; Ray, S.S. Homogeneous synthesis of cellulose fatty esters in ionic liquid (1-butyl-3-methylimidazolium chloride) and study of their comparative antifriction property. J. Ind. Eng. Chem. 2015, 24, 14–19. [Google Scholar] [CrossRef]
- Peng, X.; Ren, J.; Sun, R. Homogeneous esterification of xylan-rich hemicelluloses with maleic anhydride in ionic liquid. Biomacromolecules 2010, 11, 3519–3524. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, W.; Feng, Y.; Wu, J.; Yu, J.; He, J.; Zhang, J. Homogeneous esterification of cellulose in room temperature ionic liquids. Polym. Int. 2015, 64, 963–970. [Google Scholar] [CrossRef]
- Lacroix, C.; Sultan, E.; Fleury, E.; Charlot, A. Functional galactomannan platform from convenient esterification in imidazolium-based ionic liquids. Polym. Chem. 2012, 3, 538–546. [Google Scholar] [CrossRef]
- Zweckmair, T.; Hettegger, H.; Abushammala, H.; Bacher, M.; Potthast, A.; Laborie, M.-P.; Rosenau, T. On the mechanism of the unwanted acetylation of polysaccharides by 1, 3-dialkylimidazolium acetate ionic liquids: Part 1—Analysis, acetylating agent, influence of water, and mechanistic considerations. Cellulose 2015, 22, 3583–3596. [Google Scholar] [CrossRef]
- Mine, S.; Izawa, H.; Kaneko, Y.; Kadokawa, J. Acetylation of α-chitin in ionic liquids. Carbohydr. Res. 2009, 344, 2263–2265. [Google Scholar] [CrossRef]
- Ren, J.; Sun, R.; Liu, C.; Cao, Z.; Luo, W. Acetylation of wheat straw hemicelluloses in ionic liquid using iodine as a catalyst. Carbohydr. Polym. 2007, 70, 406–414. [Google Scholar] [CrossRef]
- Mormann, W.; Wezstein, M. Trimethylsilylation of cellulose in ionic liquids. Macromol. Biosci. 2009, 9, 369–375. [Google Scholar] [CrossRef]
- Gömez, J.A.C.; Erler, U.W.; Klemm, D.O. Physics. 4-methoxy substituted trityl groups in 6-O protection of cellulose: Homogeneous synthesis, characterization, detritylation. Macromol. Chem. 1996, 197, 953–964. [Google Scholar] [CrossRef]
- Gericke, M.; Liebert, T.; Heinze, T. Interaction of ionic liquids with polysaccharides, 8–synthesis of cellulose sulfates suitable for polyelectrolyte complex formation. Macromol. Biosci. 2009, 9, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Liebert, T.F.; Heinze, T.J. Exploitation of reactivity and selectivity in cellulose functionalization using unconventional media for the design of products showing new superstructures. Biomacromolecules 2001, 2, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Liebert, T.F.; Heinze, T. Tailored cellulose esters: Synthesis and structure determination. Biomacromolecules 2005, 6, 333–340. [Google Scholar] [CrossRef]
- Ebner, G.; Schiehser, S.; Potthast, A.; Rosenau, T. Side reaction of cellulose with common 1-alkyl-3-methylimidazolium-based ionic liquids. Tetrahedron Lett. 2008, 49, 7322–7324. [Google Scholar] [CrossRef]
- Liebner, F.; Patel, I.; Ebner, G.; Becker, E.; Horix, M.; Potthast, A.; Rosenau, T. Thermal aging of 1-alkyl-3-methylimidazolium ionic liquids and its effect on dissolved cellulose. Holzforschung 2010, 64, 161–166. [Google Scholar] [CrossRef]
- Böhmdorfer, S.; Hosoya, T.; Röder, T.; Potthast, A.; Rosenau, T. A cautionary note on thermal runaway reactions in mixtures of 1-alkyl-3-methylimidazolium ionic liquids and N-methylmorpholine-N-oxide. Cellulose 2017, 24, 1927–1932. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Bi, W.; Sun, M.; Wang, F.; Shen, J.; Okamoto, Y. Chiral recognition ability of amylose derivatives bearing regioselectively different carbamate pendants at 2, 3-and 6-positions. Carbohydr. Polym. 2019, 218, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Kaida, Y.; Okamoto, Y. Optical resolution on regioselectively carbamoylated cellulose and amylose with 3, 5-dimethylphenyl and 3, 5-dichlorophenyl isocyanates. Bull. Chem. Soc. Jpn. 1993, 66, 2225–2232. [Google Scholar] [CrossRef]
- Hoffmann, C.V.; Pell, R.; Lämmerhofer, M.; Lindner, W. Synergistic effects on enantioselectivity of zwitterionic chiral stationary phases for separations of chiral acids, bases, and amino acids by HPLC. Anal. Chem. 2008, 80, 8780–8789. [Google Scholar] [CrossRef] [PubMed]



| Calculated (wt%) | Found (wt%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | Cl | S | C | H | O | N | Cl | S | |
| 10 | 63.15 | 6.18 | 24.53 | 6.14 | - | - | 59.90 ± 0.07 | 6.27 ± 0.05 | 25.96 ± 0.50 | 5.66 ± 0.07 | - | - |
| 11b | 60.93 | 5.11 | 23.57 | 4.58 | 5.80 | - | 59.43 ± 0.14 | 4.86 ± 0.18 | 25.96 ± 0.50 | 4.28 ± 0.12 | 6.11 ± 0.05 | - |
| 11c | 64.57 | 5.59 | 24.97 | 4.86 | - | - | 61.27 ± 0.10 | 5.08 ± 0.14 | 25.16 ± 0.34 | 4.48 ± 0.04 | - | - |
| 13aB | 58.84 | 5.82 | 27.99 | 7.35 | - | - | 57.79 ± 0.05 | 5.98 ± 0.09 | 28.34 ± 0.09 | 5.56 ± 0.03 | - | - |
| 13aC | 59.96 ± 0.18 | 5.98 ± 0.12 | 26.39 ± 0.50 | 6.03 ± 0.05 | - | - | ||||||
| 13aD | 55.04 ± 0.19 | 5.56 ± 0.12 | 29.48 ± 0.64 | 6.81 ± 0.06 | - | - | ||||||
| 13bB | 53.37 | 5.47 | 28.96 | 6.92 | - | 5.28 | 59.89 ± 0.14 | 6.13 ± 0.03 | 26.01 ± 0.72 | 6.11 ± 0.02 | - | 0.08 ± 0.04 |
| 13bC | 49.75 ± 0.19 | 5.15 ± 0.03 | 23.48 ± 0.52 | 5.41 ± 0.03 | - | 1.03 ± 0.02 | ||||||
| 13bD | 46.58 ± 0.02 | 5.48 ± 0.08 | 32.70 ± 1.17 | 6.27 ± 0.03 | - | 4.86 ± 0.04 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, C.V.; Rosenau, T.; Hettegger, H. Synthesis of Polyanionic Cellulose Carbamates by Homogeneous Aminolysis in an Ionic Liquid/DMF Medium. Molecules 2022, 27, 1384. https://doi.org/10.3390/molecules27041384
Bui CV, Rosenau T, Hettegger H. Synthesis of Polyanionic Cellulose Carbamates by Homogeneous Aminolysis in an Ionic Liquid/DMF Medium. Molecules. 2022; 27(4):1384. https://doi.org/10.3390/molecules27041384
Chicago/Turabian StyleBui, Cuong Viet, Thomas Rosenau, and Hubert Hettegger. 2022. "Synthesis of Polyanionic Cellulose Carbamates by Homogeneous Aminolysis in an Ionic Liquid/DMF Medium" Molecules 27, no. 4: 1384. https://doi.org/10.3390/molecules27041384






