Ocimum basilicum L. Methanol Extract Enhances Mitochondrial Efficiency and Decreases Adipokine Levels in Maturing Adipocytes Which Regulate Macrophage Systemic Inflammation
Abstract
:1. Introduction
2. Materials and Method
2.1. Chemicals and Cell Culture
Plant Material
2.2. Preparation of Basil Seed Hexane (Non-Polar) and Methanol (Polar) Extract
2.3. Gas Chromatography and Mass Spectroscopy Analysis
2.4. Isolation of Monocytes from Whole Blood and Bacterial LPS-Stimulated Macrophage Formation
2.5. Differentiation of Human Mesenchymal Stem Cells into Preadipocytes
2.6. Cytotoxicity Assay
2.7. Experimental Design
2.8. Light (Oil Red O) and Fluorescent (Nile Red) Staining Analysis for Lipid Accumulation
2.9. Mitochondrial Membrane Potential (JC-1 Staining) Assay
2.10. Biochemical Parameters Analysis
2.11. Quantitative Polymerase Chain Reaction (qPCR) Analysis
2.12. Quantification of Protein Using ELISA
2.13. Statistical Analysis
3. Results
3.1. Identification of Phytochemicals by Gas Chromatography–Mass Spectrometry
3.2. Effect of Basil Seed Methanol Extract on Adipocyte and Macrophage Viability
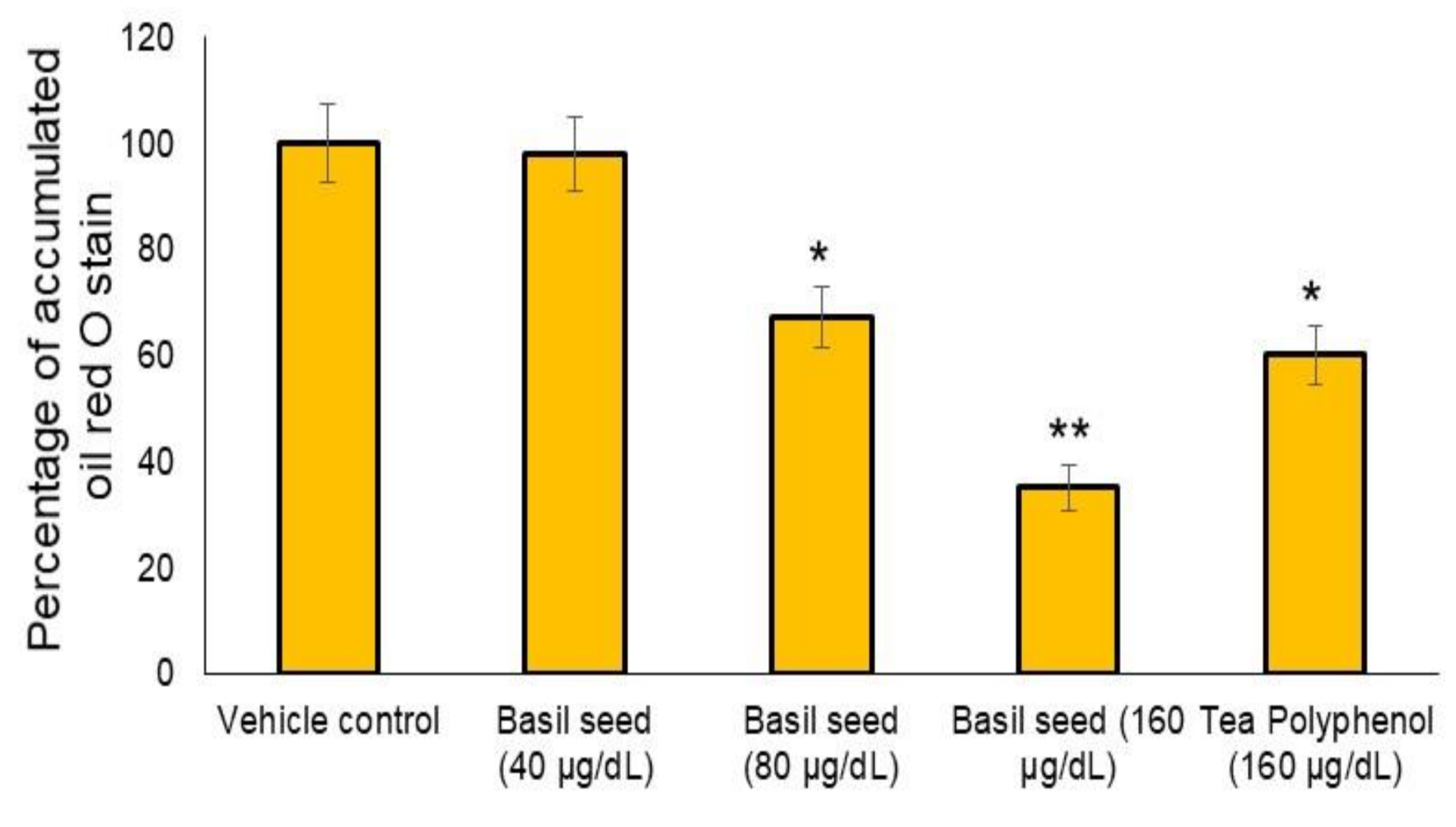
3.3. Dose Determination Based on Lipid Accumulation Inhibitory Potential Using Oil Red’ O Staining Analysis
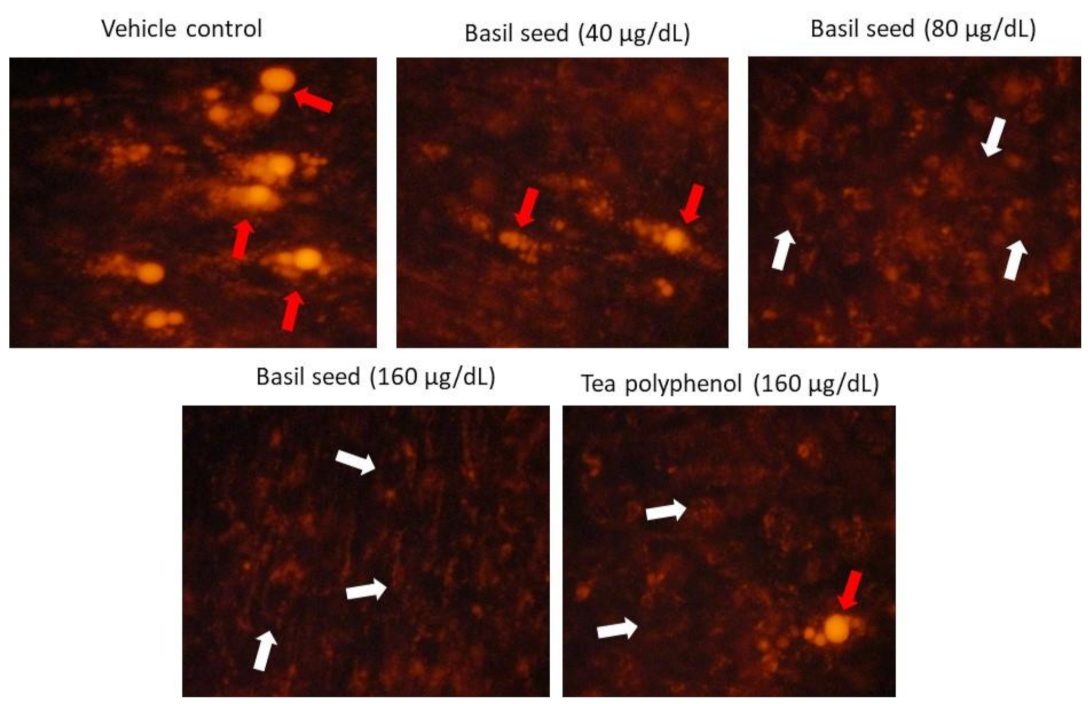
3.4. Determination of Lipid Accumulation in Adipocyte Using Nile Red Fluorescence Staining
3.5. Activity of Glycerol Phosphate Dehydrogenase, LDH and Level of Triglyceride in Vehicle Control and BSME-Treated Adipocyte after 14 Days
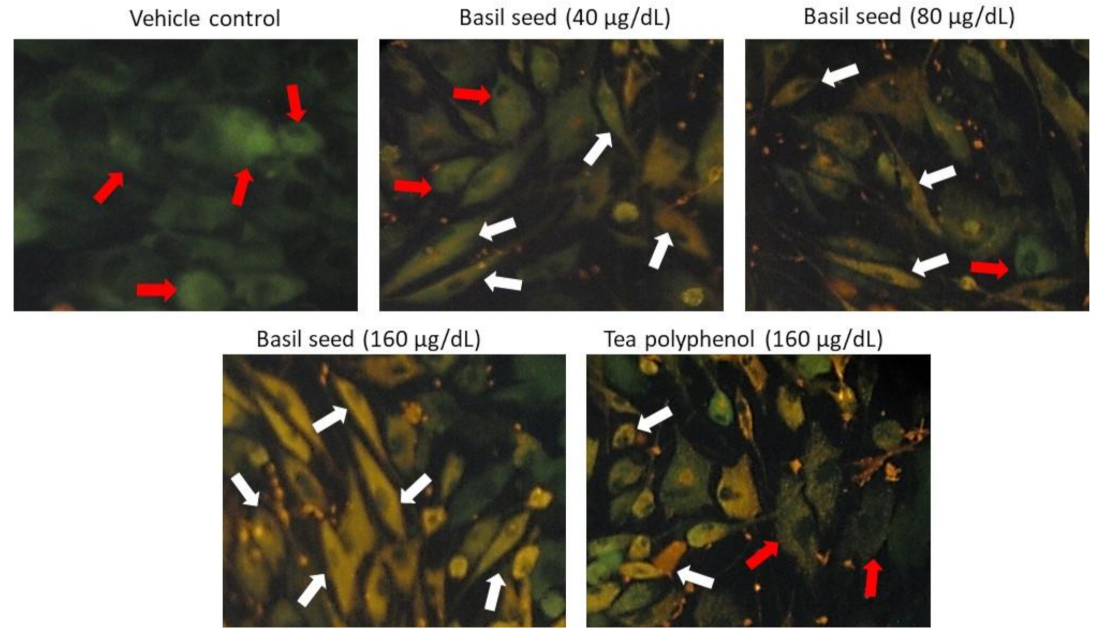
3.6. Mitochondrial Membrane Potential (JC-1) and Oxidative Efficiency Analysis
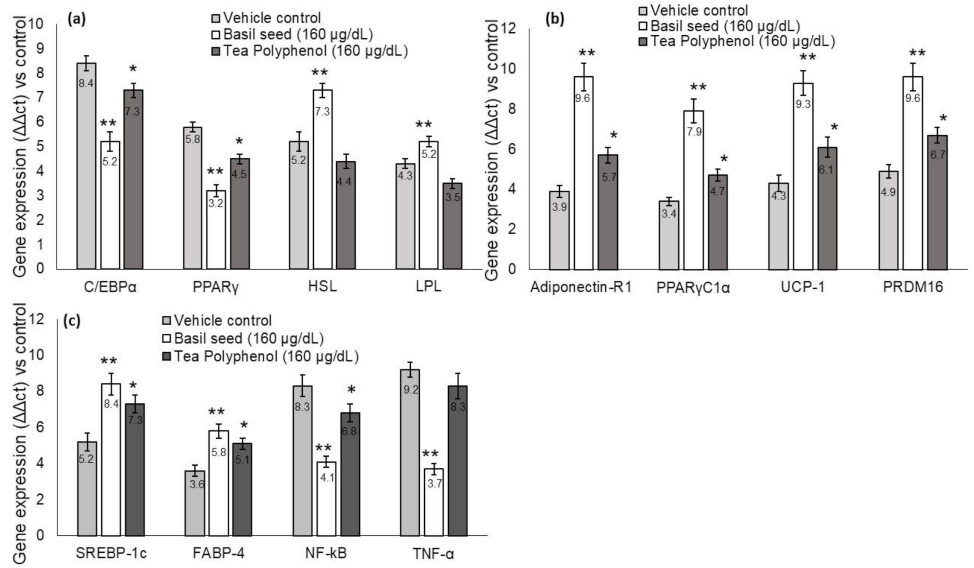
3.7. Quantification of Adipogenesis, Mitochondrial Thermogenesis and Inflammation-Related Gene Expression Levels in BSME-Treated Adipocytes
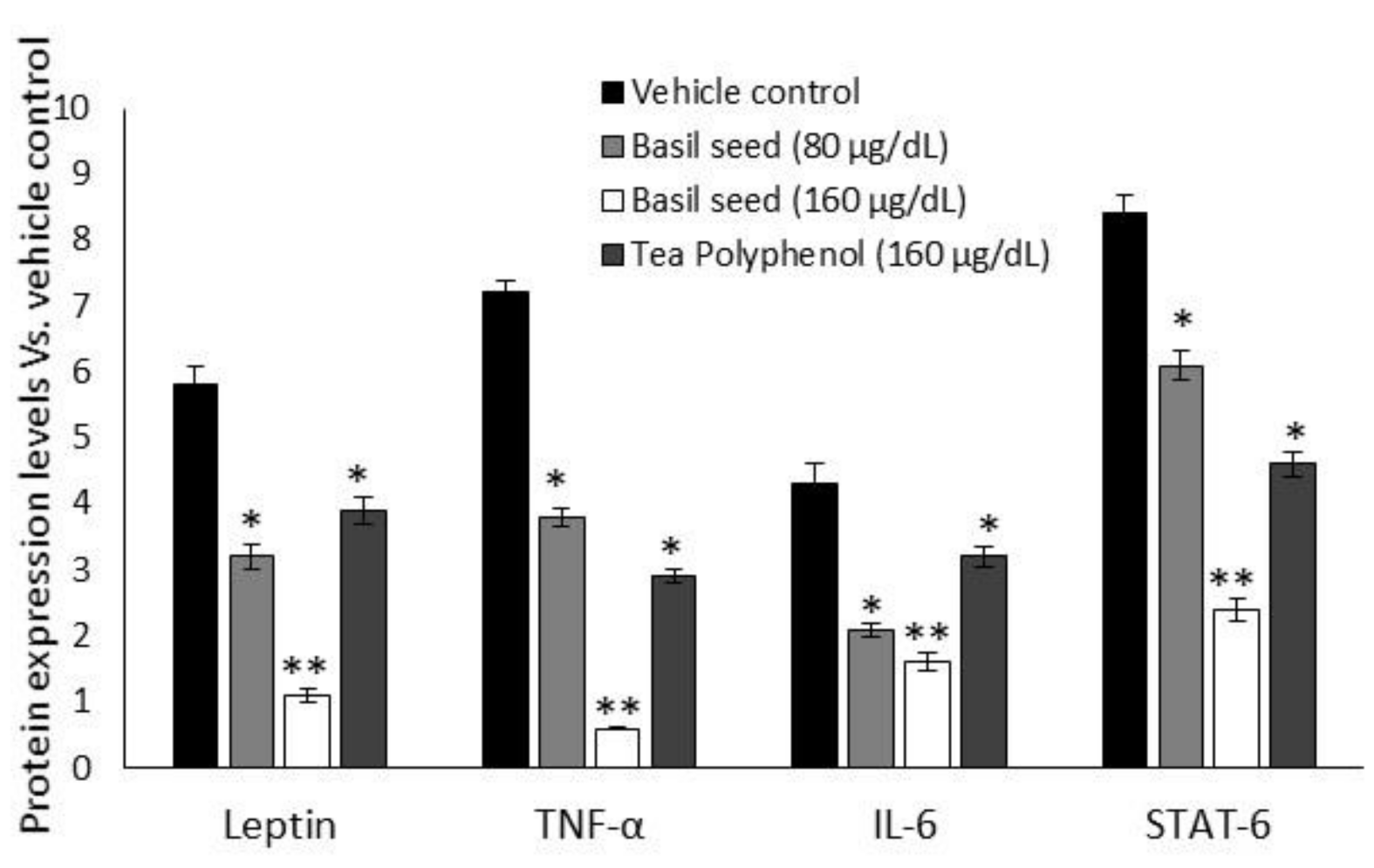
3.8. Intracellular Protein Levels in BSME-Treated Adipocyte after 14 Days
3.9. Results for Macrophage Treated with “BSME-Treated Adipocyte Condition Media”
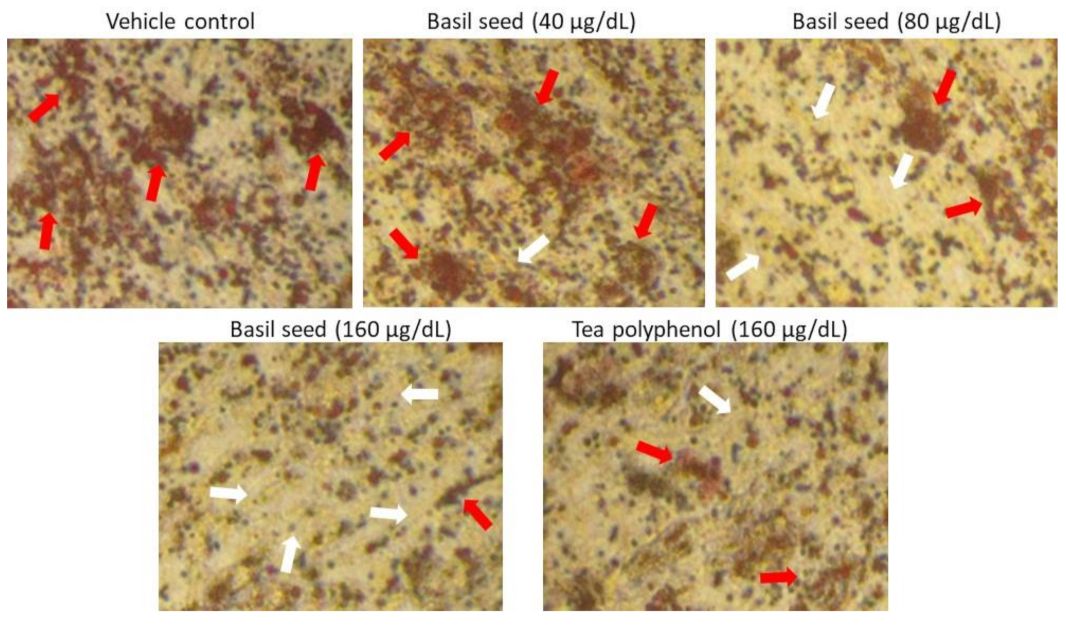
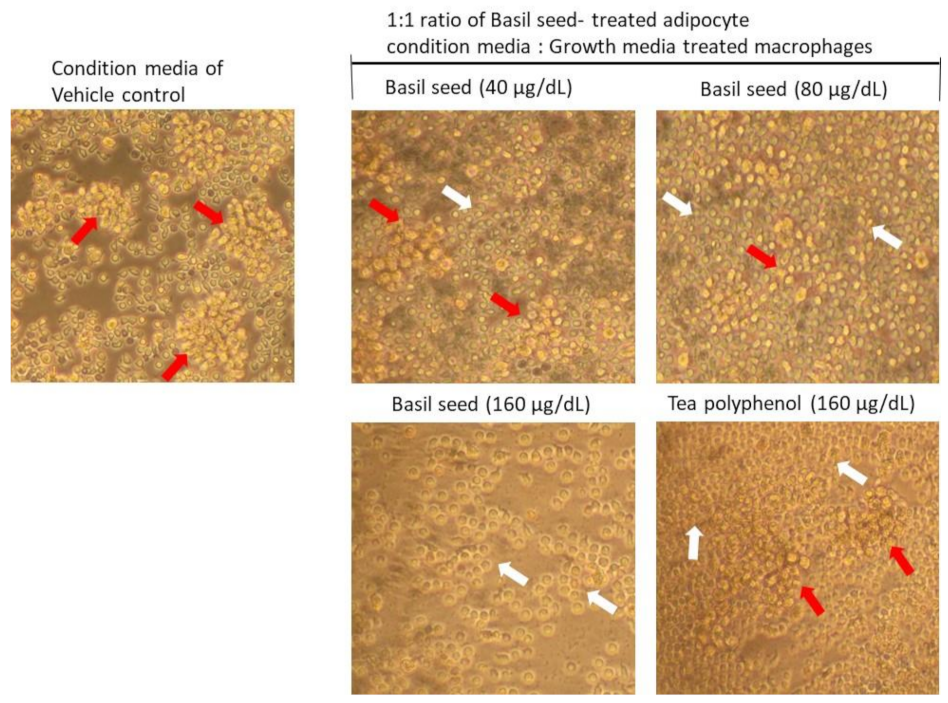
3.9.1. Determination of Lipid Accumulation and Foam Cell Development in Macrophage Using Oil Red O Staining
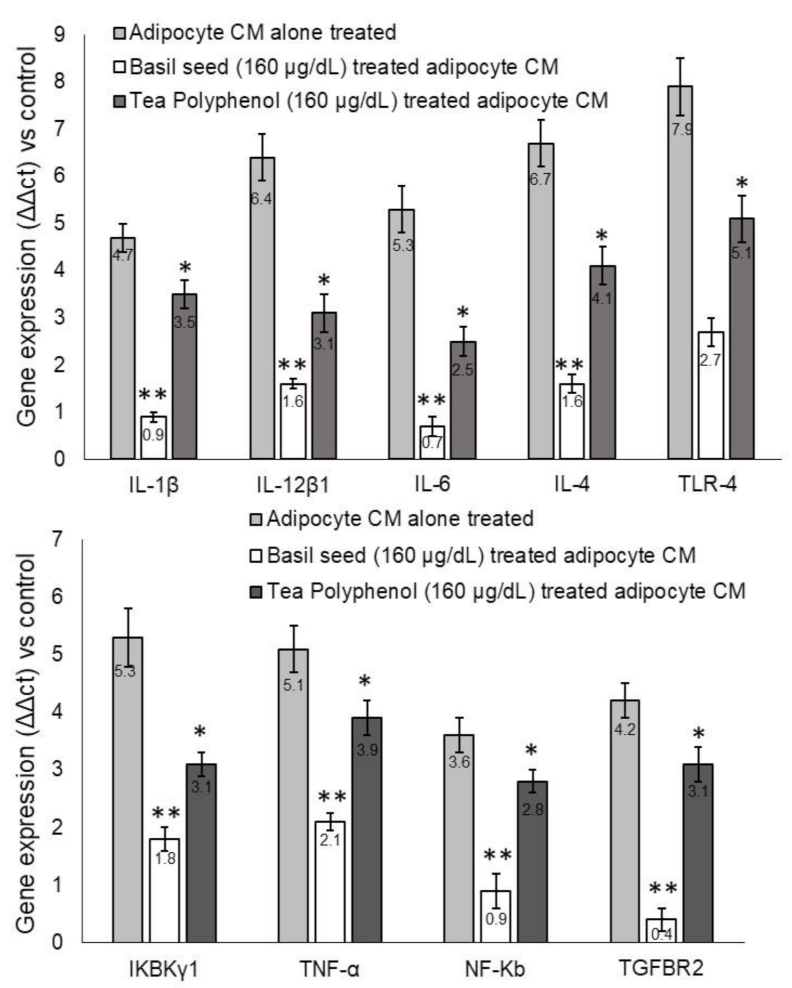
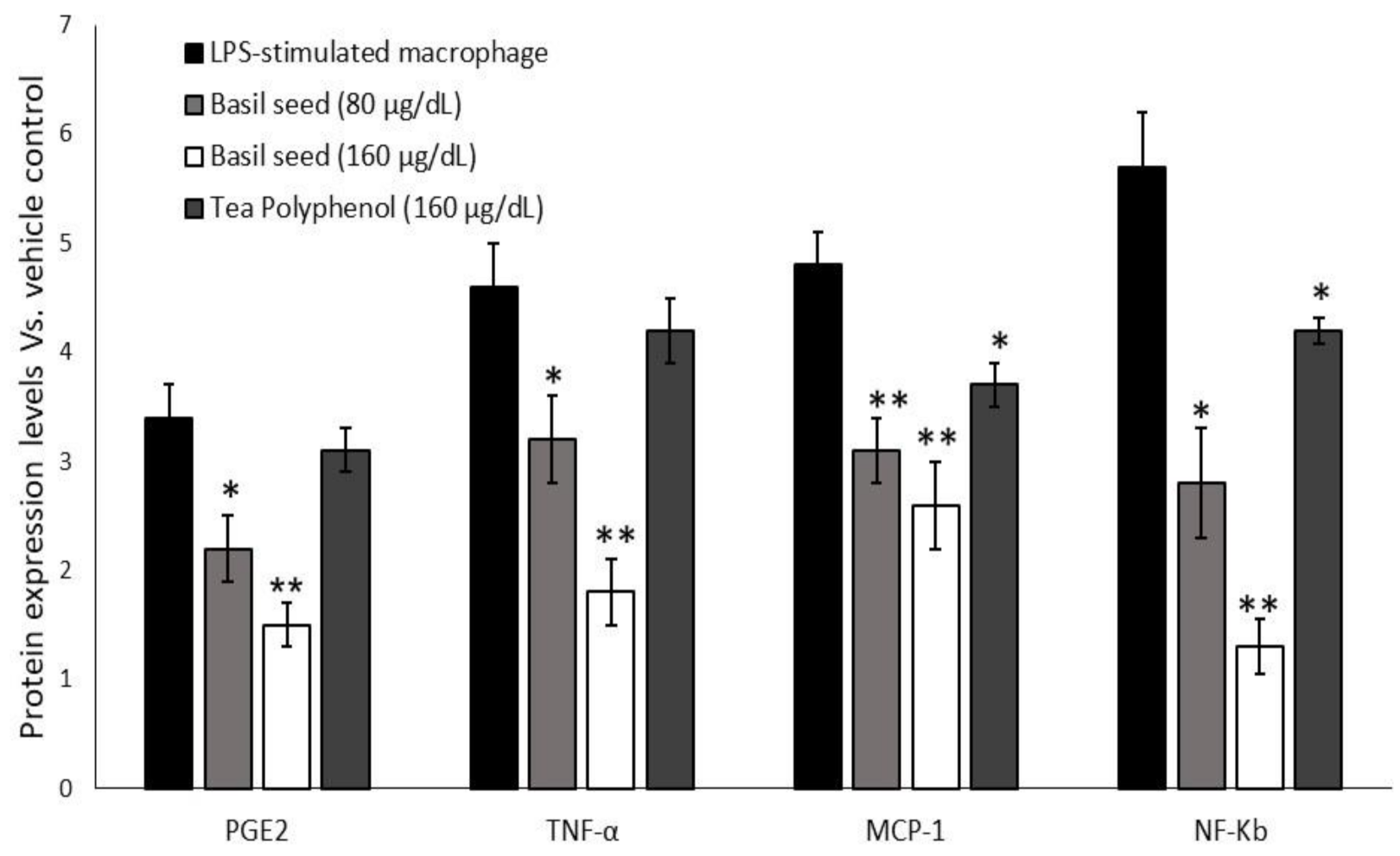
3.9.2. Metabolic-Inflammation-related Gene Expression Levels in Macrophage after Treatment with BSME-Treated Adipocyte Condition Media
3.9.3. Quantification of Intracellular Protein Levels in Macrophage after Treatment with “BSME-Treated Adipocyte Condition Media”
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; de Mejia, E.G. Cocoa Shell Aqueous Phenolic Extract Preserves Mitochondrial Function and Insulin Sensitivity by Attenuating Inflammation between Macrophages and Adipocytes In Vitro. Mol. Nutr. Food Res. 2019, 63, e1801413. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.P.; Nam, S.H.; Friedman, M. Mechanism of the antiadipogenic-antiobesity effects of a rice hull smoke extract in 3T3-L1 preadipocyte cells and in mice on a high-fat diet. Food Funct. 2015, 6, 2939–2948. [Google Scholar] [CrossRef] [PubMed]
- Hammarstedt, A.; Gogg, S.; Hedjazifar, S.; Nerstedt, A.; Smith, U. Impaired adipogenesis and dysfunctional adipose tissue in human hypertrophic obesity. Physiol. Rev. 2018, 98, 1911–1941. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Park, J.; Choi, J.H. Revisiting PPARγ as a target for the treatment of metabolic disorders. BMB Rep. 2014, 47, 599–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meissburger, B.; Ukropec, J.; Roeder, E.; Beaton, N.; Geiger, M.; Teupser, D.; Civan, B.; Langhans, W.; Nawroth, P.P.; Gasperikova, D.; et al. Adipogenesis and insulin sensitivity in obesity are regulated by retinoid-related orphan receptor gamma. EMBO Mol. Med. 2011, 3, 637–651. [Google Scholar] [CrossRef]
- Neeland, I.J.; Turer, A.T.; Ayers, C.R.; Berry, J.D.; Rohatgi, A.; Das, S.R.; Khera, A.; Vega, G.L.; McGuire, D.K.; Grundy, S.M.; et al. Body fat distribution and incident cardiovascular disease in obese adults. J. Am. Coll. Cardiol. 2015, 65, 2150–2151. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Wang, N.L.; Sun, B.; Cai, G.P. Estrogen receptor mediates the effects of pseudoprotodiocsin on adipogenesis in 3T3-L1 cells. Am. J. Physiol. Cell Physiol. 2010, 299, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, T.; Church, C.; Baker, D.J.; Simon, W.J. The role of adipokines in skeletal muscle inflammation and insulin sensitivity. J. Inflamm. 2018, 15, 9. [Google Scholar] [CrossRef] [Green Version]
- Mancuso, P. The role of adipokines in chronic inflammation. Immunotargets Ther. 2016, 23, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; Lanza, I.R.; Swain, J.M.; Sarr, M.G.; Nair, K.S.; Jensen, M.D. Adipocyte mitochondrial function is reduced in human obesity independent of fat cell size. J. Clin. Endocrinol. Metab. 2014, 99, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Brand, M.D.; Orr, A.L.; Perevoshchikova, I.V.; Quinlan, C.L. The role of mitochondrial function and cellular bioenergetics in ageing and disease. Br. J. Dermatol. 2013, 169, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louet, J.F.; Chatelain, F.; Decaux, J.F.; Park, E.A.; Kohl, C.; Pineau, T.; Girard, J.; Pegorier, J.P. Long-chain fatty acids regulate liver carnitine palmitoyltransferase I gene (L-CPT I) expression through a peroxisome-proliferator-activated receptor alpha (PPARalpha)-independent pathway. Biochem. J. 2001, 354, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS crosstalk in inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxidative Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [Green Version]
- Lumeng, C.N.; Deyoung, S.M.; Bodzin, J.L.; Saltiel, A.R. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 2007, 56, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, H.; Takahashi-Muto, C.; Nagase, M.; Kassai, M.; Tanaka-Yachi, R.; Kiyose, C. Anti-inflammatory effects of extracts of sweet basil (Ocimum basilicum L.) on a co-culture of 3T3-L1 adipocytes and RAW264.7 macrophages. J. Oleo Sci. 2020, 69, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Noor, Z.I.; Ahmed, D.; Rehman, H.M.; Qamar, M.T.; Froeyen, M.; Ahmad, S.; Mirza, M.U. In Vitro antidiabetic, anti-obesity and antioxidant analysis of Ocimum basilicum aerial biomass and in silico molecular docking simulations with alpha-amylase and lipase enzymes. Biology 2019, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Calderón Bravo, H.; Vera Céspedes, N.; Zura-Bravo, L.; Muñoz, L.A. Basil seeds as a novel food, source of nutrients and functional ingredients with beneficial properties: A Review. Foods 2021, 10, 1467. [Google Scholar] [CrossRef]
- Subash-Babu, P.; Al-Maiman, S.A.; Al-Harbi, L.N.; Alshatwi, A.A. Beneficial fatty acid ratio of Salvia hispanica L. (Chia Seed) potentially inhibits adipocyte hypertrophy, and decreases adipokines expression and inflammation in macrophage. Foods 2020, 9, 368. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, G.H.; Shim, S.M. Different effect of methanol extracts and bioaccessible fraction of S. milax china on triglyceride accumulation in adipocytes. J. Food Biochem. 2014, 38, 1–5. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.K.; Kwon, D.Y.; Park, R. Anti-adipogenic effects of Garcinia extract on the lipid droplet accumulation and the expression of transcription factor. Biofactors 2004, 22, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. An. Biochem. 1967, 72, 248–254. [Google Scholar] [CrossRef]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7, 85. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.Y. Analysis of variance (ANOVA) comparing means of more than two groups. Restor. Dent. Endod. 2014, 39, 74–77. [Google Scholar] [CrossRef] [Green Version]
- Filip, S. Basil (Ocimum basilicum L.) a source of valuable phytonutrients. Int. J. Clin. Nutr. Diet. 2017, 3, 118. [Google Scholar] [CrossRef] [Green Version]
- Vieira, C.; Fetzer, S.; Sauer, S.K.; Evangelista, S.; Averbeck, B.; Kress, M.; Reeh, P.W.; Cirillo, A.L.; Maggi, C.A.; Manzini, S. Pro-and anti-inflammatory actions of ricinoleic acid: Similarities and differences with capsaicin. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2001, 364, 87–95. [Google Scholar] [CrossRef]
- Yu, Z.; Guo, W.; Ma, X.; Zhang, B.; Dong, P.; Huang, L.; Wang, X.; Wang, C.; Huo, X.; Yu, W.; et al. Gamabufotalin, a bufadienolide compound from toad venom, suppresses COX-2 expression through targeting IKKβ/NF-κB signaling pathway in lung cancer cells. Mol. Cancer 2014, 13, 203. [Google Scholar] [CrossRef] [Green Version]
- Ben-Chetrit, E.; Bergmann, S.; Sood, R. Mechanism of the anti-inflammatory effect of colchicine in rheumatic diseases: A possible new outlook through microarray analysis. Rheumatology 2006, 45, 274–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robroeks, C.M.; Van de Kant, K.D.; Van Vliet, D.; Kester, A.D.; Hendriks, H.J.; Damoiseaux, J.G.; Wodzig, W.K.W.H.; Rijkers, G.T.; Dompeling, E.; Jobsis, Q. Comparison of the anti-inflammatory effects of extra-fine hydrofluoroalkane-beclomethasone Vs fluticasone dry powder inhaler on exhaled inflammatory markers in childhood asthma. Ann. Allergy Asthma Immunol. 2008, 100, 601–607. [Google Scholar] [CrossRef]
- Yan, S.X.; Deng, X.M.; Wang, Q.T.; Sun, X.J.; Wei, W. Prednisone treatment inhibits the differentiation of B lymphocytes into plasma cells in MRL/MpSlac-lpr mice. Acta Pharmacol. Sin. 2015, 36, 1367–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciccone, M.M.; Cortese, F.; Gesualdo, M.; Carbonara, S.; Zito, A.; Ricci, G.; De Pascalis, F.; Scicchitano, P.; Riccioni, G. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediat. Inflamm. 2013, 2013, 782137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maccarrone, M.; Gubellini, P.; Bari, M.; Picconi, B.; Battista, N.; Centonze, D.; Bernardi, G.; Finazzi-Agrò, A.; Calabresi, P. Levodopa treatment reverses endocannabinoid system abnormalities in experimental parkinsonism. J. Neurochem. 2003, 85, 1018–1025. [Google Scholar] [CrossRef]
- Botella-Carretero, J.I.; Balsa, J.A.; Vázquez, C.; Peromingo, R.; Díaz-Enriquez, M.; Escobar-Morreale, H.F. Retinol and α-tocopherol in morbid obesity and nonalcoholic fatty liver disease. Obes. Surg. 2010, 20, 69–76. [Google Scholar] [CrossRef]
- Huang, C.H.; Kuo, H.S.; Liu, J.W.; Lin, Y.L. Synthesis and antitumor evaluation of novel bis-triaziquone derivatives. Molecules 2009, 14, 2306–2316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fayed, A.H.A. Brain trace element concentration of rats treated with the plant alkaloid, vincamine. Biol. Trace Elem. Res. 2010, 136, 314–319. [Google Scholar] [CrossRef]
- Baliga, M.S.; Jimmy, R.; Thilakchand, K.R.; Sunitha, V.; Bhat, N.R.; Saldanha, E.; Rao, S.; Arora, R.; Palatty, P.L. Ocimum sanctum L (Holy Basil or Tulsi) and its phytochemicals in the prevention and treatment of cancer. Nutr. Cancer 2013, 65, 26–35. [Google Scholar] [CrossRef]
- Bucktowar, K.; Bucktowar, M.; Bholoa, L.D. A review on sweet basil seeds: Ocimum basilicum. World J. Pharm. Pharm. Sci. 2016, 5, 554–567. [Google Scholar]
- Yang, Y.; Wang, J.; Zhang, Y.; Li, J.; Sun, W. Black sesame seeds ethanol extract ameliorates hepatic lipid accumulation, oxidative stress, and insulin resistance in fructose-induced nonalcoholic fatty liver disease. J. Agric. Food Chem. 2018, 66, 10458–10469. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, L.; Li, B.; Jiang, H.; Duan, Y.; Xie, Z.; Shuai, L.; Li, J.; Li, J. AMP-activated protein kinase (AMPK) regulates energy metabolism through modulating thermogenesis in adipose tissue. Front. Physiol. 2018, 21, 122. [Google Scholar] [CrossRef] [Green Version]
- Laura, L.G.; Stuart, A.M.; Jeremy, W.T. Hormonal Regulation of Lipogenesis. Vitam. Horm. 2013, 91, 1–27. [Google Scholar]
- Luo, J.; Qi, J.; Wang, W.; Luo, Z.; Liu, L.; Zhang, G.; Zhou, Q.; Liu, J.; Peng, X. Antiobesity effect of flaxseed polysaccharide via inducing satiety due to leptin resistance removal and promoting lipid metabolism through the AMP-activated protein kinase (AMPK) signaling pathway. J. Agric. Food Chem. 2019, 67, 7040–7049. [Google Scholar] [CrossRef] [PubMed]
- Berndt, J.; Kralisch, S.; Klöting, N.; Ruschke, K.; Kern, M.; Fasshauer, M.; Schön, M.R.; Stumvoll, M.; Blüher, M. Adipose triglyceride lipase gene expression in human visceral obesity. Exp. Clin. Endocrinol. Diabetes 2008, 116, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, K.; Hui, X.; Kong, X.; Sweeney, G.; Wang, Y.; Xu, A.; Teng, M.; Liu, P.; Wu, D. Carnitine palmitoyltransferase 1A prevents fatty acid-induced adipocyte dysfunction through suppression of c-Jun N-terminal kinase. Biochem. J. 2011, 435, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.; Mera, P.; Malandrino, M.I.; Mir, J.F.; Herrero, L. Mitochondrial fatty acid oxidation in obesity. Antioxid. Redox Signal. 2013, 9, 269–284. [Google Scholar] [CrossRef] [Green Version]
- Chani, E.M.M.; Pacheco, S.O.S.; Martínez, G.A.; Freitas, M.R.; Ivona, J.G.; Ivona, J.A.; Craig, W.J.; Pacheco, F.J. Long-Term dietary intake of chia seed is associated with increased bone mineral content and improved hepatic and intestinal morphology in Sprague-Dawley Rats. Nutrients 2018, 10, 922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppack, S.W. Pro-inflammatory cytokines and adipose tissue. Proc. Nutr. Soc. 2001, 60, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Fève, B.; Bastard, J.P. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2009, 1, 305–311. [Google Scholar] [CrossRef]
- Vozarova, B.; Weyer, C.; Hanson, K.; Tataranni, P.A.; Bogardus, C.; Pratley, R.E. Circulating interleukin-6 in relation to adiposity P.A., insulin action, and insulin secretion. Obes. Res. 2001, 9, 414–417. [Google Scholar] [CrossRef]
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose tissue in obesity-related inflammation and insulin resistance: Cells, cytokines, and chemokines. ISRN Inflamm. 2013, 2013, 139239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrêa, L.H.; Corrêa, R.; Farinasso, C.M.; de Sant’Ana, D.L.P.; Magalhães, K.G. Adipocytes and Macrophages Interplay in the Orchestration of Tumor Microenvironment: New Implications in Cancer Progression. Front. Immunol. 2017, 19, 1129. [Google Scholar] [CrossRef] [PubMed]
- Plomgaard, P.; Bouzakri, K.; Krogh-Madsen, R.; Mittendorfer, B.; Zierath, J.R.; Pedersen, B.K. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 2005, 54, 2939–2945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Deyoung, S.M.; Saltiel, A.R. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am. J. Physiol. -Endocrinol. Metab. 2007, 292, 166–174. [Google Scholar] [CrossRef] [Green Version]


| Peak No. | List of Compounds | Molecular Formula | Molecular Weight | Retention Time | Reported Biological Activity |
|---|---|---|---|---|---|
| 1 | Ricinoleic acid | C18H34O3 | 298 | 51.979 | Anti-inflammatory [27] |
| 2 | Gamabufotalin | C24H34O5 | 402 | 52.658 | Anti-inflammatory [28] |
| 3 | Colchicine | C22H25NO6 | 399 | 53.938 | Anti-inflammatory [29] |
| 4 | Beclomethasone | C22H29CIO5 | 408 | 53.938 | Anti-inflammatory [30] |
| 5 | Prednisone | C21H26O5 | 358 | 56.624 | Anti-inflammatory [31] |
| 6 | ß Carotene | C40H56 | 536 | 57.838 | Antioxidant and anti-inflammatory [32] |
| 7 | Levodopa | C9H11NO4 | 197 | 57.838 | Anti-inflammatory [33] |
| 8 | Retinol | C20H30O | 286 | 59.240 | Anti-obesity [34] |
| 9 | Triaziquone | C12H13N3O2 | 231 | 61.136 | Anti-inflammatory, Anti-cancer [35] |
| 10 | Retinyl acetate | C22H32O2 | 328 | 69.637 | Anti-proliferation [34] |
| 11 | Vincamine | C21H26N2O3 | 354 | 71.044 | Anti-aging, Anti-inflammatory [36] |
| Groups | Glycerol Phosphate Dehydrogenase (Unit */mg Protein) | Triglyceride (mg/dL) | Lactate Dehydrogenase † |
|---|---|---|---|
| Vehicle Control | 105.98 ± 44.56 | 5.29 ± 1.74 | 0.18 ± 0.01 |
| BSME (80 µg/dL) | 54.6 ± 6.43 * | 3.65 ± 1.05 * | 0.11 ± 0.01 * |
| BSME (160 µg/dL) | 25.7 ± 2.47 ** | 3.24 ± 1.09 ** | 0.10 ± 0.01 ** |
| Tea Polyphenol (160 µg/dL) | 88.7 ± 7.26 * | 3.85 ± 1.16 * | 0.13 ± 0.02 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subash-Babu, P.; Mohammed Alowaidh, H.; Al-Harbi, L.N.; Shamlan, G.; Aloud, A.A.; AlSedairy, S.A.; Alshatwi, A.A. Ocimum basilicum L. Methanol Extract Enhances Mitochondrial Efficiency and Decreases Adipokine Levels in Maturing Adipocytes Which Regulate Macrophage Systemic Inflammation. Molecules 2022, 27, 1388. https://doi.org/10.3390/molecules27041388
Subash-Babu P, Mohammed Alowaidh H, Al-Harbi LN, Shamlan G, Aloud AA, AlSedairy SA, Alshatwi AA. Ocimum basilicum L. Methanol Extract Enhances Mitochondrial Efficiency and Decreases Adipokine Levels in Maturing Adipocytes Which Regulate Macrophage Systemic Inflammation. Molecules. 2022; 27(4):1388. https://doi.org/10.3390/molecules27041388
Chicago/Turabian StyleSubash-Babu, Pandurangan, Hussah Mohammed Alowaidh, Laila Naif Al-Harbi, Ghalia Shamlan, Amal A. Aloud, Sahar Abdulaziz AlSedairy, and Ali Abdullah Alshatwi. 2022. "Ocimum basilicum L. Methanol Extract Enhances Mitochondrial Efficiency and Decreases Adipokine Levels in Maturing Adipocytes Which Regulate Macrophage Systemic Inflammation" Molecules 27, no. 4: 1388. https://doi.org/10.3390/molecules27041388
APA StyleSubash-Babu, P., Mohammed Alowaidh, H., Al-Harbi, L. N., Shamlan, G., Aloud, A. A., AlSedairy, S. A., & Alshatwi, A. A. (2022). Ocimum basilicum L. Methanol Extract Enhances Mitochondrial Efficiency and Decreases Adipokine Levels in Maturing Adipocytes Which Regulate Macrophage Systemic Inflammation. Molecules, 27(4), 1388. https://doi.org/10.3390/molecules27041388







