Recovery Techniques Enabling Circular Chemistry from Wastewater
Abstract
1. Introduction
2. Volatile Fatty Acids (VFAs) from Wastewater
2.1. Recovery of the VFA from Fermentation Broth
2.1.1. Liquid-Liquid Extraction for Recovery of the VFAs
2.1.2. VFAs Recovery via Adsorption-Desorption Technique
Activated Carbon
Synthetic Polyaromatic Resins
2.1.3. Functionalized Synthetic Polyaromatic Resins
Anion Exchange Resins
2.1.4. Comparative Assessment of Various Desorption Techniques
3. Medium-Chain Carboxylic Acid from Wastewater
3.1. Recovery of Bio-Based Medium-Chain Carboxylic Acids
Membrane-Based Liquid-Liquid Extraction
3.2. Adsorption Using Anion Exchange Resins
3.3. Considerations on MCCA Recovery Techniques
4. Long-Chain Dicarboxylic Acids from Wastewater
5. Unsaturated Short-Chain Carboxylic Acids from Wastewater
5.1. Crotonic Acid
5.2. Polyhydroxyalkanoates Production from Waste/Wastewater
5.3. Crotonic Acid Synthesis by PHB/PHBV Pyrolysis
5.4. Purification of the Crotonic Acid Obtained by PHB/PHBV Pyrolysis
6. Extracellular Polymeric Substances (EPS)
6.1. Production of EPS from Wastewater
6.2. Concentration and Fractionation Techniques for EPS
6.2.1. Solvent Precipitation
6.2.2. Ultrafiltration
| Membrane Technology | Target Biopolymer | Matrix | Membrane Features | Key Findings | Ref. |
|---|---|---|---|---|---|
| Ultrafiltration (diafiltration) | Polysaccharide | Culture broth (Porphyridium cruentum) | Polyethersulfone Flat sheet MWCO: 300 kDa | 64% of the polysaccharide recovered in the permeate Final purity in retentate fraction: 57% Purity 10-fold higher than initial | [167] |
| Ultrafiltration | Polysaccharide | Culture broth (Sphingomonas pituitose) | Polyethersulfone Hollow fiber MWCO: 55 kDa | Stream was concentrated by 5-fold 90% of polysaccharides in the retentate fraction | [168] |
| Ultrafiltration | Polysaccharide | Synthetic solution | Polysulfone Hollow fiber MWCO: 6 kDa | 56% of arabinoxylan was in the retentate fraction, and stream was concentrated by 2-fold 76% of rhamnogalacturonan was in the retentate, and stream was concentrated by 5-fold | [183] |
| Ultrafiltration | Polysaccharide Protein | Culture broth (Spirulina platensis) | Polyether sulfone Flat sheet MWCO: 5 kDa | Concentration factor was 40-fold 0.2 g/L EPS solution was obtained in the retentate | [184] |
| Ultrafiltration | Protein | Poultry processing wastewater | Polysulfone Flat sheet MWCO: 30 kDa | Stream was concentrated by 3-fold 100% recovery of protein in the retentate Loss of protein in retentate due to fouling | [135] |
| Ultrafiltration | Protein | Poultry processing wastewater | Regenerated cellulose Spiral-wound MWCO: 30 kDa | Stream was concentrated by 7-fold concentration 100% recovery of protein in the retentate | [181] |
| Ultrafiltration | Protein | Fermentation broth (Ethanol production from corn) | Regenerated cellulose Flat sheet MWCO: 5 kDa | Concentration factor was 2-fold 80% of protein was in retentate 2-fold purification factor | [185] |
6.2.3. Aqueous Two-Phase Systems (ATPS)
Polymer/Polymer ATPS
Polymer/Salt ATPS
Alcohol/Salt ATPS
Ionic Liquid (IL)-Based ATPS
Complex Coacervates
6.2.4. Back-Extraction and Recycling of Compounds
| ATPS | Target | Composition wt% | Matrix | Key Findings | Ref. |
|---|---|---|---|---|---|
| Polymer/polymer | Protein (amyloglucosidase) | PEG 4000: 13% NaPA 15000: 11% | Synthetic solution | 86% of the enzyme was present in PEG-rich phase Improvement on purity by a factor of 2 | [223] |
| Polymer/polymer | Protein (interferon α-2b) | PEG 600: 30% PPG 400: 30% | Culture broth (Escherichia coli) | 90% of the protein in the PEG-rich phase Improvement on purity by a factor of 2 | [224] |
| Polymer/salt | Protein (Rubisco) | PEG 400: 39% Citrate: 25% | Synthetic solution | 97% of rubisco is extracted to PEG-rich phase Protein kept native form after extraction | [207] |
| Polymer/salt | Protein (β-lactoglobulin α-lactalbumin) | PEG 1500: 14% (NH4)2SO4: 26% | Cheese whey | 95% of protein recovered as precipitate Purity equal to 80% | [225] |
| Polymer/salt | Polysaccharide (mannose, glucose, galactose units) | PEG 600: 23% NaH2PO4: 17% | Culture broth (EPS from Lactobacillus plantarum) | 72% of polysaccharides in salt-rich phase No protein was observed in salt-rich phase | [209] |
| Alcohol/salt | Protein (recombinant green fluorescence protein) | 1-PrOH: 33% Citrate: 18% | Culture broth (Escherichia coli) | 92% of protein is in citrate-rich phase | [226] |
| Alcohol/salt | Protein (pectinase) | EtOH: 19% K3PO4: 22% | Crude extract from Mango wastes | 97% of pectinase was extracted to ethanol-rich phase, and purity increased by a factor of 11 | [227] |
| Alcohol/salt | Polysaccharide | EtOH: 15% Na2CO3: 20% | Crude extract from Cordyceps sinensis | 97% of polysaccharide extracted to salt-rich phase Purity > 70% | [228] |
| IL/salt | Polysaccharide protein | EMIMCl: 15% K3PO4: 22% | Crude extract from Isochrysis galbana (microalgae) | 100% of the protein extracted to IL-rich phase 61% of the polysaccharides in salt-rich phase | [229] |
| IL/salt | Protein (BSA) | C8MIMCl:21% K2HPO4: 28% | Synthetic solution | 100% of BSA is extracted into IL-rich phase Protein kept native form after extraction | [230] |
| IL/salt | Polysaccharide protein | BMIMCl:16% K3PO4: 22% | Fermentation broth (Cordyceps sinensis) | 89% of polysaccharides in salt-rich phase 88% of proteins extracted into IL-rich phase | [217] |
| IL/polymer | Protein (amyloglucosidase) | PPG400: 30% [Ch][DHCit]: 30% | Fetal bovine serum matrix | 100% extraction of protein to PPG-rich phase, even at high protein concentrations (10 g/L) Protein kept native form after extraction | [231] |
| IL/polymer | Protein (interferon α-2b) | PEG400: 37% [Ch][DHP]: 36% | Synthetic solution | 80% of rubisco is extracted to PEG-rich phase Protein kept native form after extraction | [207] |
6.3. Three-Phase Partitioning Systems (TPPs)
6.4. Final Considerations on EPS Recovery
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jess, A.; Wasserscheid, P. Chemical Technology: From Principles to Products, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2020; p. 591. [Google Scholar]
- Straathof, A.J.J. Transformation of Biomass into Commodity Chemicals Using Enzymes or Cells. Chem. Rev. 2014, 114, 1871–1908. [Google Scholar] [CrossRef] [PubMed]
- Salusjärvi, L.; Havukainen, S.; Koivistoinen, O.; Toivari, M. Biotechnological production of glycolic acid and ethylene glycol: Current state and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 2525–2535. [Google Scholar] [CrossRef] [PubMed]
- Wagemann, K.; Tippkötter, N. Biorefineries: A Short Introduction. In Biorefineries; Wagemann, K., Tippkötter, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–11. [Google Scholar]
- Tamis, J.; Joosse, B.; Loosdrecht, M.v.; Kleerebezem, R. High-rate volatile fatty acid (VFA) production by a granular sludge process at low pH. Biotechnol. Bioeng. 2015, 112, 2248–2255. [Google Scholar] [CrossRef] [PubMed]
- Reyhanitash, E.; Fufachev, E.; van Munster, K.D.; van Beek, M.B.M.; Sprakel, L.M.J.; Edelijn, C.N.; Weckhuysen, B.M.; Kersten, S.R.A.; Bruijnincx, P.C.A.; Schuur, B. Recovery and conversion of acetic acid from a phosphonium phosphinate ionic liquid to enable valorization of fermented wastewater. Green Chem. 2019, 21, 2023–2034. [Google Scholar] [CrossRef]
- Fufachev, E.V.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Toward Catalytic Ketonization of Volatile Fatty Acids Extracted from Fermented Wastewater by Adsorption. ACS Sustain. Chem. Eng. 2020, 8, 11292–11298. [Google Scholar]
- Carvajal-Arroyo, J.M.; Andersen, S.J.; Ganigué, R.; Rozendal, R.A.; Angenent, L.T.; Rabaey, K. Production and extraction of medium chain carboxylic acids at a semi-pilot scale. Chem. Eng. J. 2021, 416, 127886. [Google Scholar] [CrossRef]
- Xu, J.; Bian, B.; Angenent, L.T.; Saikaly, P.E. Long-Term Continuous Extraction of Medium-Chain Carboxylates by Pertraction With Submerged Hollow-Fiber Membranes. Front. Bioeng. Biotechnol. 2021, 9, 716. [Google Scholar] [CrossRef]
- Xu, J.; Guzman, J.J.; Andersen, S.J.; Rabaey, K.; Angenent, L.T. In-line and selective phase separation of medium-chain carboxylic acids using membrane electrolysis. Chem. Commun. 2015, 51, 6847–6850. [Google Scholar] [CrossRef]
- Xu, J.; Guzman, J.J.L.; Angenent, L.T. Direct medium-chain carboxylic-acid oil separation from a bioreactor by an electrodialysis/phase separation cell. Environ. Sci. Technol. 2021, 55, 634–644. [Google Scholar] [CrossRef]
- Bauwelinck, J.; Caluwé, M.; Wijnants, M.; Wittner, N.; Broos, W.; Dries, J.; Akkermans, V.; Tavernier, S.; Cornet, I. Chocolate industry side streams as a valuable feedstock for microbial long-chain dicarboxylic acid production. Biochem. Eng. J. 2021, 167, 107888. [Google Scholar] [CrossRef]
- Ariffin, H.; Nishida, H.; Shirai, Y.; Hassan, M.A. Determination of multiple thermal degradation mechanisms of poly(3-hydroxybutyrate). Polym. Degrad. Stab. 2008, 93, 1433–1439. [Google Scholar] [CrossRef]
- Ariffin, H.; Nishida, H.; Shirai, Y.; Hassan, M.A. Highly selective transformation of poly[(R)-3-hydroxybutyric acid] into trans-crotonic acid by catalytic thermal degradation. Polym. Degrad. Stab. 2010, 95, 1375–1381. [Google Scholar] [CrossRef]
- Xiang, H.; Wen, X.; Miu, X.; Li, Y.; Zhou, Z.; Zhu, M. Thermal depolymerization mechanisms of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Prog. Nat. Sci. 2016, 26, 58–64. [Google Scholar] [CrossRef]
- Mamat, M.R.Z.; Ariffin, H.; Hassan, M.A.; Mohd Zahari, M.A.K. Bio-based production of crotonic acid by pyrolysis of poly(3-hydroxybutyrate) inclusions. J. Clean. Prod. 2014, 83, 463–472. [Google Scholar] [CrossRef]
- Patel, A.; Sarkar, O.; Rova, U.; Christakopoulos, P.; Matsakas, L. Valorization of volatile fatty acids derived from low-cost organic waste for lipogenesis in oleaginous microorganisms-A review. Bioresour. Technol. 2021, 321, 124457. [Google Scholar] [CrossRef]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Yang, Y.-H. Microbial production of volatile fatty acids: Current status and future perspectives. Rev. Environ. Sci. Bio/Technol. 2017, 16, 327–345. [Google Scholar] [CrossRef]
- Acetic Acid Market–Growth, Trends, COVID-19 Impact, and Forecasts (2021–2026). Available online: https://www.mordorintelligence.com/industry-reports/acetic-acid-market (accessed on 8 December 2021).
- Bruni, C.; Foglia, A.; Eusebi, A.L.; Frison, N.; Akyol, Ç.; Fatone, F. Targeted Bio-Based Volatile Fatty Acid Production from Waste Streams through Anaerobic Fermentation: Link between Process Parameters and Operating Scale. ACS Sustain. Chem. Eng. 2021, 9, 9970–9987. [Google Scholar] [CrossRef]
- Bengtsson, S.; Hallquist, J.; Werker, A.; Welander, T. Acidogenic fermentation of industrial wastewaters: Effects of chemostat retention time and pH on volatile fatty acids production. Biochem. Eng. J. 2008, 40, 492–499. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, X.; Zhang, P.; Wan, J.; Guo, H.; Ghasimi, D.S.M.; Morera, X.C.; Zhang, T. Overview of key operation factors and strategies for improving fermentative volatile fatty acid production and product regulation from sewage sludge. J. Environ. Sci. 2020, 87, 93–111. [Google Scholar] [CrossRef]
- Dai, K.; Wen, J.-L.; Zhang, F.; Zeng, R.J. Valuable biochemical production in mixed culture fermentation: Fundamentals and process coupling. Appl. Microbiol. Biotechnol. 2017, 101, 6575–6586. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Battista, F.; Frison, N.; Pavan, P.; Cavinato, C.; Gottardo, M.; Fatone, F.; Eusebi, A.L.; Majone, M.; Zeppilli, M.; Valentino, F.; et al. Food Wastes and Sewage Sludge as Feedstock for an Urban Biorefinery Producing Biofuels and Added-Value Bioproducts. J. Chem. Technol. Biotechnol. 2020, 95, 328–338. [Google Scholar] [CrossRef]
- De Groof, V.; Coma, M.; Arnot, T.; Leak, D.J.; Lanham, A.B. Medium Chain Carboxylic Acids from Complex Organic Feedstocks by Mixed Culture Fermentation. Molecules 2019, 24, 398. [Google Scholar] [CrossRef] [PubMed]
- Reyhanitash, E.; Kersten, S.R.A.; Schuur, B. Recovery of Volatile Fatty Acids from Fermented Wastewater by Adsorption. ACS Sustain. Chem. Eng. 2017, 5, 9176–9184. [Google Scholar] [CrossRef]
- Gössi, A.; Burgener, F.; Kohler, D.; Urso, A.; Kolvenbach, B.A.; Riedl, W.; Schuur, B. In-situ recovery of carboxylic acids from fermentation broths through membrane supported reactive extraction using membrane modules with improved stability. Sep. Purif. Technol. 2020, 241, 116694. [Google Scholar] [CrossRef]
- Marták, J.; Schlosser, Š. Extraction of lactic acid by phosphonium ionic liquids. Sep. Purif. Technol. 2007, 57, 483–494. [Google Scholar] [CrossRef]
- Rodríguez-Llorente, D.; Bengoa, A.; Pascual-Muñoz, G.; Navarro, P.; Águeda, V.I.; Delgado, J.A.; Álvarez-Torrellas, S.; García, J.; Larriba, M. Sustainable Recovery of Volatile Fatty Acids from Aqueous Solutions Using Terpenoids and Eutectic Solvents. ACS Sustain. Chem. Eng. 2019, 7, 16786–16794. [Google Scholar] [CrossRef]
- Sprakel, L.M.J.; Schuur, B. Solvent developments for liquid-liquid extraction of carboxylic acids in perspective. Sep. Purif. Technol. 2019, 211, 935–957. [Google Scholar] [CrossRef]
- Pan, X.-R.; Li, W.-W.; Huang, L.; Liu, H.-Q.; Wang, Y.-K.; Geng, Y.-K.; Kwan-Sing Lam, P.; Yu, H.-Q. Recovery of high-concentration volatile fatty acids from wastewater using an acidogenesis-electrodialysis integrated system. Bioresour. Technol. 2018, 260, 61–67. [Google Scholar] [CrossRef]
- Ravishankar, H.; Dessì, P.; Trudu, S.; Asunis, F.; Lens, P.N.L. Silicone membrane contactor for selective volatile fatty acid and alcohol separation. Process Saf. Environ. Prot. 2021, 148, 125–136. [Google Scholar] [CrossRef]
- Bóna, Á.; Bakonyi, P.; Galambos, I.; Bélafi-Bakó, K.; Nemestóthy, N. Separation of Volatile Fatty Acids from Model Anaerobic Effluents Using Various Membrane Technologies. Membranes 2020, 10, 252. [Google Scholar] [CrossRef]
- Brouwer, T.; Dielis, B.C.; Bock, J.M.; Schuur, B. Hydrophobic Deep Eutectic Solvents for the Recovery of Bio-Based Chemicals: Solid–Liquid Equilibria and Liquid–Liquid Extraction. Processes 2021, 9, 796. [Google Scholar] [CrossRef]
- Jomnonkhaow, U.; Uwineza, C.; Mahboubi, A.; Wainaina, S.; Reungsang, A.; Taherzadeh, M.J. Membrane bioreactor-assisted volatile fatty acids production and in situ recovery from cow manure. Bioresour. Technol. 2021, 321, 124456. [Google Scholar] [CrossRef]
- Sukphun, P.; Sittijunda, S.; Reungsang, A. Volatile Fatty Acid Production from Organic Waste with the Emphasis on Membrane-Based Recovery. Fermentation 2021, 7, 159. [Google Scholar] [CrossRef]
- Zhu, X.; Leininger, A.; Jassby, D.; Tsesmetzis, N.; Ren, Z.J. Will Membranes Break Barriers on Volatile Fatty Acid Recovery from Anaerobic Digestion? ACS ES&T Eng. 2021, 1, 141–153. [Google Scholar]
- Aghapour Aktij, S.; Zirehpour, A.; Mollahosseini, A.; Taherzadeh, M.J.; Tiraferri, A.; Rahimpour, A. Feasibility of membrane processes for the recovery and purification of bio-based volatile fatty acids: A comprehensive review. J. Ind. Eng. Chem. 2020, 81, 24–40. [Google Scholar] [CrossRef]
- te Brinke, E.; Reurink, D.M.; Achterhuis, I.; de Grooth, J.; de Vos, W.M. Asymmetric polyelectrolyte multilayer membranes with ultrathin separation layers for highly efficient micropollutant removal. Appl. Mater. Today 2020, 18, 100471. [Google Scholar] [CrossRef]
- Durmaz, E.N.; Sahin, S.; Virga, E.; de Beer, S.; de Smet, L.C.P.M.; de Vos, W.M. Polyelectrolytes as Building Blocks for Next-Generation Membranes with Advanced Functionalities. ACS Appl. Polym. Mater 2021, 3, 4347–4374. [Google Scholar] [CrossRef]
- Reyhanitash, E.; Brouwer, T.; Kersten, S.R.A.; van der Ham, A.G.J.; Schuur, B. Liquid–liquid extraction-based process concepts for recovery of carboxylic acids from aqueous streams evaluated for dilute streams. Chem. Eng. Res. Des. 2018, 137, 510–533. [Google Scholar] [CrossRef]
- Aşçı, Y.S.; Lalikoglu, M. Development of New Hydrophobic Deep Eutectic Solvents Based on Trioctylphosphine Oxide for Reactive Extraction of Carboxylic Acids. Ind. Eng. Chem. Res. 2021, 60, 1356–1365. [Google Scholar] [CrossRef]
- Darwish, A.S.; Warrag, S.E.E.; Lemaoui, T.; Alseiari, M.K.; Hatab, F.A.; Rafay, R.; Alnashef, I.; Rodríguez, J.; Alamoodi, N. Green Extraction of Volatile Fatty Acids from Fermented Wastewater Using Hydrophobic Deep Eutectic Solvents. Fermentation 2021, 7, 226. [Google Scholar] [CrossRef]
- Bruinhorst, A.V.D.; Raes, S.; Maesara, S.A.; Kroon, M.C.; Esteves, A.C.C.; Meuldijk, J. Hydrophobic eutectic mixtures as volatile fatty acid extractants. Sep. Purif. Technol. 2019, 216, 147–157. [Google Scholar] [CrossRef]
- Reyhanitash, E.; Zaalberg, B.; Ijmker, H.M.; Kersten, S.R.A.; Schuur, B. CO2-enhanced extraction of acetic acid from fermented wastewater. Green Chem. 2015, 17, 4393–4400. [Google Scholar] [CrossRef]
- Gausmann, M.; Kocks, C.; Doeker, M.; Eggert, A.; Maßmann, T.; Jupke, A. Recovery of succinic acid by integrated multi-phase electrochemical pH-shift extraction and crystallization. Sep. Purif. Technol. 2020, 240, 116489. [Google Scholar] [CrossRef]
- Gausmann, M.; Kocks, C.; Pastoors, J.; Büchs, J.; Wierckx, N.; Jupke, A. Electrochemical pH-T-Swing Separation of Itaconic Acid for Zero Salt Waste Downstream Processing. ACS Sustain. Chem. Eng. 2021, 9, 9336–9347. [Google Scholar]
- Rizzioli, F.; Battista, F.; Bolzonella, D.; Frison, N. Volatile Fatty Acid Recovery from Anaerobic Fermentate: Focusing on Adsorption and Desorption Performances. Ind. Eng. Chem. Res. 2021, 60, 13701–13709. [Google Scholar] [CrossRef]
- Ukanwa, K.S.; Patchigolla, K.; Sakrabani, R.; Anthony, E.; Mandavgane, S. A Review of Chemicals to Produce Activated Carbon from Agricultural Waste Biomass. Sustainability 2019, 11, 6204. [Google Scholar] [CrossRef]
- Yousuf, A.; Bonk, F.; Bastidas-Oyanedel, J.R.; Schmidt, J.E. Recovery of carboxylic acids produced during dark fermentation of food waste by adsorption on Amberlite IRA-67 and activated carbon. Bioresour. Technol. 2016, 217, 137–140. [Google Scholar] [CrossRef]
- Tharani, D.; Ananthasubramanian, M. Process intensification in separation and recovery of biogenic volatile fatty acid obtained through acidogenic fermentation of organics-rich substrates. Chem. Eng. Process. 2021, 169, 108592. [Google Scholar] [CrossRef]
- Belhamdi, B.; Merzougui, Z.; Trari, M.; Addoun, A. A kinetic, equilibrium and thermodynamic study of l-phenylalanine adsorption using activated carbon based on agricultural waste (date stones). J. Appl. Res. Technol. 2016, 14, 354–366. [Google Scholar] [CrossRef]
- López-Garzón, C.S.; Straathof, A.J.J. Recovery of carboxylic acids produced by fermentation. Biotechnol. Adv. 2014, 32, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Rodríguez, C.I.; Paltrinieri, L.; de Smet, L.C.P.M.; van der Wielen, L.A.M.; Straathof, A.J.J. Recovery and esterification of aqueous carboxylates by using CO2-expanded alcohols with anion exchange. Green Chem. 2017, 19, 729–738. [Google Scholar] [CrossRef]
- Fernando-Foncillas, C.; Cabrera-Rodríguez, C.I.; Caparrós-Salvador, F.; Varrone, C.; Straathof, A.J.J. Highly selective recovery of medium chain carboxylates from co-fermented organic wastes using anion exchange with carbon dioxide expanded methanol desorption. Bioresour. Technol. 2021, 319, 124178. [Google Scholar] [CrossRef]
- Cabrera-Rodríguez, C.I.; Moreno-González, M.; de Weerd, F.A.; Viswanathan, V.; van der Wielen, L.A.M.; Straathof, A.J.J. Esters production via carboxylates from anaerobic paper mill wastewater treatment. Bioresour. Technol. 2017, 237, 186–192. [Google Scholar] [CrossRef]
- Rebecchi, S.; Pinelli, D.; Bertin, L.; Zama, F.; Fava, F.; Frascari, D. Volatile fatty acids recovery from the effluent of an acidogenic digestion process fed with grape pomace by adsorption on ion exchange resins. Chem. Eng. J. 2016, 306, 629–639. [Google Scholar] [CrossRef]
- Steinbusch, K.J.; Hamelers, H.V.; Plugge, C.M.; Buisman, C.J. Biological formation of caproate and caprylate from acetate: Fuel and chemical production from low grade biomass. Energy Environ. Sci. 2011, 4, 216–224. [Google Scholar] [CrossRef]
- Eregowda, T.; Rene, E.R.; Rintala, J.; Lens, P.N.L. Volatile fatty acid adsorption on anion exchange resins: Kinetics and selective recovery of acetic acid. Sep. Sci. Technol. 2020, 55, 1449–1461. [Google Scholar] [CrossRef]
- Shi, X.; Wu, L.; Wei, W.; Ni, B.-J. Insights into the microbiomes for medium-chain carboxylic acids production from biowastes through chain elongation. Crit. Rev. Environ. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Cavalcante, W.d.A.; Leitão, R.C.; Gehring, T.A.; Angenent, L.T.; Santaella, S.T. Anaerobic fermentation for n-caproic acid production: A review. Process Biochem. 2017, 54, 106–119. [Google Scholar] [CrossRef]
- Chen, W.; Ye, Y.; Steinbusch, K.; Strik, D.; Buisman, C. Methanol as an alternative electron donor in chain elongation for butyrate and caproate formation. Biomass Bioenergy 2016, 93, 201–208. [Google Scholar] [CrossRef]
- Xu, J.; Hao, J.; Guzman, J.J.; Spirito, C.M.; Harroff, L.A.; Angenent, L.T. Temperature-phased conversion of acid whey waste into medium-chain carboxylic acids via lactic acid: No external e-donor. Joule 2018, 2, 280–295. [Google Scholar] [CrossRef]
- Ding, H.-B.; Tan, G.-Y.A.; Wang, J.-Y. Caproate formation in mixed-culture fermentative hydrogen production. Bioresour. Technol. 2010, 101, 9550–9559. [Google Scholar] [CrossRef]
- Drake, B.D.H.; Howel, C.J.; Chen, P.N.; Wakefield, A. High Boiling Solvent System for Recovery of Acetic Acid from Aqueous Solutions. EU Patent 0134650A1, 20 March 1985. [Google Scholar]
- Saboe, P.O.; Manker, L.P.; Monroe, H.R.; Michener, W.E.; Haugen, S.; Tan, E.C.D.; Prestangen, R.L.; Beckham, G.T.; Karp, E.M. Energy and techno-economic analysis of bio-based carboxylic acid recovery by adsorption. Green Chem. 2021, 23, 4386–4402. [Google Scholar] [CrossRef]
- Lambrecht, J.; Cichocki, N.; Schattenberg, F.; Kleinsteuber, S.; Harms, H.; Müller, S.; Sträuber, H. Key sub-community dynamics of medium-chain carboxylate production. Microb. 2019, 18, 92. [Google Scholar] [CrossRef]
- Červeňanský, I.; Mihaľ, M.; Markoš, J. Pertraction-adsorption in situ product removal system: Design and mathematical modeling. Chem. Eng. Process. 2019, 143, 107604. [Google Scholar] [CrossRef]
- Yu, J.; Liao, J.; Huang, Z.; Wu, P.; Zhao, M.; Liu, C.; Ruan, W. Enhanced Anaerobic Mixed Culture Fermentation with Anion-Exchange Resin for Caproate Production. Processes 2019, 7, 404. [Google Scholar] [CrossRef]
- Ge, S.; Usack, J.G.; Spirito, C.M.; Angenent, L.T. Long-Term n-Caproic Acid Production from Yeast-Fermentation Beer in an Anaerobic Bioreactor with Continuous Product Extraction. Environ. Sci. Technol. 2015, 49, 8012–8021. [Google Scholar] [CrossRef]
- Saboe, P.O.; Manker, L.P.; Michener, W.E.; Peterson, D.J.; Brandner, D.G.; Deutch, S.P.; Kumar, M.; Cywar, R.M.; Beckham, G.T.; Karp, E.M. In situ recovery of bio-based carboxylic acids. Green Chem. 2018, 20, 1791–1804. [Google Scholar] [CrossRef]
- Tanaka, K.; Takiyama, H. Effect of Oiling-Out during Crystallization on Purification of an Intermediate Compound. Org. Process Res. Dev. 2019, 23, 2001–2008. [Google Scholar] [CrossRef]
- Buathong, P.; Boonvitthya, N.; Truan, G.; Chulalaksananukul, W. Whole-Cell Biotransformation of 1,12-Dodecanedioic Acid from Coconut Milk Factory Wastewater by Recombinant CYP52A17SS Expressing Saccharomyces cerevisiae. Processes 2020, 8, 969. [Google Scholar] [CrossRef]
- Green, K.D.; Turner, M.K.; Woodley, J.M. Candida cloacae oxidation of long-chain fatty acids to dioic acids. Enzyme Microb. Technol. 2000, 27, 205–211. [Google Scholar] [CrossRef]
- Werner, N.; Zibek, S. Biotechnological production of bio-based long-chain dicarboxylic acids with oleogenious yeasts. World J. Microbiol. Biotechnol. 2017, 33, 194. [Google Scholar] [CrossRef] [PubMed]
- Huf, S.; Krügener, S.; Hirth, T.; Rupp, S.; Zibek, S. Biotechnological synthesis of long-chain dicarboxylic acids as building blocks for polymers. Eur. J. Lipid Sci. Technol. 2011, 113, 548–561. [Google Scholar] [CrossRef]
- Buathong, P.; Boonvitthya, N.; Truan, G.; Chulalaksananukul, W. Biotransformation of lauric acid into 1,12-dodecanedioic acid using CYP52A17 expressed in Saccharomyces cerevisiae and its application in refining coconut factory wastewater. Int. Biodeterior. Biodegradation 2019, 139, 70–77. [Google Scholar] [CrossRef]
- Blumenstein, J.; Albert, J.; Schulz, R.P.; Kohlpaintner, C. Crotonaldehyde and Crotonic Acid. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 1–20. [Google Scholar]
- Koch, D.; Meurer, G. Means and Methods for Producing Crotonic Acid. EU Patent EP 11003157A, 17 October 2012. [Google Scholar]
- Kunasundari, B.; Sudesh, K. Isolation and recovery of microbial polyhydroxyalkanoates. Express Polym. Lett. 2011, 5, 620–634. [Google Scholar] [CrossRef]
- Chee, J.Y.; Yoga, S.; Lau, N.-S.; Ling, S.; Abed, R.; Sudesh, K. Bacterially produced polyhydroxyalkanoate (PHA): Converting renewable resources into bioplastic. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Formatex Research Center: Badajoz, Spain, 2010; pp. 1395–1404. [Google Scholar]
- Farid, N.F.S.M.; Ariffin, H.; Mamat, M.R.Z.; Mohd Zahari, M.A.K.; Hassan, M.A. Non-solvent-based pretreatment of poly(3-hydroxybutyrate) for improved bio-based crotonic acid production. RSC Adv. 2015, 5, 33546–33553. [Google Scholar] [CrossRef][Green Version]
- Koller, M.; Niebelschütz, H.; Braunegg, G. Strategies for recovery and purification of poly[(R)-3-hydroxyalkanoates] (PHA) biopolyesters from surrounding biomass. Eng. Life Sci. 2013, 13, 549–562. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Tan, G.-Y.A.; Chen, C.-L.; Li, L.; Ge, L.; Wang, L.; Razaad, I.M.; Li, Y.; Zhao, L.; Mo, Y.; Wang, J.-Y. Start a Research on Biopolymer Polyhydroxyalkanoate (PHA): A Review. Polymers 2014, 6, 706–754. [Google Scholar] [CrossRef]
- Khajuria, R. Polyhydroxyalkanoates: Biosynthesis to commercial production-A review. J. Microbiol. Biotechnol. Food Sci. 2017, 6, 1098–1106. [Google Scholar]
- Jacquel, N.; Lo, C.-W.; Wei, Y.-H.; Wu, H.-S.; Wang, S.S.; Wang, S.S. Isolation and purification of bacterial poly(3-hydroxyalkanoates). Biochem. Eng. J. 2008, 39, 15–27. [Google Scholar] [CrossRef]
- Nielsen, C.; Rahman, A.; Rehman, A.U.; Walsh, M.K.; Miller, C.D. Food waste conversion to microbial polyhydroxyalkanoates. Microb. Biotechnol. 2017, 10, 1338–1352. [Google Scholar] [CrossRef]
- Coats, E.R.; Watson, B.S.; Brinkman, C.K. Polyhydroxyalkanoate synthesis by mixed microbial consortia cultured on fermented dairy manure: Effect of aeration on process rates/yields and the associated microbial ecology. Water Res. 2016, 106, 26–40. [Google Scholar] [CrossRef]
- Bluemink, E.D.; van Nieuwenhuijzen, A.F.; Wypkema, E.; Uijterlinde, C.A. Bio-plastic (poly-hydroxy-alkanoate) production from municipal sewage sludge in the Netherlands: A technology push or a demand driven process? Water Sci. Technol. 2016, 74, 353–358. [Google Scholar] [CrossRef]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef]
- de Souza Reis, G.A.; Michels, M.H.A.; Fajardo, G.L.; Lamot, I.; de Best, J.H. Optimization of Green Extraction and Purification of PHA Produced by Mixed Microbial Cultures from Sludge. Water 2020, 12, 1185. [Google Scholar] [CrossRef]
- Koller, M. Established and advanced approaches for recovery of microbial polyhydroxyalkanoate (PHA) biopolyesters from surrounding microbial biomass. EuroBiotech J. 2020, 4, 113–126. [Google Scholar] [CrossRef]
- Samorì, C.; Basaglia, M.; Casella, S.; Favaro, L.; Galletti, P.; Giorgini, L.; Marchi, D.; Mazzocchetti, L.; Torri, C.; Tagliavini, E. Dimethyl carbonate and switchable anionic surfactants: Two effective tools for the extraction of polyhydroxyalkanoates from microbial biomass. Green Chem. 2015, 17, 1047–1056. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Jong-Min, J.; Yi, D.; Kim, J.-H.; Seo, H.-M.; Rha, C.; Sinskey, A.; Brigham, C. Application of a non-halogenated solvent, methyl ethyl ketone (MEK) for recovery of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) [P(HB-co-HV)] from bacterial cells. Biotechnol. Bioprocess Eng. 2015, 20, 291–297. [Google Scholar] [CrossRef]
- Prajapati, K.; Nayak, R.; Shukla, A.; Parmar, P.; Goswami, D.; Saraf, M. Polyhydroxyalkanoates: An Exotic Gleam in the Gloomy Tale of Plastics. J. Polym. Environ. 2021, 29, 2013–2032. [Google Scholar] [CrossRef]
- Kalia, V.C.; Singh Patel, S.K.; Shanmugam, R.; Lee, J.-K. Polyhydroxyalkanoates: Trends and advances toward biotechnological applications. Bioresour. Technol. 2021, 326, 124737. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.B.; Pereira, J.R.; Marreiros, B.C.; Reis, M.A.; Freitas, F. Microbial production of medium-chain length polyhydroxyalkanoates. Process Biochem. 2021, 102, 393–407. [Google Scholar] [CrossRef]
- Gecim, G.; Aydin, G.; Tavsanoglu, T.; Erkoc, E.; Kalemtas, A. Review on extraction of polyhydroxyalkanoates and astaxanthin from food and beverage processing wastewater. J. Water Process. Eng. 2021, 40, 101775. [Google Scholar] [CrossRef]
- Pagliano, G.; Galletti, P.; Samorì, C.; Zaghini, A.; Torri, C. Recovery of polyhydroxyalkanoates from single and mixed microbial cultures: A review. Front. Bioeng. Biotechnol. 2021, 9, 54. [Google Scholar] [CrossRef]
- Gonzalez, K.; Navia, R.; Liu, S.; Cea, M. Biological Approaches in Polyhydroxyalkanoates Recovery. Curr. Microbiol. 2020, 78, 1–10. [Google Scholar] [CrossRef]
- Yang, X.; Odelius, K.; Hakkarainen, M. Microwave-Assisted Reaction in Green Solvents Recycles PHB to Functional Chemicals. ACS Sustain. Chem. Eng. 2014, 2, 2198–2203. [Google Scholar] [CrossRef]
- Mullen, C.A.; Boateng, A.A.; Schweitzer, D.; Sparks, K.; Snell, K.D. Mild pyrolysis of P3HB/switchgrass blends for the production of bio-oil enriched with crotonic acid. J. Anal. Appl. Pyrolys. 2014, 107, 40–45. [Google Scholar] [CrossRef]
- Samorì, C.; Kiwan, A.; Torri, C.; Conti, R.; Galletti, P.; Tagliavini, E. Polyhydroxyalkanoates and Crotonic Acid from Anaerobically Digested Sewage Sludge. ACS Sustain. Chem. Eng. 2019, 7, 10266–10273. [Google Scholar] [CrossRef]
- Parodi, A.; Jorea, A.; Fagnoni, M.; Ravelli, D.; Samorì, C.; Torri, C.; Galletti, P. Bio-based crotonic acid from polyhydroxybutyrate: Synthesis and photocatalyzed hydroacylation. Green Chem. 2021, 23, 3420–3427. [Google Scholar] [CrossRef]
- Fernández-Dacosta, C.; Posada, J.A.; Ramirez, A. Techno-economic and carbon footprint assessment of methyl crotonate and methyl acrylate production from wastewater-based polyhydroxybutyrate (PHB). J. Clean. Prod. 2016, 137, 942–952. [Google Scholar] [CrossRef]
- Spekreijse, J.; Le Nôtre, J.; Sanders, J.P.M.; Scott, E.L. Conversion of polyhydroxybutyrate (PHB) to methyl crotonate for the production of biobased monomers. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Li, Y.; Strathmann, T.J. Kinetics and mechanism for hydrothermal conversion of polyhydroxybutyrate (PHB) for wastewater valorization. Green Chem. 2019, 21, 5586–5597. [Google Scholar] [CrossRef]
- Torri, C.; Weme, T.D.O.; Samorì, C.; Kiwan, A.; Brilman, D.W.F. Renewable Alkenes from the Hydrothermal Treatment of Polyhydroxyalkanoates-Containing Sludge. Environ. Sci. Technol. 2017, 51, 12683–12691. [Google Scholar] [CrossRef]
- Kang, S.; Chen, H.; Zheng, Y.; Xiao, Y.; Xu, Y.; Wang, Z. One-Pot Catalytic Conversion of Poly(3-hydroxybutyrate) to Propylene at 240 °C. Chem. Select 2019, 4, 403–406. [Google Scholar] [CrossRef]
- Song, X.; Liu, F.; Wang, H.; Wang, C.; Yu, S.; Liu, S. Methanolysis of microbial polyester poly(3-hydroxybutyrate) catalyzed by Brønsted-Lewis acidic ionic liquids as a new method towards sustainable development. Polym. Degrad. Stab. 2018, 147, 215–221. [Google Scholar] [CrossRef]
- Nishida, H.; Ariffin, H.; Shirai, Y.; Hassan, M.A. Precise depolymerization of poly (3-hydroxybutyrate) by pyrolysis. Biopolymers 2010, 19, 370–386. [Google Scholar]
- Kang, S.; Yu, J. One-pot production of hydrocarbon oil from poly(3-hydroxybutyrate). RSC Adv. 2014, 4, 14320–14327. [Google Scholar] [CrossRef]
- Morikawa, H.; Marchessault, R.H. Pyrolysis of bacterial polyalkanoates. Can. J. Chem. 1981, 59, 2306–2313. [Google Scholar] [CrossRef]
- Al-Haj Ibrahim, H. Introductory Chapter: Pyrolysis. In Recent Advances in Pyrolysis; IntechOpen: London, UK, 2020. [Google Scholar]
- Fraga, A.; Ruseckaite, R.A.; Jiménez, A. Thermal degradation and pyrolysis of mixtures based on poly(3-hydroxybutyrate-8%-3-hydroxyvalerate) and cellulose derivatives. Polym. Test. 2005, 24, 526–534. [Google Scholar] [CrossRef]
- Ariffin, H.; Nishida, H.; Hassan, M.A.; Shirai, Y. Chemical recycling of polyhydroxyalkanoates as a method towards sustainable development. Biotechnol. J. 2010, 5, 484–492. [Google Scholar] [CrossRef]
- Kim, K.J.; Doi, Y.; Abe, H. Effects of residual metal compounds and chain-end structure on thermal degradation of poly(3-hydroxybutyric acid). Polym. Degrad. Stab. 2006, 91, 769–777. [Google Scholar] [CrossRef]
- Kim, K.J.; Doi, Y.; Abe, H. Effect of metal compounds on thermal degradation behavior of aliphatic poly(hydroxyalkanoic acid)s. Polym. Degrad. Stab. 2008, 93, 776–785. [Google Scholar] [CrossRef]
- Hocking, M.B. The Effect of Heat on cis- and trans-Crotonic Acids: Alternatives to Direct cis–trans Isomerism. Can. J. Chem. 1972, 50, 1224–1232. [Google Scholar] [CrossRef]
- Brouwer, T.; van Lin, R.; ten Kate, A.J.B.; Schuur, B.; Bargeman, G. Influence of Solvent and Acid Properties on the Relative Volatility and Separation Selectivity for Extractive Distillation of Close-Boiling Acids. Ind. Eng. Chem. Res. 2021, 60, 7406–7416. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/crotonic_acid (accessed on 8 December 2021).
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2-Pentenoic-acid#section=Computed-Properties (accessed on 8 December 2021).
- Chemispider. Available online: http://www.chemspider.com/Chemical-Structure.553682.html (accessed on 8 December 2021).
- Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/NL/en/product/aldrich/113018 (accessed on 8 December 2021).
- Sigma-Aldrich. Available online: https://www.sigmaaldrich.cn/CN/zh (accessed on 8 December 2021).
- Zeng, Y.; Qian, H.; Chen, X.; Li, Z.; Yu, S.; Xiao, X. Thermodynamic Estimate of pKa Values of the Carboxylic Acids in Aqueous Solution with the Density Functional Theory. Chin. J. Chem. 2010, 28, 727–733. [Google Scholar] [CrossRef]
- Moradali, M.F.; Rehm, B.H.A. Bacterial biopolymers: From pathogenesis to advanced materials. Nat. Rev. Microbiol. 2020, 18, 195–210. [Google Scholar] [CrossRef]
- Balannec, B.; Vourch, M.; Rabiller-Baudry, M.; Chaufer, B. Comparative study of different nanofiltration and reverse osmosis membranes for dairy effluent treatment by dead-end filtration. Sep. Purif. Technol. 2005, 42, 195–200. [Google Scholar] [CrossRef]
- Baskaran, K.; Palmowski, L.M.; Watson, B.M. Wastewater reuse and treatment options for the dairy industry. Water Supply 2003, 3, 85–91. [Google Scholar] [CrossRef]
- Kushwaha, J.P.; Srivastava, V.C.; Mall, I.D. An overview of various technologies for the treatment of dairy wastewaters. Crit. Rev. Food Sci. Nutr. 2011, 51, 442–452. [Google Scholar] [CrossRef]
- Gopinatha Kurup, G.; Adhikari, B.; Zisu, B. Recovery of proteins and lipids from dairy wastewater using food grade sodium lignosulphonate. Water Resour. Ind. 2019, 22, 100114. [Google Scholar] [CrossRef]
- Lo, Y.M.; Cao, D.; Argin-Soysal, S.; Wang, J.; Hahm, T.S. Recovery of protein from poultry processing wastewater using membrane ultrafiltration. Bioresour. Technol. 2005, 96, 687–698. [Google Scholar] [CrossRef]
- Avula, R.Y.; Nelson, H.M.; Singh, R.K. Recycling of poultry process wastewater by ultrafiltration. Innov. Food Sci. Emerg. Technol. 2009, 10, 1–8. [Google Scholar] [CrossRef]
- Chua, J.Y.; Liu, S.Q. Soy whey: More than just wastewater from tofu and soy protein isolate industry. Trends Food Sci. Technol. 2019, 91, 24–32. [Google Scholar] [CrossRef]
- Li, X.; Long, J.; Hua, Y.; Chen, Y.; Kong, X.; Zhang, C. Protein recovery and anti-nutritional factor removal from soybean wastewater by complexing with a high concentration of polysaccharides in a novel quick-shearing system. J. Food Eng. 2019, 241, 1–9. [Google Scholar] [CrossRef]
- Garcia-Castello, E.; Cassano, A.; Criscuoli, A.; Conidi, C.; Drioli, E. Recovery and concentration of polyphenols from olive mill wastewaters by integrated membrane system. Water Res. 2010, 44, 3883–3892. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, H.H.P. Influences of Extracellular Polymeric Substances (EPS) on Flocculation, Settling, and Dewatering of Activated Sludge. Crit. Rev. Environ. Sci. Technol. 2003, 33, 237–273. [Google Scholar] [CrossRef]
- Jindal, N.; Singh Khattar, J. Microbial Polysaccharides in Food Industry. In Biopolymers for Food Design; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 95–123. [Google Scholar]
- Nielsen, P.H.; Jahn, A. Extraction of EPS. In Microbial Extracellular Polymeric Substances; Wingender, J.W., Neu, T.R., Flemming, H.C., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 49–72. [Google Scholar]
- Wingender, J.; Neu, T.R.; Flemming, H.-C. What are Bacterial Extracellular Polymeric Substances? In Microbial Extracellular Polymeric Substances; Wingender, J., Neu, T.R., Flemming, H.-C., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 1–19. [Google Scholar]
- More, T.T.; Yadav, J.S.S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Extracellular polymeric substances of bacteria and their potential environmental applications. J. Environ. Manag. 2014, 144, 1–25. [Google Scholar] [CrossRef]
- Barcelos, M.C.S.; Vespermann, K.A.C.; Pelissari, F.M.; Molina, G. Current status of biotechnological production and applications of microbial exopolysaccharides. Crit. Rev. Food. Sci. Nutr. 2020, 60, 1475–1495. [Google Scholar] [CrossRef]
- Sheng, G.-P.; Yu, H.-Q.; Li, X.-Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef]
- Ding, Z.; Bourven, I.; Guibaud, G.; van Hullebusch, E.D.; Panico, A.; Pirozzi, F.; Esposito, G. Role of extracellular polymeric substances (EPS) production in bioaggregation: Application to wastewater treatment. Appl. Microbiol. Biotechnol. 2015, 99, 9883–9905. [Google Scholar] [CrossRef] [PubMed]
- Felz, S.; Al-Zuhairy, S.; Aarstad, O.A.; van Loosdrecht, M.C.M.; Lin, Y.M. Extraction of Structural Extracellular Polymeric Substances from Aerobic Granular Sludge. J. Vis. Exp. 2016, 115, 54534. [Google Scholar] [CrossRef] [PubMed]
- Boleij, M.; Seviour, T.; Li, L.; Loosdrecht, M.C.M.V.; Lin, Y. Solubilization and characterization of extracellular proteins from anammox granular sludge. Water Res. 2019, 164, 114952. [Google Scholar] [CrossRef] [PubMed]
- Izadi, P.; Izadi, P.; Eldyasti, A. Holistic insights into extracellular polymeric substance (EPS) in anammosx bacterial matrix and the potential sustainable biopolymer recovery: A review. Chemosphere 2021, 274, 129703. [Google Scholar] [CrossRef] [PubMed]
- Nouha, K.; Kumar, R.S.; Balasubramanian, S.; Tyagi, R.D. Critical review of EPS production, synthesis and composition for sludge flocculation. J. Environ. Sci. 2018, 66, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Ates, O. Systems Biology of Microbial exopolysaccharides Production. Front. Bioeng. Biotechnol. 2015, 3, 200. [Google Scholar] [CrossRef] [PubMed]
- Donot, F.; Fontana, A.; Baccou, J.C.; Schorr-Galindo, S. Microbial exopolysaccharides: Main examples of synthesis, excretion, genetics and extraction. Carbohydr. Polym. 2012, 87, 951–962. [Google Scholar] [CrossRef]
- Selbmann, L.; Stingele, F.; Petruccioli, M. Exopolysaccharide production by filamentous fungi: The example of Botryosphaeria rhodina. Antonie van Leeuwenhoek 2003, 84, 135–145. [Google Scholar] [CrossRef]
- Yildiz, H.; Karatas, N. Microbial exopolysaccharides: Resources and bioactive properties. Process Biochem. 2018, 72, 41–46. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.D.; Reis, M.A.M. Advances in bacterial exopolysaccharides: From production to biotechnological applications. Trends Biotechnol. 2011, 29, 388–398. [Google Scholar] [CrossRef]
- Ajao, V.; Millah, S.; Gagliano, M.C.; Bruning, H.; Rijnaarts, H.; Temmink, H. Valorization of glycerol/ethanol-rich wastewater to bioflocculants: Recovery, properties, and performance. J. Hazard. Mater. 2019, 375, 273–280. [Google Scholar] [CrossRef]
- Durmaz, B.; Sanin, F.D. Effect of carbon to nitrogen ratio on the physical and chemical properties of activated sludge. Environ. Technol. 2003, 24, 1331–1340. [Google Scholar] [CrossRef]
- Erkan, H.S.; Onkal Engin, G.; Ince, M.; Bayramoglu, M.R. Effect of carbon to nitrogen ratio of feed wastewater and sludge retention time on activated sludge in a submerged membrane bioreactor. Environ. Sci. Pollut. Res. Int. 2016, 23, 10742–10752. [Google Scholar] [CrossRef]
- Ye, F.; Ye, Y.; Li, Y. Effect of C/N ratio on extracellular polymeric substances (EPS) and physicochemical properties of activated sludge flocs. J. Hazard Mater. 2011, 188, 37–43. [Google Scholar] [CrossRef]
- Feng, C.; Lotti, T.; Canziani, R.; Lin, Y.; Tagliabue, C.; Malpei, F. Extracellular biopolymers recovered as raw biomaterials from waste granular sludge and potential applications: A critical review. Sci. Total Environ. 2021, 753, 142051. [Google Scholar] [CrossRef]
- Palaniraj, A.; Jayaraman, V. Production, recovery and applications of xanthan gum by Xanthomonas campestris. J. Food Eng. 2011, 106, 1–12. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Springer, J.; Kooy, F.K.; van den Broek, L.A.M.; Eggink, G. Production Methods for Hyaluronan. Int. J. Carbohydr. Chem. 2013, 2013, 624967. [Google Scholar] [CrossRef]
- Leathers, T.D. Biotechnological production and applications of pullulan. Appl. Microbiol. Biotechnol. 2003, 62, 468–473. [Google Scholar] [CrossRef]
- Marcati, A.; Ursu, A.V.; Laroche, C.; Soanen, N.; Marchal, L.; Jubeau, S.; Djelveh, G.; Michaud, P. Extraction and fractionation of polysaccharides and B-phycoerythrin from the microalga Porphyridium cruentum by membrane technology. Algal Res. 2014, 5, 258–263. [Google Scholar] [CrossRef]
- Hu, X.; Go, H.D. Fractionation of polysaccharides by gradient non-solvent precipitation: A review. Trends Food Sci. Technol. 2018, 81, 108–115. [Google Scholar] [CrossRef]
- Patel, A.K.; Laroche, C.; Marcati, A.; Ursu, A.V.; Jubeau, S.; Marchal, L.; Petit, E.; Djelveh, G.; Michaud, P. Separation and fractionation of exopolysaccharides from Porphyridium cruentum. Bioresour. Technol. 2013, 145, 345–350. [Google Scholar] [CrossRef]
- Bahl, M.A.; Schultheis, E.; Hempel, D.C.; Nörtemann, B.; Franco-Lara, E. Recovery and purification of the exopolysaccharide PS-EDIV from Sphingomonas pituitosa DSM 13101. Carbohydr. Polym. 2010, 80, 1037–1041. [Google Scholar] [CrossRef]
- Novák, P.; Havlíček, V. Protein Extraction and Precipitation. In Proteomic Profiling and Analytical Chemistry; Ciborowski, P., Silberring, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 79–90. [Google Scholar]
- Burgess, R.R. Protein Precipitation Techniques. In Methods in Enzymology; Burgess, R.R., Deutscher, M.P., Eds.; Academic Press: Cambridge, MA, USA, 2009; Volume 463, pp. 331–342. [Google Scholar]
- Manzoni, M.; Rollini, M. Isolation and characterization of the exopolysaccharide produced by Streptococcus thermophilus SFi20. Biotechnol. Lett. 2001, 23, 1491–1497. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, H.; Wu, D.; Linhardt, R.J.; Zhi, Z.; Yan, L.; Chen, S.; Ye, X. Green recovery of pectic polysaccharides from citrus canning processing water. J. Clean. Prod. 2017, 144, 459–469. [Google Scholar] [CrossRef]
- Crowell, A.M.J.; Wall, M.J.; Doucette, A.A. Maximizing recovery of water-soluble proteins through acetone precipitation. Anal. Chim. Acta J. 2013, 796, 48–54. [Google Scholar] [CrossRef]
- Kreyenschulte, D.; Krull, R.; Margaritis, A. Recent Advances in Microbial Biopolymer Production and Purification. Crit. Rev. Biotechnol. 2012, 34, 1–15. [Google Scholar] [CrossRef]
- Bruggen, B.V.D.; Vandecasteele, C.; Gestel, T.V.; Doyenb, W.; Leysenb, R. Review of Pressure-Driven Membrane Processes in wastewater treatment and drinking water production. Environ. Prog. 2003, 22, 46–56. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Ng, C.Y.; Lim, Y.P.; Ng, G.H. Ultrafiltration in Food Processing Industry: Review on Application, Membrane Fouling, and Fouling Control. Food Bioprocess Technol. 2012, 5, 1143–1156. [Google Scholar] [CrossRef]
- Rajendran, S.R.C.K.; Mason, B.; Doucette, A.A. Review of Membrane Separation Models and Technologies: Processing Complex Food-Based Biomolecular Fractions. Food Bioprocess Technol. 2021, 14, 415–428. [Google Scholar] [CrossRef]
- Luo, J.; Ding, L.; Qi, B.; Jaffrin, M.Y.; Wan, Y. A two-stage ultrafiltration and nanofiltration process for recycling dairy wastewater. Bioresour. Technol. 2011, 102, 7437–7442. [Google Scholar] [CrossRef]
- Giacobbo, A.; Oliveira, M.; Duarte, E.C.N.F.; Mira, M.C.; Bernardes, A.M.; Pinho, M.N.D. Ultrafiltration Based Process for the Recovery of Polysaccharides and Polyphenols from Winery Effluents Ultrafiltration Based Process for the Recovery of Polysaccharides and Polyphenols from Winery Effluents. Sep. Sci. Technol. 2013, 48, 438–444. [Google Scholar] [CrossRef]
- Lakra, R.; Choudhury, S.; Basu, S. Recovery of protein and carbohydrate from dairy wastewater using ultrafiltration and forward osmosis processes. Mater. Today Proc. 2021, 47, 1400–1403. [Google Scholar] [CrossRef]
- Białas, W.; Stangierski, J.; Konieczny, P. Protein and water recovery from poultry processing wastewater integrating microfiltration, ultrafiltration and vacuum membrane distillation. Int. J. Environ. Sci. Technol. 2015, 12, 1875–1888. [Google Scholar] [CrossRef]
- Zhao, Z.; Cuellar, S.; Ilyas, A.; Muylaert, K. Optimization of negatively charged polysulfone membranes for concentration and puri fi cation of extracellular polysaccharides from Arthrospira platensis using the response surface methodology. Sep. Purif. Technol. 2020, 252, 117385. [Google Scholar] [CrossRef]
- Jorda, J.; Marechal, P.; Rigal, L.; Pontalier, P.Y. Biopolymer purification by ultrafiltration. Desalination 2002, 148, 187–191. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Xiong, S.; Zhang, H.; Li, N.; Zhou, S.; Liu, Y.; Huang, Z. Pilot-scale isolation of bioactive extracellular polymeric substances from cell-free media of mass microalgal cultures using tangential-flow ultrafiltration. Process Biochem. 2011, 46, 1104–1109. [Google Scholar] [CrossRef]
- Leberknight, J.; Wielenga, B.; Lee-Jewett, A.; Menkhaus, T.J. Recovery of high value protein from a corn ethanol process by ultrafiltration and an exploration of the associated membrane fouling. J. Membr. Sci. 2011, 366, 405–412. [Google Scholar] [CrossRef]
- Grilo, A.L.; Aires-Barros, M.R.; Azevedo, A.M. Partitioning in Aqueous Two-Phase Systems: Fundamentals, Applications and Trends. Sep. Purif. Rev. 2016, 45, 68–80. [Google Scholar] [CrossRef]
- Pereira, J.F.B.; Freire, M.G.; Coutinho, J.A.P. Aqueous two-phase systems: Towards novel and more disruptive applications. Fluid Phase Equilib. 2020, 505, 112341. [Google Scholar] [CrossRef]
- Mayolo-Deloisa, K.; Benavides, J.; Rito-Palomares, M. General Concepts and Definitions of Aqueous Two-Phase Systems. In Aqueous Two-Phase Systems for Bioprocess Development for the Recovery of Biological Products; Rito-Palomares, M., Benavides, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–18. [Google Scholar]
- Diamond, A.D.; Hsu, J.T. Phase diagrams for dextran-PEG aqueous two-phase systems at 22 °C. Biotechnol. Tech. 1989, 3, 119–124. [Google Scholar] [CrossRef]
- da Silva, L.H.M.; Meirelles, A.J.A. Phase equilibrium and protein partitioning in aqueous mixtures of maltodextrin with polypropylene glycol. Carbohydr. Polym. 2001, 46, 267–274. [Google Scholar] [CrossRef]
- Johansson, H.-O.; Feitosa, E.; Junior, A.P. Phase Diagrams of the Aqueous Two-Phase Systems of Poly(ethylene glycol)/Sodium Polyacrylate/Salts. Polymers 2011, 3, 587–601. [Google Scholar] [CrossRef]
- Zhao, X.; Xie, X.; Yan, Y. Liquid–liquid equilibrium of aqueous two-phase systems containing poly(propylene glycol) and salt ((NH4)2SO4, MgSO4, KCl, and KAc): Experiment and correlation. Thermochim. Acta 2011, 516, 46–51. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Willauer, H.D.; Rogers, R.D. Phase Diagram Data for Several PEG + Salt Aqueous Biphasic Systems at 25 °C. J. Chem. Eng. Data 2003, 48, 1230–1236. [Google Scholar] [CrossRef]
- Wu, C.; Wang, J.; Pei, Y.; Wang, H.; Li, Z. Salting-Out Effect of Ionic Liquids on Poly(propylene glycol) (PPG): Formation of PPG + Ionic Liquid Aqueous Two-Phase Systems. J. Chem. Eng. Data 2010, 55, 5004–5008. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Silva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef]
- Passos, H.; Ferreira, A.R.; Cláudio, A.F.M.; Coutinho, J.A.P.; Freire, M.G. Characterization of aqueous biphasic systems composed of ionic liquids and a citrate-based biodegradable salt. Biochem. Eng. J. 2012, 67, 68–76. [Google Scholar] [CrossRef]
- Sintra, T.E.; Cruz, R.; Ventura, S.P.M.; Coutinho, J.A.P. Phase diagrams of ionic liquids-based aqueous biphasic systems as a platform for extraction processes. J. Chem. Thermodyn. 2014, 77, 206–213. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Tabanez, N.L.; Farias, F.O.; Kurnia, K.A.; Mafra, M.R.; Pereira, J.F.B. Determination, characterization and modeling of aqueous biphasic systems composed of propylammonium-based ionic liquids and phosphate salts. Chem. Phys. Lett. 2020, 754, 137623. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, Y.; Hu, S.; Han, J.; Xu, X. Phase Diagrams of Ammonium Sulfate + Ethanol/1-Propanol/2-Propanol + Water Aqueous Two-Phase Systems at 298.15 K and Correlation. J. Chem. Eng. Data 2010, 55, 876–881. [Google Scholar] [CrossRef]
- Arzideh, S.M.; Movagharnejad, K.; Pirdashti, M. Influence of the Temperature, Type of Salt, and Alcohol on Phase Diagrams of 2-Propanol + Inorganic Salt Aqueous Two-Phase Systems: Experimental Determination and Correlation. J. Chem. Eng. Data 2018, 63, 2813–2824. [Google Scholar] [CrossRef]
- Ferreira-Faria, D.; Aires-Barros, M.R.; Azevedo, A.M. Continuous aqueous two-phase extraction: From microfluidics to integrated biomanufacturing. Fluid Phase Equilib. 2019, 508, 112438. [Google Scholar] [CrossRef]
- Albertsson, P.Å. Fractionation of particles and macromolecules in aqueous two-phase systems. Biochem. Pharmacol. 1961, 5, 351–358. [Google Scholar] [CrossRef]
- Asenjo, J.A.; Andrews, B.A. Aqueous two-phase systems for protein separation: A perspective. J. Chromatogr. A 2011, 1218, 8826–8835. [Google Scholar] [CrossRef]
- Iqbal, M.; Tao, Y.; Xie, S.; Zhu, Y.; Chen, D.; Wang, X.; Huang, L.; Peng, D.; Sattar, A.; Shabbir, M.A.B.; et al. Aqueous two-phase system (ATPS): An overview and advances in its applications. Biol. Proced. Online 2016, 18, 1–18. [Google Scholar] [CrossRef]
- Badhwar, P.; Kumar, P.; Dubey, K.K. Extractive Fermentation for Process integration and amplified pullulan production by A. pullulans in Aqueous Two Phase Systems. Sci. Rep. 2019, 9, 32. [Google Scholar] [CrossRef]
- Singh, S.; Tavana, H. Collagen Partition in Polymeric Aqueous Two-Phase Systems for Tissue Engineering. Front. Chem. 2018, 6, 379. [Google Scholar] [CrossRef]
- Ruiz, C.A.S.; van den Berg, C.; Wijffels, R.H.; Eppink, M.H.M. Rubisco separation using biocompatible aqueous two-phase systems. Sep. Purif. Technol. 2018, 196, 254–261. [Google Scholar] [CrossRef]
- Flores-Gatica, M.; Castañeda-Aponte, H.; Gil-Garzon, M.R.; Mora-Galvez, L.M.; Banda-Magaña, M.P.; Jáuregui-Jáuregui, J.A.; Torres-Acosta, M.A.; Mayolo-Deloisa, K.; Licona-Cassani, C. Primary recovery of hyaluronic acid produced in Streptococcus equi subsp. zooepidemicus using PEG–citrate aqueous two-phase systems. AMB Express 2021, 11, 123. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, L.; Zhu, M.; Wu, S.; Wang, X.; Li, D.; Liu, C.; Feng, Z.; Tian, B. Separation, structural characteristics and biological activity of lactic acid bacteria exopolysaccharides separated by aqueous two-phase system. LWT 2021, 147, 111617. [Google Scholar] [CrossRef]
- Iyyaswami, R.; Belur, P.D.; Girish, B.; Nagaraj, V.H. Development and Evaluation of PEG-Lithium Citrate Salt Based Aqueous Two Phase System and Its Application in Partitioning of Proteins from Fish Industry Effluent. Sep. Sci. Technol. 2012, 47, 591–598. [Google Scholar] [CrossRef]
- Chow, Y.H.; Yap, Y.J.; Tan, C.P.; Anuar, M.S.; Tejo, B.A.; Show, P.L.; Ariff, A.B.; Ng, E.-p.; Ling, T.C. Characterization of bovine serum albumin partitioning behaviors in polymer-salt aqueous two-phase systems. J. Biosci. Bioeng. 2015, 120, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.K.; Ooi, C.W.; Tan, J.S.; Show, P.L.; Ariff, A.; Ling, T.C. Recovery of human interferon alpha-2b from recombinant Escherichia coli using alcohol/salt-based aqueous two-phase systems. Sep. Purif. Technol. 2013, 120, 362–366. [Google Scholar] [CrossRef]
- Cheong, K.L.; Xia, L.X.; Liu, Y. Isolation and Characterization of Polysaccharides from Oysters (Crassostrea gigas) with Anti-Tumor Activities Using an Aqueous Two-Phase System. Mar. Drugs 2017, 15, 338. [Google Scholar] [CrossRef]
- Li, Z.; Pei, Y.; Wang, H.; Fan, J.; Wang, J. Ionic liquid-based aqueous two-phase systems and their applications in green separation processes. TRAC Trends Anal. Chem. 2010, 29, 1336–1346. [Google Scholar] [CrossRef]
- Freire, M.G.; Claudio, F.M.; Araújo, J.M.M.; Coutinho, J.A.P.; Marrucho, I.M.; Lopes, J.N.C.; Rebelo, L.P.N. Aqueous biphasic systems: A boost brought about by using ionic liquids. Chem. Soc. Rev. 2012, 41, 4966–4995. [Google Scholar] [CrossRef]
- Du, Z.; Yu, Y.-l.; Wang, J.-h. Extraction of Proteins from Biological Fluids by Use of an Ionic Liquid/Aqueous Two-Phase System. Chem.-A Eur. J. 2007, 13, 2130–2137. [Google Scholar] [CrossRef]
- Yan, J.-k.; Ma, H.-l.; Pei, J.-j.; Wang, Z.-b.; Wu, J.-y. Facile and effective separation of polysaccharides and proteins from Cordyceps sinensis mycelia by ionic liquid aqueous two-phase system. Sep. Purif. Technol. 2014, 135, 278–284. [Google Scholar] [CrossRef]
- Van Lente, J.; Pazos Urrea, M.; Brouwer, T.; Schuur, B.; Lindhoud, S. Complex coacervates as extraction media. Green Chem. 2021, 23, 5812–5824. [Google Scholar] [CrossRef]
- Lindhoud, S.; Claessens, M.M.A.E. Accumulation of small protein molecules in a macroscopic complex coacervate. Soft Matter 2016, 12, 408–413. [Google Scholar] [CrossRef]
- Cláudio, A.F.M.; Marques, C.F.C.; Boal-Palheiros, I.; Freire, M.G.; Coutinho, J.A.P. Development of back-extraction and recyclability routes for ionic-liquid-based aqueous two-phase systems. Green Chem. 2014, 16, 259–268. [Google Scholar] [CrossRef]
- Ruiz, C.A.S.; Kwaijtaal, J.; Peinado, O.C.; Van Den Berg, C.; Wijffels, R.H.; Eppink, M.H.M. Multistep Fractionation of Microalgal Biomolecules Using Selective Aqueous Two-Phase Systems. ACS Sustain. Chem. Eng. 2020, 8, 2441–2452. [Google Scholar]
- Li, Z.; Liu, X.; Pei, Y.; Wang, J.; He, M. Design of environmentally friendly ionic liquid aqueous two-phase systems for the efficient and high activity extraction of proteins. Green Chem. 2012, 14, 2941–2950. [Google Scholar] [CrossRef]
- Alcântara, L.A.P.; do Nascimento, K.S.; Mourão, C.A.; Minim, V.P.R.; Minim, L.A. Aqueous two-phase poly(ethylene glycol)–sodium polyacrylate system for amyloglucosidase purification: Equilibrium diagrams and partitioning studies. Sep. Purif. Technol. 2013, 118, 888–894. [Google Scholar] [CrossRef]
- Castro, L.S.; Pereira, P.; Passarinha, L.A.; Freire, M.G.; Pedro, A.Q. Enhanced performance of polymer-polymer aqueous two-phase systems using ionic liquids as adjuvants towards the purification of recombinant proteins. Sep. Purif. Technol. 2020, 248, 117051. [Google Scholar] [CrossRef]
- González-Amado, M.; Tavares, A.P.; Freire, M.G.; Soto, A.; Rodríguez, O. Recovery of lactose and proteins from cheese whey with poly (ethylene) glycol/sulfate aqueous two-phase systems. Sep. Purif. Technol. 2021, 255, 117686. [Google Scholar] [CrossRef]
- Lo, S.C.; Ramanan, R.N.; Tey, B.T.; Tan, W.S.; Show, P.L.; Ling, T.C.; Ooi, C.W. Purification of the recombinant enhanced green fluorescent protein from Escherichia coli using alcohol+salt aqueous two-phase systems. Sep. Purif. Technol. 2018, 192, 130–139. [Google Scholar] [CrossRef]
- Amid, M.; Abdul Manap, M.Y.; Mustafa, S. Purification of pectinase from mango (Mangifera indica L. cv. Chokanan) waste using an aqueous organic phase system: A potential low cost source of the enzyme. J. Chromatogr. B 2013, 931, 17–22. [Google Scholar] [CrossRef]
- Li, Z.; Chen, A.; Li, Z.; Qu, M.; Chen, H.; Yang, B.; Wang, Y. A novel and environmentally friendly bioprocess for separation and partial purification of polysaccharides from: Cordyceps sinensis mycelia by an aqueous two-phase system. RSC Adv. 2017, 7, 37659–37665. [Google Scholar] [CrossRef]
- Santos, J.H.P.M.; Trigo, J.P.; Maricato, E.; Nunes, C.; Coimbra, M.; Ventura, S.P.M. Fractionation of Isochrysis galbana Proteins, Arabinans, and Glucans Using Ionic-Liquid-Based Aqueous Biphasic Systems. ACS Sustain. Chem. 2018, 6, 14042–14053. [Google Scholar] [CrossRef]
- Pei, Y.; Wang, J.; Wu, K.; Xuan, X.; Lu, X. Ionic liquid-based aqueous two-phase extraction of selected proteins. Sep. Purif. Technol. 2009, 64, 288–295. [Google Scholar] [CrossRef]
- Quental, M.V.; Caban, M.; Pereira, M.M.; Stepnowski, P.; Coutinho, J.A.P.; Freire, M.G. Enhanced extraction of proteins using cholinium-based ionic liquids as phase-forming components of aqueous biphasic systems. Biotechnol. J. 2015, 10, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-k.; Wang, Y.-y.; Qiu, W.-y.; Ma, H.; Wang, Z.-b. Three-phase partitioning as an elegant and versatile platform applied to nonchromatographic bioseparation processes. Crit. Rev. Food Sci. Nutr. 2017, 58, 2416–2431. [Google Scholar] [CrossRef] [PubMed]
- Dennison, C.; Lovrien, R. Three Phase Partitioning: Concentration and Purification of Proteins. Protein Expr. Purify. 1997, 11, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Ma, H.; Yan, J.-K.; Wang, K.-D.; Yang, Y.; Wang, W.-H.; Zhang, H.-N. Three-phase partitioning system with dimethyl carbonate as organic phase for partitioning of exopolysaccharides from Phellinus baumii. Int. J. Biol. Macromol. 2019, 131, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gupta, M.N. Three phase partitioning of carbohydrate polymers: Separation and purification of alginates. Carbohydr. Polym. 2002, 48, 391–395. [Google Scholar] [CrossRef]
- Belchior, D.C.V.; Freire, M.G. Simultaneous separation of egg white proteins using aqueous three-phase partitioning systems. J. Mol. Liq. 2021, 336, 116245. [Google Scholar] [CrossRef]

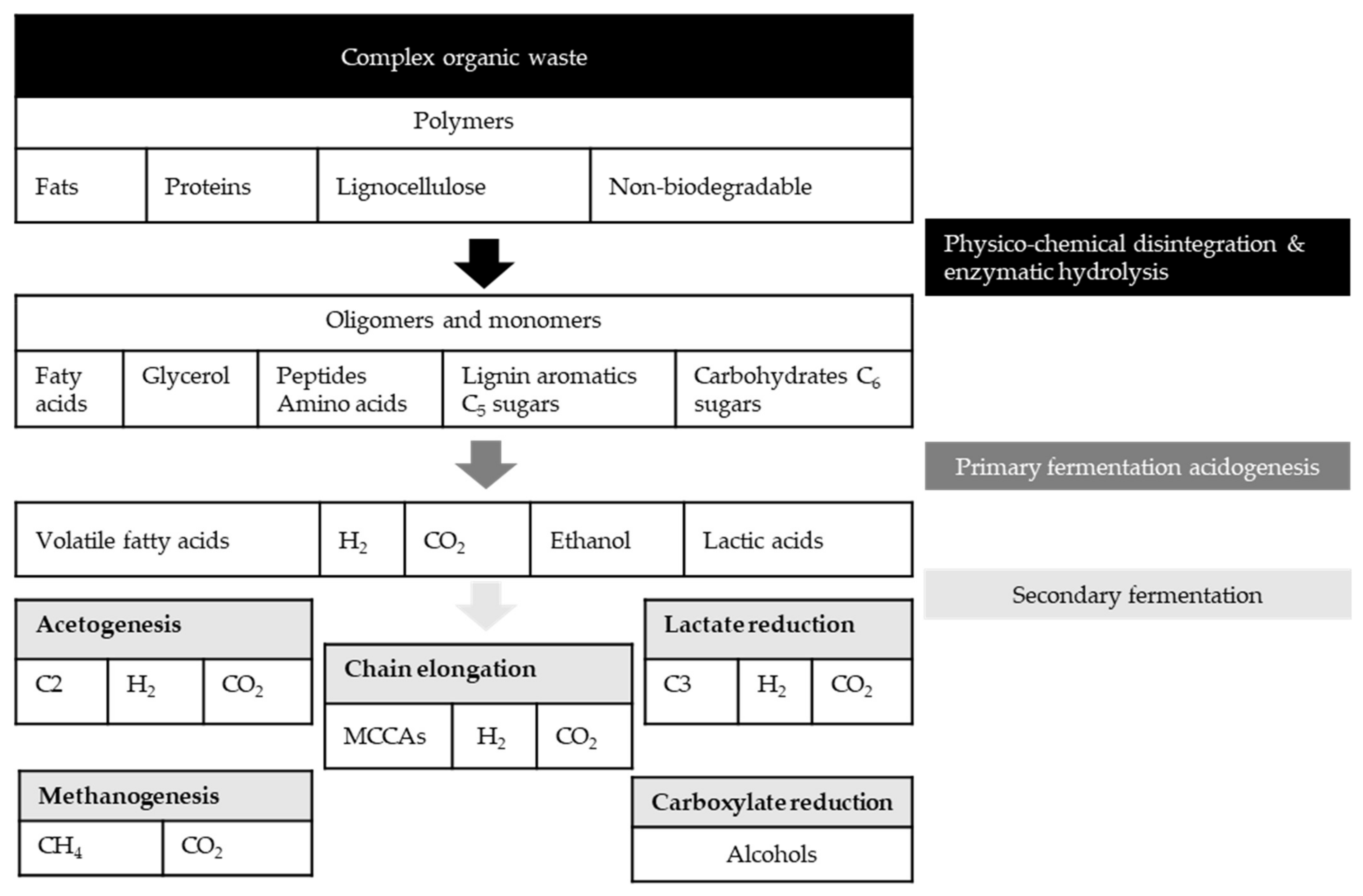
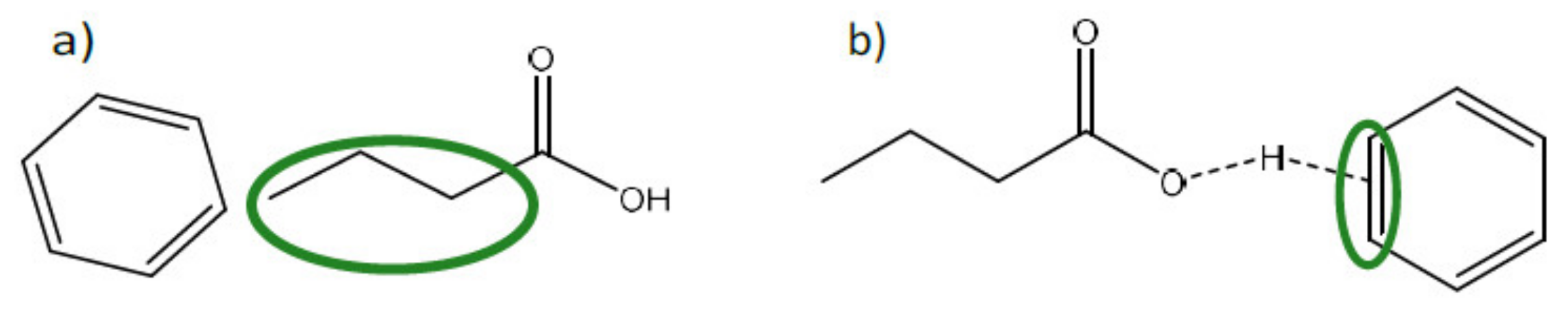

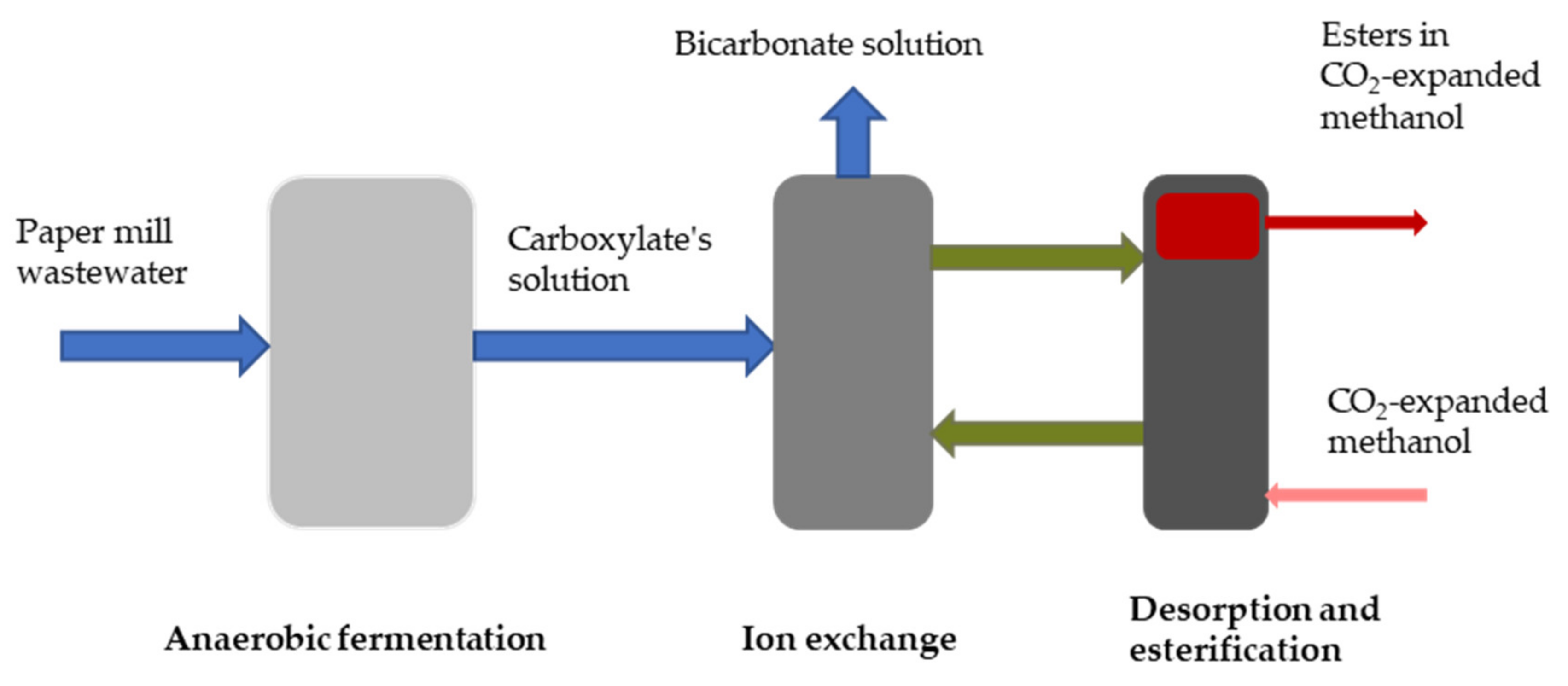


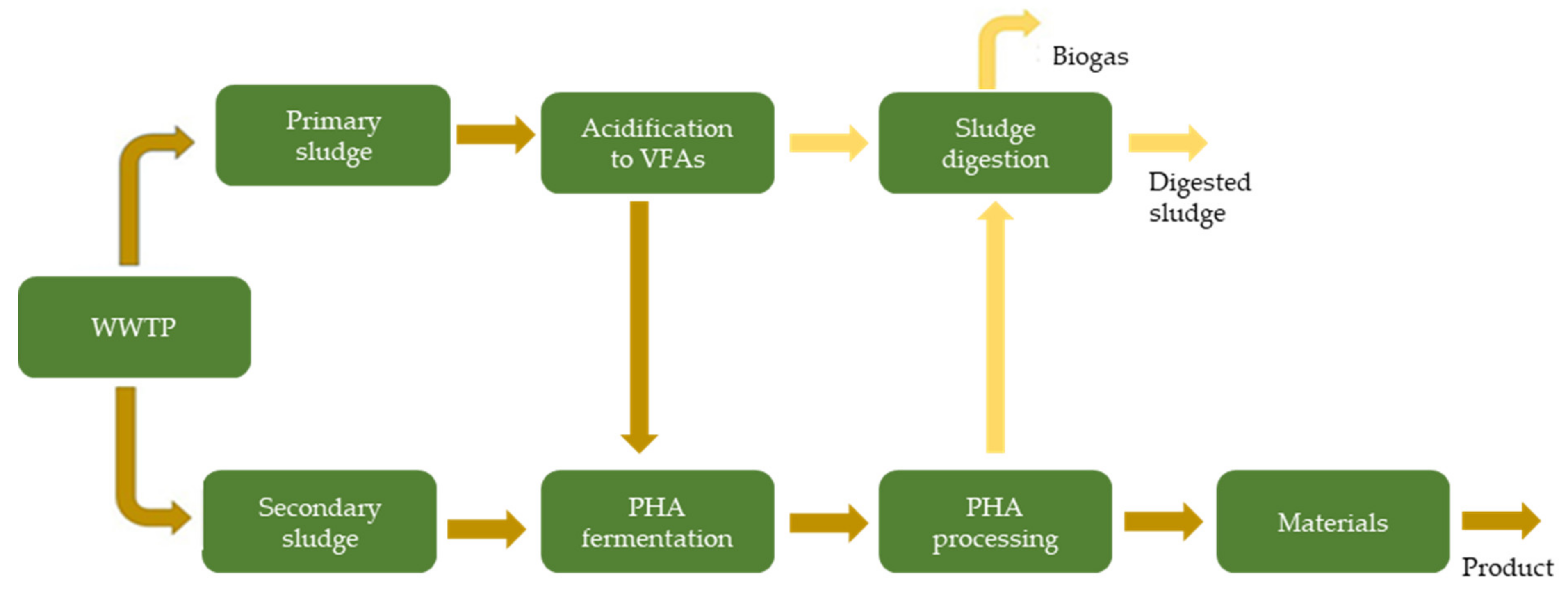

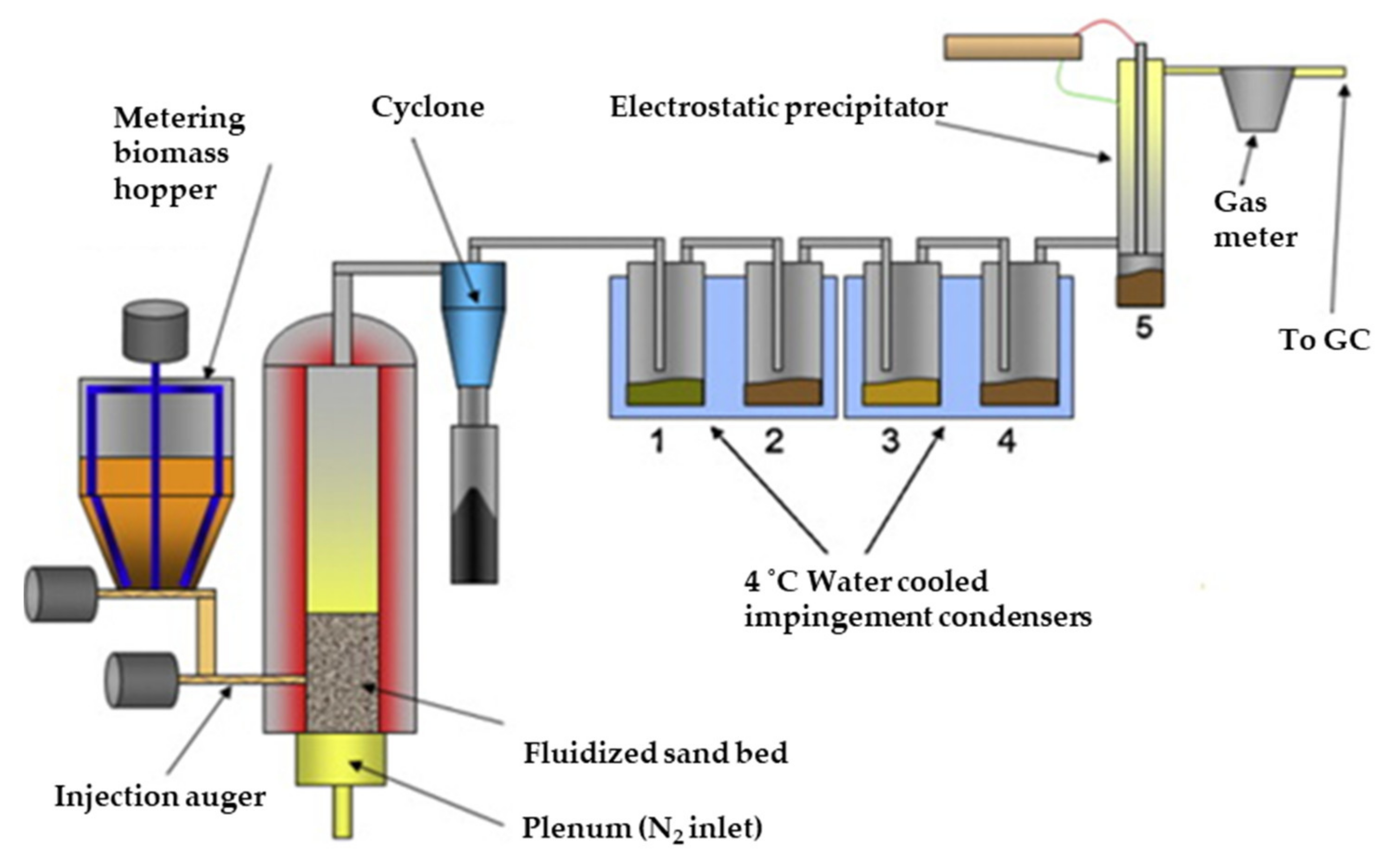

| Fermentation pH | Main Carboxylate Species | Amine B or Ammonium Compound Q | Interaction Mechanism | Remarks |
|---|---|---|---|---|
| pH < pKa | HA | Primary, secondary, or tertiary amine (B) | • Acid-base reaction leading to BH + A− ion pairing • H-bonding B:HA | Ion pairing prevails over H-bonding for strong basic B H-bonding between the amine and hydroxyl moiety of the acid |
| Primary, secondary, or tertiary ammonium salt (QH + X−) | • H-bonding QH + X−:HA • Anion exchange reaction leading to QH + A− | Anion exchange will occur only for A− being weaker base than X− | ||
| Quaternary ammonium hydroxide (Q + OH-) | • Anion exchange reaction leading to Q + A− | |||
| Quaternary ammonium salt (Q + X−) | • H-bonding Q + X−:HA • Anion exchange Reaction leading to Q + A− | Anion exchange will occur only for A− being weaker base than X− | ||
| pH < pKa | A− | Primary or secondary amine (B) | • H-bonding B:A | H-bonding between the amine and carbonyl group of the acid |
| Primary, secondary, or tertiary ammonium salt (QH + X−) | • Anion exchange reaction leading to QH + A− | |||
| Quaternary ammonium hydroxide (Q + OH-) | • Anion exchange reaction leading to Q + A− | |||
| Quaternary ammonium salt (Q + X−) | anion exchange reaction leading to Q + A− |
| Adsorbent | Functional Group | Adsorbent Regeneration Technique | Remarks | Limitations | Ref. |
|---|---|---|---|---|---|
| Activated carbon | Basified organic solvents as eluent | Carboxylates are recovered. Energy duty not in this stage, but with regeneration from the basified solvent | Requires extra distillation step to recover the organic solvent and carboxylate salts from the eluent Recovers the acids as a carboxylate salt | [50] | |
| Synthetic polyaromatic resins | Non | Thermal desorption | Recover the VFAs in their acid form Enables the fractionation of the acids High concentration factor can be achieved | Energy demand in regeneration can be high, depending on the acid to water ratio in the adsorbent pores | [28] |
| Functionalized synthetic polyaromatic resins | Pyridine, imidazole, and primary, secondary or tertiary amine (weak base) | Base eluents Mineral acid eluents | Carboxylates are recovered. Energy duty not in this stage, but with regeneration from the basified solvent | Stochiometric waste salt coproduction Requires extra agent to protonate the carboxylates Requires extra separation method to recover the acids as a carboxylate salt from the eluent | [55] |
| Organic solvents as eluent | Requires extra step to recover carboxylate salts from the eluent | ||||
| Functionalized synthetic polyaromatic resins | Quaternary ammonium (strong base) | Base eluents | Stochiometric waste salt coproduction when carboxylates are targeted Requires extra step to recover carboxylate salts from the eluent | [55] | |
| Functionalized synthetic polyaromatic resins | Quaternary ammonium (strong base) | CO2 expanded alcohol | No stochiometric waste salt production Combined desorption and esterification of the acids | High pressure required for acid desorption Requires extra step to recover carboxylic acids from the eluent (e.g., distillation) | [56] |
| HB Content PHBV (mol%) | Catalyst | Degradation Temperature (°C) | Pyrolyis Temperature (°C) | Crotonic Acid Yield (wt%) | Pyrolyzates Composition (wt%) | Ref. |
|---|---|---|---|---|---|---|
| 100 | No | 260–290 | 260 | N/A | Crotonic acid 67.7 Isocrotonic acid 3.1 Oligomers 29.2 | [13] |
| 100 | No | 290 | 290 | 62.5 | Crotonic acid 63.8 Isocrotonic acid 1.0 Oligomers 33.7 3-hydroxybutyric acid 1.5 | [16] |
| 88 | No | 280–290 | 280 | N/A | Crotonic acid 60.34 2-pentenoic acid 7.13 Oligomers 32.53% | [119] |
| 88 | Yes (MgOH2) | 240–250 | 260 | N/A | Crotonic acid 85.31 2-pentenoic acid 10.92 Oligomers 3.77 | [119] |
| 100 | No | 280 | 280 | 57.0 | Crotonic acid 57.1 Isocrotonic acid 3.6 Oligomers 39.3 | [14] |
| 100 | Yes (MgOH2) | 240 | 240 | 83.0 | Crotonic acid 97.7 Isocrotonic acid 0.6 Oligomers 1.7 | [14] |
| 98.95 | No | 280–290 | 290 | N/A | Crotonic acid 58.09 2-pentenoic acid 0.51 Isopropyl-2-crotonic acid 38.38 Butyric-2-crotonic acid 2.9 | [15] |
| 100 | No | 300–310 | 310 | 65 | Crotonic acid 57.1 Isocrotonic acid 5.0 Oligomers 37.9 | [84] |
| 100 | No, NaOH pretreament | 300–310 | 310 | 80 | Crotonic acid 86.6 Isocrotonic acid 1.9 Oligomers 11.5 | [84] |
| 100 | No | N/A | 250 | N/A | Crotonic acid 64.4 Oligomer 8.4 | [116] |
| 100 | No | 290 | 170 at 150 mbar | 58 | 92% Crotonic acid | [107] |
| Property | Crotonic Acid | 2-Pentenoic Acid |
|---|---|---|
| Molecular weight (g mol−1) | 86.0892 [124] | 100.117 [125] |
| Boiling point (°C @ 760 mmHg) | 184.7 [124] | 200–203 [126] |
| Melting point (°C) | 72 [124] | 8–10 [126] |
| Water solubility (g L−1 @ 25 °C) | 94 [124] | 62.9 [125] |
| Density (g Ml−1 @ 25 °C) | 1.027 [127] | 0.99 [128] |
| pKa (@ 25 °C) | 4.817 [124] | 5.02 [129] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhami, V.; Antunes, E.C.; Temmink, H.; Schuur, B. Recovery Techniques Enabling Circular Chemistry from Wastewater. Molecules 2022, 27, 1389. https://doi.org/10.3390/molecules27041389
Elhami V, Antunes EC, Temmink H, Schuur B. Recovery Techniques Enabling Circular Chemistry from Wastewater. Molecules. 2022; 27(4):1389. https://doi.org/10.3390/molecules27041389
Chicago/Turabian StyleElhami, Vahideh, Evelyn C. Antunes, Hardy Temmink, and Boelo Schuur. 2022. "Recovery Techniques Enabling Circular Chemistry from Wastewater" Molecules 27, no. 4: 1389. https://doi.org/10.3390/molecules27041389
APA StyleElhami, V., Antunes, E. C., Temmink, H., & Schuur, B. (2022). Recovery Techniques Enabling Circular Chemistry from Wastewater. Molecules, 27(4), 1389. https://doi.org/10.3390/molecules27041389






