In Vivo Antistress Effects of Synthetic Flavonoids in Mice: Behavioral and Biochemical Approach
Abstract
:1. Introduction
2. Results
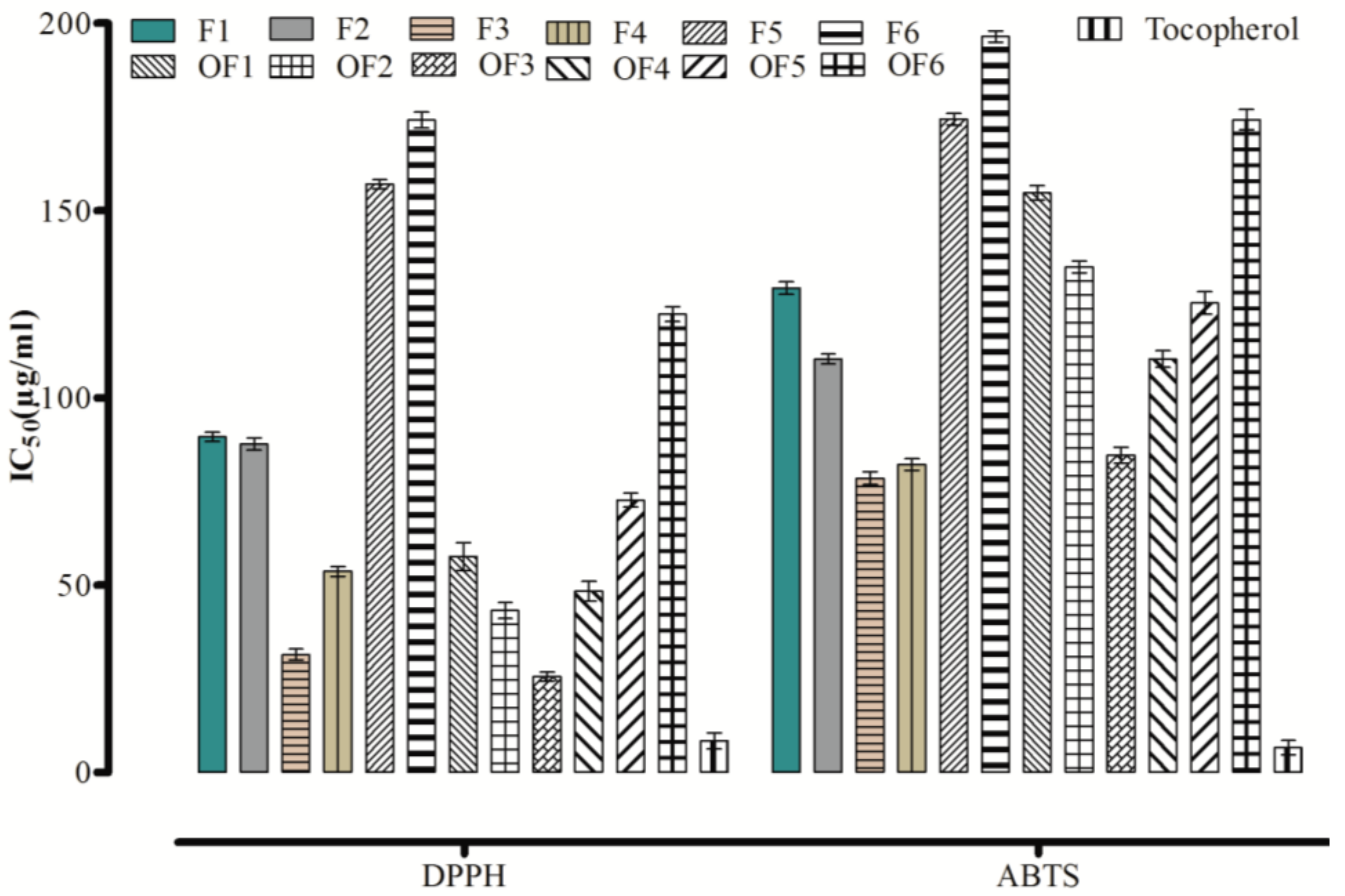
2.1. In Vitro Antioxidant Activity
2.2. Acute Toxicity Study
2.3. Chemical Induced Stress
2.4. Swimming Endurance Test
2.5. Restraint Stress
2.6. Assessment of Biochemical Parameters and Biomarker Study
3. Discussion
4. Materials and Methods
4.1. Chemicals and Animals
4.2. In Vitro Antioxidant Activity
4.3. Acute Toxicity Study
4.4. Anti-Stress Activity
4.4.1. Chemical Induced Stress
4.4.2. Swimming Endurance Test
4.4.3. Restraint Stress
4.5. Assessment of Biochemical Parameters and Biomarker Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Doreddula, S.K.; Bonam, S.R.; Gaddam, D.P.; Desu, B.S.R.; Ramarao, N.; Pandy, V. Phytochemical analysis, antioxidant, antistress, and nootropic activities of aqueous and methanolic seed extracts of ladies finger (Abelmoschus esculentus L.) in mice. Sci. World J. 2014, 2014, 519848. [Google Scholar] [CrossRef] [Green Version]
- Şahin, E.; Gümüşlü, S. Alterations in brain antioxidant status, protein oxidation and lipid peroxidation in response to different stress models. Behav. Brain Res. 2004, 155, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Sulakhiya, K.; Patel, V.K.; Saxena, R.; Dashore, J.; Srivastava, A.K.; Rathore, M. Effect of Beta vulgaris Linn. leaves extract on anxiety-and depressive-like behavior and oxidative stress in mice after acute restraint stress. Pharmacogn. Res. 2016, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Kalogeris, T.; Bao, Y.; Korthuis, R.J. Mitochondrial reactive oxygen species: A double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014, 2, 702–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Garg, R.; Prakash, A.K. Effect of St. John’s Wort (Hypericum perforatum) treatment on restraint stress-induced behavioral and biochemical alteration in mice. BMC Complementary Altern. Med. 2010, 10, 18. [Google Scholar] [CrossRef] [Green Version]
- de Souza Balk, R.; Bridi, J.C.; de Lima Portella, R.; Carvalho, N.R.; Dobrachinski, F.; Da Silva, M.H.; Amaral, G.P.; Dias, G.R.M.; de Vargas Barbosa, N.; Soares, F.A.A. Clomipramine treatment and repeated restraint stress alter parameters of oxidative stress in brain regions of male rats. Neurochem. Res. 2010, 35, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, M.; Castilla-Ortega, E.; Pedraza, C.; Blanco, E.; Hurtado-Guerrero, I.; Barbancho, M.A.; Chun, J.; Rodríguez-de-Fonseca, F.; Estivill-Torrús, G.; Núñez, L.J.S. Chronic Immobilization in the ma lpar1 Knockout Mice Increases Oxidative Stress in the Hippocampus. Int. J. Neurosci. 2012, 122, 583–589. [Google Scholar] [CrossRef]
- Nade, V.S.; Kawale, L.A.; Naik, R.A.; Yadav, A.V. Adaptogenic effect of Morus alba on chronic footshock-induced stress in rats. Indian J. Pharmacol. 2009, 41, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habbu, P.; Mahadevan, K.; Kulkarni, P.; Daulatsingh, C.; Veerapur, V.; Shastry, R. Adaptogenic and in vitro antioxidant activity of flavanoids and other fractions of Argyreia speciosa (Burm. f) Boj. in acute and chronic stress paradigms in rodents. Indian J. Exp. Biol. 2010, 48, 53–60. [Google Scholar]
- Sandhya, K.D.; Soniya, M.D.; Navdeep, S.; Pooja, T. Antistress activity of Boerhaavia diffusa root extract and a polyherbal formulation containing Boerhaavia diffusa using cold restraint stress model. Int. J. Pharm. Pharm. Sci. 2011, 3, 130–132. [Google Scholar]
- Ben Othman, M.; Han, J.; El Omri, A.; Ksouri, R.; Neffati, M.; Isoda, H. Antistress effects of the ethanolic extract from Cymbopogon schoenanthus growing wild in Tunisia. Evid. Based Complementary Altern. Med. 2013, 2013, 737401. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Singh, N.; Jaggi, A.S. Anti-stress effects of cilnidipine and nimodipine in immobilization subjected mice. Physiol. Behav. 2012, 105, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Alves-Silva, J.M.; Pereira, O.R.; Cardoso, S.M. Antioxidant capacities of flavones and benefits in oxidative-stress related diseases. Curr. Top. Med. Chem. 2015, 15, 105–119. [Google Scholar] [CrossRef]
- Indap, M.; Radhika, S.; Motiwale, L.; Rao, K. Quercetin: Antitumor activity and pharmacological manipulations for increased therapeutic gains. Indian J. Pharm. Sci. 2006, 68, 456–489. [Google Scholar]
- Lipinski, B. Hydroxyl radical and its scavengers in health and disease. Oxidative Med. Cell. Longev. 2011, 2011, 809696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooqui, T.; Farooqui, A.A. Neuroprotective Effects of Phytochemicals in Neurological Disorders; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Shoaib, M.; Ali Shah, S.W.; Ali, N.; Shah, I.; Naveed Umar, M.; Ayaz, M.; Tahir, M.N.; Akhtar, S. In vitro enzyme inhibition potentials and antioxidant activity of synthetic flavone derivatives. J. Chem. 2015, 2015, 516878. [Google Scholar] [CrossRef] [Green Version]
- Shoaib, M.; Shah, S.; Ali, N.; Shah, I.; Umar, M.; Shafiullah; Tahir, M.; Ghias, M. Synthetic flavone derivatives. An antibacterial evaluation and structure-activity relationship study. Bulg. Chem. Commun. 2016, 48, 414–421. [Google Scholar]
- Ghias, M.; Shoaib, M.; SW, A.S.; Umar, M.N.; Ullah, S.; Ali, N.; Shah, I. Nootropic effects of synthetic flavonoid derivatives on scopolamine induced memory impairment in mice via cholinesterase inhibition and antioxidant system. Pak. J. Pharm. Sci. 2019, 32, 2325–2332. [Google Scholar]
- Shinohara, H.; Fukumitsu, H.; Seto, A.; Furukawa, S. Medium-chain fatty acid-containing dietary oil alleviates the depression-like behaviour in mice exposed to stress due to chronic forced swimming. J. Funct. Foods 2013, 5, 601–606. [Google Scholar] [CrossRef]
- Patel, N.; Galani, V.; Patel, B. Antistress activity of Argyreia speciosa roots in experimental animals. J. Ayurveda Integr. Med. 2011, 2, 129. [Google Scholar]
- Debnath, J.; Prakash, T.; Karki, R.; Kotresha, D.; Sharma, P. An experimental evaluation of anti-stress effects of Terminalia chebula. J. Physiol. Biomed. Sci. 2011, 24, 13–19. [Google Scholar]
- Azmathulla, S.; Hule, A.; Naik, S.R. Evaluation of adaptogenic activity profile of herbal preparation. Indian J. Exp. Biol. 2006, 44, 574–579. [Google Scholar] [PubMed]
- Shoaib, M.; Shah, I.; Ali, N.; Shah, S.W.A. In vitro acetylcholinesterase and butyrylcholinesterase inhibitory potentials of essential oil of Artemisia macrocephala. Bangladesh J. Pharm. 2015, 10, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Lorke, D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983, 54, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.P.; Juvekar, A.R. Attenuation of Acute and Chronic Restraint Stress-induced Perturbations in Experimental Animals by Nelumbo nucifera Gaertn. Indian J. Pharm. Sci. 2008, 70, 327–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, T.; Sah, S.P.; Singh, A. Antistress activity of ethanolic extract of Asparagus racemosus Willd roots in mice. Indian J. Exp. Biol. 2012, 50, 419–424. [Google Scholar]
- Foyet, H.S.; Abaïssou, H.H.N.; Wado, E.; Acha, E.A.; Alin, C. Emilia coccinae (SIMS) G Extract improves memory impairment, cholinergic dysfunction, and oxidative stress damage in scopolamine-treated rats. BMC Complementary Altern. Med. 2015, 15, 333. [Google Scholar] [CrossRef] [Green Version]

| Group | Number of Writhes | Antistress Response (%) |
|---|---|---|
| Control (2% Tween80) | 47.38 ± 2.66 | - |
| F1 | 19.45 ± 1.89 * | 58.94 |
| F2 | 13.12 ± 2.21 *** | 72.30 |
| F3 | 11.18 ± 1.56 *** | 74.40 |
| F4 | 16.21 ± 2.09 ** | 65.78 |
| F5 | 16.31 ± 2.58 ** | 64.57 |
| F6 | 17.01 ± 1.89 ** | 64.09 |
| OF1 | 17.32 ± 2.33 * | 63.44 |
| OF2 | 12.19 ± 2.98 *** | 74.27 |
| OF3 | 9.03 ± 1.79 *** | 80.94 |
| OF4 | 14.18 ± 2.17 ** | 67.07 |
| OF5 | 16.44 ± 3.04 ** | 63.30 |
| OF6 | 15.88 ± 2.11 ** | 66.48 |
| Standard | 4.02 ± 1.87 *** | 91.51 |
| Group | Immobility Time (Min) |
|---|---|
| Control (2% Tween80) | 12.56 ± 1.31 |
| F1 | 4.05 ± 0.45 ** |
| F2 | 3.12 ± 0.38 ** |
| F3 | 2.88 ± 0.26 *** |
| F4 | 3.21 ± 0.19 ** |
| F5 | 3.64 ± 0.21 * |
| F6 | 3.41 ± 0.23 ** |
| OF1 | 4.32 ± 0.31 * |
| OF2 | 2.95 ± 0.37 *** |
| OF3 | 2.33 ± 0.28 *** |
| OF4 | 3.44 ± 0.25 ** |
| OF5 | 3.25 ± 0.29 ** |
| OF6 | 3.34 ± 0.25 ** |
| Standard | 6.12 ± 0.47 |
| Group | Weight in mg | ||
|---|---|---|---|
| Adrenal Gland | Spleen | Brain | |
| Control | 5.45 ± 1.07 | 249.3 ± 2.21 | 453.3 ± 3.11 |
| Stress control | 15.02 ± 1.78 ††† | 101.8 ± 2.56 ††† | 364.7 ± 2.44 ††† |
| F1 | 8.13 ± 1.69 * | 171.5 ± 2.71 * | 398.1 ± 2.57 * |
| F2 | 7.63 ± 1.11 ** | 180.4 ± 2.38 * | 401.6 ± 3.11 ** |
| F3 | 7.22 ± 1.51 ** | 181.7 ± 2.06 ** | 411.3 ± 2.93 *** |
| F4 | 8.03 ± 1.98 * | 173.1 ± 2.66 * | 402.4 ± 3.19 ** |
| F5 | 8.12 ± 1.81 * | 170.3 ± 2.61 * | 412.7 ± 2.81 ** |
| F6 | 8.09 ± 1.77 * | 171.1 ± 2.40 * | 408.3 ± 2.60 ** |
| OF1 | 8.09 ± 1.34 * | 188.4 ± 2.24 ** | 397.4 ± 3.76 |
| OF2 | 7.21 ± 1.87 *** | 190.5 ± 2.76 ** | 414.2 ± 2.51 *** |
| OF3 | 6.79 ± 1.10 *** | 194.8 ± 2.11 *** | 413.6 ± 2.66 *** |
| OF4 | 7.91 ± 1.91 * | 183.9 ± 2.56 ** | 401.8 ± 2.34 ** |
| OF5 | 8.04 ± 1.18 * | 186.3 ± 2.74 ** | 400.5 ± 2.21 * |
| OF6 | 8.11 ± 1.31 * | 179.1 ± 2.30 ** | 403.9 ± 2.239 * |
| Standard | 10.58 ± 5.77 ns | 158.3 ± 2.89 * | 390.8 ± 2.75 * |
| Group | Biochemical Parameters (Blood) | ||
|---|---|---|---|
| Glucose | TGs | Total Ch | |
| Control | 96.43 ± 1.89 | 52.31 ± 0.98 | 79.05 ± 0.54 |
| Stress control | 139.62 ± 2.24 ††† | 101.22 ± 1.34 ††† | 141.72 ± 1.75 ††† |
| F1 | 105.22 ± 2.15 ns | 54.39 ± 0.87 * | 81.33 ± 0.61 * |
| F2 | 99.23 ± 1.69 * | 45.09 ± 0.67 *** | 78.61 ± 0.78 ** |
| F3 | 98.45 ± 1.51 ** | 44.69 ± 0.37 *** | 78.43 ± 0.93 *** |
| F4 | 100.92 ± 1.91 * | 47.51 ± 0.38 *** | 80.76 ± 0.91 ** |
| F5 | 101.33 ± 1.72 * | 46.72 ± 0.51 *** | 81.82 ± 0.85 * |
| F6 | 102.09 ± 1.62 * | 47.26 ± 0.44 *** | 82.01 ± 0.79 * |
| OF1 | 104.55 ± 1.77 ns | 51.22 ± 0.66 ** | 80.81 ± 0.72 ** |
| OF2 | 97.56 ± 1.61 *** | 42.88 ± 0.41 *** | 76.82 ± 0.86 *** |
| OF3 | 97.06 ± 1.71 *** | 43.11 ± 0.36 *** | 76.09 ± 0.64 *** |
| OF4 | 101.15 ± 1.66 * | 46.92 ± 0.44 *** | 78.33 ± 0.76 ** |
| OF5 | 101.09 ± 1.14 * | 46.24 ± 0.39 *** | 77.91 ± 0.97 *** |
| OF6 | 100.32 ± 1.29 * | 44.02 ± 0.30 *** | 77.02 ± 0.97 *** |
| Standard | 108.22 ± 1.06 ns | 38.52 ± 0.57 *** | 75.62 ± 0.65 *** |
| Group | Biochemical Parameters (Brain) | ||
|---|---|---|---|
| Catalase (IU/dL) | SOD (IU/dL) | TBARS (nmol/g) | |
| Control | 23.65 ± 0.89 | 15.75 ± 0.58 | 14.21 ± 0.41 |
| Stress control | 6.71 ± 0.46 ††† | 4.11 ± 0.54 ††† | 27.92 ± 0.75 ††† |
| F1 | 16.33 ± 0.69 *** | 7.96 ± 0.60 *** | 19.42 ± 0.51 *** |
| F2 | 18.21 ± 0.61 | 8.77 ± 0.59 | 18.72 ± 0.62 *** |
| F3 | 18.92 ± 0.51 *** | 9.42 ± 0.87 *** | 18.44 ± 0.63 *** |
| F4 | 17.88 ± 0.59 *** | 9.31 ± 0.78 *** | 19.09 ± 0.49 *** |
| F5 | 17.11 ± 0.51 *** | 8.04 ± 0.57 *** | 19.02 ± 0.71 ** |
| F6 | 17.36 ± 0.62 *** | 8.13 ± 0.51 *** | 18.96 ± 0.60 ** |
| OF1 | 17.02 ± 0.71 *** | 8.46 ± 0.76 *** | 19.25 ± 0.51 *** |
| OF2 | 18.74 ± 0.67 | 9.02 ± 0.54 | 17.74 ± 0.56 *** |
| OF3 | 19.02 ± 0.77 *** | 9.58 ± 0.66 *** | 17.49 ± 0.61 *** |
| OF4 | 17.45 ± 0.66 *** | 8.35 ± 0.64 *** | 18.67 ± 0.76 *** |
| OF5 | 17.06 ± 0.61 *** | 8.11 ± 0.51 *** | 18.56 ± 0.51 * |
| OF6 | 17.01 ± 0.52 *** | 8.03 ± 0.49 *** | 18.10 ± 0.51 * |
| Standard | 17.25 ± 0.66 *** | 6.56 ± 0.57 *** | 18.22 ± 0.65 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghias, M.; Shah, S.W.A.; Al-Joufi, F.A.; Shoaib, M.; Shah, S.M.M.; Ahmed, M.N.; Zahoor, M. In Vivo Antistress Effects of Synthetic Flavonoids in Mice: Behavioral and Biochemical Approach. Molecules 2022, 27, 1402. https://doi.org/10.3390/molecules27041402
Ghias M, Shah SWA, Al-Joufi FA, Shoaib M, Shah SMM, Ahmed MN, Zahoor M. In Vivo Antistress Effects of Synthetic Flavonoids in Mice: Behavioral and Biochemical Approach. Molecules. 2022; 27(4):1402. https://doi.org/10.3390/molecules27041402
Chicago/Turabian StyleGhias, Mehreen, Syed Wadood Ali Shah, Fakhria A. Al-Joufi, Mohammad Shoaib, Syed Muhammad Mukarram Shah, Muhammad Naeem Ahmed, and Muhammad Zahoor. 2022. "In Vivo Antistress Effects of Synthetic Flavonoids in Mice: Behavioral and Biochemical Approach" Molecules 27, no. 4: 1402. https://doi.org/10.3390/molecules27041402






