Long-Term Potentiodynamic Testing and Tribometric Properties of Amorphous Alloy Coatings under Saline Environment
Abstract
:1. Introduction
2. Results and Discussion
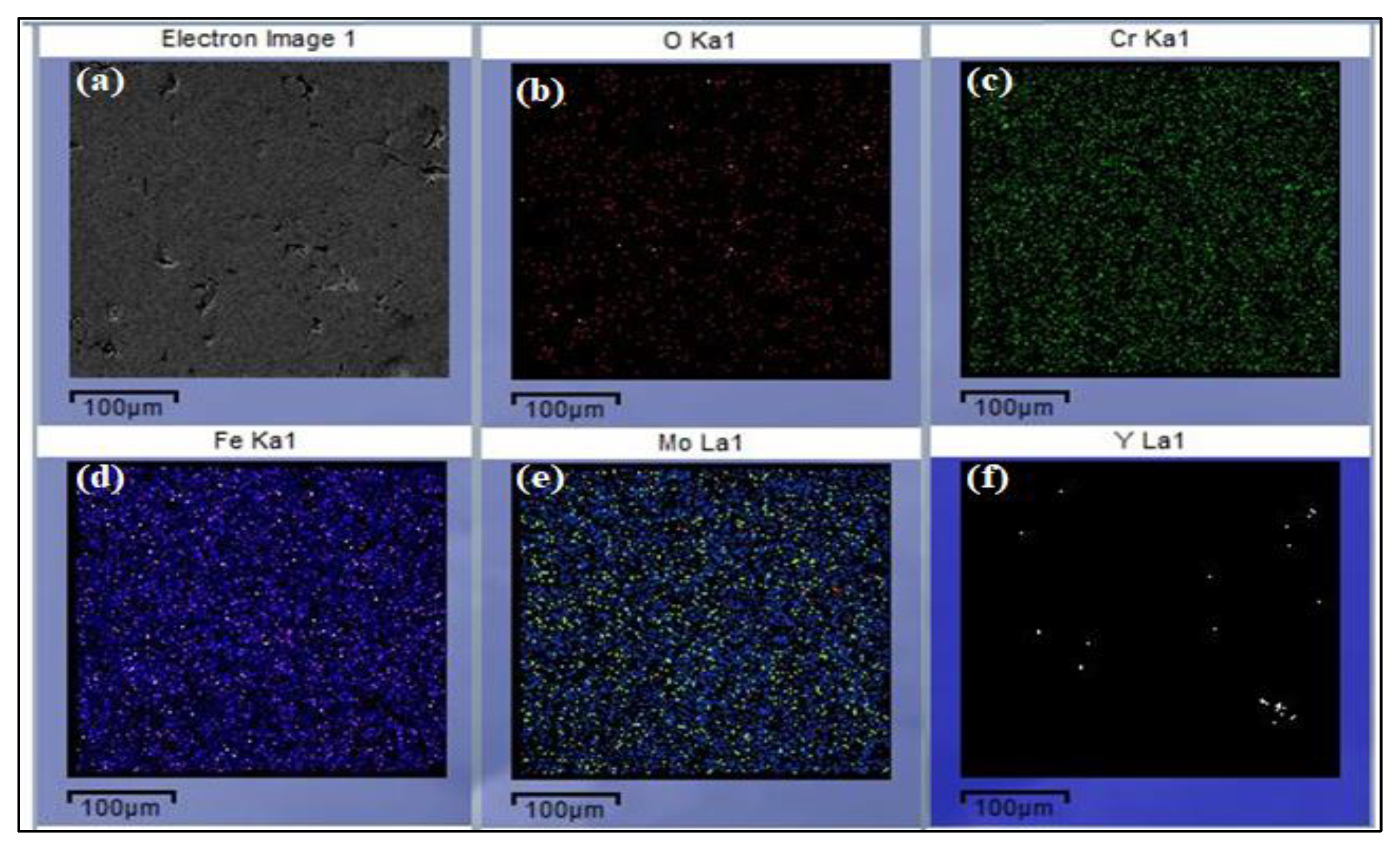
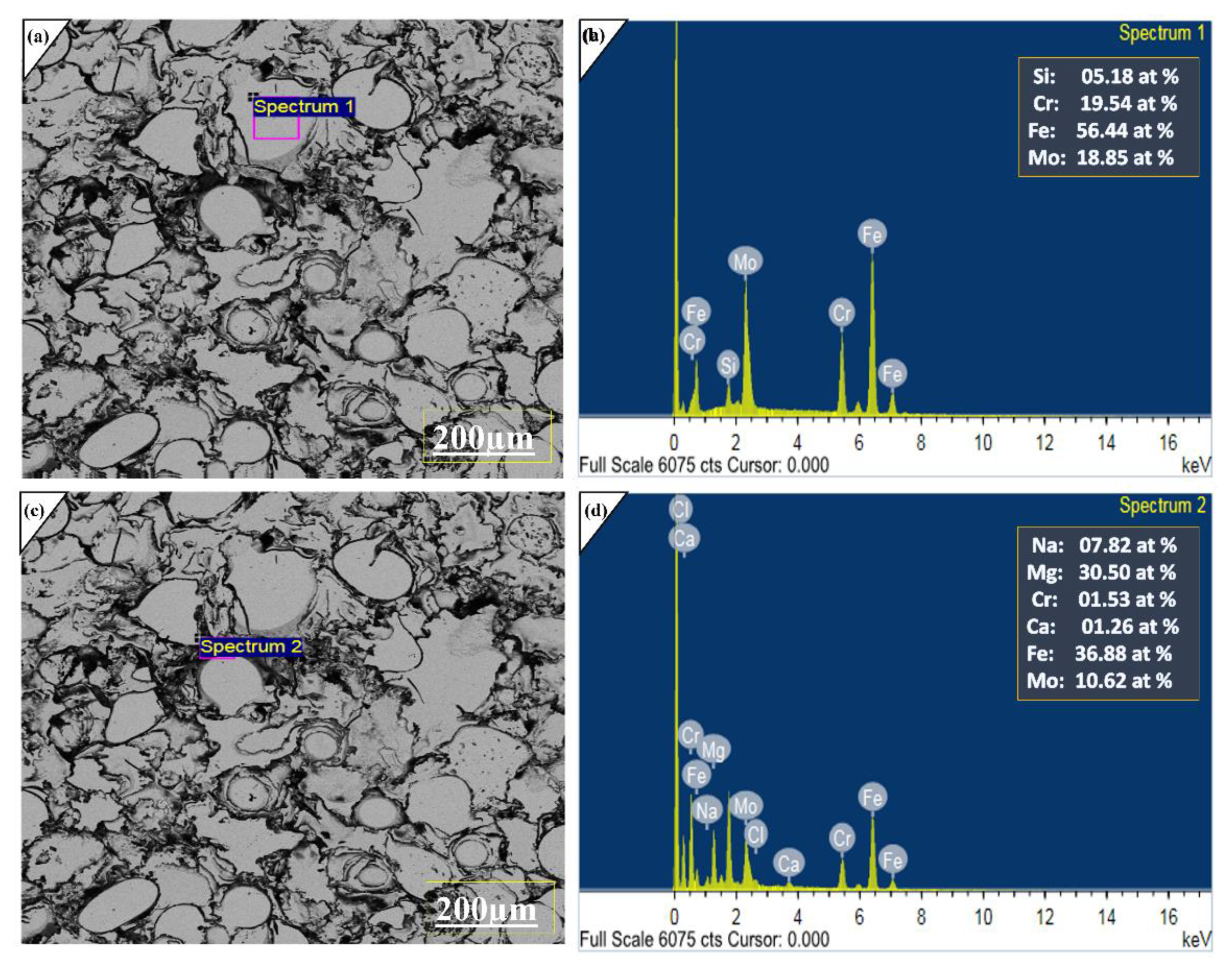
2.1. Microstructure and Characterization Analysis
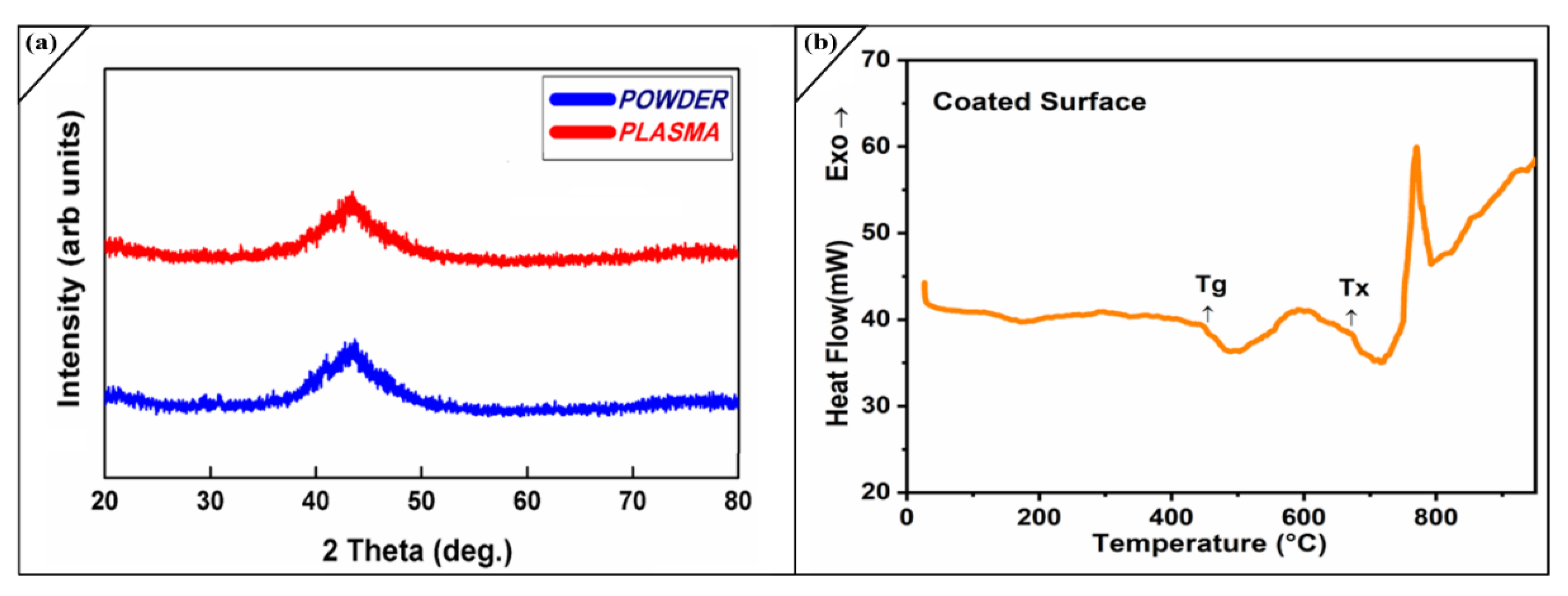
2.2. Phase Composition of the Coatings
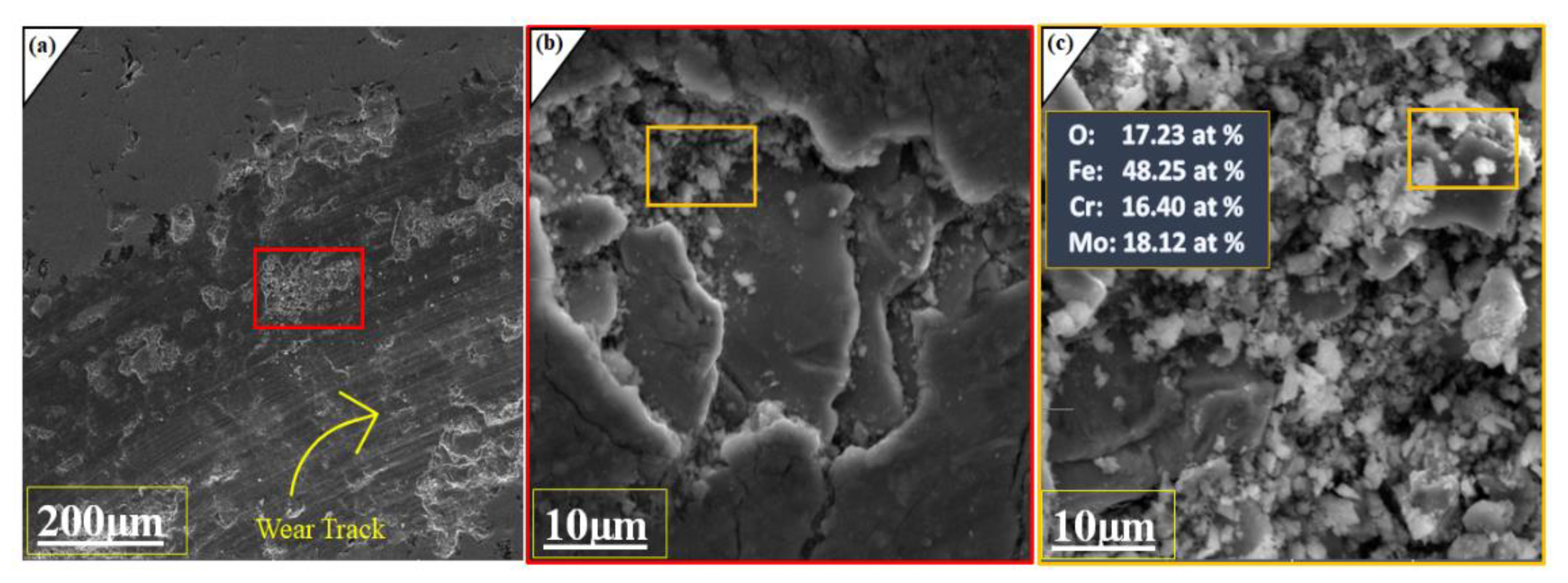
2.3. Mechanical Studies of Coated Layer
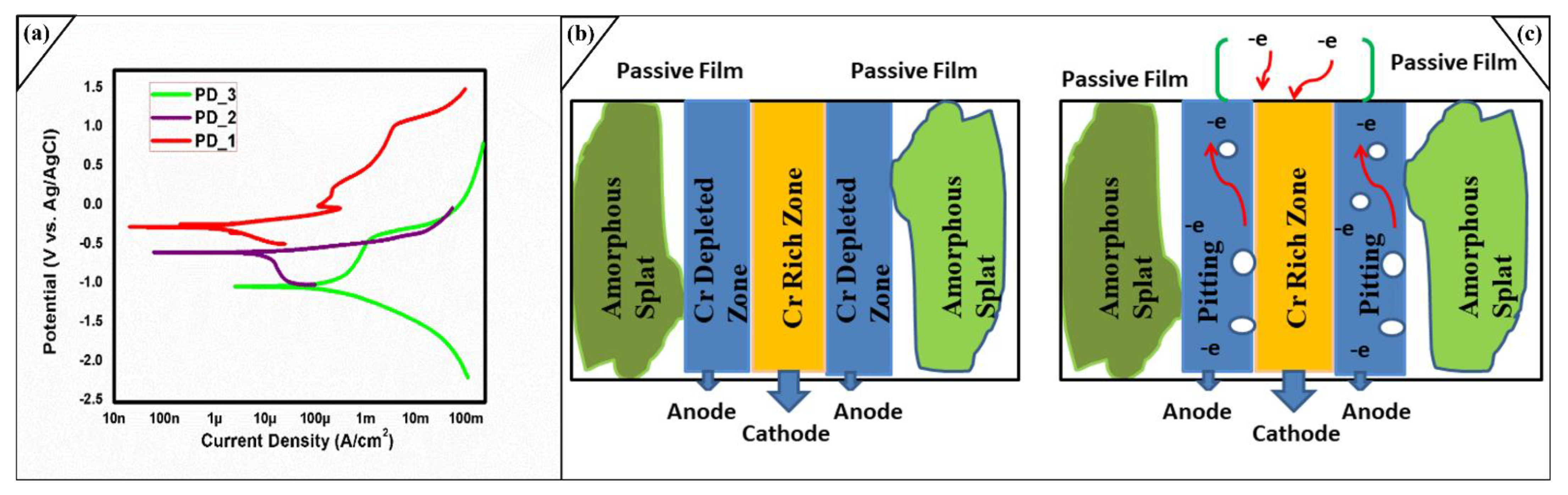
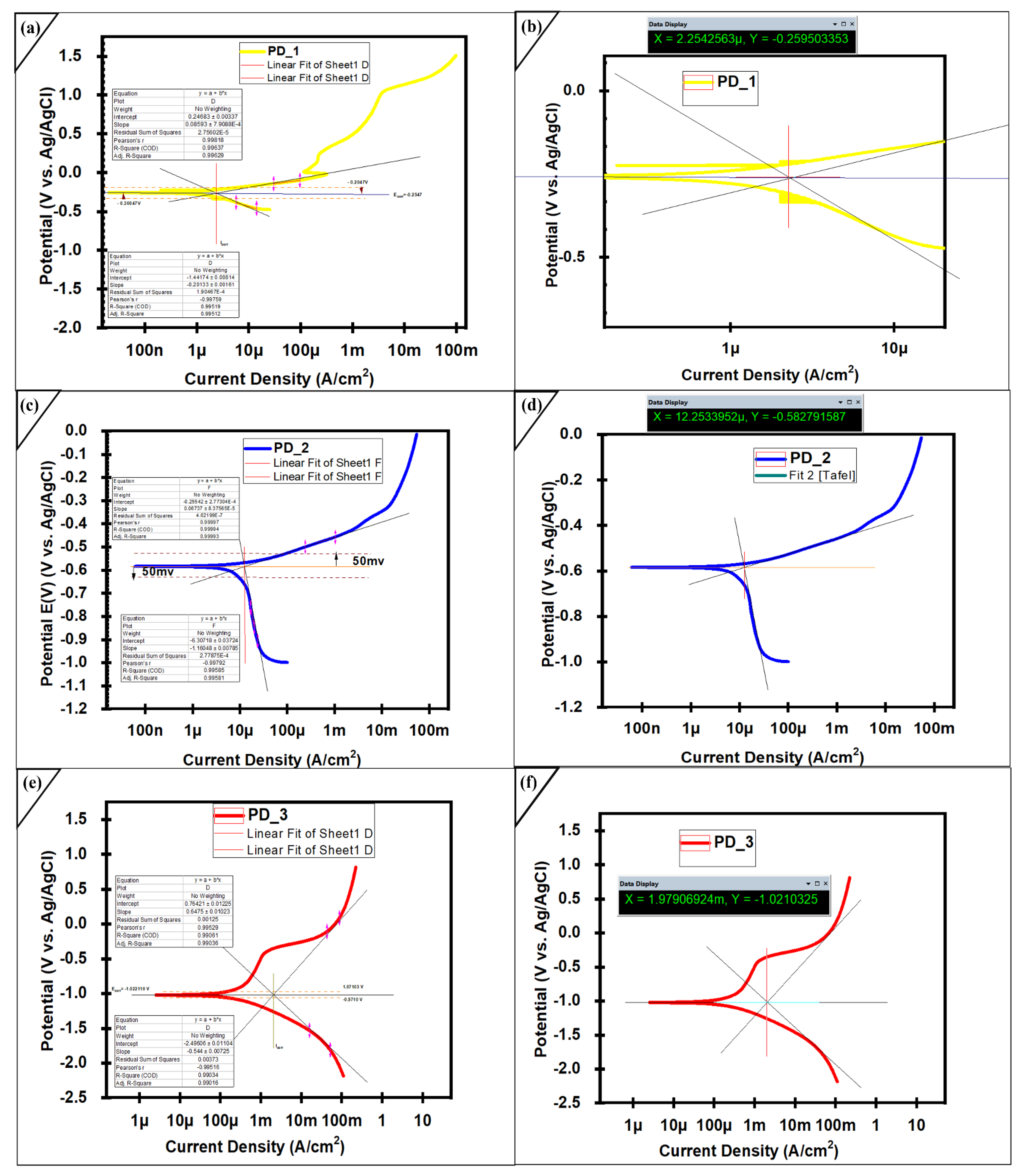
2.4. Electrochemical Studies of Coated Layer
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Amiya, K.; Urata, A.; Nishiyama, N.; Inoue, A. Fe-B-Si-Nb Bulk Metallic Glasses with High Strength above 4000 MPa and Distinct Plastic Elongation. Mater. Trans. 2004, 45, 1214–1218. [Google Scholar] [CrossRef] [Green Version]
- Babilas, R.; Nowosielski, R. Iron-based bulk amorphous alloys. Arch. Mater. Sci. Eng. 2010, 44, 5–27. [Google Scholar]
- Suryanarayana, C.; Inoue, A. Iron-based bulk metallic glasses. Int. Mater. Rev. 2013, 58, 131–166. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, X.; Chen, Y.; Xu, B. Abrasion Resistance of Al-Ni-Mm-Fe Amorphous and Nanocrystalline Composite Coating on the Surface of AZ91 Magnesium Alloy. Phys. Procedia 2013, 50, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Louzguine-Luzgin, D.V.; Inoue, A. Bulk Metallic Glasses. Encycl. Glass Sci. Technol. Hist. Cult. 2021, 2, 919–936. [Google Scholar]
- Proceedings of the 19th Chemnitz Seminar on Materials Engineering—19. Werkstofftechnisches Kolloquium, Chemnitz, Germany, 16–17 March 2017; IOP Publishing: Bristol, UK, 2017; Volume 181, p. 012012.
- Si, C.; Zhang, Z.; Zhang, Q.; Cai, J. Influence of mechanical alloying on the particle size, microstructure and soft magnetic properties of coarse Fe-based amorphous powders prepared by gas atomization. J. Non-Crystalline Solids 2021, 559, 120675. [Google Scholar] [CrossRef]
- Sistaninia, M.; Kasberger, R.; Kolednik, O. To the design of highly fracture-resistant composites by the application of the yield stress inhomogeneity effect. Compos. Struct. 2018, 185, 113–122. [Google Scholar] [CrossRef]
- Huang, F.; Kang, J.-J.; Yue, W.; Fu, Z.-Q.; Zhu, L.-N.; She, D.-S.; Liang, J.; Wang, C.-B. Corrosion Behavior of FeCrMoCBY Amorphous Coating Fabricated by High-Velocity Air Fuel Spraying. J. Therm. Spray Technol. 2019, 28, 842–850. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.G.; Ke, W.; Sun, W.H.; Hou, W.L.; Chang, X.C.; Wang, J.Q. Slurry erosion–corrosion behaviour of high-velocity oxy-fuel (HVOF) sprayed Fe-based amorphous metallic coatings for marine pump in sand-containing NaCl solutions. Corros. Sci. 2011, 53, 3177–3185. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, Y.; Fan, H.; Shen, J. Synthesis of Fe–Cr–Mo–C–B amorphous coating with high corrosion resistance. Mater. Lett. 2012, 89, 229–232. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Zhang, X.; Yi, S. Effects of Cr contents in Fe-based bulk metallic glasses on the glass forming ability and the corrosion resistance. Mater. Chem. Phys. 2009, 113, 878–883. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, C.; Yang, Y.; Peng, Y.; Liu, L. Corrosion and wear resistance of a Fe-based amorphous coating in underground environment. Intermetallics 2012, 30, 94–99. [Google Scholar] [CrossRef]
- Ma, H.R.; Chen, X.Y.; Li, J.W.; Chang, C.T.; Wang, G.; Li, H.; Wang, X.M.; Li, R.W. Fe-based amorphous coating with high corrosion and wear resistance. Surf. Eng. 2016, 33, 56–62. [Google Scholar] [CrossRef]
- Xie, L.; Xiong, X.; Zeng, Y.; Wang, Y. The wear properties and mechanism of detonation sprayed iron-based amor-phous coating. Surf. Coat. Technol. 2019, 366, 146–155. [Google Scholar] [CrossRef]
- Cao, S.; Liang, J.; Zhou, J.; Wang, L. Microstructure evolution and wear resistance of in-situ nanoparticles reinforcing Fe-based amorphous composite coatings. Surf. Interfaces 2020, 21, 100652. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, L.; Chan, K.C.; Chen, Q.; Tang, C.Y. Wear behavior of HVOF-sprayed Fe-based amorphous coatings. Intermetallics 2012, 29, 80–85. [Google Scholar] [CrossRef]
- Ibrahim, M.Z.; Sarhan, A.A.; Kuo, T.Y.; Yusof, F.; Hamdi, M.; Lee, T.M. Developing a new laser cladded FeCrMoCB metallic glass layer on nickel-free stainless-steel as a potential superior wear-resistant coating for joint replacement implants. Surf. Coat. Technol. 2020, 392, 125755. [Google Scholar] [CrossRef]
- Farmer, J.; Choi, J.-S.; Saw, C.; Haslam, J.; Day, D.; Hailey, P.; Lian, T.; Rebak, R.; Perepezko, J.; Payer, J.; et al. Iron-Based Amorphous Metals: High-Performance Corrosion-Resistant Material Development. Met. Mater. Trans. A 2009, 40, 1289–1305. [Google Scholar] [CrossRef]
- Yuting, D.; Guofeng, M. Research Progress of Fe-based Amorphous/Nanocrystalline Alloys. In IOP Con-ference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 565, p. 012048. [Google Scholar]
- Mondal, K.; Nuñez, L., III; Downey, C.M.; van Rooyen, I.J. Recent advances in the thermal barrier coatings for extreme environments. Mater. Sci. Energy Technol. 2021, 4, 208–210. [Google Scholar] [CrossRef]
- Material Performance, NACE Area & Section News, East and Pacific Area. Available online: https://nace.mydigitalpublication.com/publication/?i=667826&article_id=3726115&view=articleBrowser (accessed on 2 February 2022).
- Daniyan, I.; Mpofu, K.; Adeodu, A.; Azeez, T. Investigating the Quality and Conformity of Carbon Steel AISI 1035 under Varying Heat Treatment Conditions. Procedia Manuf. 2019, 35, 1111–1116. [Google Scholar] [CrossRef]
- Chaorun, S.; Weichao, W. Preparation of Fe-Based Amorphous Powders and Its Application in Corrosion Resistant Coating. Sci. Adv. Mater. 2018, 10, 1505–1508. [Google Scholar] [CrossRef]
- Zhou, Z.; Han, F.-X.; Yao, H.-H.; Li, Y.-Z.; Yang, Y.-G.; Guo, X.-Y.; Tan, Z.; Xue, Y.-F.; He, D.-Y.; Wang, L. Novel Fe-Based Amorphous Composite Coating with a Unique Interfacial Layer Improving Thermal Barrier Application. ACS Appl. Mater. Interfaces 2021, 13, 23057–23066. [Google Scholar] [CrossRef]
- Iqbal, A.; Siddique, S.; Maqsood, M.; Rehman, M.A.U.; Yasir, M. Comparative Analysis on the Structure and Properties of Iron-Based Amorphous Coating Sprayed with the Thermal Spraying Techniques. Coatings 2020, 10, 1006. [Google Scholar] [CrossRef]
- Figueroa, I.; Betancourt, I.; Lara, G.; Verduzco, J. Effect of B, Si and Cr on the mechanical properties of Fe-based amorphous metallic ribbons. J. Non-Crystalline Solids 2005, 351, 3075–3080. [Google Scholar] [CrossRef]
- Li, X.; Zhai, H.; Li, W.; Cui, S.; Ning, W.; Qiu, X. Dry sliding wear behaviors of Fe-based amorphous metallic coating synthesized by d-gun spray. J. Non-Crystalline Solids 2020, 537, 120018. [Google Scholar] [CrossRef]
- Koga, G.; Schulz, R.; Savoie, S.; Nascimento, A.; Drolet, Y.; Bolfarini, C.; Kiminami, C.; Botta, W. Microstructure and wear behavior of Fe-based amorphous HVOF coatings produced from commercial precursors. Surf. Coatings Technol. 2016, 309, 938–944. [Google Scholar] [CrossRef]
- Soleymanibrojeni, M.; Shi, H.; Udoh, I.I.; Liu, F.; Han, E.-H. Microcontainers with 3-amino-1,2,4-triazole-5-thiol for Enhancing Anticorrosion Waterborne Coatings for AA2024-TProg. Org. Coatings 2019, 137, 105336. [Google Scholar] [CrossRef]
- Qi, J.; Ye, Z.; Gong, N.; Qu, X.; Mercier, D.; Światowska, J.; Skeldon, P.; Marcus, P. Formation of a trivalent chromium con-version coating on AZ91D magnesium alloy. Corros. Sci. 2021, 186, 109459. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, R.; Yang, Y.; Wu, Y.; Liu, L. Influence of the size of spraying powders on the microstructure and corrosion resistance of Fe-based amorphous coating. Electrochim. Acta 2011, 56, 6380–6388. [Google Scholar] [CrossRef]
- Katakam, S.; Santhanakrishnan, S.; Dahotre, N.B. Fe-Based Amorphous Coatings on AISI 4130 Structural Steel for Corrosion Resistance. JOM 2012, 64, 709–715. [Google Scholar] [CrossRef]
- Zheng, Z.; Zheng, Y.; Sun, W.; Wang, J. Effect of applied potential on passivation and erosion–corrosion of a Fe-based amorphous metallic coating under slurry impingement. Corros. Sci. 2014, 82, 115–124. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, Y.; Chu, Z.; Yang, Y.; Li, Y.; Yan, D. Corrosion behavior of plasma sprayed ceramic and metallic coatings on carbon steel in simulated seawater. Mater. Des. 2013, 52, 630–637. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Lin, T.-J.; Sheu, H.-H.; Lee, H.-B. A study on corrosion and corrosion-wear behavior of Fe-based amorphous alloy coating prepared by high velocity oxygen fuel method. J. Mater. Res. Technol. 2021, 15, 4880–4895. [Google Scholar] [CrossRef]
- Ning, W.; Zhai, H.; Xiao, R.; He, D.; Liang, G.; Wu, Y.; Li, W.; Li, X. The corrosion resistance mechanism of Fe-based amorphous coatings synthesized by detonation gun spraying. J. Mater. Eng. Perform. 2020, 29, 3921–3929. [Google Scholar] [CrossRef]
- Tian, W.-P.; Yang, H.-W.; Zhang, S.-D. Synergistic Effect of Mo, W, Mn and Cr on the Passivation Behavior of a Fe-Based Amorphous Alloy Coating. Acta Met. Sin. 2017, 31, 308–320. [Google Scholar] [CrossRef]
- Chu, Z.; Deng, W.; Zheng, X.; Zhou, Y.; Zhang, C.; Xu, J.; Gao, L. Corrosion Mechanism of Plasma-Sprayed Fe-Based Amorphous Coatings with High Corrosion Resistance. J. Therm. Spray Technol. 2020, 29, 1111–1118. [Google Scholar] [CrossRef]
- Si, C.; Duan, B.; Zhang, Q.; Cai, J.; Wu, W. Microstructure, corrosion-resistance, and wear-resistance properties of subsonic flame sprayed amorphous Fe–Mo–Cr–Co coating with extremely high amorphous rate. J. Mater. Res. Technol. 2020, 9, 3292–3303. [Google Scholar] [CrossRef]

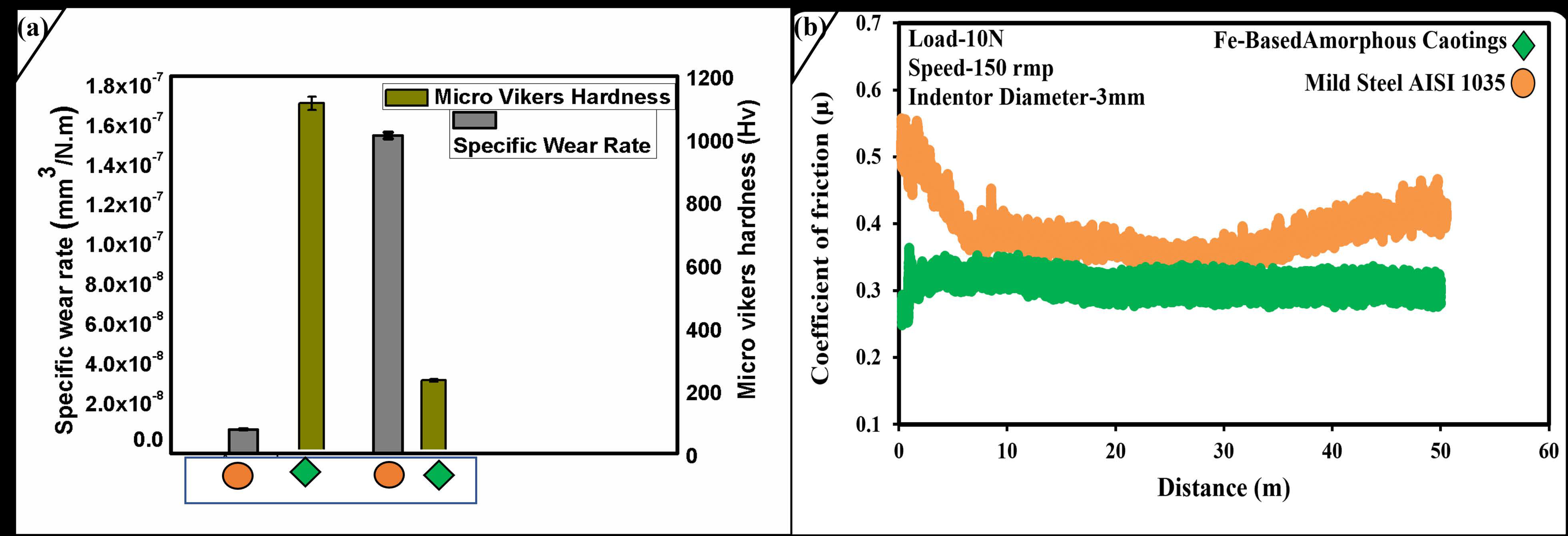

| Samples | Hardness (Hv) | Force (N) | Wear Rate mm3N−1m−1 |
|---|---|---|---|
| Amorphous | 1140 ± 1% | 10 | 0.0000000122124 (±2%) |
| AISI 1035 | 227 ± 1% | 10 | 0.000000159716 (±2%) |
| Tested Samples and Hours | Corrosion Potential(V) | Corrosion Current Density | Beta Anode (V/Decade) | Beta Cathode (V/Decade) | Rate of Corrosion (mpy) |
|---|---|---|---|---|---|
| 1 h Immersion PD-3 | −1.0210325 | 1979.07 μA/cm2 | 0.6475 ± (0.01023) | −0.544 ± (0.00725) | 9.25 × 10−4 (±1%) |
| 24 h Immersion PD-1 | −0.259503 | 2.25425 μA/cm2 | 0.08593 ± 7.9088 × 10−4 | −0.20133 ± 0.00161 | 0.0343 × 10−4 (±1%) |
| 48 h Immersion PD-2 | −0.583870009 | 12.38734 μA/cm2 | 0.06737 ± 8.3756 × 10−5 | −1.1648 ± 0.00785 | 1.88 × 10−4 (±1%) |
| Elements | Fe | Mo | Cr | Y | C | B |
|---|---|---|---|---|---|---|
| wt % | 48 | 14 | 15 | 2 | 15 | 6 |
| Elements | C | p | Mn | S | Fe |
|---|---|---|---|---|---|
| wt % | 0.350 | 0.040 | 0.90 | 0.050 | 98.66 |
| Voltage | Spray Thickness | Current | Feeding Rate | Spray Distance | Power |
|---|---|---|---|---|---|
| 50 V−60 V | 0.2 mm | 200–800 A | 25 gmin−1 | 150 mm | 40 KW |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, A.; Iqbal, A.; Moskal, G.; Yasir, M.; Al-Mansour, A.I.; Khan, M.A.; Alam, S.; Shahbaz, M.; Zia, A.; Ejaz, A. Long-Term Potentiodynamic Testing and Tribometric Properties of Amorphous Alloy Coatings under Saline Environment. Molecules 2022, 27, 1421. https://doi.org/10.3390/molecules27041421
Iqbal A, Iqbal A, Moskal G, Yasir M, Al-Mansour AI, Khan MA, Alam S, Shahbaz M, Zia A, Ejaz A. Long-Term Potentiodynamic Testing and Tribometric Properties of Amorphous Alloy Coatings under Saline Environment. Molecules. 2022; 27(4):1421. https://doi.org/10.3390/molecules27041421
Chicago/Turabian StyleIqbal, Amjad, Ayesha Iqbal, Grzegorz Moskal, Muhammad Yasir, Abdullah I. Al-Mansour, Mohammad Amir Khan, Shamshad Alam, Muhammad Shahbaz, Adeel Zia, and Ahsan Ejaz. 2022. "Long-Term Potentiodynamic Testing and Tribometric Properties of Amorphous Alloy Coatings under Saline Environment" Molecules 27, no. 4: 1421. https://doi.org/10.3390/molecules27041421
APA StyleIqbal, A., Iqbal, A., Moskal, G., Yasir, M., Al-Mansour, A. I., Khan, M. A., Alam, S., Shahbaz, M., Zia, A., & Ejaz, A. (2022). Long-Term Potentiodynamic Testing and Tribometric Properties of Amorphous Alloy Coatings under Saline Environment. Molecules, 27(4), 1421. https://doi.org/10.3390/molecules27041421







