Saprophytic Bacillus Accelerates the Release of Effective Components in Agarwood by Degrading Cellulose
Abstract
:1. Introduction
2. Results
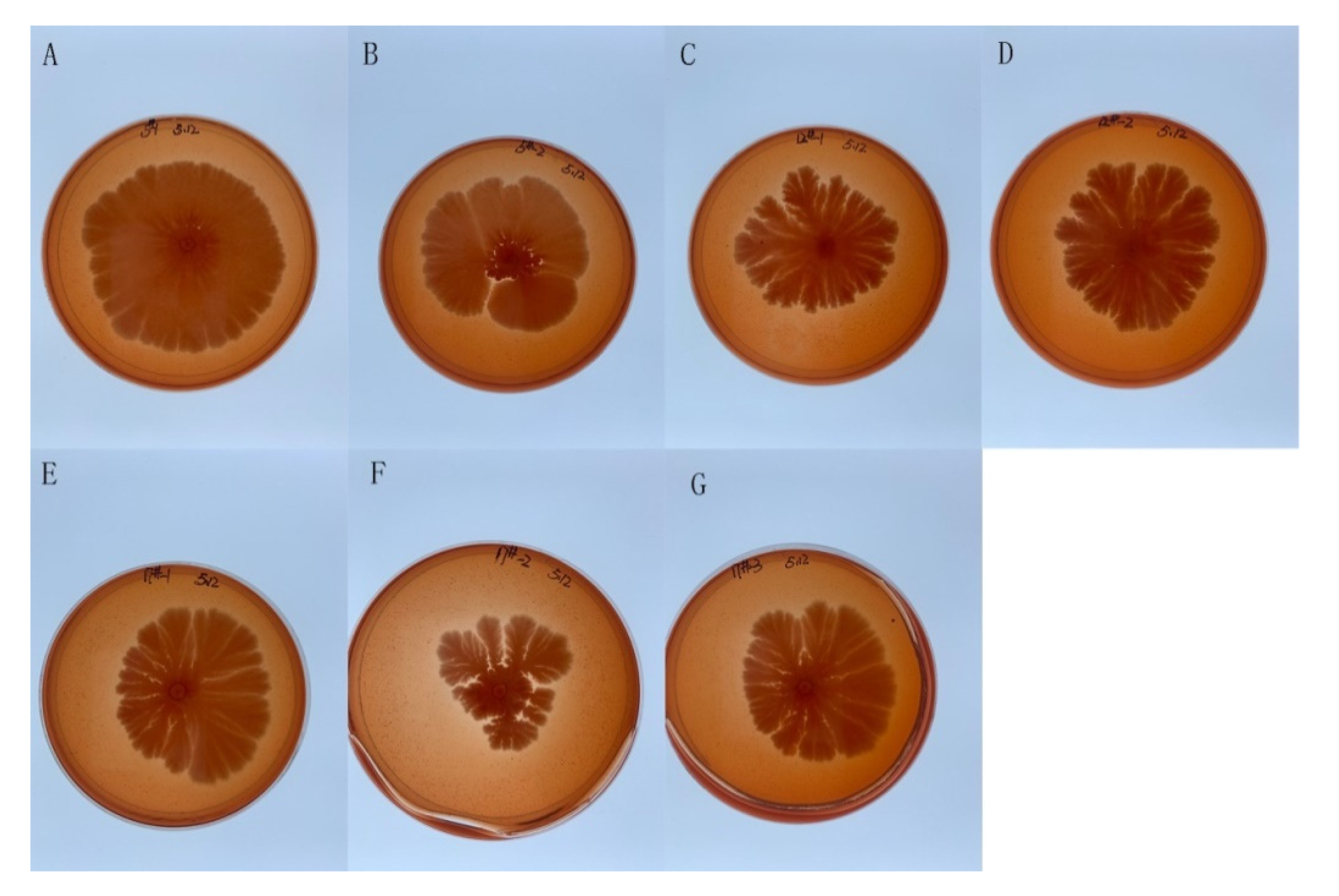
2.1. Isolation and Characterization of Saprophytic Bacteria from 10-Year-Old Agarwood
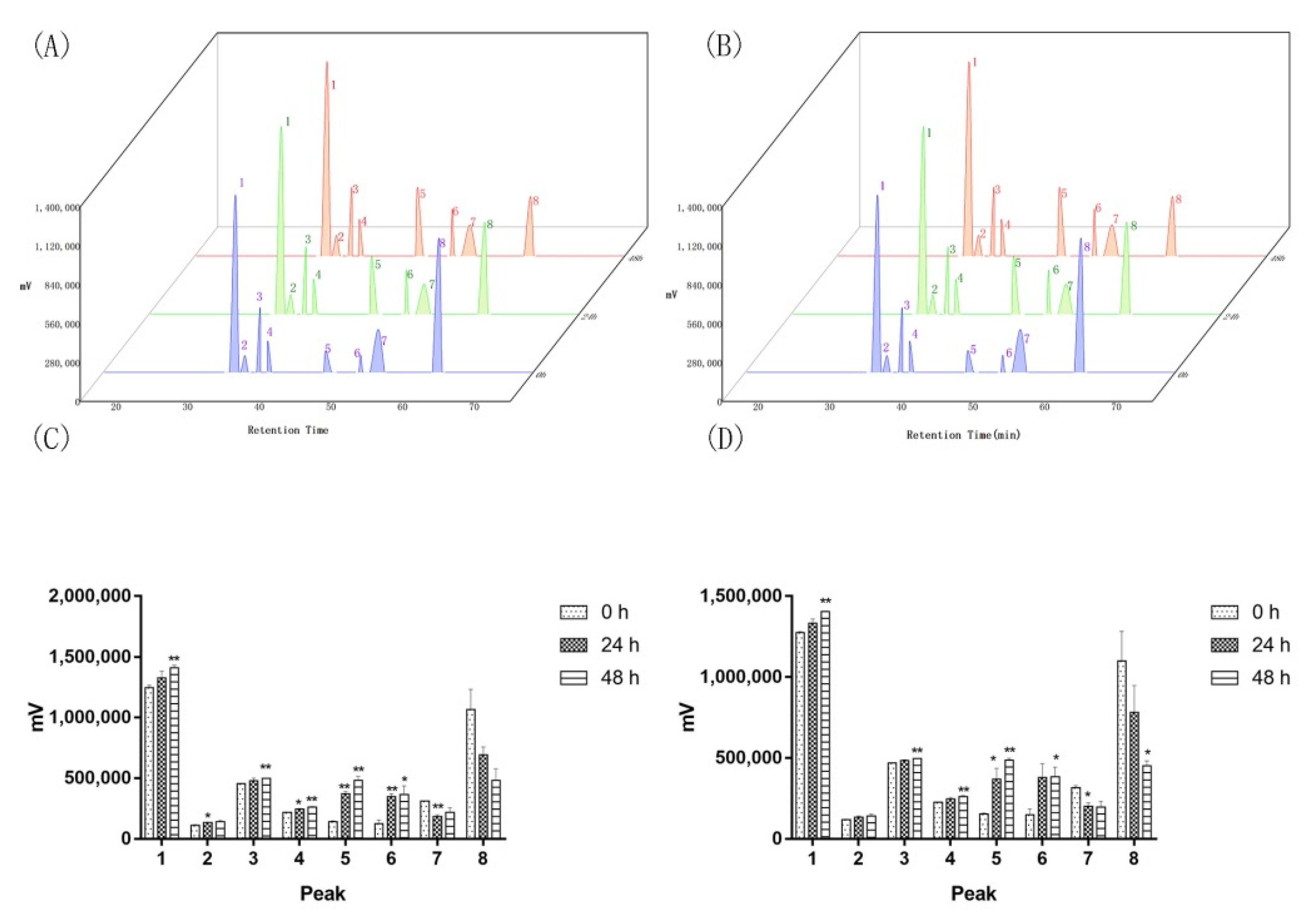
2.2. Two of the Saprophytic Bacillus Promotes the Release of Major Components of Agarwood
2.3. Saprophytic Bacilli Are Cellulase-Producing Strains
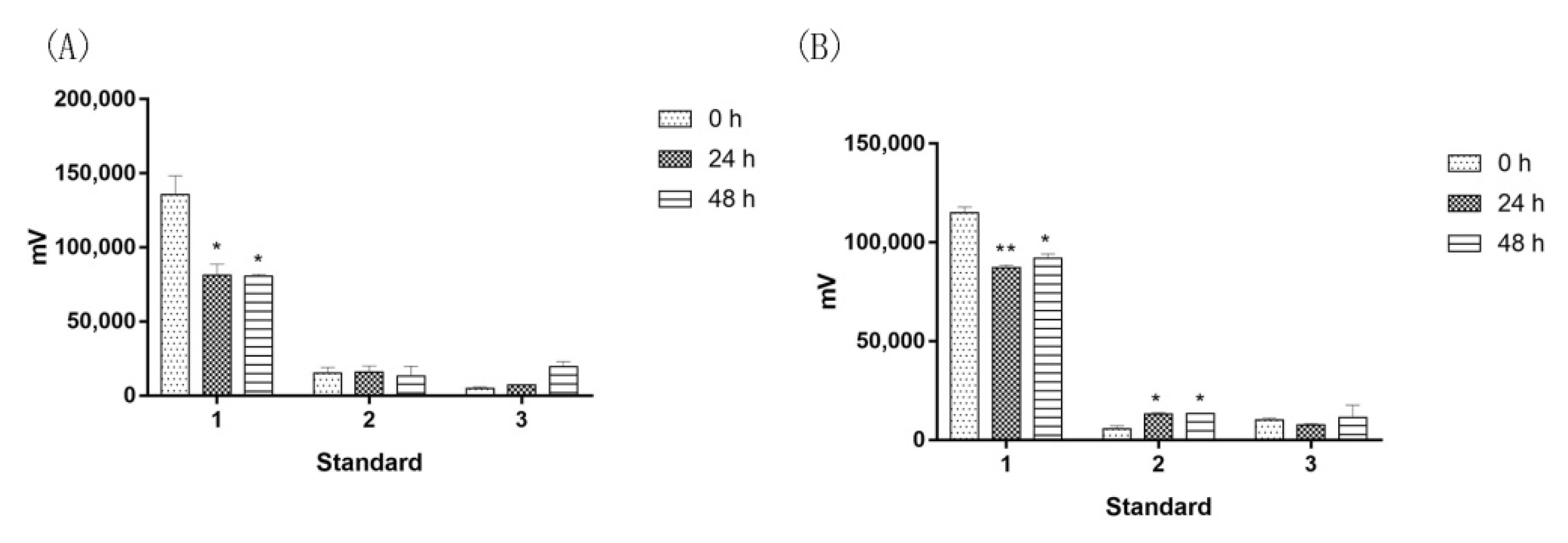
2.4. Saprophytic Bacillus Had a Limited Effect on Degrading Major Effective Components of Agarwood
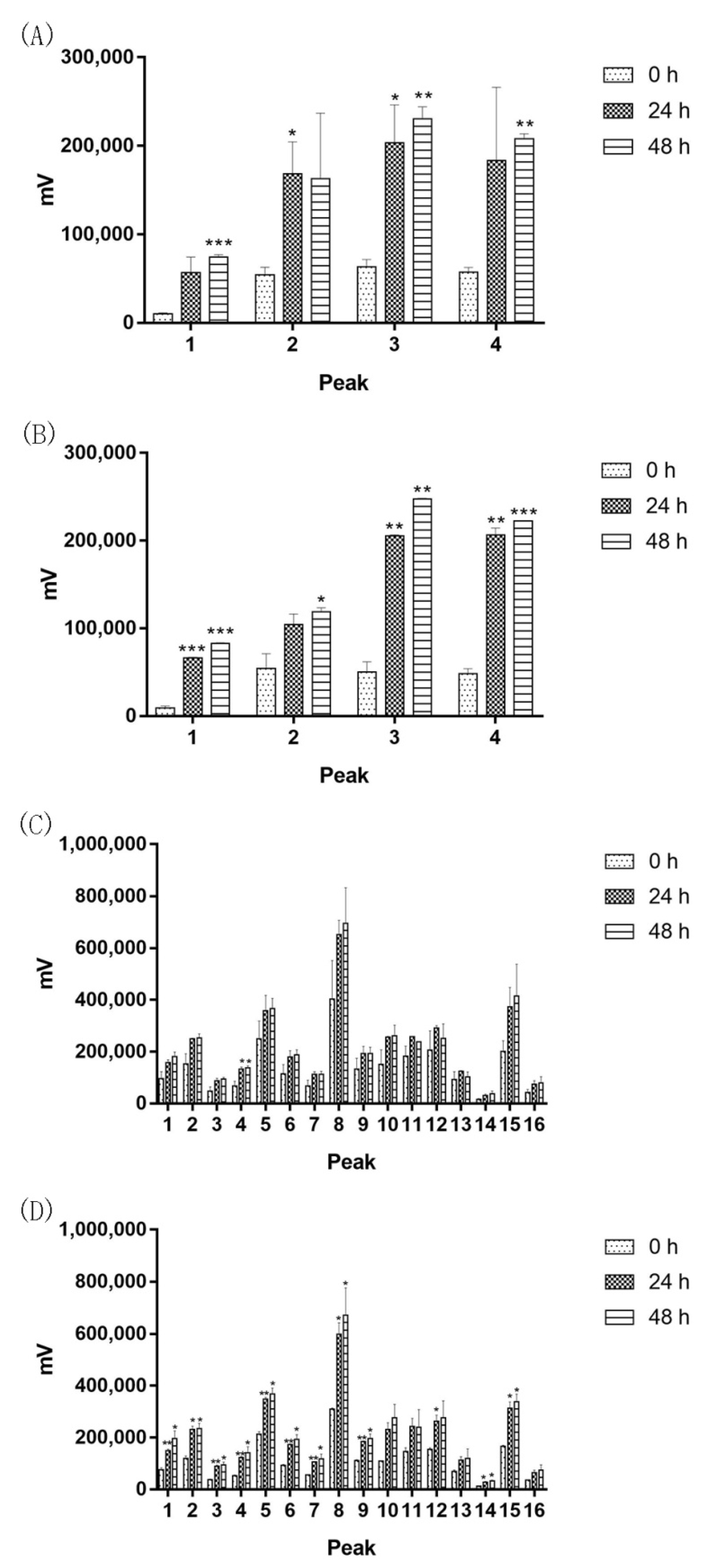
2.5. Common Chinese Herbal Medicine Powder Fermentation
2.6. Saprophytic Bacilli Had a Limited Effect in Aquilaria sinensis
3. Discussion
4. Materials and Methods
4.1. Isolation of Saprophytic Microorganism
4.2. Agarwood Powder Fermentation
4.3. Cellulose Degradation
4.4. Cellulase Activity Detection
4.5. Degradation of Effective Components of Agarwood
4.6. Effect of Saprophytic Bacteria on the Other Traditional Medicines
4.7. Replicon Sequencing in Aquilaria sinensis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A
Appendix A.1. Isolation and Characterization of Saprophytic Bacteria from 10-Years Agarwood
Appendix A.2. Common Chinese Herbal Medicine Powder Fermentation
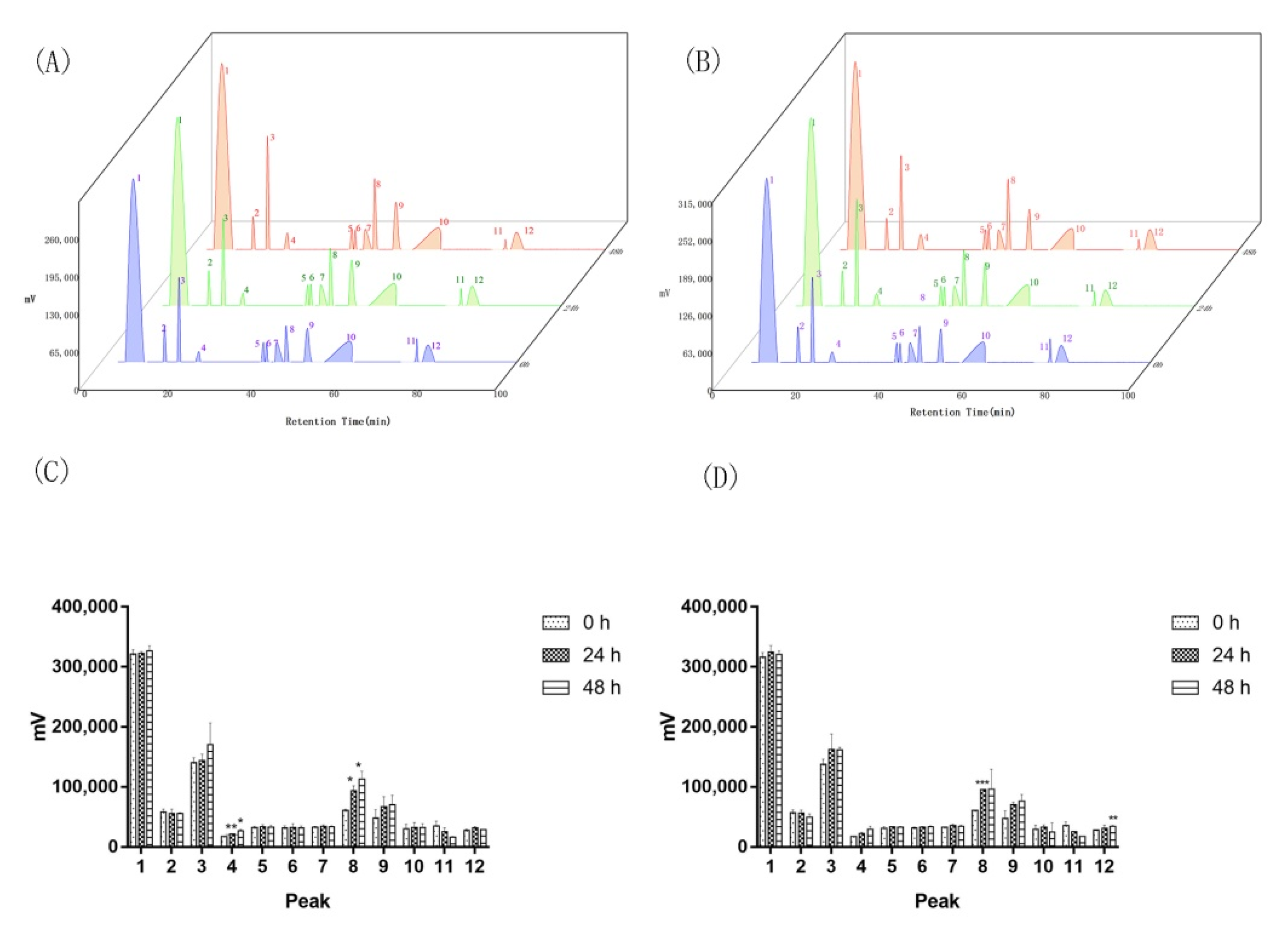
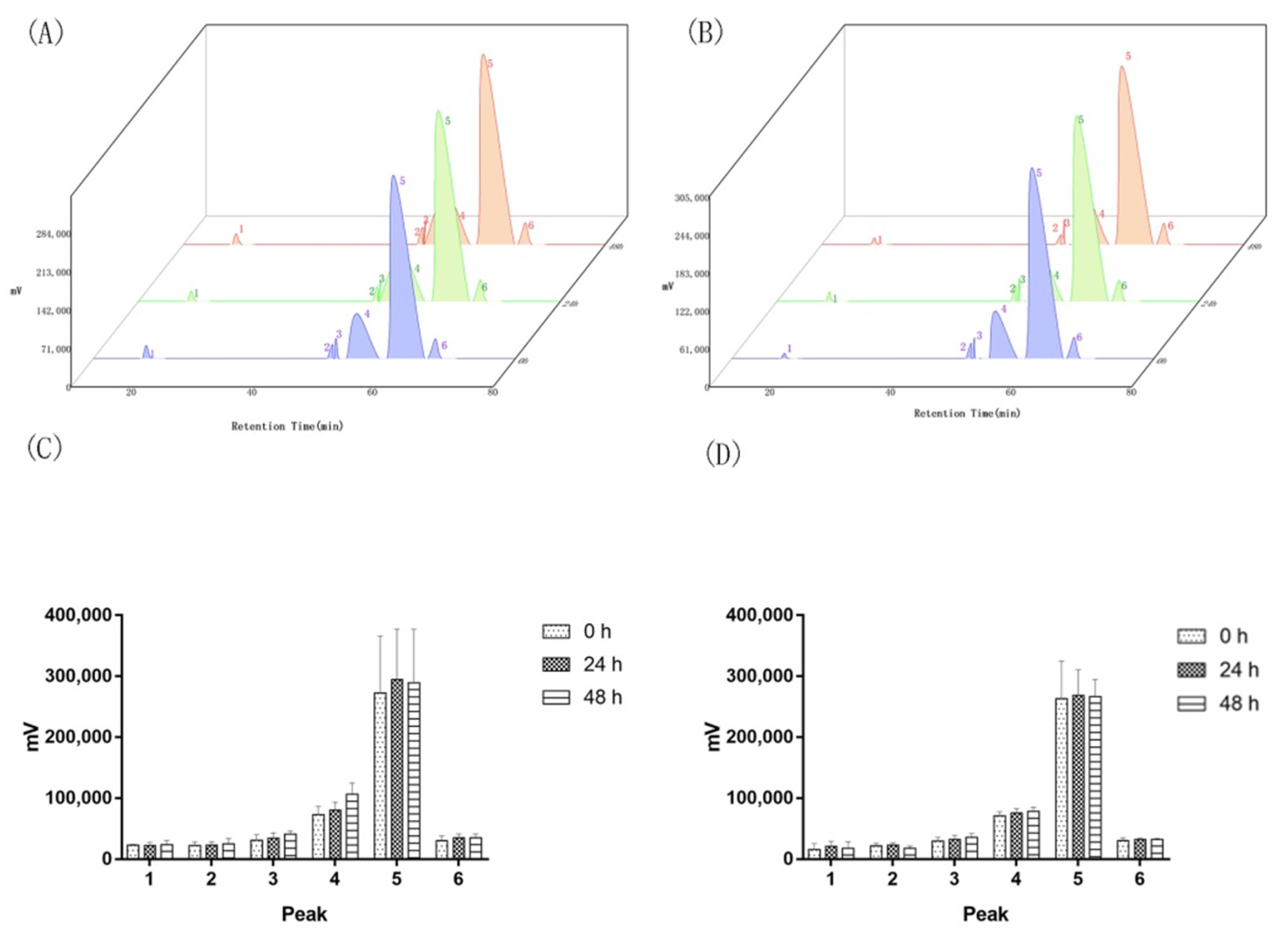
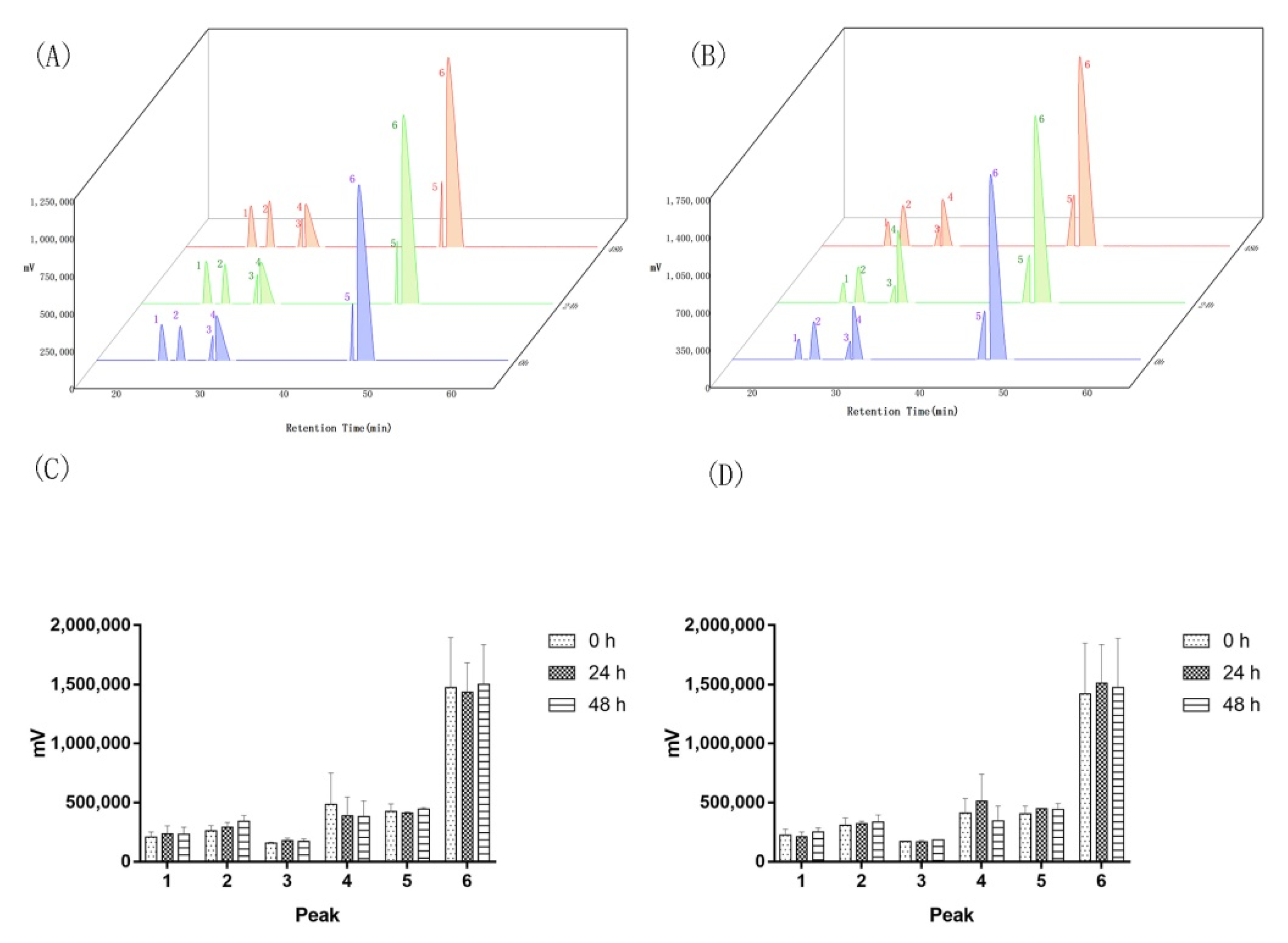
Appendix A.3. Replicon Sequencing in Aquilaria sinensis
| Content | Control | Bs-2 | Bs-4 | Bs-5 |
|---|---|---|---|---|
| Bacillus in root(%) | 0.0037 | 0.0006 | 0.0012 | 0.0013 |
| Bacillus in root(%) | 0.0031 | 0.0021 | 0.0014 | 0.0011 |
| Bacillus in root(%) | 0.0035 | 0.0011 | 0.0012 | 0.0023 |
| Content | Control | Bs-2 | Bs-4 | Bs-5 |
|---|---|---|---|---|
| Bacillus in stem(%) | 0.0039 | 0.0029 | 0.0026 | 0.0285 |
| Bacillus in stem(%) | 0.0085 | 0.0000 | 0.0004 | 0.0009 |
| Bacillus in stem(%) | 0.0000 | 0.0000 | 0.0000 | 0.0057 |
| Content | Control | Bs-2 | Bs-4 | Bs-5 |
|---|---|---|---|---|
| Bacillus in leaf(%) | 0.0000 | 0.0001 | 0.0000 | 0.0000 |
| Bacillus in leaf(%) | 0.0000 | 0.0003 | 0.0000 | 0.0000 |
| Bacillus in leaf(%) | 0.0000 | 0.0050 | 0.0000 | 0.0000 |
References
- Liu, Y.Y.; Wei, J.H.; Gao, Z.H.; Zhang, Z.; Lyu, J.C. A review of quality assessment and grading for agarwood. Chin. Herb. Med. 2017, 9, 22–30. [Google Scholar] [CrossRef]
- Li, W.; Chen, H.Q.; Wang, H.; Mei, W.L.; Dai, H.F. Natural products in agarwood and Aquilaria plants: Chemistry, biological activities and biosynthesis. Nat. Prod. Rep. 2021, 38, 528–565. [Google Scholar] [CrossRef]
- Moissl-Eichinger, C.; Pausan, M.; Taffner, J.; Berg, G.; Bang, C.; Schmitz, R.A. Archaea are interactive components of complex microbiomes. Trends. Microbiol. 2018, 26, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Aguiar-Pulido, V.; Huang, W.; Suarez-Ulloa, V.; Cickovski, T.; Mathee, K.; Narasimhan, G. Metagenomics, metatranscriptomics, and metabolomics approaches for microbiome analysis: Supplementary issue: Bioinformatics methods and applications for big metagenomics data. Evol. Bioinform. Online 2016, 12s1, S36436. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Chaturvedi, P.; Kulkarni, M.G.; van Staden, J. A critical review on exploiting the pharmaceutical potential of plant endophytic fungi. Biotechnol. Adv. 2020, 39, 107462. [Google Scholar] [CrossRef] [PubMed]
- Depetris, M.B.; Acuña, C.A.; Gutierrez, S.; Marcón, F.; Felitti, S.A. Fungal endophyte diversity in Paspalum and effects on plant growth. Grass Forage Sci. 2020, 75, 316–325. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Exploring the potentialities of beneficial endophytes for improved plant growth. Saudi J. Biol. Sci. 2020, 27, 3622–3633. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, A.; Essack, M.; Lafi, F.F.; Andres-Barrao, C.; Jalal, R.; Alamoudi, S.; Razali, R.; Alzubaidy, H.; Shah, K.H.; Siddique, S.; et al. Bioprospecting desert plant Bacillus endophytic strains for their potential to enhance plant stress tolerance. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Jiao, R.; Munir, S.; He, P.; Yang, H.; Wu, Y.; Wang, J.; He, P.; Cai, Y.; Wang, G.; He, Y. Biocontrol potential of the endophytic Bacillus amyloliquefaciens YN201732 against tobacco powdery mildew and its growth promotion. Biol. Control 2020, 143, 104160. [Google Scholar] [CrossRef]
- Haleckerab, S.; Wennricha, J.; Rodrigoc, S.; Andréed, N.; Rabschd, L.; Baschiene, C.; Steinertd, M.; Stadlerab, M.; Surupab, F.; Schulzd, B. Fungal endophytes for biocontrol of ash dieback: The antagonistic potential of Hypoxylon rubiginosum. Fungal. Ecol. 2020, 45, 100918. [Google Scholar] [CrossRef]
- Luzmaria, R.; Morales-Cedeño, M.D.C.O. Plant growth-promoting bacterial endophytes as biocontrol agents of pre- and post-harvest diseases: Fundamentals, methods of application and future perspectives. Microbiol. Res. 2021, 242, 126612. [Google Scholar]
- Edupuganti, S.A.; Krishna, P.H.A.; Ula, R.G.G.B. A review on isolation of endophytes from Indian medicinal plants. Int. J. Pharm. Res. Alli. 2019, 11, 301–308. [Google Scholar]
- Osama Abdalla, A.M.; Jin-Biao, M.; Yong-Hong, L.; Daoyuan, Z.; Shao, H.; Shrikant, B.; Brian, P.H.; Wen-Jun, L.; Li, L. Beneficial Endophytic Bacterial Populations Associated with Medicinal Plant Thymus vulgaris Alleviate Salt Stress and Confer Resistance to Fusarium oxysporum. Front. Plant. Sci. 2020, 11, 47. [Google Scholar]
- Chen, X.Y.; Yang, Y.; Liu, Y.Y.; Wei, J.H.; Zhang, Z.; Gan, B.C. Isolation and identification of endophytic fungi which promote agarwood formation in Aquilaria sinensis. Chin. Pharm. J. 2014, 49, 1118–1120. [Google Scholar]
- Bhore, S.J.; Preveena, J.; Kandasamy, K.I. Isolation and identification of bacterial endophytes from pharmaceutical agarwood-producing Aquilaria species. Pharmacogn. Res. 2013, 5, 134–137. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.L.; Kuang, Z.Y.; Song, M.W.; Zhang, R. Community structure and difference of endophtic bacteria in Aquilaria sinensis with and without agarwood. China J. Chin. Mater. Med. 2015, 40, 63–67. [Google Scholar]
- Xiang, Z.; Gao, J.Q.; Yuan, Y.; Quan, Y.; Zhou, L.Y.; Liu, J.; Huang, L.Q. Aanalysis of bacteria distribution characteristics in different layers of agarwood based on hiseq sequencing. China J. Chin. Mater. Med. 2020, 45, 2374–2381. [Google Scholar]
- Gao, X.X.; Zhou, W.P.; Wang, L.; Zhang, W.M.; Yan, H.J. Effects of agarwood formation induced by Fusarium sp. A2 on distribution and community composition of endophytic fungi in leaves of Aquilaria sinensis. China J. Chin. Mater. Med. 2014, 39, 197–203. [Google Scholar]
- Xiao, W.J.; Chen, H.Q.; Wang, H.; Cai, C.H.; Mei, W.L.; Dai, H.F. New secondary metabolites from the endophytic fungus Fusarium sp. HP-2 isolated from “Qi-Nan” agarwood. Fitoterapia 2018, 130, 180–183. [Google Scholar] [CrossRef]
- Monggoot, S.; Popluechai, S.; Gentekaki, E.; Pripdeevech, P. Fungal Endophytes: An Alternative Source for Production of Volatile Compounds from Agarwood Oil of Aquilaria subintegra. Microb. Ecol. 2017, 74, 54–61. [Google Scholar] [CrossRef]
- Li, B.; Wei, C.; Wen, T.; Shi, J.; Dong, L.; Guo, P. Screening of agricultural waste straw-degrading strains and their enzyme-producing conditions. Acta Sci. Circumstantiae 2012, 32, 3095–3100. [Google Scholar]
- Luciano Silveira, M.H.; Rau, M.; Pinto Da Silva Bon, E.; Andreaus, J. A simple and fast method for the determination of endo- and exo-cellulase activity in cellulase preparations using filter paper. Enzym. Microb. Tech. 2012, 51, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, S.; Ito, M. Agarotetrol in agarwood: Its use in evaluation of agarwood quality. J. Nat. Med. 2020, 74, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Chen, D.L.; Yu, Z.X.; Wang, C.H.; Jian, F.; Yu, M.; Wei, J.H. New 2-(2-phenylethyl)chromone derivatives from agarwood and their inhibitory effects on tumor cells. Nat. Prod. Res. 2020, 34, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Schulz, B.; Guske, S.; Dammann, U.; Boyle, C. Endophyte-host interactions. II. Defining symbiosis of the endophyte-host interaction. Symbiosis 1998, 25, 213–227. [Google Scholar]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Microbiology 2008, 6, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Hartley, S.E.; Eschen, R.; Horwood, J.M.; Gange, A.C.; Hill, E.M. Infection by a foliar endophyte elicits novel arabidopside-based plant defence reactions in its host, Cirsium arvense. New Phytol. 2015, 205, 816–827. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Schillaci, M.; Walker, R.; Smith, P.M.C.; Watt, M.; Roessner, U. Alleviation of salinity stress in plants by endophytic plant-fungal symbiosis: Current knowledge, perspectives and future directions. Plant Soil 2021, 461, 219–244. [Google Scholar] [CrossRef]
- Wang, Z.H.; Skidmore, A.K.; Wang, T.J.; Darvishzadeh, R.; Hearne, J. Applicability of the PROSPECT model for estimating protein and cellulose + lignin in fresh leaves. Remote Sens. Environ. 2015, 168, 205–218. [Google Scholar] [CrossRef]
- Borukanlu, M.R.; Ca, O.H.Z.; Moradpour, P.; Khedive, E. Effects of growth rate of eastern poplar trees on the chemical and morphological characteristics of wood fibers. Eur. J. Wood Wood Prod. 2021, 79, 1479–1494. [Google Scholar] [CrossRef]
- Zhang, X.M.; Miao, F.Y.; Niu, L.L.; Wei, Y. Berberine carried gelatin/sodium alginate hydrogels with antibacterial and EDTA-induced detachment performances. Int. J. Biol. Macromol. 2021, 181, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Hao, C.Q.; Qin, Y.; Zhang, W.J.; Gang, L.; Liu, S.G.; Xin, H. Inhibition of Haemophilus parasuis by berberine and proteomic studies of its mechanism of action. Res. Vet. Sci. 2021, 138, 62–68. [Google Scholar]
- Ma, Y.; Li, X.; Hou, L.X.; Ca, A.Z. Extraction solvent affects the antioxidant, antimicrobial, cholinesterase and HepG2 human hepatocellular carcinoma cell inhibitory activities of Zanthoxylum bungeanum pericarps and the major chemical components. Ind. Crop. Prod. 2019, 142, 111872. [Google Scholar] [CrossRef]
- Xie, H.; Shao, J.M.; Wang, G.L. Antimicrobial effects of total alkaloids extracted from Zanthoxylum Bungeanum Maxim. Chengde Shiyou Gaodeng Zhuanke Xuexiao Xuebao 2013, 15, 24–26. [Google Scholar]


| Strains | Bs-1 | Bs-2 | Bs-3 | Bs-4 | Bs-5 | Bs-6 | Bs-7 |
|---|---|---|---|---|---|---|---|
| FPA (U/mL) | 0.15 | 0.20 | 0.11 | 0.22 | 0.14 | 0.12 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; He, R.; Cui, Y.; Li, Y.; Ge, X. Saprophytic Bacillus Accelerates the Release of Effective Components in Agarwood by Degrading Cellulose. Molecules 2022, 27, 1428. https://doi.org/10.3390/molecules27041428
Yang H, He R, Cui Y, Li Y, Ge X. Saprophytic Bacillus Accelerates the Release of Effective Components in Agarwood by Degrading Cellulose. Molecules. 2022; 27(4):1428. https://doi.org/10.3390/molecules27041428
Chicago/Turabian StyleYang, Huizhen, Runying He, Yao Cui, Ying Li, and Xizhen Ge. 2022. "Saprophytic Bacillus Accelerates the Release of Effective Components in Agarwood by Degrading Cellulose" Molecules 27, no. 4: 1428. https://doi.org/10.3390/molecules27041428
APA StyleYang, H., He, R., Cui, Y., Li, Y., & Ge, X. (2022). Saprophytic Bacillus Accelerates the Release of Effective Components in Agarwood by Degrading Cellulose. Molecules, 27(4), 1428. https://doi.org/10.3390/molecules27041428





