Polysaccharide-Rich Red Algae (Gelidium amansii) Hot-Water Extracts Alleviate Abnormal Hepatic Lipid Metabolism without Suppression of Glucose Intolerance in a Streptozotocin/Nicotinamide-Induced Diabetic Rat Model
Abstract
1. Introduction
2. Results and Discussion
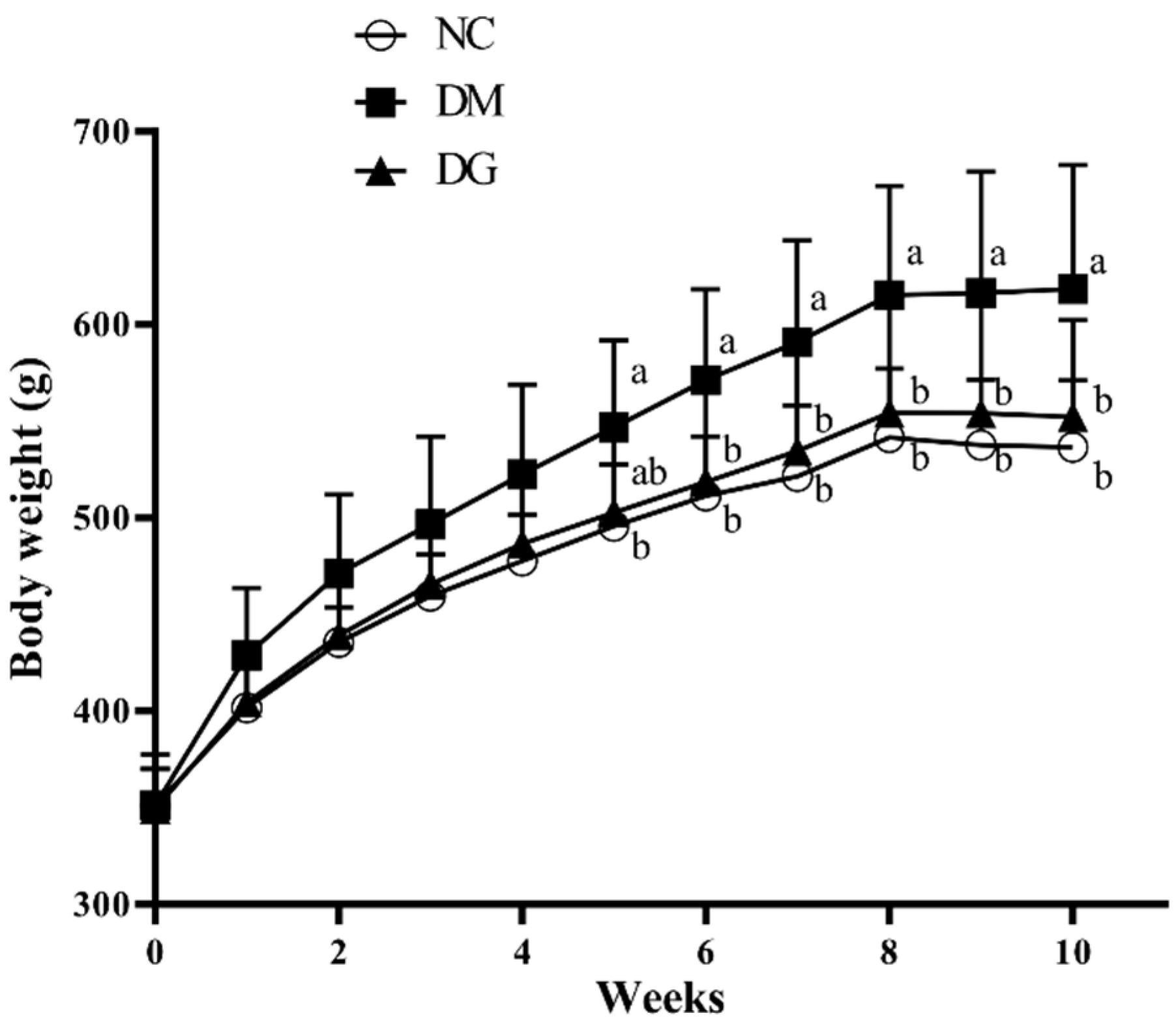
2.1. Changes in Body Weight, Food Intake, and Tissue Weight
2.2. Changes in Blood Glucose and Insulin Levels, Glucose Tolerance, Hepatic Lipids, Glycogen Content, Glycometabolism-Related Enzymes, TBARS, and Adipose Tissue Lipolysis Rate
3. Materials and Methods
3.1. Animals and Experimental Diets
3.2. Blood Glucose and OGTT Assay
3.3. Blood and Tissue Collection
3.4. Liver Lipid Measurement
3.5. Hepatic Thiobarbituric Acid Reactive Substances (TBARS) Determination
3.6. Lipolysis Rate Analysis
3.7. Glycogen Content and Measurement of Activities of Hexokinase, Glucose-6-Phosphatase (G6Pase), and Glucose-6-Phosphate Dehydrogenase (G6PD)
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Reaven, G.M. Pathophysiology of insulin resistance in human disease. Physiol. Rev. 1995, 75, 473–486. [Google Scholar] [CrossRef]
- Weyer, C.; Funahashi, T.; Tanaka, S.; Hotta, K.; Matsuzawa, Y.; Pratley, R.E.; Tataranni, P.A. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001, 86, 1930–1935. [Google Scholar] [CrossRef]
- Anderson, J.W.; Kendall, C.W.; Jenkins, D.J. Importance of weight management in type 2 diabetes: Review with meta-analysis of clinical studies. J. Am. Coll. Nutr. 2003, 22, 331–339. [Google Scholar] [CrossRef]
- Eckel, R.H.; Kahn, S.E.; Ferrannini, E.; Goldfine, A.B.; Nathan, D.M.; Schwartz, M.W.; Smith, R.J.; Smith, S.R. Obesity and type 2 diabetes: What can be unified and what needs to be individualized. J. Clin. Endocrinol. Metab. 2011, 96, 1654–1663. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2012, 35, 64–71. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, X.; Tang, F.; Zuo, S.H. An experimental study on the hypoglycemic effect of agar polysaccharide in diabetic rats. Health Med. Res. Pract. 2011, 4, 8–10. [Google Scholar]
- Maeda, H.; Yamamoto, R.; Hirao, K.; Tochikubo, O. Effects of agar (kanten) diet on obese patients with impaired glucose tolerance and type 2 diabetes. Diabetes Obes. Metabol. 2005, 7, 40–46. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, H.C.; Vitek, L.; Nam, C.M. Algae consumption and risk of type 2 diabetes: Korean National Health and Nutrition Examination Survey in 2005. J. Nutr. Sci. Vitaminol. 2009, 56, 13–18. [Google Scholar] [CrossRef]
- Labropoulos, K.C.; Niesz, D.E.; Danforth, S.C.; Kevrekidis, P.G. Dynamic rheology of agar gels: Theory and experiments. Part I. Development of a rheological model. Carbohyd. Polym. 2002, 50, 393–406. [Google Scholar] [CrossRef]
- Chen, Y.H.; Tu, C.J.; Wu, H.T. Growth-inhibitory effects of the red alga Gelidium amansii on cultured cells. Biol. Pharm. Bull. 2004, 27, 180–184. [Google Scholar] [CrossRef]
- Liu, H.C.; Chang, C.J.; Yang, T.H.; Chiang, M.T. Long-term feeding of red algae (Gelidium amansii) ameliorates glucose and lipid metabolism in a high-fructose-diet-impaired glucose-tolerance rat model. J. Food Drug Anal. 2017, 25, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.H.; Yao, H.T.; Chiang, M.T. Red algae (Gelidium amansii) reduces adiposity via activation of lipolysis in rats with diabetes induced by streptozotocin-nicotinamide. J. Food Drug Anal. 2015, 23, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.H.; Yao, H.T.; Chiang, M.T. Red algae (Gelidium amansii) hot-water extract ameliorates lipid metabolism in hamsters fed a high-fat diet. J. Food Drug Anal. 2017, 25, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.H.; Chiu, C.Y.; Lu, T.J.; Liu, S.H.; Chiang, M.T. The anti-obesity effect of polysaccharide-rich red algae (Gelidium amansii) hot-water extracts in high-fat diet-induced obese hamsters. Mar. Drugs. 2019, 17, 532. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Hung, H.I.; Yang, T.H.; Pan, C.L.; Chiang, M.T. Polysaccharide-rich red algae (Gelidium amansii) hot-water extracts ameliorate the altered plasma cholesterol and hepatic lipid homeostasis in high-fat diet-fed rats. J. Food Drug Anal. 2021, 29, 46–56. [Google Scholar] [CrossRef]
- Watt, M.J.; Holmes, A.G.; Pinnamaneni, S.K.; Garnham, A.P.; Steinberg, G.R.; Kemp, B.E.; Febbraio, M.A. Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E500e8. [Google Scholar] [CrossRef]
- Clark, M.J.; Slavin, J.L. The Effect of fiber on satiety and food intake: A systematic review. J. Am. Coll. Nutr. 2013, 32, 200–211. [Google Scholar] [CrossRef]
- Chandalia, M.; Garg, A.; Lutjohann, D.; Bergmann, K.; Grundy, S.M.; Brinkley, L.J. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N. Engl. J. 2000, 342, 1392–1398. [Google Scholar] [CrossRef]
- Shen, K.P.; Lin, H.L.; Yen, H.W.; Hsieh, S.L.; An, L.M.; Wu, B.N. Eugenosedin-A improves glucose metabolism and inhibits MAPKs expression in streptozotocin/nicotinamide-induced diabetic rats. Kaohsiung J. Med. Sci. 2018, 34, 142–149. [Google Scholar] [CrossRef]
- Iftikhar, A.; Aslam, B.; Iftikhar, M.; Majeed, W.; Batool, M.; Zahoor, B.; Amna, N.; Gohar, H.; Latif, I. Effect of Caesalpinia Bonduc polyphenol extract on alloxan-induced diabetic rats in attenuating hyperglycemia by upregulating insulin secretion and inhibiting JNK signaling pathway. Oxid. Med. Cell. Longev. 2020, 2020, 9020219. [Google Scholar] [CrossRef]
- Chadt, A.; Al-Hasani, H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflugers Arch. 2020, 472, 1273–1298. [Google Scholar] [CrossRef]
- Salvesen, G.S.; Duckett, C.S. IAP proteins: Blocking the road to death’s door. Nat. Rev. Mol. Cell. Biol. 2002, 3, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Xia, P. Cellular inhibitor of apoptosis protein-1 (cIAP1) plays a critical role in β-cell survival under endoplasmic reticulum stress: Promoting ubiquitination and degradation of C/EBP homologous protein (CHOP). J. Biol. Chem. 2012, 287, 32236–32245. [Google Scholar] [CrossRef] [PubMed]
- Plesner, A.; Liston, P.; Tan, R.; Korneluk, R.G.; Verchere, C.B. The X-linked inhibitor of apoptosis protein enhances survival of murine islet allografts. Diabetes 2005, 54, 2533–2540. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahmadi, S.; Mainali, R.; Nagpal, R.; Sheikh-Zeinoddin, M.; Soleimanian-Zad, S.; Wang, S.; Deep, G.; Kumar Mishra, S.; Yadav, H. Dietary polysaccharides in the amelioration of gut microbiome dysbiosis and metabolic diseases. Obes. Control Ther. 2017, 4. [Google Scholar] [CrossRef]
- Kim, K.; Nam, K.; Kurihara, H.; Kim, S. Potent α-glucosidase inhibitors purified from the red alga Grateloupia elliptica. Phytochemistry 2008, 69, 2820–2825. [Google Scholar] [CrossRef]
- Noce, A.; Di Lauro, M.; Di Daniele, F.; Pietroboni Zaitseva, A.; Marrone, G.; Borboni, P.; Di Daniele, N. Natural bioactive compounds useful in clinical management of metabolic syndrome. Nutrients 2021, 13, 630. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Morbini, M.; Colletti, A.; Bove, M.; Veronesi, M.; Giovannini, M.; Borghi, C. Nutraceutical effects on glucose and lipid metabolism in patients with impaired fasting glucose: A pilot, double-blind, placebo-controlled, randomized clinical trial on a combined product. High Blood Press Cardiovasc. Prev. 2017, 24, 283–288. [Google Scholar] [CrossRef][Green Version]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Carlson, S.E.; Goldfarb, S. sensitive enzymatic method of determination of free and esterified tissue cholesterol. Clin. Chim. Acta 1977, 79, 575–582. [Google Scholar] [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Berger, J.J.; Barnard, R.J. Effect of diet on fat cell size and hormone-sensitive lipase activity. J. Appl. Physiol. 1999, 87, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Murat, J.C.; Serfaty, A. Simple enzymatic determination of polysaccharide (glycogen) content of animal tissues. Clin. Chem. 1974, 20, 1576–1577. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, F.; Ohshima, H.; Umezawa, K. Distribution of glucose-6-phosphate metabolizing enzymes in fish. Bull. Jpn. Soc. Sci. Fish. 1972, 38, 589–593. [Google Scholar] [CrossRef]
- DeWaal, D.; Nogueira, V.; Terry, A.R.; Patra, K.C.; Jeon, S.M.; Guzman, G.; Au, J.; Long, C.P.; Antoniewicz, M.R.; Hay, N. Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nat. Commun. 2018, 9, 446. [Google Scholar] [CrossRef] [PubMed]
- Taussky, H.H.; Shorr, E. A microcolorimetric method for the determination of inorganic phosphorus. J. Biol. Chem. 1953, 202, 675–685. [Google Scholar] [CrossRef]



| Diet | NC | DM | DG |
|---|---|---|---|
| Initial body weight (g) | 351.0 ± 19.0 a | 351.2 ± 26.3 a | 351.3 ± 20.6 a |
| Final body weight (g) | 500.8 ± 30.0 b | 591.0 ± 57.7 a | 530.0 ± 50.6 b |
| Body weight gain (g) | 160.4 ± 20.3 c | 232.2 ± 35.0 a | 187.5 ± 36.0 bc |
| Food intake (g/day) | 29.8 ± 1.99 ab | 30.5 ± 2.43 a | 27.6 ± 2.12 b |
| Feed efficiency (%) | 5.38 ± 0.43 b | 7.61 ± 0.92 a | 6.79 ± 1.22 a |
| Diet | NC | DM | DG |
|---|---|---|---|
| Liver weight (g) | 13.3 ± 0.92 c | 30.7 ± 4.94 a | 24.0 ± 3.24 b |
| Relative liver weight (g/100 g B.W.) | 2.62 ± 0.12 c | 5.18 ± 0.58 a | 4.53 ± 0.39 b |
| Total adipose tissue weight (g) | 12.9 ± 2.81 b | 20.6 ± 5.30 a | 14.2 ± 4.17 b |
| Relative adipose tissue weight (g/100 g B.W.) | 2.31 ± 0.43 b | 3.47 ± 0.76 a | 2.66 ± 0.67 b |
| Perirenal adipose weight (g) | 5.95 ± 1.70 b | 10.9 ± 2.49 a | 7.39 ± 2.29 b |
| Relative perirenal adipose weight (g/100 g B.W.) | 1.16 ± 0.29 b | 1.83 ± 0.37 a | 1.38 ± 0.37ab |
| Epididymal adipose weight (g) | 5.91 ± 1.44 b | 9.76 ± 2.88 a | 6.80 ± 2.19 b |
| Relative epididymal adipose weight (g/100 g B.W.) | 1.15 ± 0.22 b | 1.63 ± 0.41 a | 1.28 ± 0.37 ab |
| Gastrocnemius muscle weight (g) | 6.18 ± 0.40 a | 6.08 ± 0.51 a | 6.03 ± 0.53 a |
| Relative gastrocnemius muscle weight (g/100 g B.W.) | 1.21 ± 0.08 a | 1.03 ± 0.06 c | 1.14 ± 0.10 ab |
| Soleus muscle weight (g) | 0.41 ± 0.08 a | 0.44 ± 0.07 a | 0.42 ± 0.07 a |
| Relative soleus muscle weight (g/100 g B.W.) | 0.08 ± 0.01 a | 0.07 ± 0.01 b | 0.08 ± 0.01 a |
| Diet | NC | DM | DG |
|---|---|---|---|
| Triglyceride | |||
| (mg/g liver) | 12.5 ± 5.58 b | 67.6 ± 31.7 a | 56.1 ± 20.6 a |
| (g/liver) | 0.17 ± 0.08 c | 2.13 ± 1.23 a | 1.32 ± 0.42 b |
| Total cholesterol | |||
| (mg/g liver) | 3.67 ± 1.42 c | 96.3 ± 11.6 a | 81.2 ± 20.9 b |
| (g/liver) | 0.05 ± 0.02 c | 2.98 ± 0.64 a | 1.96 ± 0.60 b |
| Diet | NC | DM | DG |
|---|---|---|---|
| Hexokinase (nmol/min/mg protein) | 5.96 ± 1.48 a | 4.44 ± 1.33 a | 5.94 ± 3.12 a |
| Glucose-6-phosphatase (nmol/min/mg protein) | 0.32 ± 0.03 c | 0.5 ± 0.07 a | 0.44 ± 0.04 b |
| Glucose-6-phosphatase /Hexokinase ratio | 0.06 ± 0.02 c | 0.12 ± 0.03 a | 0.09 ± 0.03 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-H.; Ku, C.-Y.; Chiang, M.-T. Polysaccharide-Rich Red Algae (Gelidium amansii) Hot-Water Extracts Alleviate Abnormal Hepatic Lipid Metabolism without Suppression of Glucose Intolerance in a Streptozotocin/Nicotinamide-Induced Diabetic Rat Model. Molecules 2022, 27, 1447. https://doi.org/10.3390/molecules27041447
Liu S-H, Ku C-Y, Chiang M-T. Polysaccharide-Rich Red Algae (Gelidium amansii) Hot-Water Extracts Alleviate Abnormal Hepatic Lipid Metabolism without Suppression of Glucose Intolerance in a Streptozotocin/Nicotinamide-Induced Diabetic Rat Model. Molecules. 2022; 27(4):1447. https://doi.org/10.3390/molecules27041447
Chicago/Turabian StyleLiu, Shing-Hwa, Chia-Yu Ku, and Meng-Tsan Chiang. 2022. "Polysaccharide-Rich Red Algae (Gelidium amansii) Hot-Water Extracts Alleviate Abnormal Hepatic Lipid Metabolism without Suppression of Glucose Intolerance in a Streptozotocin/Nicotinamide-Induced Diabetic Rat Model" Molecules 27, no. 4: 1447. https://doi.org/10.3390/molecules27041447
APA StyleLiu, S.-H., Ku, C.-Y., & Chiang, M.-T. (2022). Polysaccharide-Rich Red Algae (Gelidium amansii) Hot-Water Extracts Alleviate Abnormal Hepatic Lipid Metabolism without Suppression of Glucose Intolerance in a Streptozotocin/Nicotinamide-Induced Diabetic Rat Model. Molecules, 27(4), 1447. https://doi.org/10.3390/molecules27041447







