Mechanisms, Anti-Quorum-Sensing Actions, and Clinical Trials of Medicinal Plant Bioactive Compounds against Bacteria: A Comprehensive Review
Abstract
:1. Introduction
2. Natural Products from Medicinal Plants as Antibacterial Drugs
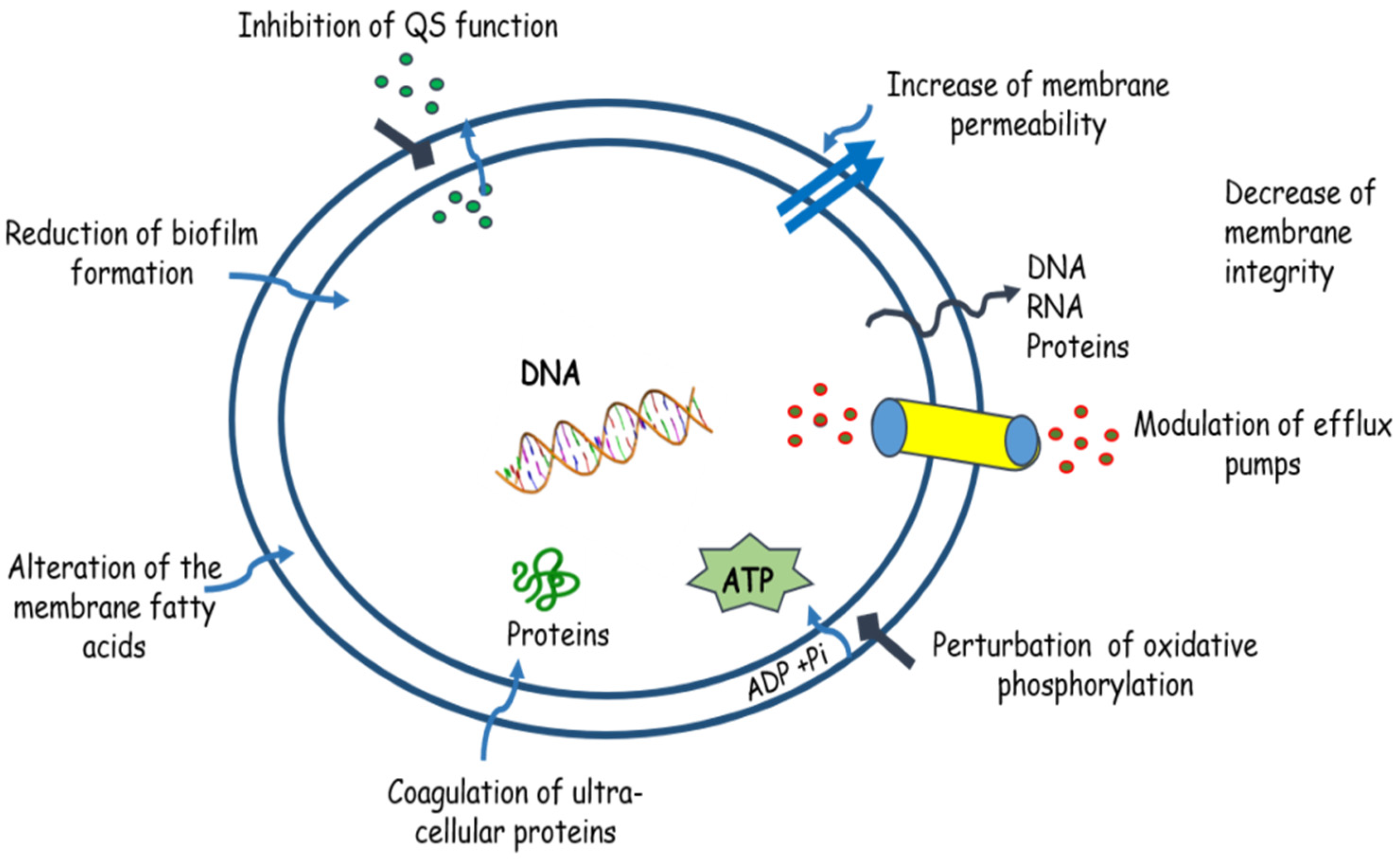
3. Classical Antibacterial Mechanisms of Natural Products Isolated from Medicinal Plants
3.1. Terpenoids
3.2. Antibacterial Actions of Flavonoids
3.2.1. Inhibition of Cell Envelope (Wall) Synthesis
3.2.2. Inhibition of Nucleic Acid Synthesis
3.2.3. Inhibition of Bacterial Motility
3.2.4. Inhibition of Biofilm Formation
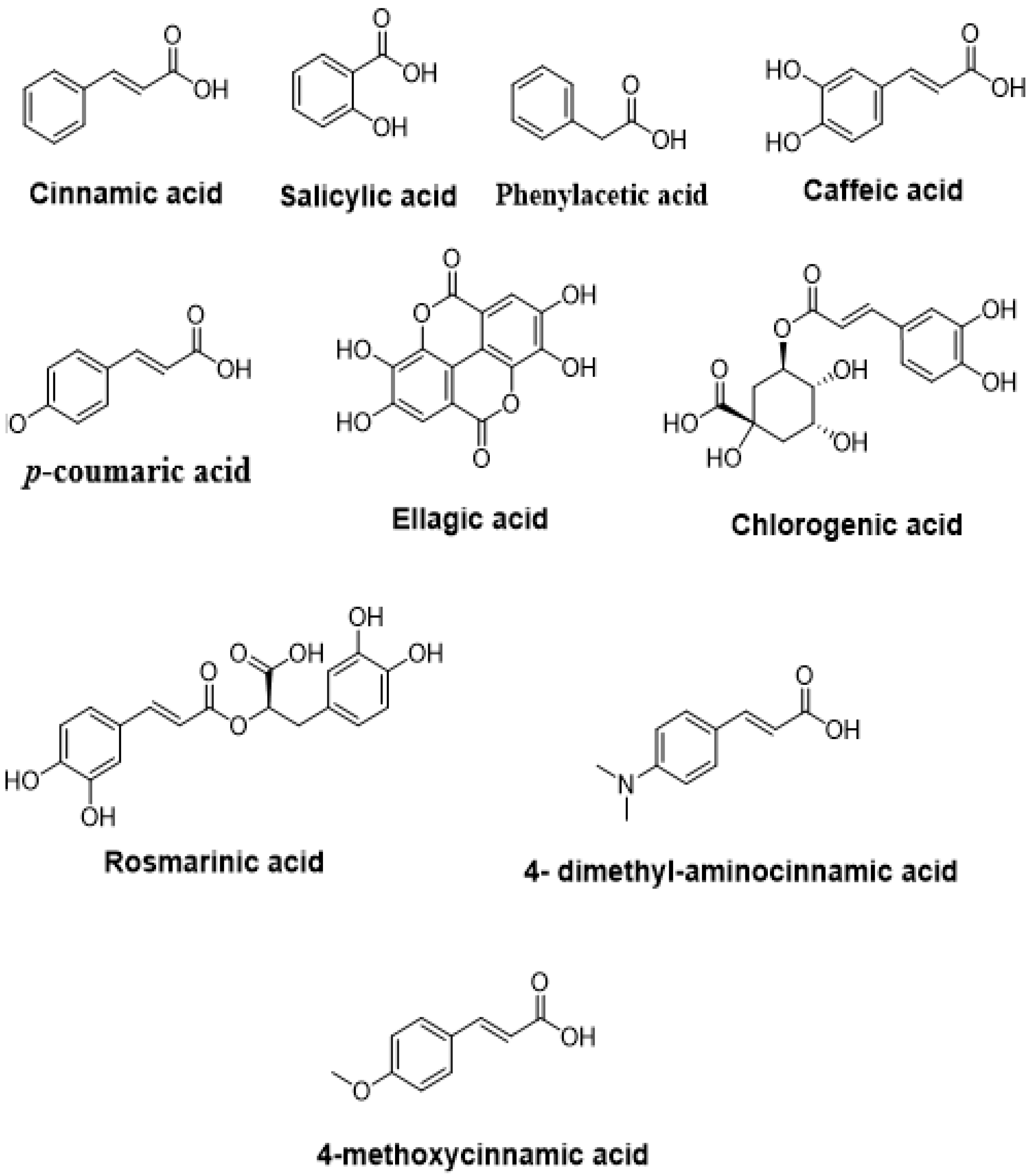
3.3. Antibacterial Actions of Phenolic Acids
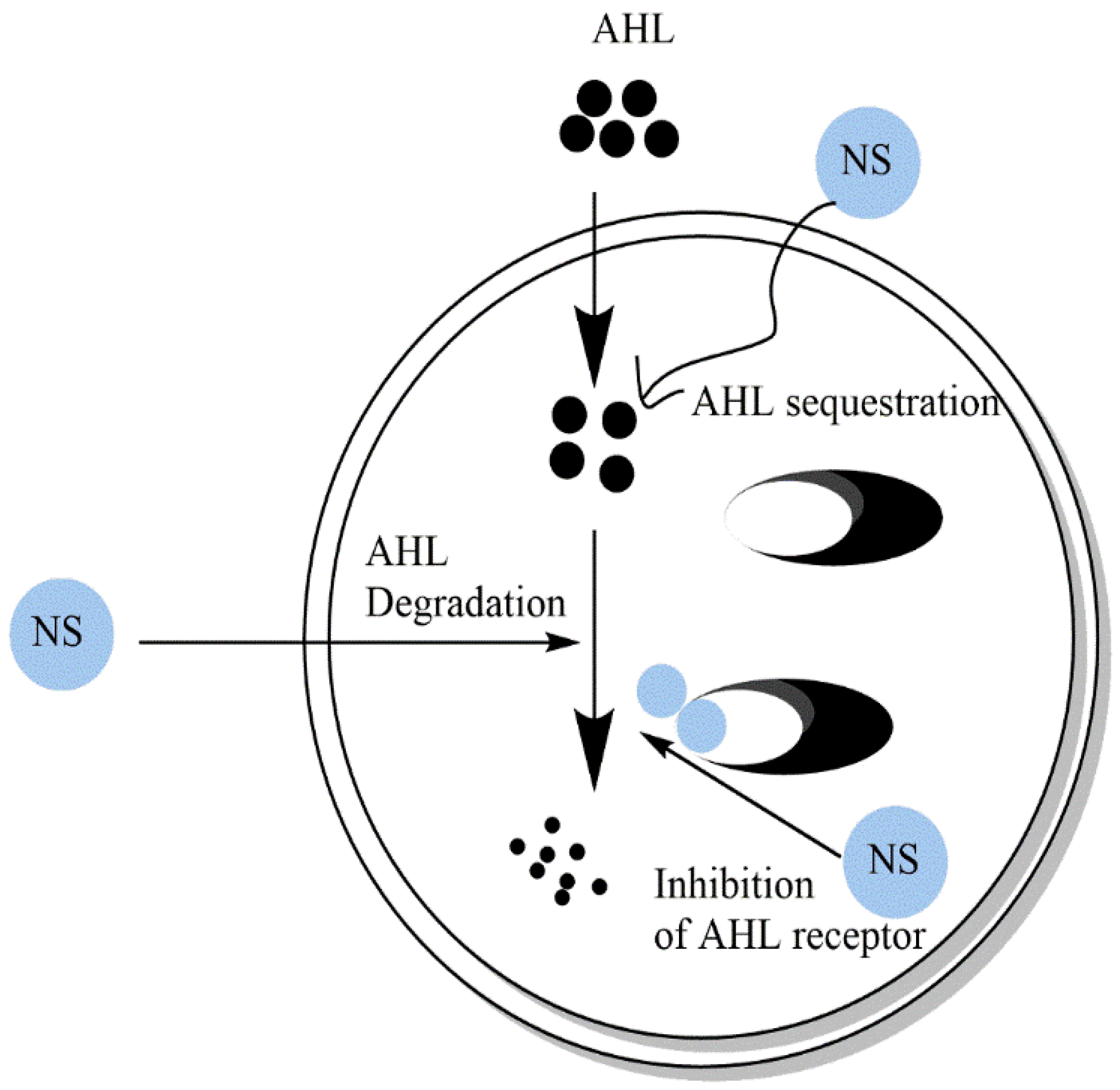
4. Secondary Metabolites of Medicinal Plants as Anti-Quorum-Sensing Agents
4.1. Quorum-Sensing Systems in Bacteria
4.2. Action of Secondary Metabolites on QS
4.2.1. Terpenoids
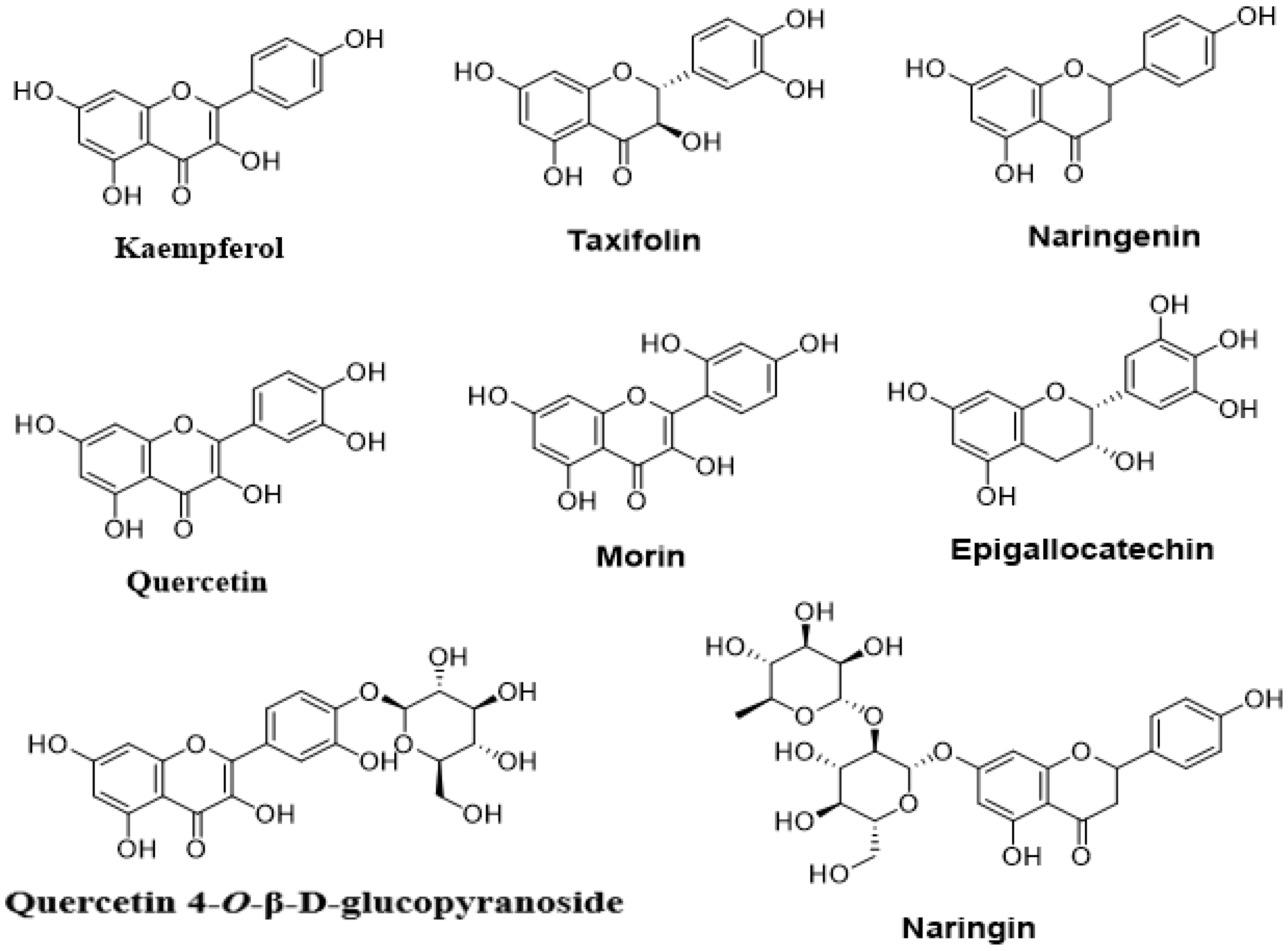
4.2.2. Flavonoids
4.2.3. Phenolic Acids
5. Clinical Investigations of Natural Compounds Isolated from Medicinal Plants
5.1. Clinical Investigations of Terpenoids
5.2. Clinical Investigations of Flavonoids
6. Techno-Economic Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Balahbib, A.; El Omari, N.; Hachlafi, N.E.L.; Lakhdar, F.; El Menyiy, N.; Salhi, N.; Mrabti, H.N.; Bakrim, S.; Zengin, G.; Bouyahya, A. Health Beneficial and Pharmacological Properties of P-Cymene. Food Chem. Toxicol. 2021, 153, 112259. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Belmehdi, O.; Benjouad, A.; El Hassani, R.A.; Amzazi, S.; Dakka, N.; Bakri, Y. Pharmacological Properties and Mechanism Insights of Moroccan Anticancer Medicinal Plants: What Are the next Steps? Ind. Crops Prod. 2020, 147, 112198. [Google Scholar] [CrossRef]
- Bouyahya, A.; El Allam, A.; Zeouk, I.; Taha, D.; Zengin, G.; Goh, B.H.; Catauro, M.; Montesano, D.; El Omari, N. Pharmacological Effects of Grifolin: Focusing on Anticancer Mechanisms. Molecules 2022, 27, 284. [Google Scholar] [CrossRef]
- Bouyahya, A.; El Menyiy, N.; Oumeslakht, L.; El Allam, A.; Balahbib, A.; Rauf, A.; Muhammad, N.; Kuznetsova, E.; Derkho, M.; Thiruvengadam, M.; et al. Preclinical and Clinical Antioxidant Effects of Natural Compounds against Oxidative Stress-Induced Epigenetic Instability in Tumor Cells. Antioxidants 2021, 10, 1553. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; El Omari, N.; Belmehdi, O.; Lagrouh, F.; El Jemli, M.; Marmouzi, I.; Faouzi, M.E.A.; Taha, D.; Bourais, I.; Zengin, G.; et al. Pharmacological Investigation of Ajuga iva Essential Oils Collected at Three Phenological Stages. Flavour Fragr. J. 2021, 36, 75–83. [Google Scholar] [CrossRef]
- Bouyahya, A.; El Omari, N.; Elmenyiy, N.; Guaouguaou, F.-E.; Balahbib, A.; Belmehdi, O.; Salhi, N.; Imtara, H.; Mrabti, H.N.; El-Shazly, M.; et al. Moroccan Antidiabetic Medicinal Plants: Ethnobotanical Studies, Phytochemical Bioactive Compounds, Preclinical Investigations, Toxicological Validations and Clinical Evidences; Challenges, Guidance and Perspectives for Future Management of Diabetes Worldwide. Trends Food Sci. Technol. 2021, 115, 147–254. [Google Scholar] [CrossRef]
- Bouyahya, A.; El Omari, N.; Hakkur, M.; El Hachlafi, N.; Charfi, S.; Balahbib, A.; Guaouguaou, F.-E.; Rebezov, M.; Maksimiuk, N.; Shariati, M.A.; et al. Sources, Health Benefits, and Biological Properties of Zeaxanthin. Trends Food Sci. Technol. 2021, 118, 519–538. [Google Scholar] [CrossRef]
- Bouyahya, A.; Guaouguaou, F.-E.; El Omari, N.; El Menyiy, N.; Balahbib, A.; El-Shazly, M.; Bakri, Y. Anti-Inflammatory and Analgesic Properties of Moroccan Medicinal Plants: Phytochemistry, in vitro and in vivo Investigations, Mechanism Insights, Clinical Evidences and Perspectives. J. Pharm. Anal. 2021. [Google Scholar] [CrossRef]
- Bouyahya, A.; Mechchate, H.; Benali, T.; Ghchime, R.; Charfi, S.; Balahbib, A.; Burkov, P.; Shariati, M.A.; Lorenzo, J.M.; Omari, N.E. Health Benefits and Pharmacological Properties of Carvone. Biomolecules 2021, 11, 1803. [Google Scholar] [CrossRef]
- Bouyahya, A.; Zengin, G.; Belmehdi, O.; Bourais, I.; Chamkhi, I.; Taha, D.; Benali, T.; Dakka, N.; Bakri, Y. Origanum compactum Benth.; from Traditional Use to Biotechnological Applications. J. Food Biochem. 2020, 44, e13251. [Google Scholar] [CrossRef]
- El Omari, N.; Bakha, M.; Imtara, H.; Guaouguaoua, F.-E.; Balahbib, A.; Zengin, G.; Bouyahya, A. Anticancer Mechanisms of Phytochemical Compounds: Focusing on Epigenetic Targets. Environ. Sci. Pollut. Res. 2021, 28, 47869–47903. [Google Scholar] [CrossRef] [PubMed]
- El Omari, N.; El Menyiy, N.; Zengin, G.; Goh, B.H.; Gallo, M.; Montesano, D.; Naviglio, D.; Bouyahya, A. Anticancer and Anti-Inflammatory Effects of Tomentosin: Cellular and Molecular Mechanisms. Separations 2021, 8, 207. [Google Scholar] [CrossRef]
- Hachlafi, N.E.; Aanniz, T.; Menyiy, N.E.; Baaboua, A.E.; Omari, N.E.; Balahbib, A.; Shariati, M.A.; Zengin, G.; Fikri-Benbrahim, K.; Bouyahya, A. In vitro and in vivo Biological Investigations of Camphene and Its Mechanism Insights: A Review. Food Rev. Int. 2021, 1–28. [Google Scholar] [CrossRef]
- Khouchlaa, A.; Talbaoui, A.; El Idrissi, A.E.Y.; Bouyahya, A.; Ait Lahsen, S.; Kahouadji, A.; Tijane, M. Determination of phenol content and evaluation of in vitro litholytic effects on urolithiasis of moroccan zizyphus lotus L. extract. Phytothérapie 2017, 16, 14–19. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Chamkhi, I.; El Omari, N.; Balahbib, A.; Sharifi-Rad, J.; Bouyahya, A.; Akram, M.; Iqbal, M.; Docea, A.O.; et al. Pharmacological Properties of Chalcones: A Review of Preclinical Including Molecular Mechanisms and Clinical Evidence. Front. Pharmacol. 2021, 11, 2068. [Google Scholar] [CrossRef] [PubMed]
- Calina, D.; Buga, A.M.; Mitroi, M.; Buha, A.; Caruntu, C.; Scheau, C.; Bouyahya, A.; El Omari, N.; El Menyiy, N.; Docea, A.O. The Treatment of Cognitive, Behavioural and Motor Impairments from Brain Injury and Neurodegenerative Diseases through Cannabinoid System Modulation—Evidence from In Vivo Studies. J. Clin. Med. 2020, 9, 2395. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Bakri, Y.; Et-Touys, A.; Talbaoui, A.; Khouchlaa, A.; Charfi, S.; Abrini, J.; Dakka, N. Résistance aux antibiotiques et mécanismes d’action des huiles essentielles contre les bactéries. Phytothérapie 2017, 1–11. [Google Scholar] [CrossRef]
- Bouyahya, A.; Lagrouh, F.; El Omari, N.; Bourais, I.; El Jemli, M.; Marmouzi, I.; Salhi, N.; Faouzi, M.E.A.; Belmehdi, O.; Dakka, N.; et al. Essential Oils of Mentha viridis Rich Phenolic Compounds Show Important Antioxidant, Antidiabetic, Dermatoprotective, Antidermatophyte and Antibacterial Properties. Biocatal. Agric. Biotechnol. 2020, 23, 101471. [Google Scholar] [CrossRef]
- Bouyahya, A.; Abrini, J.; Dakka, N.; Bakri, Y. Essential Oils of Origanum compactum Increase Membrane Permeability, Disturb Cell Membrane Integrity, and Suppress Quorum-Sensing Phenotype in Bacteria. J. Pharm. Anal. 2019, 9, 301–311. [Google Scholar] [CrossRef]
- Lee, J.; Wu, J.; Deng, Y.; Wang, J.; Wang, C.; Wang, J.; Chang, C.; Dong, Y.; Williams, P.; Zhang, L.-H. A Cell-Cell Communication Signal Integrates Quorum Sensing and Stress Response. Nat. Chem. Biol. 2013, 9, 339–343. [Google Scholar] [CrossRef]
- Bouyahya, A.; El Omari, N.; Guaouguaou, F.-E.; Benali, T.; Chamkhi, I. Quorum Sensing as Molecular Target to Fight Against Infectious Diseases. In Quorum Sensing: Microbial Rules of Life; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2020; Volume 1374, pp. 67–85. [Google Scholar] [CrossRef]
- Chamkhi, I.; El Omari, N.; Benali, T.; Bouyahya, A. Quorum Sensing and Plant-Bacteria Interaction: Role of Quorum Sensing in the Rhizobacterial Community Colonization in the Rhizosphere. In Quorum Sensing: Microbial Rules of Life; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2020; Volume 1374, pp. 139–153. [Google Scholar] [CrossRef]
- Trosko, J.E. Evolution of Microbial Quorum Sensing to Human Global Quorum Sensing: An Insight into How Gap Junctional Intercellular Communication Might Be Linked to the Global Metabolic Disease Crisis. Biology 2016, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhu, P.; Jiang, J.; Zhu, H.; Tan, S.; Li, R. Signaling Molecules of Quorum Sensing in Bacteria. Rev. Biotechnol. Biochem. 2020, 1–5. [Google Scholar]
- Wang, S.; Payne, G.F.; Bentley, W.E. Quorum Sensing Communication: Molecularly Connecting Cells, Their Neighbors, and Even Devices. Annu. Rev. Chem. Biomol. Eng. 2020, 11, 447–468. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Dakka, N.; Et-Touys, A.; Abrini, J.; Bakri, Y. Medicinal Plant Products Targeting Quorum Sensing for Combating Bacterial Infections. Asian Pac. J. Trop. Med. 2017, 10, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wang, R.; An, M.-M.; Bei-Bei, L. Iron-Depletion Prevents Biofilm Formation in Pseudomonas aeruginosa through Twitching Motility and Quorum Sensing. Braz. J. Microbiol. 2010, 41, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Frederick, M.R.; Kuttler, C.; Hense, B.A.; Eberl, H.J. A Mathematical Model of Quorum Sensing Regulated EPS Production in Biofilm Communities. Theor. Biol. Med. Model. 2011, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Kurnia, D.; Rachmawati, P.; Satari, M.H. Antibacterial of Dibenzo-p-Dioxi-2,8-Dicarboxylic Acid Against Pathogenic Oral Bacteria, E. faecalis ATCC 29212 Peptide Pheromones: Quorum Sensing of in vitro and in silico Study. Drug Des. Devel. Ther. 2020, 14, 3079–3086. [Google Scholar] [CrossRef] [PubMed]
- Satari, M.H.; Apriyanti, E.; Dharsono, H.D.A.; Nurdin, D.; Gartika, M.; Kurnia, D. Effectiveness of Bioactive Compound as Antibacterial and Anti-Quorum Sensing Agent from Myrmecodia pendans: An in silico Study. Molecules 2021, 26, 2465. [Google Scholar] [CrossRef]
- Apriyanti, E.; Satari, M.H.; Kurnia, D. Potential of MurA Enzyme and GBAP in Fsr Quorum Sensing System as Antibacterial Drugs Target: In vitro and in silico Study of Antibacterial Compounds from Myrmecodia Pendans. Comb. Chem. High Throughput Screen. 2021, 24, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Vattem, D.A.; Mihalik, K.; Crixell, S.H.; McLean, R.J.C. Dietary Phytochemicals as Quorum Sensing Inhibitors. Fitoterapia 2007, 78, 302–310. [Google Scholar] [CrossRef]
- Musk, D.J., Jr.; Hergenrother, P.J. Chemical Countermeasures for the Control of Bacterial Biofilms: Effective Compounds and Promising Targets. Curr. Med. Chem. 2006, 13, 2163–2177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevrot, R.; Rosen, R.; Haudecoeur, E.; Cirou, A.; Shelp, B.J.; Ron, E.; Faure, D. GABA Controls the Level of Quorum-Sensing Signal in Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA 2006, 103, 7460–7464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asif, M. Natural Anti-Quorum Sensing Agents Against Pseudomonas aeruginosa. J. Chem. Rev. 2020, 2, 57–69. [Google Scholar] [CrossRef]
- Nascimento, G.G.; Locatelli, J.; Freitas, P.C.; Silva, G.L. Antibacterial Activity of Plant Extracts and Phytochemicals on Antibiotic-Resistant Bacteria. Braz. J. Microbiol. 2000, 31, 247–256. [Google Scholar] [CrossRef]
- Sakagami, Y.; Kajimura, K. Bactericidal Activities of Disinfectants against Vancomycin-Resistant Enterococci. J. Hosp. Infect. 2002, 50, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.M.; Levy, S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011, 24, 718. [Google Scholar] [CrossRef] [Green Version]
- Chartone-Souza, E. Bactérias Ultra-Resistentes: Uma Guerra Quase Perdida. Cienc. Hoje 1998, 23, 27–35. [Google Scholar]
- Alves, S.; Duarte, A.; Sousa, S.; Domingues, F.C. Study of the Major Essential Oil Compounds of Coriandrum sativum against Acinetobacter baumannii and the Effect of Linalool on Adhesion, Biofilms and Quorum Sensing. Biofouling 2016, 32, 155–165. [Google Scholar] [CrossRef]
- Liu, X.; Cai, J.; Chen, H.; Zhong, Q.; Hou, Y.; Chen, W.; Chen, W. Antibacterial Activity and Mechanism of Linalool against Pseudomonas aeruginosa. Microb. Pathog. 2020, 141, 103980. [Google Scholar] [CrossRef]
- Prakash, A.; Vadivel, V.; Rubini, D.; Nithyanand, P. Antibacterial and Antibiofilm Activities of Linalool Nanoemulsions against Salmonella typhimurium. Food Biosci. 2019, 28, 57–65. [Google Scholar] [CrossRef]
- Espina, L.; Gelaw, T.K.; de Lamo-Castellví, S.; Pagán, R.; García-Gonzalo, D. Mechanism of Bacterial Inactivation by (+)-Limonene and Its Potential Use in Food Preservation Combined Processes. PLoS ONE 2013, 8, e56769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Sun, Z.; Chen, W. Antimicrobial Susceptibility and Antibacterial Mechanism of Limonene against Listeria monocytogenes. Molecules 2020, 25, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramenium, G.A.; Vijayakumar, K.; Pandian, S.K. Limonene Inhibits Streptococcal Biofilm Formation by Targeting Surface-Associated Virulence Factors. J. Med. Microbiol. 2015, 64, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Woo, E.-R.; Lee, D.G. Phytol Has Antibacterial Property by Inducing Oxidative Stress Response in Pseudomonas aeruginosa. Free Radic. Res. 2016, 50, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Mei, Y.; He, B.; Sun, X.; Li, J. Reducing Quorum Sensing-Mediated Virulence Factor Expression and Biofilm Formation in Hafnia Alvei by Using the Potential Quorum Sensing Inhibitor L-Carvone. Front. Microbiol. 2019, 9, 3324. [Google Scholar] [CrossRef] [PubMed]
- Nostro, A.; Scaffaro, R.; D’Arrigo, M.; Botta, L.; Filocamo, A.; Marino, A.; Bisignano, G. Study on Carvacrol and Cinnamaldehyde Polymeric Films: Mechanical Properties, Release Kinetics and Antibacterial and Antibiofilm Activities. Appl. Microbiol. Biotechnol. 2012, 96, 1029–1038. [Google Scholar] [CrossRef]
- Trevisan, D.A.C.; da Silva, A.F.; Negri, M.; de Abreu Filho, B.A.; Machinski, M., Jr.; Patussi, E.V.; Campanerut-Sá, P.A.Z.; Mikcha, J.M.G.; Trevisan, D.A.C.; da Silva, A.F.; et al. Antibacterial and Antibiofilm Activity of Carvacrol against Salmonella enterica Serotype Typhimurium. Braz. J. Pharm. Sci. 2018, 54, 1–8. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, F.; Ji, B.-P.; Pei, R.-S.; Xu, N. The Antibacterial Mechanism of Carvacrol and Thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Churklam, W.; Chaturongakul, S.; Ngamwongsatit, B.; Aunpad, R. The Mechanisms of Action of Carvacrol and Its Synergism with Nisin against Listeria monocytogenes on Sliced Bologna Sausage. Food Control 2020, 108, 106864. [Google Scholar] [CrossRef]
- Pontes, E.K.U.; Melo, H.M.; Nogueira, J.W.A.; Firmino, N.C.S.; de Carvalho, M.G.; Catunda, F.E.A., Jr.; Cavalcante, T.T.A. Antibiofilm Activity of the Essential Oil of Citronella (Cymbopogon nardus) and Its Major Component, Geraniol, on the Bacterial Biofilms of Staphylococcus aureus. Food Sci. Biotechnol. 2019, 28, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, A.; Jayasree, T.; Valliammai, A.; Pandian, S.K. Myrtenol Attenuates MRSA Biofilm and Virulence by Suppressing SarA Expression Dynamism. Front. Microbiol. 2019, 10, 2027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordeiro, L.; Figueiredo, P.; Souza, H.; Sousa, A.; Andrade-Júnior, F.; Barbosa-Filho, J.; Lima, E. Antibacterial and Antibiofilm Activity of Myrtenol against Staphylococcus aureus. Pharmaceuticals 2020, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cai, X.; Cao, J.; Wu, Z.; Pan, D. Effects of 1,8-Cineole on Carbohydrate Metabolism Related Cell Structure Changes of Salmonella. Front. Microbiol. 2018, 9, 1078. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.-J.; Jwa, S.-K. Inhibitory Effects of β-Caryophyllene on Streptococcus mutans Biofilm. Arch. Oral Biol. 2018, 88, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Moo, C.-L.; Yang, S.-K.; Osman, M.-A.; Yuswan, M.H.; Loh, J.-Y.; Lim, W.-M.; Lim, S.-H.-E.; Lai, K.-S. Antibacterial Activity and Mode of Action of β-Caryophyllene on Bacillus Cereus. Pol. J. Microbiol. 2020, 69, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Viana, E.S.; Campos, M.E.M.; Ponce, A.R.; Mantovani, H.C.; Vanetti, M.C.D. Biofilm Formation and Acyl Homoserine Lactone Production in Hafnia alvei Isolated from Raw Milk. Biol. Res. 2009, 42, 427–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, F.; Peng, F.; Xiong, L.; Chen, J.; Peng, C.; Dai, M. In Vitro and In Vivo Antibacterial Activity of Patchouli Alcohol from Pogostemon cablin. Chin. J. Integr. Med. 2021, 27, 125–130. [Google Scholar] [CrossRef]
- Yu, X.-D.; Xie, J.-H.; Wang, Y.-H.; Li, Y.-C.; Mo, Z.-Z.; Zheng, Y.-F.; Su, J.-Y.; Liang, Y.; Liang, J.-Z.; Su, Z.-R. Selective Antibacterial Activity of Patchouli Alcohol against Helicobacter pylori Based on Inhibition of Urease. Phytother. Res. 2015, 29, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Parai, D.; Chattopadhyay, S.; Mukherjee, S.K. Andrographolide: Antibacterial Activity against Common Bacteria of Human Health Concern and Possible Mechanism of Action. Folia Microbiol. 2017, 62, 237–244. [Google Scholar] [CrossRef]
- Wolska, K.I.; Grudniak, A.M.; Fiecek, B.; Kraczkiewicz-Dowjat, A.; Kurek, A. Antibacterial Activity of Oleanolic and Ursolic Acids and Their Derivatives. Cent. Eur. J. Biol. 2010, 5, 543–553. [Google Scholar] [CrossRef]
- Zhou, L.; Ding, Y.; Chen, W.; Zhang, P.; Chen, Y.; Lv, X. The in Vitro Study of Ursolic Acid and Oleanolic Acid Inhibiting Cariogenic Microorganisms as Well as Biofilm. Oral Dis. 2013, 19, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Alseekh, S.; Fernie, A.R. Conservation and Diversification of Flavonoid Metabolism in the Plant Kingdom. Curr. Opin. Plant Biol. 2020, 55, 100–108. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in Flavonoid Research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Wallace, J.; Bowlin, N.O.; Mills, D.M.; Saenkham, P.; Kwasny, S.M.; Opperman, T.J.; Williams, J.D.; Rock, C.O.; Bowlin, T.L.; Moir, D.T. Discovery of Bacterial Fatty Acid Synthase Type II Inhibitors Using a Novel Cellular Bioluminescent Reporter Assay. Antimicrob. Agents Chemother. 2015, 59, 5775–5787. [Google Scholar] [CrossRef] [Green Version]
- Pearson, J.P.; Feldman, M.; Iglewski, B.H.; Prince, A. Pseudomonas aeruginosa Cell-to-Cell Signaling Is Required for Virulence in a Model of Acute Pulmonary Infection. Infect. Immun. 2000, 68, 4331–4334. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Kong, Y.; Wu, D.; Zhang, H.; Wu, J.; Chen, J.; Ding, J.; Hu, L.; Jiang, H.; Shen, X. Three Flavonoids Targeting the β-Hydroxyacyl-Acyl Carrier Protein Dehydratase from Helicobacter pylori: Crystal Structure Characterization with Enzymatic Inhibition Assay. Protein Sci. 2008, 17, 1971–1978. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.-H.; Hu, Z.-Q.; Okubo, S.; Hara, Y.; Shimamura, T. Mechanism of Synergy between Epigallocatechin Gallate and β-Lactams against Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1737–1742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.H.; Tian, W.X. Inhibitory Effects of Flavonoids on Animal Fatty Acid Synthase. J. Biochem. 2004, 135, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Ulanowska, K.; Tkaczyk, A.; Konopa, G.; Węgrzyn, G. Differential Antibacterial Activity of Genistein Arising from Global Inhibition of DNA, RNA and Protein Synthesis in Some Bacterial Strains. Arch. Microbiol. 2006, 184, 271–278. [Google Scholar] [CrossRef]
- Ohemeng, K.A.; Schwender, C.F.; Fu, K.P.; Barrett, J.F. DNA Gyrase Inhibitory and Antibacterial Activity of Some Flavones (1). Bioorg. Med. Chem. Lett. 1993, 3, 225–230. [Google Scholar] [CrossRef]
- Plaper, A.; Golob, M.; Hafner, I.; Oblak, M.; Šolmajer, T.; Jerala, R. Characterization of Quercetin Binding Site on DNA Gyrase. Biochem. Biophys. Res. Commun. 2003, 306, 530–536. [Google Scholar] [CrossRef]
- Spina, M.; Cuccioloni, M.; Mozzicafreddo, M.; Montecchia, F.; Pucciarelli, S.; Eleuteri, A.M.; Fioretti, E.; Angeletti, M. Mechanism of Inhibition of Wt-Dihydrofolate Reductase from E. coli by Tea Epigallocatechin-Gallate. Proteins Struct. Funct. Bioinform. 2008, 72, 240–251. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef] [Green Version]
- Harshey, R.M. Bacterial Motility on a Surface: Many Ways to a Common Goal. Annu. Rev. Microbiol. 2003, 57, 249–273. [Google Scholar] [CrossRef] [PubMed]
- Rütschlin, S.; Böttcher, T. Inhibitors of Bacterial Swarming Behavior. Chemistry 2020, 26, 964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, L.N.; Zimmer, K.R.; Macedo, A.J.; Trentin, D.S. Plant Natural Products Targeting Bacterial Virulence Factors. Chem. Rev. 2016, 116, 9162–9236. [Google Scholar] [CrossRef] [PubMed]
- Pejin, B.; Ciric, A.; Glamoclija, J.; Nikolic, M.; Stanimirovic, B.; Sokovic, M. Quercetin Potently Reduces Biofilm Formation of the Strain Pseudomonas aeruginosa PAO1 in Vitro. Curr. Pharm. Biotechnol. 2015, 16, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms and Device-Associated Infections. Emerg. Infect. Dis. 2001, 7, 277. [Google Scholar] [CrossRef]
- Ding, Y.; Avramova, Z.; Fromm, M. The Arabidopsis Trithorax-like Factor ATX1 Functions in Dehydration Stress Responses via ABA-Dependent and ABA-Independent Pathways. Plant J. 2011, 66, 735–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjarnsholt, T.; Ciofu, O.; Molin, S.; Givskov, M.; Høiby, N. Applying Insights from Biofilm Biology to Drug Development—Can a New Approach Be Developed? Nat. Rev. Drug Discov. 2013, 12, 791–808. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.R.; Pillai, S.D.; Patil, B.S. Suppression of Bacterial Cell–Cell Signalling, Biofilm Formation and Type III Secretion System by Citrus flavonoids. J. Appl. Microbiol. 2010, 109, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Asahi, Y.; Noiri, Y.; Miura, J.; Maezono, H.; Yamaguchi, M.; Yamamoto, R.; Azakami, H.; Hayashi, M.; Ebisu, S. Effects of the Tea Catechin Epigallocatechin Gallate on P Orphyromonas Gingivalis Biofilms. J. Appl. Microbiol. 2014, 116, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Tan, K.S. Effects of Epigallocatechin Gallate against Enterococcus faecalis Biofilm and Virulence. Arch. Oral Biol. 2015, 60, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Croft, K.D. The Chemistry and Biological Effects of Flavonoids and Phenolic Acids a. Ann. N. Y. Acad. Sci. 1998, 854, 435–442. [Google Scholar] [CrossRef]
- Campos, F.M.; Couto, J.A.; Figueiredo, A.R.; Tóth, I.V.; Rangel, A.O.; Hogg, T.A. Cell Membrane Damage Induced by Phenolic Acids on Wine Lactic Acid Bacteria. Int. J. Food Microbiol. 2009, 135, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Jeon, B. Contribution of Surface Polysaccharides to the Resistance of Campylobacter jejuni to Antimicrobial Phenolic Compounds. J. Antibiot. 2015, 68, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Diniz-Silva, H.T.; da Silva Cirino, I.C.; dos Santos Falcão-Silva, V.; Magnani, M.; de Souza, E.L.; Siqueira-Júnior, J.P. Tannic Acid as a Potential Modulator of Norfloxacin Resistance in Staphylococcus aureus Overexpressing NorA. Chemotherapy 2016, 61, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Kępa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wąsik, T.J. Antimicrobial Potential of Caffeic Acid against Staphylococcus aureus Clinical Strains. BioMed Res. Int. 2018, 2018, 7413504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanhueza, L.; Melo, R.; Montero, R.; Maisey, K.; Mendoza, L.; Wilkens, M. Synergistic Interactions between Phenolic Compounds Identified in Grape Pomace Extract with Antibiotics of Different Classes against Staphylococcus aureus and Escherichia coli. PLoS ONE 2017, 12, e0172273. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Sun, X.; Xie, Q.; Liu, H.; Zhao, Y.; Pan, Y.; Hwang, C.-A.; Wu, V.C. Antimicrobial Effect of Blueberry (Vaccinium corymbosum L.) Extracts against the Growth of Listeria monocytogenes and Salmonella enteritidis. Food Control 2014, 35, 159–165. [Google Scholar] [CrossRef]
- Díaz-Gómez, R.; López-Solís, R.; Obreque-Slier, E.; Toledo-Araya, H. Comparative Antibacterial Effect of Gallic Acid and Catechin against Helicobacter pylori. LWT-Food Sci. Technol. 2013, 54, 331–335. [Google Scholar] [CrossRef]
- Díaz-Gómez, R.; Toledo-Araya, H.; López-Solís, R.; Obreque-Slier, E. Combined Effect of Gallic Acid and Catechin against Escherichia coli. LWT-Food Sci. Technol. 2014, 59, 896–900. [Google Scholar] [CrossRef]
- Salaheen, S.; Peng, M.; Joo, J.; Teramoto, H.; Biswas, D. Eradication and Sensitization of Methicillin Resistant Staphylococcus aureus to Methicillin with Bioactive Extracts of Berry Pomace. Front. Microbiol. 2017, 8, 253. [Google Scholar] [CrossRef]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial Activity of Phenolic Acids against Commensal, Probiotic and Pathogenic Bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Merkl, R.; Hrádková, I.; Filip, V.; Šmidrkal, J. Antimicrobial and Antioxidant Properties of Phenolic Acids Alkyl Esters. Czech J. Food Sci. 2010, 28, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Maldonado, A.F.; Schieber, A.; Gänzle, M.G. Structure–Function Relationships of the Antibacterial Activity of Phenolic Acids and Their Metabolism by Lactic Acid Bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef]
- Chibane, L.B.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant Antimicrobial Polyphenols as Potential Natural Food Preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [Green Version]
- Duggirala, S.; Nankar, R.P.; Rajendran, S.; Doble, M. Phytochemicals as Inhibitors of Bacterial Cell Division Protein FtsZ: Coumarins Are Promising Candidates. Appl. Biochem. Biotechnol. 2014, 174, 283–296. [Google Scholar] [CrossRef]
- Oh, E.; Jeon, B. Synergistic Anti-Campylobacter jejuni Activity of Fluoroquinolone and Macrolide Antibiotics with Phenolic Compounds. Front. Microbiol. 2015, 6, 1129. [Google Scholar] [CrossRef] [Green Version]
- Almajano, M.P.; Carbo, R.; Delgado, M.E.; Gordon, M.H. Effect of PH on the Antimicrobial Activity and Oxidative Stability of Oil-in-Water Emulsions Containing Caffeic Acid. J. Food Sci. 2007, 72, C258–C263. [Google Scholar] [CrossRef]
- Bassler, B.L. Small Talk: Cell-to-Cell Communication in Bacteria. Cell 2002, 109, 421–424. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.-M. Relationships between the Regulatory Systems of Quorum Sensing and Multidrug Resistance. Front. Microbiol. 2016, 7, 958. [Google Scholar] [CrossRef] [PubMed]
- Reading, N.C.; Sperandio, V. Quorum Sensing: The Many Languages of Bacteria. FEMS Microbiol. Lett. 2006, 254, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Burt, S.A.; Ojo-Fakunle, V.T.A.; Woertman, J.; Veldhuizen, E.J.A. The Natural Antimicrobial Carvacrol Inhibits Quorum Sensing in Chromobacterium violaceum and Reduces Bacterial Biofilm Formation at Sub-Lethal Concentrations. PLoS ONE 2014, 9, e93414. [Google Scholar] [CrossRef] [Green Version]
- Tapia-Rodriguez, M.R.; Hernandez-Mendoza, A.; Gonzalez-Aguilar, G.A.; Martinez-Tellez, M.A.; Martins, C.M.; Ayala-Zavala, J.F. Carvacrol as Potential Quorum Sensing Inhibitor of Pseudomonas aeruginosa and Biofilm Production on Stainless Steel Surfaces. Food Control 2017, 75, 255–261. [Google Scholar] [CrossRef]
- Tapia-Rodriguez, M.R.; Bernal-Mercado, A.T.; Gutierrez-Pacheco, M.M.; Vazquez-Armenta, F.J.; Hernandez-Mendoza, A.; Gonzalez-Aguilar, G.A.; Martinez-Tellez, M.A.; Nazzaro, F.; Ayala-Zavala, J.F. Virulence of Pseudomonas aeruginosa Exposed to Carvacrol: Alterations of the Quorum Sensing at Enzymatic and Gene Levels. J. Cell Commun. Signal. 2019, 13, 531–537. [Google Scholar] [CrossRef]
- Amaya, S.; Pereira, J.A.; Borkosky, S.A.; Valdez, J.C.; Bardón, A.; Arena, M.E. Inhibition of Quorum Sensing in Pseudomonas aeruginosa by Sesquiterpene Lactones. Phytomedicine 2012, 19, 1173–1177. [Google Scholar] [CrossRef]
- Aliyu, A.B.; Koorbanally, N.A.; Moodley, B.; Singh, P.; Chenia, H.Y. Quorum Sensing Inhibitory Potential and Molecular Docking Studies of Sesquiterpene Lactones from Vernonia blumeoides. Phytochemistry 2016, 126, 23–33. [Google Scholar] [CrossRef]
- Aliyu, A.B.; Koorbanally, N.A.; Moodley, B.; Chenia, H.Y. Sesquiterpene Lactones from Polydora Serratuloides and Their Quorum Sensing Inhibitory Activity. Nat. Prod. Res. 2020, 35, 4517–4523. [Google Scholar] [CrossRef]
- Packiavathy, I.A.S.V.; Agilandeswari, P.; Musthafa, K.S.; Pandian, S.K.; Ravi, A.V. Antibiofilm and Quorum Sensing Inhibitory Potential of Cuminum cyminum and Its Secondary Metabolite Methyl Eugenol against Gram Negative Bacterial Pathogens. Food Res. Int. 2012, 8, 85–92. [Google Scholar] [CrossRef]
- Zhou, L.; Zheng, H.; Tang, Y.; Yu, W.; Gong, Q. Eugenol Inhibits Quorum Sensing at Sub-Inhibitory Concentrations. Biotechnol. Lett. 2013, 35, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Al-Shabib, N.A.; Husain, F.M.; Ahmad, I.; Baig, M.H. Eugenol Inhibits Quorum Sensing and Biofilm of Toxigenic MRSA Strains Isolated from Food Handlers Employed in Saudi Arabia. Biotechnol. Biotechnol. Equip. 2017, 11, 387–396. [Google Scholar] [CrossRef] [Green Version]
- Rathinam, P.; Kumar, H.S.V.; Viswanathan, P. Eugenol Exhibits Anti-Virulence Properties by Competitively Binding to Quorum Sensing Receptors. Biofouling 2017, 33, 624–639. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Letsididi, K.S.; Yu, F.; Pei, Z.; Wang, H.; Letsididi, R. Inhibitive Effect of Eugenol and Its Nanoemulsion on Quorum Sensing–Mediated Virulence Factors and Biofilm Formation by Pseudomonas aeruginosa. J. Food Prot. 2019, 82, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Pejin, B.; Ciric, A.; Glamoclija, J.; Nikolic, M.; Sokovic, M. In Vitro Anti-Quorum Sensing Activity of Phytol. Nat. Prod. Res. 2015, 29, 374–377. [Google Scholar] [CrossRef]
- Srinivasan, R.; Devi, K.R.; Kannappan, A.; Pandian, S.K.; Ravi, A.V. Piper Betle and Its Bioactive Metabolite Phytol Mitigates Quorum Sensing Mediated Virulence Factors and Biofilm of Nosocomial Pathogen Serratia marcescens in Vitro. J. Ethnopharmacol. 2016, 193, 592–603. [Google Scholar] [CrossRef]
- Ruckmani, K.; Ravi, A.V. Exploring the Anti-Quorum Sensing and Antibiofilm Efficacy of Phytol against Serratia Marcescens Associated Acute Pyelonephritis Infection in Wistar Rats. Front. Cell. Infect. Microbiol. 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Vega, P.; Xu, Y.; Chen, C.-Y.; Irudayaraj, J. Exploring the Anti-Quorum Sensing Activity of a d-Limonene Nanoemulsion for Escherichia coli O157:H7. J. Biomed. Mater. Res. A 2018, 106, 1979–1986. [Google Scholar] [CrossRef]
- Šimunović, K.; Sahin, O.; Kovač, J.; Shen, Z.; Klančnik, A.; Zhang, Q.; Možina, S.S. (-)-α-Pinene Reduces Quorum Sensing and Campylobacter jejuni Colonization in Broiler Chickens. PLoS ONE 2020, 15, e0230423. [Google Scholar] [CrossRef] [Green Version]
- Rasamiravaka, T.; Vandeputte, O.M.; Pottier, L.; Huet, J.; Rabemanantsoa, C.; Kiendrebeogo, M.; Andriantsimahavandy, A.; Rasamindrakotroka, A.; Stévigny, C.; Duez, P.; et al. Pseudomonas aeruginosa Biofilm Formation and Persistence, along with the Production of Quorum Sensing-Dependent Virulence Factors, Are Disrupted by a Triterpenoid Coumarate Ester Isolated from Dalbergia trichocarpa, a Tropical Legume. PLoS ONE 2015, 10, e0132791. [Google Scholar] [CrossRef]
- Huber, B.; Eberl, L.; Feucht, W.; Polster, J. Influence of Polyphenols on Bacterial Biofilm Formation and Quorum-Sensing. Z. Nat. C 2003, 58, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, T.; Nakahara, A.; Minnatul, K.M.; Noiri, Y.; Ebisu, S.; Kato, A.; Azakami, H. The Inhibitory Effects of Catechins on Biofilm Formation by the Periodontopathogenic Bacterium, Eikenella corrodens. Biosci. Biotechnol. Biochem. 2010, 74, 2445–2450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyila, M.A.; Leonard, C.M.; Hussein, A.A.; Lall, N. Activity of South African Medicinal Plants against Listeria Monocytogenes Biofilms, and Isolation of Active Compounds from Acacia Karroo. S. Afr. J. Bot. 2012, 78, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Castillo, S.; Heredia, N.; García, S. 2(5H)-Furanone, Epigallocatechin Gallate, and a Citric-Based Disinfectant Disturb Quorum-Sensing Activity and Reduce Motility and Biofilm Formation of Campylobacter jejuni. Folia Microbiol. 2015, 60, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Su, T.-Y.; Wang, M.-Y.; Yang, S.-F.; Mar, K.; Hung, S.-L. Inhibitory Effects of Tea Catechin Epigallocatechin-3-Gallate against Biofilms Formed from Streptococcus mutans and a Probiotic Lactobacillus strain. Arch. Oral Biol. 2018, 94, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, S.; Saei, H.D.; Ahmadi, M.; Zahraei-Salehi, T. Anti-Quorum Sensing Effects of Licochalcone A and Epigallocatechin-3-Gallate against Salmonella typhimurium Isolates from Poultry Sources. Vet. Res. Forum 2020, 11, 273–279. [Google Scholar] [CrossRef]
- Truchado, P.; Giménez-Bastida, J.-A.; Larrosa, M.; Castro-Ibáñez, I.; Espín, J.C.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Allende, A. Inhibition of Quorum Sensing (QS) in Yersinia enterocolitica by an Orange Extract Rich in Glycosylated Flavanones. J. Agric. Food Chem. 2012, 60, 8885–8894. [Google Scholar] [CrossRef]
- Yue, J.; Yang, H.; Liu, S.; Song, F.; Guo, J.; Huang, C. Influence of Naringenin on the Biofilm Formation of Streptococcus mutans. J. Dent. 2018, 76, 24–31. [Google Scholar] [CrossRef]
- Gopu, V.; Meena, C.K.; Shetty, P.H. Quercetin Influences Quorum Sensing in Food Borne Bacteria: In-Vitro and In-Silico Evidence. PLoS ONE 2015, 10, e0134684. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, J.; Sun, F.; Feng, W.; Sun, Y.; Qiu, X.; Xiong, L.; Liu, Y.; Chen, Y. Quercetin Is an Effective Inhibitor of Quorum Sensing, Biofilm Formation and Virulence Factors in Pseudomonas aeruginosa. J. Appl. Microbiol. 2016, 120, 966–974. [Google Scholar] [CrossRef] [Green Version]
- Al-Yousef, H.M.; Ahmed, A.F.; Al-Shabib, N.A.; Laeeq, S.; Khan, R.A.; Rehman, M.T.; Alsalme, A.; Al-Ajmi, M.F.; Khan, M.S.; Husain, F.M. Onion Peel Ethylacetate Fraction and Its Derived Constituent Quercetin 4′-O-β-D Glucopyranoside Attenuates Quorum Sensing Regulated Virulence and Biofilm Formation. Front. Microbiol. 2017, 8, 1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabski, H.; Hunanyan, L.; Tiratsuyan, S.; Vardapetyan, H. Interaction of Quercetin with Transcriptional Regulator LasR of Pseudomonas aeruginosa: Mechanistic Insights of the Inhibition of Virulence through Quorum Sensing. bioRxiv 2017, 239996. [Google Scholar] [CrossRef] [Green Version]
- Erdönmez, D.; Rad, A.Y.; Aksöz, N.; Erdönmez, D.; Rad, A.Y.; Aksöz, N. Anti-Quorum Sensing Potential of Antioxidant Quercetin and Resveratrol. Braz. Arch. Biol. Technol. 2018, 61, e18160756. [Google Scholar] [CrossRef]
- Ouyang, J.; Feng, W.; Lai, X.; Chen, Y.; Zhang, X.; Rong, L.; Sun, F.; Chen, Y. Quercetin Inhibits Pseudomonas aeruginosa Biofilm Formation via the Vfr-Mediated LasIR System. Microb. Pathog. 2020, 149, 104291. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, O.M.; Kiendrebeogo, M.; Rasamiravaka, T.; Stévigny, C.; Duez, P.; Rajaonson, S.; Diallo, B.; Mol, A.; Baucher, M.; El Jaziri, M. The Flavanone Naringenin Reduces the Production of Quorum Sensing-Controlled Virulence Factors in Pseudomonas aeruginosa PAO1. Microbiology 2011, 157, 2120–2132. [Google Scholar] [CrossRef] [Green Version]
- Ming, D.; Wang, D.; Cao, F.; Xiang, H.; Mu, D.; Cao, J.; Li, B.; Zhong, L.; Dong, X.; Zhong, X.; et al. Kaempferol Inhibits the Primary Attachment Phase of Biofilm Formation in Staphylococcus aureus. Front. Microbiol. 2017, 8, 2263. [Google Scholar] [CrossRef] [Green Version]
- Chemmugil, P.; Lakshmi, P.T.V.; Annamalai, A. Exploring Morin as an Anti-Quorum Sensing Agent (Anti-QSA) against Resistant Strains of Staphylococcus aureus. Microb. Pathog. 2019, 127, 304–315. [Google Scholar] [CrossRef]
- Hernando-Amado, S.; Alcalde-Rico, M.; Gil-Gil, T.; Valverde, J.R.; Martínez, J.L. Naringenin Inhibition of the Pseudomonas aeruginosa Quorum Sensing Response Is Based on Its Time-Dependent Competition With N-(3-Oxo-Dodecanoyl)-L-Homoserine Lactone for LasR Binding. Front. Mol. Biosci. 2020, 7, 25. [Google Scholar] [CrossRef]
- Walker, T.S.; Bais, H.P.; Déziel, E.; Schweizer, H.P.; Rahme, L.G.; Fall, R.; Vivanco, J.M. Pseudomonas aeruginosa-Plant Root Interactions. Pathogenicity, Biofilm Formation, and Root Exudation. Plant Physiol. 2004, 134, 320–331. [Google Scholar] [CrossRef] [Green Version]
- Corral-Lugo, A.; Daddaoua, A.; Ortega, A.; Espinosa-Urgel, M.; Krell, T. Rosmarinic Acid Is a Homoserine Lactone Mimic Produced by Plants That Activates a Bacterial Quorum-Sensing Regulator. Sci. Signal. 2016, 9, ra1. [Google Scholar] [CrossRef]
- Devi, K.R.; Srinivasan, R.; Kannappan, A.; Santhakumari, S.; Bhuvaneswari, M.; Rajasekar, P.; Prabhu, N.M.; Ravi, A.V. In Vitro and in Vivo Efficacy of Rosmarinic Acid on Quorum Sensing Mediated Biofilm Formation and Virulence Factor Production in Aeromonas hydrophila. Biofouling 2016, 32, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.F.; Corral-Lugo, A.; Krell, T. The Plant Compound Rosmarinic Acid Induces a Broad Quorum Sensing Response in Pseudomonas aeruginosa PAO1. Environ. Biol. 2018, 20, 4230–4244. [Google Scholar] [CrossRef]
- Wang, H.; Chu, W.; Ye, C.; Gaeta, B.; Tao, H.; Wang, M.; Qiu, Z. Chlorogenic Acid Attenuates Virulence Factors and Pathogenicity of Pseudomonas aeruginosa by Regulating Quorum Sensing. Appl. Microbiol. Biotechnol. 2019, 103, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.-C.; Edlind, M.P.; Liu, P.; Saenkham, P.; Banta, L.M.; Wise, A.A.; Ronzone, E.; Binns, A.N.; Kerr, K.; Nester, E.W. The Plant Signal Salicylic Acid Shuts down Expression of the Vir Regulon and Activates Quormone-Quenching Genes in Agrobacterium. Proc. Natl. Acad. Sci. USA 2007, 104, 11790–11795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, S.; Gu, K.; Jiang, L.; Nassour, A. Salicylic Acid Affects Swimming, Twitching and Swarming Motility in Pseudomonas aeruginosa, Resulting in Decreased Biofilm Formation. J. Exp. Microbiol. Immunol. 2011, 15, 8. [Google Scholar]
- Lagonenko, L.; Lagonenko, A.; Evtushenkov, A. Impact of Salicylic Acid on Biofilm Formation by Plant Pathogenic Bacteria. J. Biol. Earth Sci. 2013, 3, 176–181. [Google Scholar]
- Joshi, J.R.; Burdman, S.; Lipsky, A.; Yariv, S.; Yedidia, I. Plant Phenolic Acids Affect the Virulence of Pectobacterium aroidearum and P. carotovorum ssp. Brasiliense via Quorum Sensing Regulation. Mol. Plant Pathol. 2015, 4, 487–500. [Google Scholar] [CrossRef] [Green Version]
- Rajkumari, J.; Borkotoky, S.; Murali, A.; Suchiang, K.; Mohanty, S.K.; Busi, S. Cinnamic Acid Attenuates Quorum Sensing Associated Virulence Factors and Biofilm Formation in Pseudomonas Aeruginosa PAO1. Biotechnol. Lett. 2018, 40, 1087–1100. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Zhou, J.-W.; Zhang, P.-P.; Luo, H.-Z.; Tang, S.; Li, J.-J.; Deng, S.-M.; Jia, A.-Q. Quorum Sensing Inhibition and Tobramycin Acceleration in Chromobacterium violaceum by Two Natural Cinnamic Acid Derivatives. Appl. Microbiol. Biotechnol. 2020, 104, 5025–5037. [Google Scholar] [CrossRef]
- Bodini, S.F.; Manfredini, S.; Epp, M.; Valentini, S.; Santori, F. Quorum Sensing Inhibition Activity of Garlic Extract and P-Coumaric Acid. Lett. Appl. Microbiol. 2009, 49, 551–555. [Google Scholar] [CrossRef]
- Chen, X.; Yu, F.; Li, Y.; Lou, Z.; Toure, S.L.; Wang, H. The Inhibitory Activity of P-Coumaric Acid on Quorum Sensing and Its Enhancement Effect on Meat Preservation. CyTA-J. Food 2020, 18, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Luís, Â.; Silva, F.; Sousa, S.; Duarte, A.P.; Domingues, F. Antistaphylococcal and Biofilm Inhibitory Activities of Gallic, Caffeic, and Chlorogenic Acids. Biofouling 2014, 30, 69–79. [Google Scholar] [CrossRef]
- Musthafa, K.S.; Sivamaruthi, B.S.; Pandian, S.K.; Ravi, A.V. Quorum Sensing Inhibition in Pseudomonas aeruginosa PAO1 by Antagonistic Compound Phenylacetic Acid. Curr. Microbiol. 2012, 65, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Casetti, F.; Bartelke, S.; Biehler, K.; Augustin, M.; Schempp, C.M.; Frank, U. Antimicrobial Activity Against Bacteria with Dermatological Relevance and Skin Tolerance of the Essential Oil from Coriandrum sativum, L. Fruits. Phytother. Res. 2012, 26, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.I.; Song, D.J.; Shin, C.M.; Yoon, H.; Park, Y.S.; Kim, N.; Lee, D.H. Inhibitory effects of β-caryophyllene on Helicobacter pylori infection: A randomized double-blind, placebo-controlled study. Korean J. Gastroenterol. 2019, 74, 199–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosto, F.; Benvenuti, C.; Group, C.S. Controlled study on thymol + eugenol vaginal douche versus econazole in vaginal candidiasis and metronidazole in bacterial vaginosis. Arzneimittelforschung 2011, 61, 126–131. [Google Scholar] [CrossRef]
- Baygin, O.; Tuzuner, T.; Kusgoz, A.; Senel, A.C.; Tanriver, M.; Arslan, I. Antibacterial Effects of Fluoride Varnish Compared with Chlorhexidine plus Fluoride in Disabled Children. Oral Health Prev. Dent. 2014, 12, 373–382. [Google Scholar] [CrossRef]
- Howell, A.B.; Botto, H.; Combescure, C.; Blanc-Potard, A.-B.; Gausa, L.; Matsumoto, T.; Tenke, P.; Sotto, A.; Lavigne, J.-P. Dosage Effect on Uropathogenic Escherichia coli Anti-Adhesion Activity in Urine Following Consumption of Cranberry Powder Standardized for Proanthocyanidin Content: A Multicentric Randomized Double Blind Study. BMC Infect. Dis. 2010, 10, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afshar, K.; Stothers, L.; Scott, H.; MacNeily, A.E. Cranberry Juice for the Prevention of Pediatric Urinary Tract Infection: A Randomized Controlled Trial. J. Urol. 2012, 188, 1584–1587. [Google Scholar] [CrossRef]
- Yi, L.; Yu, J.; Han, L.; Li, T.; Yang, H.; Huang, C. Combination of Baicalein and Ethanol-Wet-Bonding Improves Dentin Bonding Durability. J. Dent. 2019, 90, 103207. [Google Scholar] [CrossRef]
- Busscher, H.J.; Rinastiti, M.; Siswomihardjo, W.; van der Mei, H.C. Biofilm Formation on Dental Restorative and Implant Materials. J. Dent. Res. 2010, 89, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Vilela, M.M.; Salvador, S.L.; Teixeira, I.G.L.; Del Arco, M.C.G.; De Rossi, A. Efficacy of Green Tea and Its Extract, Epigallocatechin-3-Gallate, in the Reduction of Cariogenic Microbiota in Children: A Randomized Clinical Trial. Arch. Oral Biol. 2020, 114, 104727. [Google Scholar] [CrossRef] [PubMed]


| Molecules | Bacterial Species | Experimental Approaches | Key Results | References |
|---|---|---|---|---|
| Linalool | Acinetobacter baumannii | Evaluation of biofilm formation Anti-QS activity assay Quantification of biofilm biomass–CV staining | Inhibited biofilm formation Modified bacterial adhesion to surfaces Interfered with the QS system | [40] |
| Linalool | Pseudomonas aeruginosa | Determination of cell membrane permeability, membrane potential, and respiratory chain dehydrogenase | Damaged the respiratory chain Destroyed the integrity of bacterial membrane Disturbed normal cell morphology | [41] |
| Linalool nanoemulsions | Salmonella typhimurium | Biofilm inhibition studies Cell membrane integrity | Destroyed the integrity of bacterial membrane Induced high antibiofilm activity | [42] |
| (+)-Limonene | Escherichia coli BJ4 | Cell permeabilization test | Induced permeabilization of bacterial membrane | [43] |
| (+)-Limonene | Escherichia coli lptD4213 | Cell permeabilization test | Induced sublethal damage in the cytoplasmic membrane (at pH 4.0) | [43] |
| Limonene | Listeria monocytogenes | SEM analysis Conductivity measurement Determination of the effect of limonene on the respiratory chain complex I–V | Increased cell membrane permeability Destroyed the cell integrity and bacterial wall structure Affected respiration and energy metabolism | [44] |
| Limonene | Streptococcus pyogenes (SF370) | Analysis of antibiofilm potential, SEM, and cell viability assay | Reduced biofilm formation in a dose-dependent manner | [45] |
| Limonene | Streptococcus mutans | Analysis of antibiofilm potential, SEM, and cell viability assay | Inhibited acid production and downregulated the vicR gene Targeted the surface-associated proteins, thus reducing surface-mediated virulence factors | [45] |
| Phytol | Pseudomonas aeruginosa | Membrane depolarization assay DNA damage detection NAD+ cycling assay ROS measurement | Increased intracellular ROS level Increased transient depletion of NADH Induced DNA damage by oxidative stress Induced membrane depolarization Triggered inhibition of cell division | [46] |
| l-carvone | Hafnia alvei | In silico analysis RT-qPCR studies QS interference approach | Inhibited QS activity by reducing AHL production (0.5 μL/mL), biofilm formation (52.41%), swarming motility (74.94%), and swinging motility (61.49%) | [47] |
| Carvacrol | Escherichia coli and Staphylococcus aureus | Antibiofilm activity SEM analysis | Reduced biofilm formation | [48] |
| Carvacrol | Salmonella enterica serotype Typhimurium | MTT assay Crystal violet assay SEM analysis | Shriveled and retracted appearance at 4 × MIC Reduced metabolic activity (0.089 OD550) Reduced biofilm biomass (1.719 OD550) | [49] |
| Carvacrol and thymol | Escherichia coli | Fluorescent dyes Flow cytometry analysis | Disturbed cytoplasmic membrane | [50] |
| Carvacrol | Listeria monocytogenes | TEM analysis Flow cytometric analysis | Disrupted the structure of bacterial cells Induced degenerative changes in the cytoplasmic membrane and cell wall Modified respiratory activity Increased membrane permeability and depolarization | [51] |
| Geraniol | Staphylococcus aureus | Antibiofilm activity Biofilm biomass quantification | Reduced biofilm biomass. Reduced cell viability | [52] |
| Myrtenol | Methicillin-resistant Staphylococcus aureus | Extraction of staphyloxanthin Autolysis assay Ring biofilm inhibition assay | Inhibited production of staphyloxanthin Inhibited the synthesis of major virulence factors Inhibited biofilm formation | [53] |
| Myrtenol | Staphylococcus aureus | Antibiofilm effect | Inhibited biofilm formation | [54] |
| 1,8-Cineole | Salmonella sp. D194-2 | TEM analysis Proteomics analysis | Damaged the structure of cell walls and membranes Downregulated the carbohydrate metabolism and membrane protein-related genes | [55] |
| β-Caryophyllene | Streptococcus mutans | Confocal laser scanning microscope Real-time RT-PCR | Inhibited biofilm formation Reduced the expression of gtf genes | [56] |
| β-caryophyllene | Bacillus cereus | Measurement of UV-absorbing materials Zeta-potential measurement | Altered the membrane permeability and integrity | [57] |
| Compounds | Bacteria | Effects | References |
|---|---|---|---|
| Carvacrol | Chromobacterium violaceum | Inhibition of biofilm formation at sublethal concentrations Reducing of cviI expression Decreasing violacein and chitinase activity | [106] |
| Pseudomonas aeruginosa | Inhibition of biofilm formation Reducing pyocyanin and violacein production | [107] | |
| Pseudomonas aeruginosa ATCC 10154 | Reducing production of AHLs Reducing the expression of lasR expression Reducing biofilm formation | [108] | |
| Sesquiterpene lactone | Pseudomonas aeruginosa ATCC 27853 | Inhibition of QS phenotypes, such as biofilm formation, elastase activity, and AHLs | [109] |
| Chromobacterium violaceum | Decreasing the affinity of CviR protein to its receptor LuxR | [110] | |
| Chromobacterium violaceum ATCC 12472 | Inhibition of QS mediators | [111] | |
| Eugenol | Pseudomonas aeruginosa, Proteus mirabilis, and Serratia marcescens | Reducing AHL and violacein formation | [112] |
| Escherichia coli Pseudomonas aeruginosa | Decreasing violacein, elastase, pyocyanin, and biofilm formation Inhibition of las and pqs QS systems | [113] | |
| Methicillin-resistant Staphylococcus aureus | Reducing production of elastase, protease, chitinase, and pyocyanin | [114] | |
| Pseudomonas aeruginosa | Inhibition of biofilm formation | [115] | |
| Pseudomonas aeruginosa | Decreasing rhlA, lasI, and rhlI expression Inhibition of biofilm formation | [116] | |
| Phytol | Pseudomonas aeruginosa PAO1 | Inhibition of biofilm formation and pyocyanin production Reducing bacterial flagella motility | [117] |
| Serratia marcescens | Inhibition of protease and biofilm production | [118] | |
| Serratia marcescens | Inhibition of biofilm, lipase, and hemolysin formation Inhibition of bacterial motility Downregulation of fimA, fimC, flhC, flhD, bsmB, pigP, and shlA genes expression Decreasing production of lipase and protease | [119] | |
| Linalool | Acinetobacter baumannii | Inhibition of biofilm formation | [40] |
| d-limonene | Escherichia coli | Inhibition of biofilm formation Suppression of curli production Decreasing swimming and swarming ability | [120] |
| (−)-α-Pinene | Campylobacter jejuni | Reducing the QS communication | [121] |
| Compounds | Organisms Tested | Key Findings | References |
|---|---|---|---|
| Epigallocatechin | Burkholderia cepacia and Staphylococcus aureus | Inhibited biofilm formation by interference with AHL production | [123] |
| Eikenella corrodens | Inhibited QS mediated by auto-inducer 2 (AI-2) Inhibited biofilm formation | [124] | |
| Listeria monocytogenes | Inhibited biofilm formation | [125] | |
| Campylobacter jejuni | Disturbed QS functionin Reduced motility and biofilm formation Decreased AI-2 activity | [126] | |
| Streptococcus mutans (Sm) and probiotic Lactobacillus casei in Yakult (LcY) | Decreased biomass and acid production Inhibited biofilm formation Acid production | [127] | |
| Salmonella typhimurium | Reduced sdiA and luxS genes expression | [128] | |
| Naringin | Chromobacterium violaceum | Inhibited biofilm formation Reduced swimming and swarming motility Inducted some gene transcription, such as yenR, flhDC, and fliA | [129] |
| Yersinia enterocolitica | Inhibited biofilm formation Decreased the synthesis of AHL | [129] | |
| Streptococcus mutans | Suppressed biofilm maturation | [130] | |
| Quercetin | Escherichia coli O157:H7 and Vibrio harveyi | Inhibited biofilm formation Blocked cell–cell signaling | [83] |
| Chromobacteriumviolaceum CV026 | Reduced violacein production, biofilm formation, EPS production, motility, and alginate production | [131] | |
| Pseudomonas aeruginosa PAO1 | Inhibited biofilm formation Inhibited the twitching motility | [79] | |
| Pseudomonas aeruginosa strain PAO1 | Inhibited biofilm formation Reduced virulence factors, including pyocyanin, protease, and elastase Reduced levels of lasI, lasR, rhlI, and rhlR genes expression | [132] | |
| Quercetin 4’-O-β-d- glucopyranoside | Chromobacteriumviolaceum CV12472 and Pseudomonas aeruginosa PAO1 | Inhibited violacein, elastase, pyocyanin, and biofilm formation | [133] |
| Pseudomonas aeruginosa | Inhibited LasR expression | [134] | |
| Chromobacterium violaceum ATCC 12,472 and Chromobacterium violaceum CV026 | Inhibited production of violacein pigment Inhibited the communication molecule, C6-AHL | [135] | |
| Pseudomonas aeruginosa | Decreased adhesion, biofilm formation, swarming motility, and expression of biofilm-associated genes Reduced pyocyanin production Inhibited the activity of protease Reducing QS via the vfr-mediated lasIR system | [136] | |
| Taxifolin | Pseudomonas aeruginosa PAO1 | Reduced production of pyocyanin and elastase Inhibited the QS-controlled genes expression | [137] |
| Kaempferol | Staphylococcus aureus | Inhibited biofilm formation Inhibition of adhesion-related gene expression | [138] |
| Morin | Staphylococcus aureus | Inhibited biofilm formation Reduced motility and spreading | [139] |
| Naringenin | Pseudomonas aeruginosa | Inhibited the QS-regulated gene expression | [140] |
| Compounds | Organisms Tested | Key Findings | References |
|---|---|---|---|
| Rosmarinic acid | Pseudomonas aeruginosa PAO1 | Inhibited biofilm formation | [141] |
| Pseudomonas aeruginosa PAO1 | Inhibited QS regulator RhlR and N-butanoyl- homoserine lactone (C4-HSL) Induced a great increase in RhlR-mediated transcription than that of C4-HSL Induced QS-dependent gene expression Inhibited biofilm formation and virulence factor production (pyocyanin and elastase) | [142] | |
| Aeromonas hydrophila | Biofilm inhibitory concentration was 750 μg/mL Reduced production of QS-mediated hemolysin, lipase, and elastase Downregulated the virulence genes, such as ahh1, aerA, lip, and ahyB | [143] | |
| Pseudomonas aeruginosa PAO1 | Induced the expression of 128 genes, including numerous virulence factor genes Induced seven sRNAs that were all encoded in regions close to QS-induced genes | [144] | |
| Chlorogenic acid | Pseudomonas aeruginosa | Inhibited biofilm formation, swarming, and virulence factors Downregulation of QS-related gene expression Inhibition of QS receptors | [145] |
| Chromobacterium violaceum | Inhibited biofilm formation, swarming motility, chitinolytic activity, and violacein production | [145] | |
| Salicylic acid | Agrobacterium tumefaciens | Decreased biofilm and AHL production via the modulation of 103 genes’ expression | [146] |
| Pseudomonas aeruginosa | Decreased swimming, twitching, and swarming motility | [147] | |
| Pectobacterium carotovorum and Pseudomonas syringae pv syringae | Inhibited biofilm formation, motility, and AHL production | [148] | |
| Pectobacterium aroidearum and Pectobacterium carotovorum ssp. brasiliense | Affected the QS machinery of the two species, consequently altering the expression of bacterial virulence factors Inhibited QS genes’ expression, such as expI, expR, PC1_1442 (luxR transcriptional regulator), and luxS (a component of the AI-2 system) Reduced AHL levels | [149] | |
| Cinnamic acid | Pseudomonas aeruginosa PAO1 | Inhibited QS-dependent virulence factors and biofilm formation | [150] |
| Pectobacterium aroidearum and Pectobacterium carotovorum ssp. brasiliense | Altered gene expression of virulence factors Inhibited genes expression of QS (expI, expR, PC1_1442 (luxR transcriptional regulator), and luxS) Decreasing the expression of AHL signal | [149] | |
| Two cinnamic acid derivatives: 4-dimethyl-aminocinnamic acid and 4-methoxycinnamic acid | Chromobacterium violaceum ATCC12472 | Inhibited the synthesis of N-decanoyl-homoserine lactone Reduced production of virulence factors (violacein, hemolysin, and chitinase) Downregulated some QS-related metabolites (ethanolamine and l-methionine) Decreased QS-related genes expression (cviI and cviR) Inhibited biofilm formation | [151] |
| p-Coumaric acid | Agrobacterium tumefaciens NTL4, Chromobacterium violaceum 5999, and Pseudomonas chlororaphis | Inhibited QS responses | [152] |
| Chromobacterium violaceum (CECT 494) | Inhibited the production of violacein | [153] | |
| Caffeic acid | Staphylococcus aureus | Reduced bacterial adhesion Decreased the production of α-hemolysin | [154] |
| Ellagic acid | Burkholderiacepacia | Inhibited biofilm formation | [123] |
| Phenylacetic acid | Pseudomonas aeruginosa | Exhibited competitive action with AHLs signaling Decreased the production of pyocyanin, protease, and elastase | [155] |
| Molecules | Treatment | Experimental Approaches | Bacterial Strains | Key Results | References |
|---|---|---|---|---|---|
| Thymol + eugenol | One douche/day for one week | A multicenter, parallel group, randomized study 221 bacterial vaginosis cases | Vaginal strain | Reduced the severity of dyspareunia, vaginal dryness, erythema, and itching Reduced vaginal pH | [158] |
| Thymol + chlorhexidine | T0, before general anesthesia; T1, one month after treatment; T2, six months after treatment; T3, twelve months after treatment | 90 patients randomly assigned into 3 groups Caries risk test Bacterial counts for each individual patient at four stages (T0, T1, T2, and T3) | Salivary mutans streptococci and lactobacilli | Decreased bacterial values compared to the control group No significant differences at T0 and T3 | [159] |
| β-caryophyllene | 126 mg/day for eight weeks | Randomized double-blind, placebo-controlled trial 33 patients received β-caryophyllene 33 patients received a placebo preparation | Helicobacter pylori | No significant change in the urea breath test Improvement of epigastralgia and nausea Decreased serum IL-1β levels | [157] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouyahya, A.; Chamkhi, I.; Balahbib, A.; Rebezov, M.; Shariati, M.A.; Wilairatana, P.; Mubarak, M.S.; Benali, T.; El Omari, N. Mechanisms, Anti-Quorum-Sensing Actions, and Clinical Trials of Medicinal Plant Bioactive Compounds against Bacteria: A Comprehensive Review. Molecules 2022, 27, 1484. https://doi.org/10.3390/molecules27051484
Bouyahya A, Chamkhi I, Balahbib A, Rebezov M, Shariati MA, Wilairatana P, Mubarak MS, Benali T, El Omari N. Mechanisms, Anti-Quorum-Sensing Actions, and Clinical Trials of Medicinal Plant Bioactive Compounds against Bacteria: A Comprehensive Review. Molecules. 2022; 27(5):1484. https://doi.org/10.3390/molecules27051484
Chicago/Turabian StyleBouyahya, Abdelhakim, Imane Chamkhi, Abdelaali Balahbib, Maksim Rebezov, Mohammad Ali Shariati, Polrat Wilairatana, Mohammad S. Mubarak, Taoufiq Benali, and Nasreddine El Omari. 2022. "Mechanisms, Anti-Quorum-Sensing Actions, and Clinical Trials of Medicinal Plant Bioactive Compounds against Bacteria: A Comprehensive Review" Molecules 27, no. 5: 1484. https://doi.org/10.3390/molecules27051484








