Electrochemical Impedance Spectroscopy in the Characterisation and Application of Modified Electrodes for Electrochemical Sensors and Biosensors
Abstract
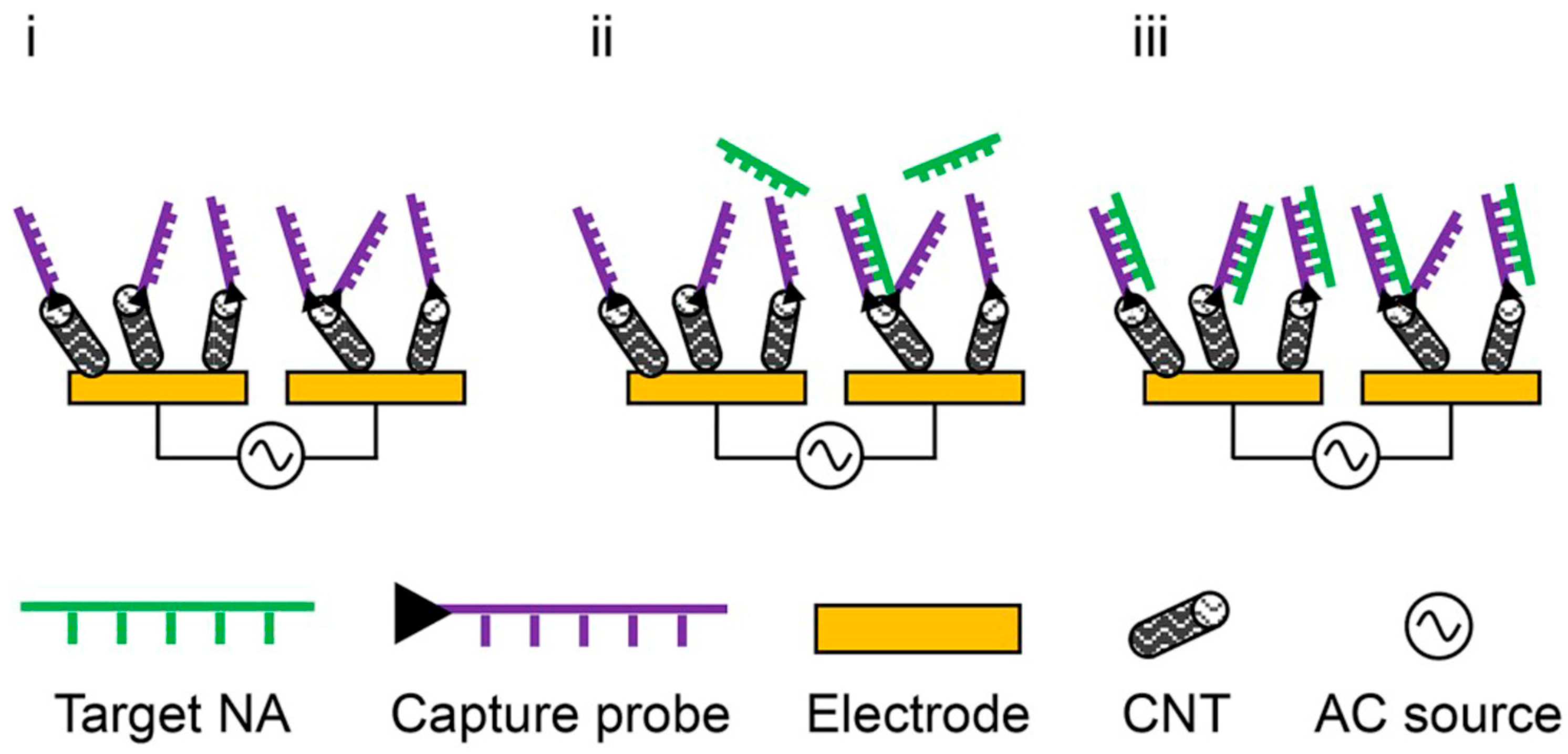
:1. Introduction
2. Fundamentals of Electrochemical Impedance Spectroscopy
2.1. Basics of Electrochemical Impedance
2.2. Physical Model of the Electrochemical Cell and Electrical Equivalent Circuits
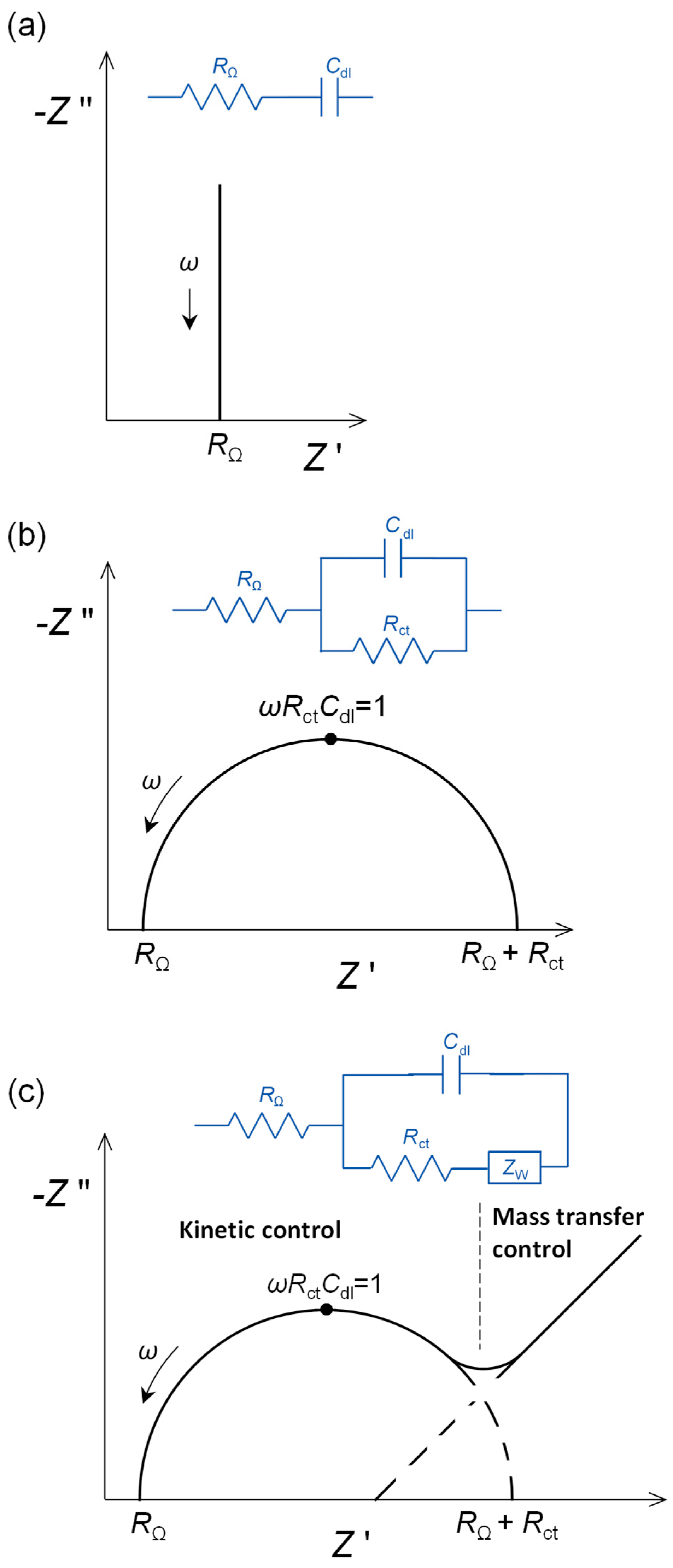
- (a)
- At applied potentials where no electrode process occurs, there is ordering of the charge at the electrode interface on the solution side, often referred to as the double layer, and this charge will be compensated by sufficient charge polarisation within the electrode. The electrical equivalent circuit element is a capacitor, and in such a case, the full equivalent circuit is RΩ in series with the double-layer capacitance, Cdl. The impedance plot is a vertical straight line, as shown in Figure 1a, with the impedance components being given by:
- (b)
- If an electrode process occurs at a particular applied potential, that is controlled by the rate of charge transfer across the electrode–solution interface over the whole frequency range, then the charge transfer is represented by a charge transfer resistance, Rct. The resistance, Rct, is placed in parallel with the double-layer capacity, Cdl, since charge transfer and charge separation happen at different sites on the electrode surface. A complex plane plot of the impedance affords a spectrum with a semicircle of diameter Rct, see Figure 1b.
- (c)
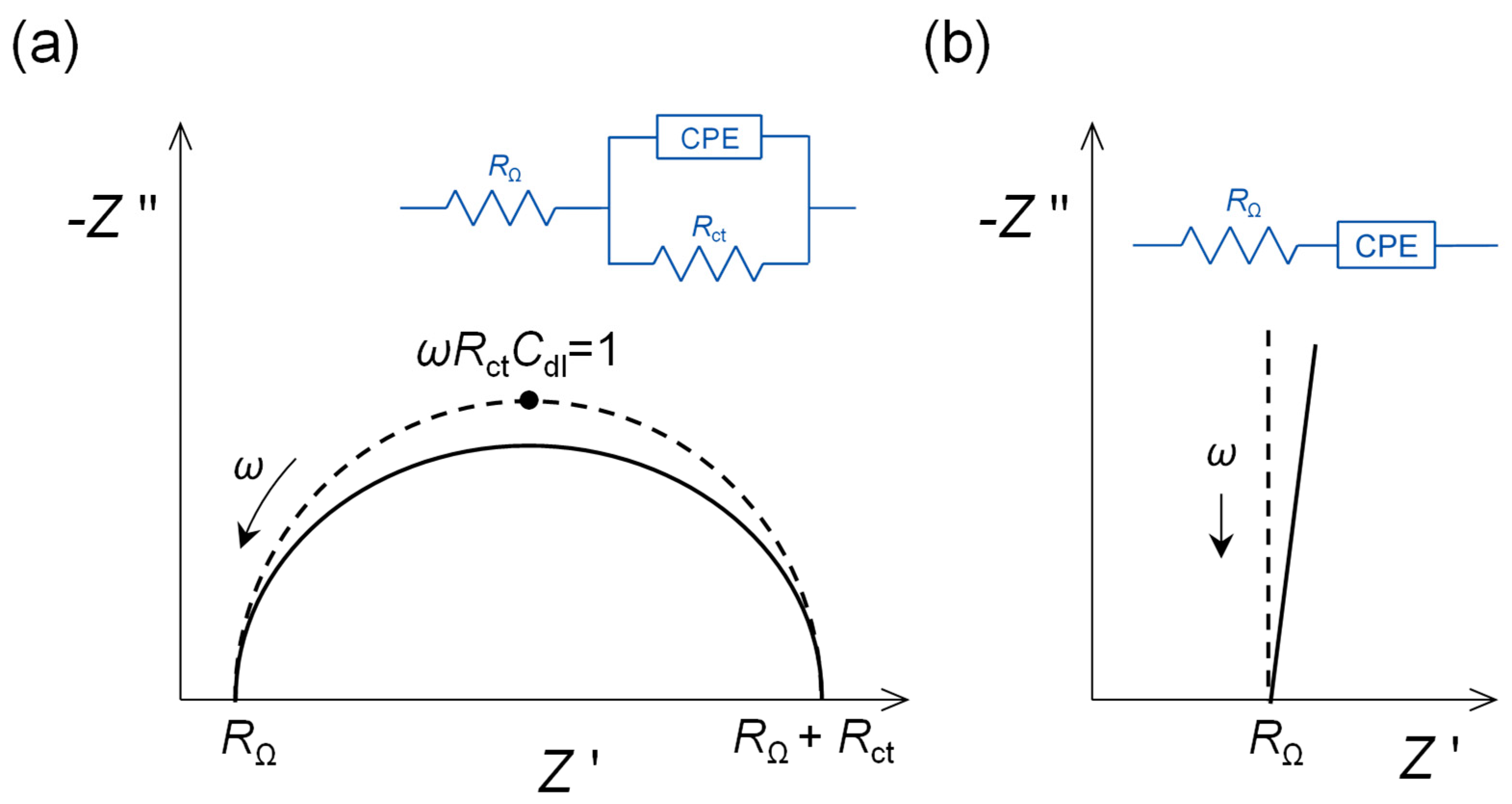
- The maximum of the semicircle corresponds to ωRctCdl = 1. The value of Rct depends on the rate of charge transfer, analyte concentration and the electroactive area of the electrode, becoming smaller in value the larger the area. The normalised values of resistances are expressed in Ω cm2. Modification of an electrode, for example by a nanomaterial, may lead to a large increase in electroactive area and, owing to this, Rct becomes smaller. In such a case, it cannot be deduced that the electron transfer rate has increased due to incorporation of the nanomaterial, without first compensating for the increase in electroactive surface area.
2.3. Analysis of Experimental Impedance Spectra
3. EIS as a Sensor Characterisation Technique
3.1. Self-Assembled Bilayer Structures
3.2. Layer-by-Layer Modified Electrode Structures
4. EIS as a Sensing Technique
4.1. EIS Using a Redox Probe
4.2. EIS without a Redox Probe
5. Future Outlook
Funding
Conflicts of Interest
References
- Brett, C.M.A.; Oliveira Brett, A.M. Electrochemistry. Principles, Methods and Applications; Oxford University Press: Oxford, UK, 1993; Chapter 11; ISBN 978-0198553885. [Google Scholar]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Orazem, M.; Tribollet, B. Electrochemical Impedance Spectroscopy, 2nd ed.; Wiley: Hoboken, NJ, USA, 2017; ISBN 978-1-119-34092-8. [Google Scholar]
- Barsoukov, E.; Macdonald, J.R. (Eds.) Impedance Spectroscopy: Theory, Experiment and Applications, 3rd ed.; Wiley: Hoboken, NJ, USA, 2018; ISBN 978-1-119-07408-3. [Google Scholar]
- Kanoun, O. Impedance spectroscopy advances and future trends: A comprehensive review. In Impedance Spectroscopy. Advanced Applications: Battery Research, Bioimpedance, System Design; Kanoun, O., Ed.; De Gruyter: Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- Srinivasan, R.; Fasmin, F. An Introduction to Electrochemical Impedance Spectroscopy; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Brett, C.M.A. Electrochemical impedance spectroscopy for characterization of electrochemical sensors and biosensors. ECS Trans. 2008, 13, 67–80. [Google Scholar] [CrossRef]
- Brett, C.M.A. Perspectives and challenges for self-assembled layer-by-layer biosensor and biomaterial architectures. Curr. Opin. Electrochem. 2018, 12, 21–26. [Google Scholar] [CrossRef]
- Brett, C.M.A.; Kresak, S.; Hianik, T.; Oliveira Brett, A.M. Studies on self-assembled alkanethiol monolayers formed at applied potential on polycrystalline gold electrodes. Electroanalysis 2003, 15, 557–565. [Google Scholar] [CrossRef] [Green Version]
- Pinto, E.M.; Barsan, M.M.; Brett, C.M.A. Mechanism of formation and construction of self-assembled myoglobin/hyaluronic acid multilayer films—An electrochemical QCM, impedance and AFM study. J. Phys. Chem. B 2010, 114, 15354–15361. [Google Scholar] [CrossRef]
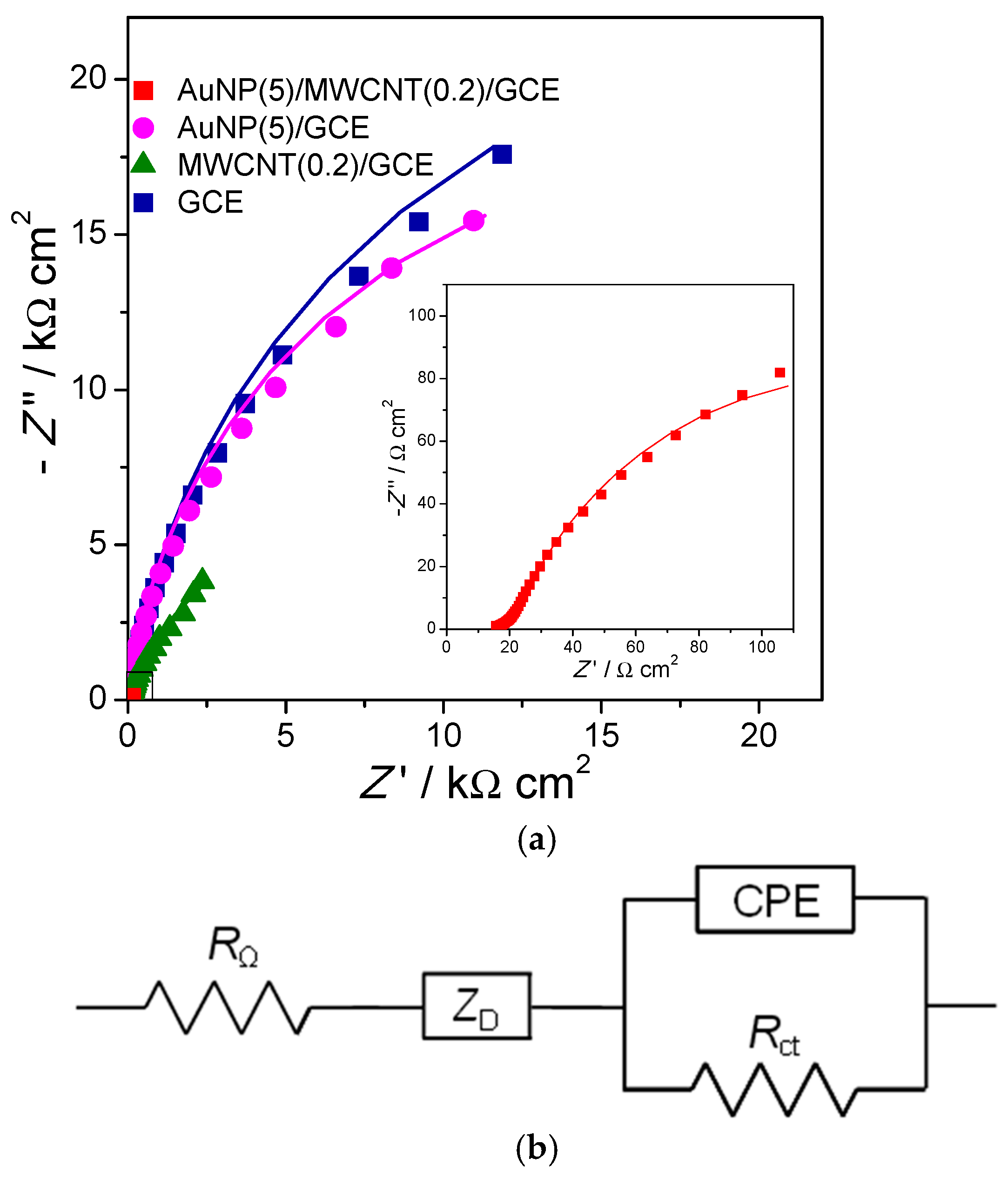
- Ben Messaoud, N.; Ghica, M.E.; Dridi, C.; Ben Ali, M.; Brett, C.M.A. Electrochemical sensor based on multiwalled carbon nanotube and gold nanoparticle modified electrode for the sensitive detection of bisphenol A. Sens. Actuators B 2017, 253, 513–522. [Google Scholar] [CrossRef]
- da Silva, W.; Queiroz, A.C.; Brett, C.M.A. Nanostructured poly(phenazine)/Fe2O3 nanoparticle film modified electrodes formed by electropolymerization in ethaline—Deep eutectic solvent. Microscopic and electrochemical characterization. Electrochim. Acta 2020, 347, 136284. [Google Scholar] [CrossRef]
- Song, Z.; Yuan, R.; Chai, Y.; Yin, B.; Fu, P.; Wang, J. Multilayer structured amperometric immunosensor based on gold nanoparticles and Prussian blue nanoparticles/nanocomposite functionalized interface. Electrochim. Acta 2010, 55, 1778–1784. [Google Scholar] [CrossRef]
- Evtugyn, G.; Porfireva, A.; Shamagsumova, R.; Hianik, T. Advances in electrochemical aptasensors based on carbon nanomaterials. Chemosensors 2020, 8, 96. [Google Scholar] [CrossRef]
- Schrattenecker, J.D.; Heer, R.; Melnik, E.; Maier, T.; Fafilek, G.; Hainberger, R. Hexaammineruthenium (II)/(III) as alternative redox-probe to hexacyanoferrat (II)/(III) for stable impedimetric biosensing with gold electrodes. Biosens. Bioelectron. 2019, 127, 25–30. [Google Scholar] [CrossRef]
- Strong, M.E.; Richards, J.R.; Torres, M.; Beck, C.M.; La Belle, J.T. Faradaic electrochemical impedance spectroscopy for enhanced analyte detection in diagnostics. Biosens. Bioelectron. 2021, 177, 112949. [Google Scholar] [CrossRef]
- Nemcekova, K.; Labuda, J. Advanced materials-integrated electrochemical sensors as promising medical diagnostics tools: A review. Mater. Sci. Eng. C 2021, 120, 111751. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, J.; Montes, R.; Baeza, M. Trends in electrochemical impedance spectroscopy involving nanocomposite transducers: Characterization, architecture surface and bio-sensing. Trac-Trends Anal. Chem. 2017, 97, 201–215. [Google Scholar] [CrossRef]
- Ito, Y.; Okuda-Shimazaki, J.; Tsugawa, W.; Loew, N.; Shitanda, I.; Lin, C.E.; la Belle, J.; Sode, K. Third generation impedimetric sensor employing direct electron transfer type glucose dehydrogenase. Biosens. Bioelectron. 2019, 129, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Qing, J.; Liu, J. A sensitive electrochemical impedance DNA biosensor based on ZnO nanorod electrodes for BCR/ABL fusion gene detection. Int. J. Electrochem. Sci. 2019, 14, 4271–4279. [Google Scholar] [CrossRef]
- Witte, C.; Lisdat, F. Direct detection of DNA and DNA-ligand interaction by impedance spectroscopy. Electroanalysis 2011, 23, 339–346. [Google Scholar] [CrossRef]
- Rushworth, J.V.; Ahmed, A.; Griffiths, H.H.; Pollock, N.M.; Hooper, N.M.; Millner, P.A. A label-free electrical impedimetric biosensor for the specific detection of Alzheimer’s amyloid-beta oligomers. Biosens. Bioelectron. 2014, 56, 83–90. [Google Scholar] [CrossRef]
- Lakard, B. Electrochemical biosensors based on conducting polymers: A review. Appl. Sci. 2020, 10, 6614. [Google Scholar] [CrossRef]
- Shaikh, M.O.; Srikanth, B.; Zhu, P.-Y.; Chuang, C.-H. Impedimetric immunosensor utilizing polyaniline/gold nanocomposite-modified screen-printed electrodes for early detection of chronic kidney disease. Sensors 2019, 19, 3990. [Google Scholar] [CrossRef] [Green Version]
- Yáñez-Sedeño, P.; Agüí, L.; Campuzano, S.; Pingarrón, J.M. What electrochemical biosensors can do for forensic science? Unique features and applications. Biosensors 2019, 9, 127. [Google Scholar] [CrossRef] [Green Version]
- Dzulkurnain, N.A.; Mokhtar, M.; Rashid, J.I.A.; Knight, V.F.; Yunus, W.M.Z.W.; Ong, K.K.; Kasim, N.A.M.; Noor, S.A.M. A review on impedimetric and voltammetric analysis based on polypyrrole conducting polymers for electrochemical sensing applications. Polymers 2021, 13, 2728. [Google Scholar] [CrossRef]
- Zheng, X.; Khaoulani, S.; Ktari, N.; Lo, M.; Khalil, A.M.; Zerrouki, C.; Fourati, N.; Chehimi, M.M. Towards clean and safe water: A review on the emerging role of imprinted polymer-based electrochemical sensors. Sensors 2021, 21, 4300. [Google Scholar] [CrossRef]
- Baghbaderani, S.S.; Noorbakhsh, A. Novel chitosan-Nafion composite for fabrication of highly sensitive impedimetric and colorimetric As(III) aptasensor. Biosens. Bioelectron. 2019, 131, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Upasham, S.; Banga, I.K.; Jagannath, B.; Paul, A.; Lin, K.-C.; Muthukumar, S.; Prasad, S. Electrochemical impedimetric biosensors, featuring the use of Room Temperature Ionic Liquids (RTILs): Special focus on non-faradaic sensing. Biosens. Bioelectron. 2021, 177, 112940. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Barsan, M.M.; Korpan, Y.; Brett, C.M.A. L-lactate selective impedimetric bienzymatic biosensor based on lactate dehydrogenase and pyruvate oxidase. Electrochim. Acta 2017, 231, 209–215. [Google Scholar] [CrossRef]
- Cao, L.; Kiely, J.; Piano, M.; Luxton, R. Nanoparticle-based 3D membrane for impedimetric biosensor applications. Bioelectrochemistry 2020, 136, 107593. [Google Scholar] [CrossRef]
- Selvam, A.P.; Vattipalli, K.M.; Prasad, S. Design of a high sensitive non-faradaic impedimetric sensor. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 3251–3254. [Google Scholar] [CrossRef] [Green Version]
- Oliveira Brett, A.M.; da Silva, L.A.; Brett, C.M.A. Adsorption of guanine, guanosine and adenine at electrodes studied by differential pulse voltammetry and electrochemical impedance. Langmuir 2002, 18, 2326–2330. [Google Scholar] [CrossRef] [Green Version]
- Trotter, M.; Borst, N.; Thewes, R.; von Stetten, F. Review: Electrochemical DNA sensing—Principles, commercial systems, and applications. Biosens. Bioelectron. 2020, 154, 112069. [Google Scholar] [CrossRef] [PubMed]
- Basuray, S.; Senapati, S.; Aijian, A.; Mahon, A.R.; Chang, H.-C. Shear and ac field enhanced carbon nanotube impedance assay for rapid, sensitive and mismatch-discriminating DNA hybridization. ACS Nano 2009, 3, 1823–1830. [Google Scholar] [CrossRef]
- Laschuk, N.O.; Easton, E.B.; Zenkina, O.V. Reducing the resistance for the use of electrochemical impedance spectroscopy analysis in materials chemistry. RSC Adv. 2021, 11, 27925. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brett, C.M.A. Electrochemical Impedance Spectroscopy in the Characterisation and Application of Modified Electrodes for Electrochemical Sensors and Biosensors. Molecules 2022, 27, 1497. https://doi.org/10.3390/molecules27051497
Brett CMA. Electrochemical Impedance Spectroscopy in the Characterisation and Application of Modified Electrodes for Electrochemical Sensors and Biosensors. Molecules. 2022; 27(5):1497. https://doi.org/10.3390/molecules27051497
Chicago/Turabian StyleBrett, Christopher M. A. 2022. "Electrochemical Impedance Spectroscopy in the Characterisation and Application of Modified Electrodes for Electrochemical Sensors and Biosensors" Molecules 27, no. 5: 1497. https://doi.org/10.3390/molecules27051497
APA StyleBrett, C. M. A. (2022). Electrochemical Impedance Spectroscopy in the Characterisation and Application of Modified Electrodes for Electrochemical Sensors and Biosensors. Molecules, 27(5), 1497. https://doi.org/10.3390/molecules27051497





