High Hydrostatic Pressure to Increase the Biosynthesis and Extraction of Phenolic Compounds in Food: A Review
Abstract
:1. Introduction
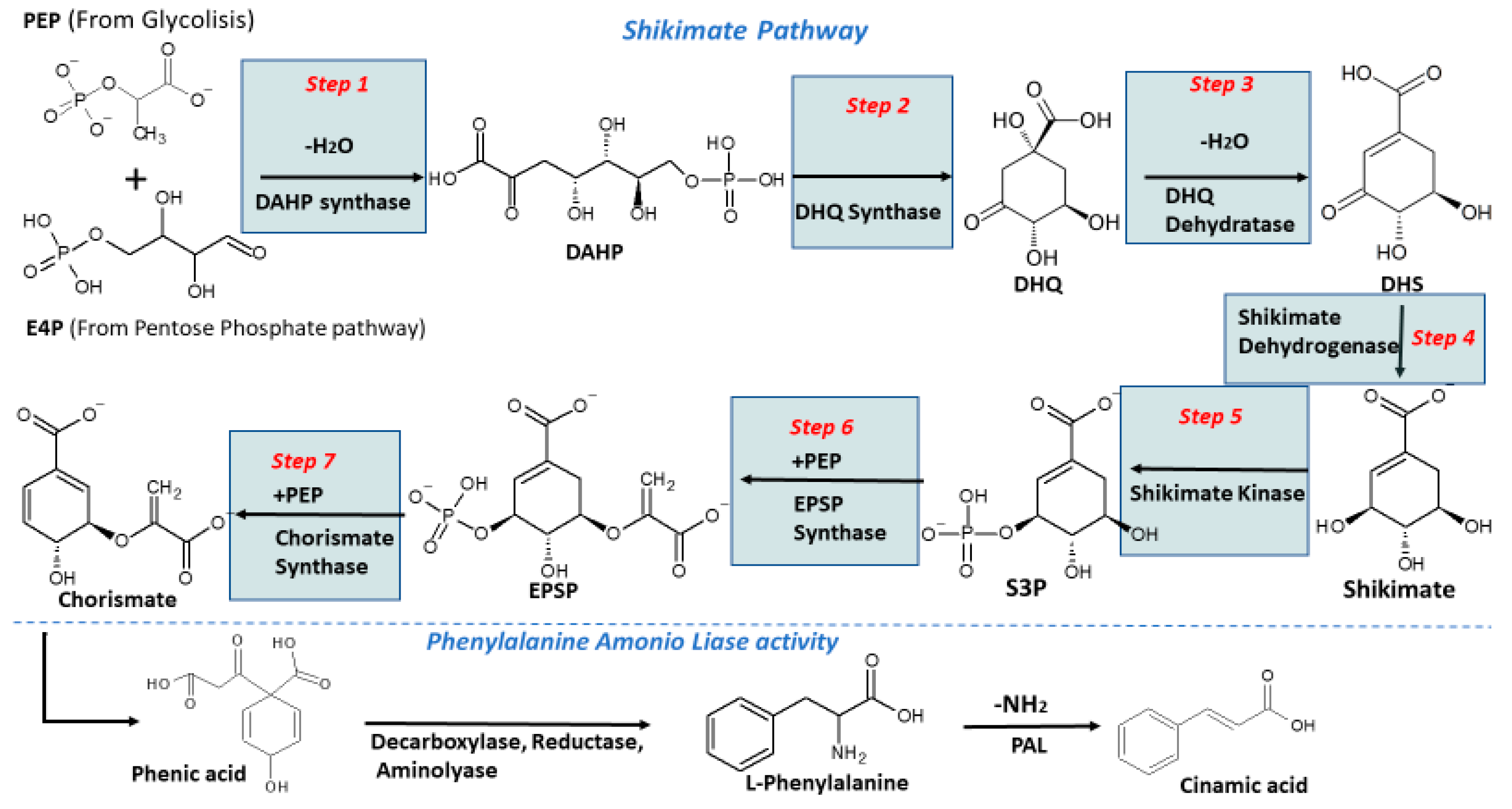
2. Biosynthesis of Phenolics in Plants
Biotic and Abiotic Factors Influencing Biosynthesis of Phenolics
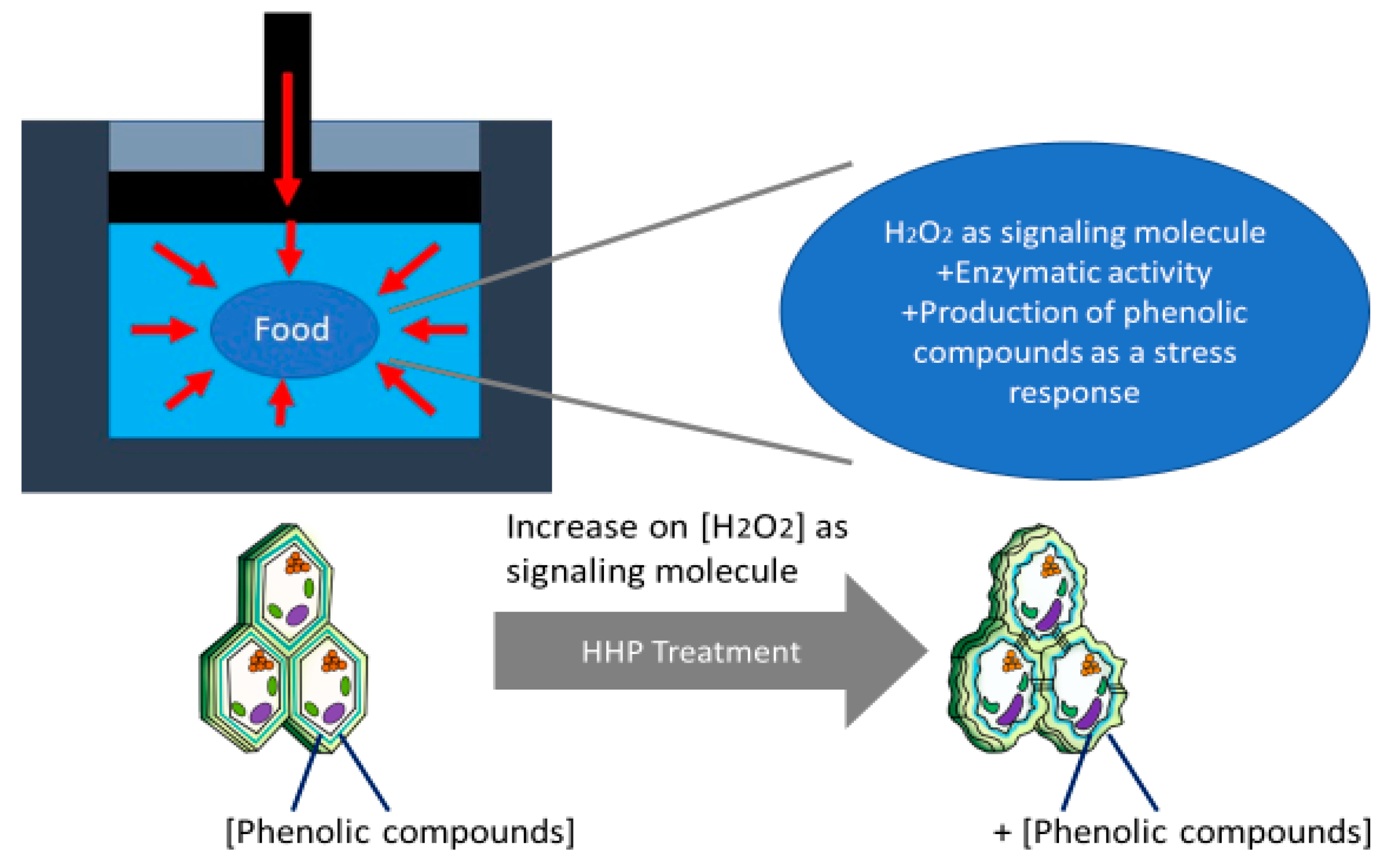
3. HHP as a Stress Factor for the Biosynthesis of Phenolics and to Increase Their Extraction Yield
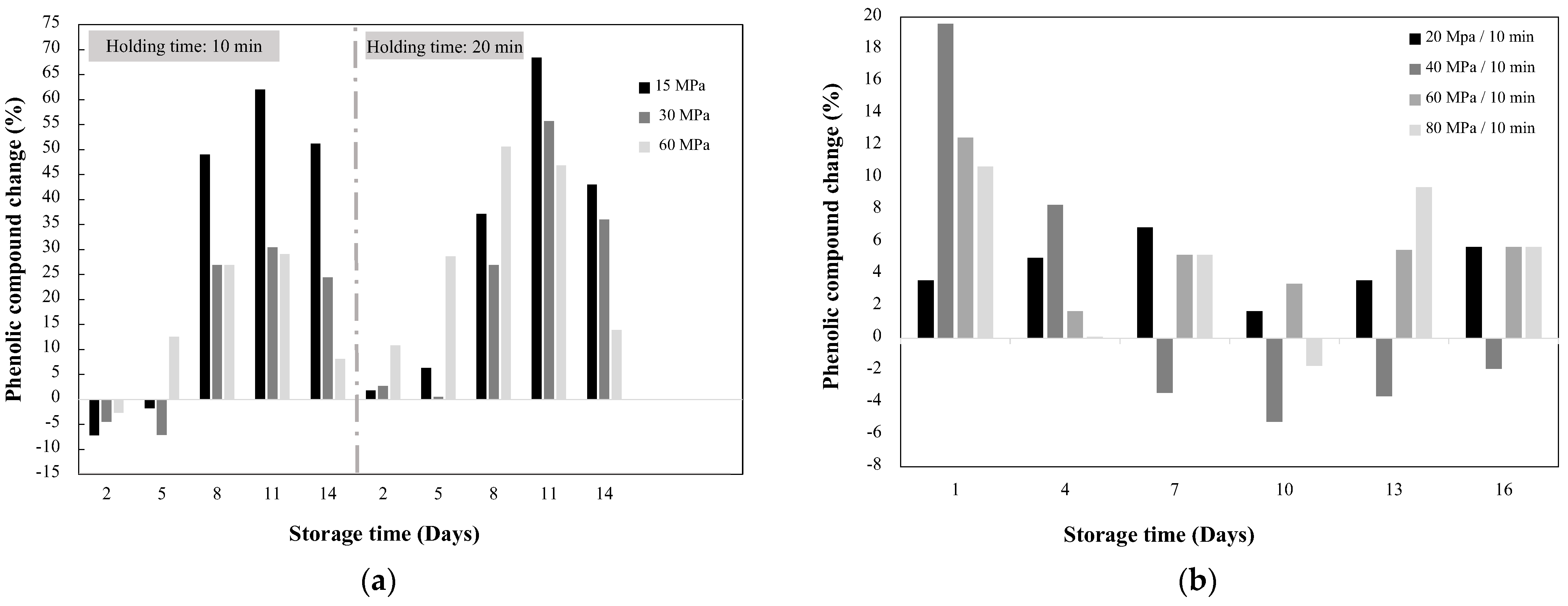
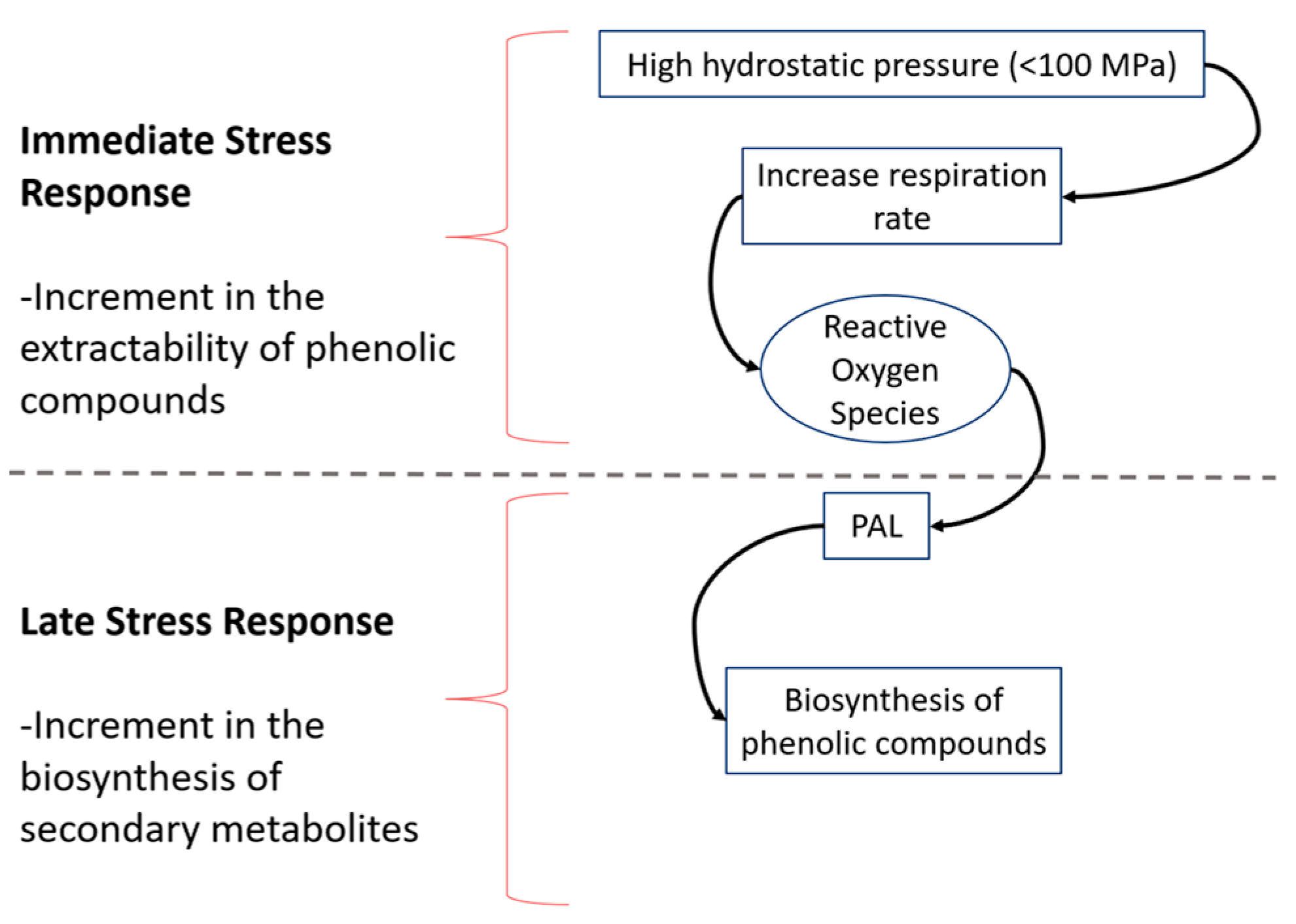
3.1. Effect of HHP on Phenolics Biosynthesis
3.2. Effect of HHP on Phenolics Extraction Yield
4. Final Remarks
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ozcan, T.; Akpinar-Bayizit, A.; Yilmaz-Ersan, L.; Delikanli, B. Phenolics in Human Health. Int. J. Chem. Eng. Appl. 2014, 5, 393–396. [Google Scholar] [CrossRef] [Green Version]
- Luna-Guevara, M.L.; Luna-Guevara, J.J.; Hernández-Carranza, P.; Ruíz-Espinosa, H.; Ochoa-Velasco, C.E. Phenolic Compounds: A Good Choice Against Chronic Degenerative Diseases. Stud. Nat. Prod. Chem. 2018, 59, 79–108. [Google Scholar] [CrossRef]
- Osorio-Tobón, J.F. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Food Chemistry Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2005, 99, 191–203. [Google Scholar] [CrossRef]
- Barba, F.J.; Putnik, P.; Kovačević, D.B.; Poojary, M.M.; Roohinejad, S.; Lorenzo, J.M.; Koubaa, M. Impact of conventional and non-conventional processing on prickly pear (Opuntia spp.) and their derived products: From preservation of beverages to valorization of by-products. Trends Food Sci. Technol. 2017, 67, 260–270. [Google Scholar] [CrossRef]
- Putnik, P.; Kovačević, D.B.; Penić, M.; Fegeš, M.; Dragović-Uzelac, V. Microwave-Assisted Extraction (MAE) of Dalmatian Sage Leaves for the Optimal Yield of Polyphenols: HPLC-DAD Identification and Quantification. Food Anal. Methods 2016, 9, 2385–2394. [Google Scholar] [CrossRef]
- Rastogi, N.K.; Raghavarao, K.S.M.S.; Balasubramaniam, V.M.; Niranjan, K.; Knorr, D. Opportunities and challenges in high pressure processing of foods. Crit. Rev. Food Sci. Nutr. 2007, 47, 69–112. [Google Scholar] [CrossRef]
- Miao, M.; Wang, Q.; Zhang, T.; Jiang, B. Effect of high hydrostatic pressure (HHP) treatment on texture changes of water bamboo shoots cultivated in China. Postharvest Biol. Technol. 2011, 59, 327–329. [Google Scholar] [CrossRef]
- Serment-Moreno, V.; Jacobo-Velázquez, D.A.; Torres, J.A.; Welti-Chanes, J. Microstructural and Physiological Changes in Plant Cell Induced by Pressure: Their Role on the Availability and Pressure-Temperature Stability of Phytochemicals. Food Eng. Rev. 2017, 9, 314–334. [Google Scholar] [CrossRef]
- Perez, M.C.P. Aplicación de Tecnologías No-Térmicas de Conservación, Pulsos Eléctricos de Alta Intensidad (PEAI), y Altas Presiones Hidrostáticas (APH), Para el Control de Cronobacter Sakazakii en Fórmula Láctea Infantil: Desarrollo de Modelos Predictivos y Valoración; Universidad Politécnica de Valencia: Valencia, Spain, 2012. [Google Scholar]
- Serment-Moreno, V.; Barbosa-Cánovas, G.; Torres, J.A.; Welti-Chanes, J. High-pressure Processing: Kinetic Models for Microbial and Enzyme Inactivation. Food Eng. Rev. 2014, 6, 56–88. [Google Scholar] [CrossRef]
- Mozhaev, V.V.; Heremans, K.; Frank, J.; Masson, P.; Balny, C. Exploiting the effects of high hydrostatic pressure in biotechnological applications. Trends Biotechnol. 1994, 12, 493–501. [Google Scholar] [CrossRef]
- Martín, J.; Asuero, A.G. High hydrostatic pressure for recovery of anthocyanins: Effects, performance, and applications. Sep. Purif. Rev. 2021, 50, 159–176. [Google Scholar] [CrossRef]
- Cheftel, J.C. Review: High-pressure, microbial inactivation and food preservation. Food Sci. Technol. Int. 1995, 1, 75–90. [Google Scholar] [CrossRef]
- Kimura, K.; Ida, M.; Yosida, Y.; Ohki, K.; Fukumoto, T.; Sakui, N. Comparison of Keeping Quality between Pressure-processed Jam and Heat-processed Jam: Changes in Flavor Components, Hue, and Nutrients during Storage. Biosci. Biotechnol. Biochem. 1994, 58, 1386–1391. [Google Scholar] [CrossRef]
- Téllez-Luis, S.J.; Ramírez, J.A.; Pérez-Lamela, C.; Vázquez, M.; Simal-Gándara, J. Aplicación de la alta presión hidrostática en la conservación de los alimentos. Ciencia y Tecnología de los Alimentos. Cienc. Tecnol. Aliment. 2001, 3, 66–80. [Google Scholar] [CrossRef] [Green Version]
- Srinivas, M.S.; Madhu, B.; Girijal, S. High Pressure Processing of Foods: A Review. 2018. Available online: https://www.researchgate.net/publication/328652367 (accessed on 7 February 2022).
- Gómez-Maqueo, J.; Welti-Chanes, M.; Cano, P. Release mechanisms of bioactive compounds in fruits submitted to high hydrostatic pressure: A dynamic microstructural analysi based on prickly pear cells. Food Res. Int. 2019, 130, 108909. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Cuéllar-Villarreal, M.D.; Welti-Chanes, J.; Cisneros-Zevallos, L.; Ramos-Parra, P.A.; Hernández-Brenes, C. Nonthermal processing technologies as elicitors to induce the biosynthesis and accumulation of nutraceuticals in plant foods. Trends Food Sci. Technol. 2017, 60, 80–87. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Vitousek, P.M. The role of polyphenols in terrestrial ecosystem nutrient cycling. Tree 2000, 15, 238–243. [Google Scholar] [CrossRef]
- Bahadur, B.; Rajam, M.V.; Sahijram, L.; Krishnamurthy, K.V. Plant Biology and Biotechnology: Plant Diversity, Organization, Function and Improvement; Springer: Berlin/Heidelberg, Germany, 2015; Volume 1, pp. 1–827. [Google Scholar] [CrossRef]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.D.; Abrahão, J.; et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Singh, S.A.; Christendat, D. Structure of Arabidopsis dehydroquinate dehydratase-shikimate dehydrogenase and implications for metabolic channeling in the shikimate pathway. Biochemistry 2006, 45, 10406. [Google Scholar] [CrossRef]
- Tzin, V.; Galili, G. New Insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef]
- Mittelstädt, G.; Negron, L.; Schofield, L.R.; Marsh, K.; Parker, E.J. Biochemical and structural characterisation of dehydroquinate synthase from the New Zealand kiwifruit Actinidia chinensis. Arch. Biochem. Biophys. 2013, 537, 185–191. [Google Scholar] [CrossRef]
- Singh, S.A.; Christendat, D. The DHQ-dehydroshikimate-SDH-shikimate-NADP(H) complex: Insights into metabolite transfer in the shikimate pathway. Cryst. Growth Des. 2007, 7, 2153–2160. [Google Scholar] [CrossRef]
- Herrmann, K.M.; Weaver, L.M. The shikimate pathway. Annu. Rev. Plant Biol. 1999, 50, 473–503. [Google Scholar] [CrossRef]
- Dudareva, H.M.N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Schönbrunn, E.; Eschenburg, S.; Shuttleworth, W.A.; Schloss, J.V.; Amrhein, N.; Evans, J.N.; Kabsch, W. Interaction of the herbicide glyphosate with its target enzyme 5-enolpyruvylshikimate 3-phosphate synthase in atomic detail. Proc. Natl. Acad. Sci. USA 2001, 98, 1376–1380. [Google Scholar] [CrossRef] [Green Version]
- Kitzing, K.; Auweter, S.; Amrhein, N.; Macheroux, P. Mechanism of chorismate synthase: Role of the two invariant histidine residues in the active site. J. Biol. Chem. 2004, 279, 9451–9461. [Google Scholar] [CrossRef] [Green Version]
- Gordo, D.A.M. Los Compuestos Fenólicos, Un Acercamiento A Su Biosíntesis, Síntesis Y Actividad Biológica. Rev. Investig. Agrar. Ambient. 2018, 9, 81–104. [Google Scholar] [CrossRef]
- Ardila, H.; Baquero, B.; Martinez, S. Inducción de la Actividad de la Enzima Fenilalanina Amonio Liasa en Clavel (Dianthus caryophyllus L.) Por Elicitores del Hongo Fusarium oxysporum f. sp. Dianthi raza 2. Rev.Colomb.Quim. 2007, 36, 151–167. [Google Scholar]
- Knaggs, R. The biosynthesis of shikimate metabolites. Nat. Prod. Rep. 2003, 18, 119–136. [Google Scholar] [CrossRef]
- Cheng, G.W.; Breen, P.J. Activity of Phenylalanine Ammonia-Lyase (PAL) and Concentrations of Anthocyanins and Phenolics in Developing Strawberry Fruit. J. Am. Soc. Hortic. Sci. 2019, 116, 865–869. [Google Scholar] [CrossRef]
- Figueiredo, C.; Barroso, J.; Pedro, L.; Scheffer, J. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavor Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Vidal, A.M. Respuestas Fisiológicas de Los Cítricos Sometidos a Condiciones de Estrés Biótico y Abiótico. Aspectos Comunes y Específicos. TDX (Tesis Doctorals en Xarxa). 2010, p. 213. Available online: http://repositori.uji.es/xmlui/handle/10234/29723 (accessed on 15 November 2021).
- Cho, M.H.; Lee, S.W. Phenolic phytoalexins in rice: Biological functions and Biosynthesis. Int. J. Mol. Sci. 2015, 16, 29120–29133. [Google Scholar] [CrossRef] [Green Version]
- Lattanzio, V. Natural Products|Phenolic Compounds: Introduction; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Hatami, M.; Badi, H.N.; Ghorbanpour, M. Nano-elicitation of secondary pharmaceutical metabolites in plant cells: A review. J. Med. Plants 2019, 18, 6–36. [Google Scholar] [CrossRef]
- Marslin, G.; Sheeba, C.J.; Franklin, G. Nanoparticles alter secondary metabolism in plants via ROS burst. Front. Plant Sci. 2017, 8, 832. [Google Scholar] [CrossRef] [Green Version]
- Rivero-Montejo, S.d.; Vargas-Hernandez, M.; Torres-Pacheco, I. Nanoparticles as novel elicitors to improve bioactive compounds in plants. Agriculture 2021, 11, 134. [Google Scholar] [CrossRef]
- Shalaby, T.A.; Bayoumi, Y.; Abdalla, N.; Taha, H.; Alshaal, T.; Shehata, S.; Amer, M.; Domokos-Szabolcsy, É.; El-Ramady, H. Nanoparticles, Soils, Plants and Sustainable Agriculture. Nanosci. Food Agric. 2016, 20, 283–312. [Google Scholar] [CrossRef]
- van Breusegem, F.; Dat, J.F.; Van, F. Reactive Oxygen Species in Plant Cell Death. Source Plant Physiol. React. Oxyg. Species 2015, 141, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Dörnenburg, H.; Knorr, D. Evaluation of Elicitor- and High-Pressure-Induced Enzymatic Browning Utilizing Potato (Solanum tuberosum) Suspension Cultures as a Model System for Plant Tissues. J. Agric. Food Chem. 1997, 45, 4173–4177. [Google Scholar] [CrossRef]
- Ramos-Parra, P.A.; García-Salinas, C.; Rodríguez-López, C.E.; García, N.; García-Rivas, G.; Hernández-Brenes, C.; de la Garza, R.I.D. High hydrostatic pressure treatments trigger de novo carotenoid biosynthesis in papaya fruit (Carica papaya cv. Maradol). Food Chem. 2018, 277, 362–372. [Google Scholar] [CrossRef]
- Hu, K.; Peng, D.; Wang, L.; Liu, H.; Xie, B.; Sun, Z. Effect of mild high hydrostatic pressure treatments on physiological and physicochemical characteristics and carotenoid biosynthesis in postharvest mango. Postharvest Biol. Technol. 2020, 172, 10. [Google Scholar] [CrossRef]
- Viacava, F.; Ortega-Hernández, E.; Welti-Chanes, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Using High Hydrostatic Pressure Processing Come-Up Time as an Innovative Tool to Induce the Biosynthesis of Free and Bound Phenolics in Whole Carrots. Food Bioprocess Technol. 2020, 13, 1717–1727. [Google Scholar] [CrossRef]
- Ortega, V.G.; Ramírez, J.A.; Velázquez, G.; Tovar, B.; Mata, M.; Montalvo, E. Effect of high hydrostatic pressure on antioxidant content of ‘Ataulfo’ mango during postharvest maturation. Food Sci. Technol. 2013, 33, 561–568. [Google Scholar] [CrossRef] [Green Version]
- Yasunaga, E.; Fukuda, S.; Takata, D.; Spreer, W.; Sardsud, V.; Nakano, K. Quality changes in fresh mango fruits (Mangifera indica L. ‘Nam Dok Mai’) under actual distribution temperature profile from Thailand to Japan. Environ. Control Biol. 2018, 56, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.E.; Gil, B.; Kim, C.T.; Cho, Y.J. Enrichment of Phenolics in Harvested Strawberries by High-Pressure Treatment. Food Bioprocess Technol. 2017, 10, 222–227. [Google Scholar] [CrossRef]
- Cai, Z.; Riedel, H.; Saw NM, M.T.; Mewis, I.; Reineke, K.; Knorr, D.; Smetanska, I. Effects of elicitors and high hydrostatic pressure on secondary metabolism of Vitis vinifera suspension culture. Process Biochem. 2011, 46, 1411–1416. [Google Scholar] [CrossRef]
- Liang, D. A salutary role of reactive oxygen species in intercellular tunnel-mediated communication. Front. Cell Dev. Biol. 2018, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Yahraus, T.; Chandra, S.; Legendre, L.; Low, P.S. Evidence for a Mechanically lnduced Oxidative Burst. 1995. Available online: www.plantphysiol.org (accessed on 7 February 2022).
- Slesak, I.; Libik, M.; Karpinska, B.; Karpinski, S.; Miszalski, Z. The role of hydrogen peroxide in regulation of plant metabolism and cellular signalling in response to environmental stresses. Acta Biochim. Pol. 2007, 54, 39–50. [Google Scholar] [CrossRef]
- Hancock, T.; Desikan, R.; Neill, S.J. Role of reactive oxygen species in cell signalling pathways. Biochem. Soc. Trans. 2001, 29, 345–350. [Google Scholar] [CrossRef]
- Jacobo-velázquez, D.A.; Santana-Galvez, J.; Cisneros-Zevallos, L. Designing Next-Generation Functional Food and Beverages: Combining Nonthermal Processing Technologies and Postharvest Abiotic Stresses. Food Eng. Rev. 2021, 13, 592–600. [Google Scholar] [CrossRef]
- Escobedo-Avellaneda, Z.; Pérez-Simón, I.; Lavilla-Martín, M.; Baranda-González, A.; Welti-Chanes, J. Enzymatic and phytochemical stabilization of orange-strawberry-banana beverages by high hydrostatic pressure and mild heat. Food Sci. Technol. Int. 2017, 23, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.E.; Anthon, G.E.; Barrett, D.M. Onion cells after high pressure and thermal processing: Comparison of membrane integrity changes using different analytical methods and impact on tissue textura. J. Food Sci. 2010, 75, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.; Yang, B.; Zhao, M.; Wei, X.; Jiang, Y.; Chen, F. High pressure extraction of corilagin from longan (Dimocarpus longan Lour.) fruit pericarp. Sep. Purif. Technol. 2009, 70, 41–45. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Torres, J.L. Analysis of nonextractable phenolic compounds in foods: The current state of the art. J. Agric. Food Chem. 2011, 59, 12713–12724. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Xu, H.; Hanna, M.; Yuan, L. Identification and quantification of free, esterified, glycosylated and insoluble-bound phenolic compounds in hawthorn berry fruit (Crataegus pinnatifida) and antioxidant activity evaluation. LWT 2020, 130, 109643. [Google Scholar] [CrossRef]
- Huang, H.W.; Hsu, C.P.; Yang, B.B.; Wang, C.Y. Advances in the extraction of natural ingredients by high pressure extraction technology. Trends Food Sci. Technol. 2013, 33, 54–62. [Google Scholar] [CrossRef]
- Koubala, B.B.; Bayang, J.P.; Wangso, H.; Kolla, M.C.; Laya, A. Variation of Phenolics (Bound and Free), Minerals, and Antioxidant Activity of Twenty-Eight Wild Edible Fruits of Twenty-Three Species from Far North Region of Cameroon. BioMed Res. Int. 2021, 2021, 4154381. [Google Scholar] [CrossRef]
- Okur, İ.; Baltacıoğlu, C.; Ağçam, E.; Baltacıoğlu, H.; Alpas, H. Evaluation of the Effect of Different Extraction Techniques on Sour Cherry Pomace Phenolic Content and Antioxidant Activity and Determination of Phenolic Compounds by FTIR and HPLC. Waste Biomass Valoriz. 2019, 10, 3545–3555. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Vejarano, R.; Bañuelos, M.A.; Sanz, P.D.; Otero, L.; Suárez-Lepe, J.A. Grape Processing by High Hydrostatic Pressure: Effect on Microbial Populations, Phenol Extraction and Wine Quality. Food Bioprocess Technol. 2014, 10, 3545–3555. [Google Scholar] [CrossRef] [Green Version]
- Torres-Ossandón, M.J.; Vega-Gálvez, A.; López, J.; Stucken, K.; Romero, J.; Di Scala, K. Effects of high hydrostatic pressure processing and supercritical fluid extraction on bioactive compounds and antioxidant capacity of Cape gooseberry pulp (Physalis peruviana L.). J. Supercrit. Fluids 2018, 318, 215–220. [Google Scholar] [CrossRef]
- Huang, W.; Bi, X.; Zhang, X.; Liao, X.; Hu, X.; Wu, J. Comparative study of enzymes, phenolics, carotenoids and color of apricot nectars treated by high hydrostatic pressure and high temperature short time. Innov. Food Sci. Emerg. Technol. 2013, 18, 74–82. [Google Scholar] [CrossRef]
- Liu, S.; Xu, Q.; Li, X.; Wang, Y.; Zhu, J.; Ning, C.; Chang, X.; Meng, X. Effects of high hydrostatic pressure on physicochemical properties, enzymes activity, and antioxidant capacities of anthocyanins extracts of wild Lonicera caerulea Berry. Innov. Food Sci. Emerg. Technol. 2016, 36, 48–58. [Google Scholar] [CrossRef]
- Tapia-Salazar, M.; Arévalo-Rivera, I.G.; Maldonado-Muñiz, M.; Garcia-Amezquita, L.E.; Nieto-López, M.G.; Ricque-Marie, D.; Cruz-Suárez, L.E.; Welti-Chanes, J. The Dietary Fiber Profile, Total Polyphenol Content, Functionality of Silvetia compressa and Ecklonia arborea, and Modifications Induced by High Hydrostatic Pressure Treatments. Food Bioprocess Technol. 2019, 12, 512–523. [Google Scholar] [CrossRef]
- Ugur, A.E.; Bolat, B.; Oztop, M.H.; Alpas, H. Effects of High Hydrostatic Pressure (HHP) Processing and Temperature on Physicochemical Characterization of Insect Oils Extracted from Acheta domesticus (House Cricket) and Tenebrio molitor (Yellow Mealworm). Waste Biomass Valoriz. 2020, 12, 4277–4286. [Google Scholar] [CrossRef]
- Clariana, M.; Valverde, J.; Wijngaard, H.; Mullen, A.M.; Marcos, B. High pressure processing of swede (Brassica napus): Impact on quality properties. Innov. Food Sci. Emerg. Technol. 2011, 12, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Kim, D.; Fan, J.P.; Chung, H.C.; Han, G.D. Changes in Extractability and Antioxidant Activity of Jerusalem Artichoke (Helianthus tuberosus L.) Tubers by Various High Hydrostatic Pressure Treatments. Food Sci. Biotechnol. 2010, 19, 1365–1371. [Google Scholar] [CrossRef]
- de Jesus AL, T.; Cristianini, M.; Dos Santos, N.M.; Júnior, M.R.M. Effects of high hydrostatic pressure on the microbial inactivation and extraction of bioactive compounds from açaí (Euterpe oleracea Martius) pulp. Food Res. Int. 2019, 130, 108856. [Google Scholar] [CrossRef]
- Xi, J.; Wang, B. Optimization of Ultrahigh-Pressure Extraction of Polyphenolic Antioxidants from Green Tea by Response Surface Methodology. Food Bioprocess Technol. 2013, 6, 2538–2546. [Google Scholar] [CrossRef]
- Qadir, S.A.; Kwon, M.C.; Han, J.G.; Ha, J.H.; Chung, H.S.; Ahn, J.; Lee, H.Y. Effect of different extraction protocols on anticancer and antioxidant activities of Berberis koreana bark extracts. J. Biosci. Bioeng. 2009, 107, 331–338. [Google Scholar] [CrossRef]
- Ghasemy-piranloo, F.; Kavousi, F.; Dadashian, S. Combination of enzyme-assisted extraction and high hydrostatic pressure for phenolic compounds recovery from grape pomace. J. Food Eng. 2020, 288, 110128. [Google Scholar] [CrossRef]
- Srinivasan, V.S. Bioavailability of nutrients: A practical approach to in vitro demonstration of the availability of nutrients in multivitamin-mineral combination products. J. Nutr. 2001, 131 (Suppl. 4), 1349–1350. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Moreno, C.; de Ancos, B.; Plaza, L.; Elez-Martinez, P.; Cano, M.P. Nutritional approaches and health-related properties of plant foods processed by high pressure and pulsed electric fields. Crit. Rev. Food Sci. Nutr. 2009, 49, 552–576. [Google Scholar] [CrossRef] [PubMed]
- Talaverano, M.I.; Pérez-Nevado, F.; Boselli, E.; Cordeiro, A.M.; Martillanes, S.; Foligni, R.; Martín-Vertedor, D. Evaluation of phenolics and acrylamide and their bioavailability in high hydrostatic pressure treated and fried table olives. J. Food Process. Preserv. 2020, 44, 1–9. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Venegas-Cubillos, G.; Ortiz-Portilla, S.; Chacana-Ojeda, M.; Maureira, H. Effects of high hydrostatic pressure (HHP) on bioaccessibility, as well as antioxidant activity, mineral and starch contents in Granny Smith apple. Food Chem. 2011, 128, 520–529. [Google Scholar] [CrossRef]


| Sample | Treatment Conditions | Storage Conditions | Analyzed Compound | Main Findings | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|
| P (MPa) | t (min) | CUT (s) | T (°C) | Approximate Change (%) | PAL Activity (%) | ||||
| Mango Mangifera indica (Whole fruit) | 15–60 | 10–20 | 3, 10 & 28 | 25 | 2–14 days at 25 °C and 85–90% RH | Total phenols | ↓7.2 up to ↑68.4 | NR | [46] |
| Flavonoids | ↓38.6 up to ↑36.8 | NR | |||||||
| Mango Mangifera indica (Whole fruit) | 20–80 | 10 | NR | 20 | 1–16 days at 13 °C with 85% RH | Total phenols | ↓5.2 up to ↑30 | NR | [47] |
| Flavonoids | ↓27.6 up to ↑69.7 | NR | |||||||
| Vitis vinifera (Suspension culture) | 40 | 10 | NR | 25 | 1–7 days at 25 °C | Anthocyanin | ↓53.9 up to ↑53.3 | NR | [48] |
| Carrots Daucus carota (Whole vegetable) | 60 & 100 | CUT | 15.33 & 20.67 | 22 | 0–3 days at 15 °C CO2 < [0.5 v/v] | Total phenols | ↓11.8 up to ↑154.9 | ↓61.4 up to ↑380 | [49] |
| Potato Solanum tuberosum (suspension culture) | 100–200 | 10 | NR | 25 | 1–24 h | Polyphenols | ↑54.0 up to ↑456.0 | ↑199 | [44] |
| Strawberry Seolhyang, Fragaria × ananassa Duch (Whole fruit) | 30–90 | 5 | NR | 25 | NR | Total phenols | ↑6.4 up to ↑23.1 | NR | [50] |
| Anthocyanin | ↓16.9 up to ↑10.0 | NR | |||||||
| Sample | Analyzed Compound | Treatment Conditions | Storage Conditions | Approximate Change (%) | Reference | |||
|---|---|---|---|---|---|---|---|---|
| P (MPa) | t (min) | CUT (min) | T (°C) | |||||
| Apricot nectar Prunus armeniaca L. | TPC (Individual phenols include: Catechin, Chlorogenic acid, Neochlorogenic acid, Epicatechin, Ferulic acid, Caffeic acid, p-Coumaric acid) | 300–500 | 5–20 | 2.5–4.2 | 34–40 | 2 days at 4 °C | ↑2.0 up to ↑12.5 | [64] |
| Sour cherry pomace Prunus cerasus L. | TPC | 400 & 500 | 1–10 | NR | 20 | −4 °C until analysis | ↑39.5 up to ↑109.9 | [65] |
| Grape by products (Skin, stems, and seeds) Vitis Vinifera | TPC | 600 | 60 | NR | 70 | NR | ↑48.0 | [66] |
| Anthocyanins | 600 | 60 | NR | 70 | NR | ↑41.4 | ||
| Jerusalem Artichoke Helianthus tuberosus L. | TPC (Pre-fermentation) | 100 | 24 h | NR | 50 | NR | ↑36.6 | [67] |
| TPC (Post-fermentation | 100 | 24 h | NR | 50 | NR | ↑61.36 | ||
| Cape gooseberry pulp Physalis peruviana L. | TPC | 300–500 | 1–5 | NR | 25 | 0 and 60 days at 4 °C | ↓32.3 up to ↑35.9 | [68] |
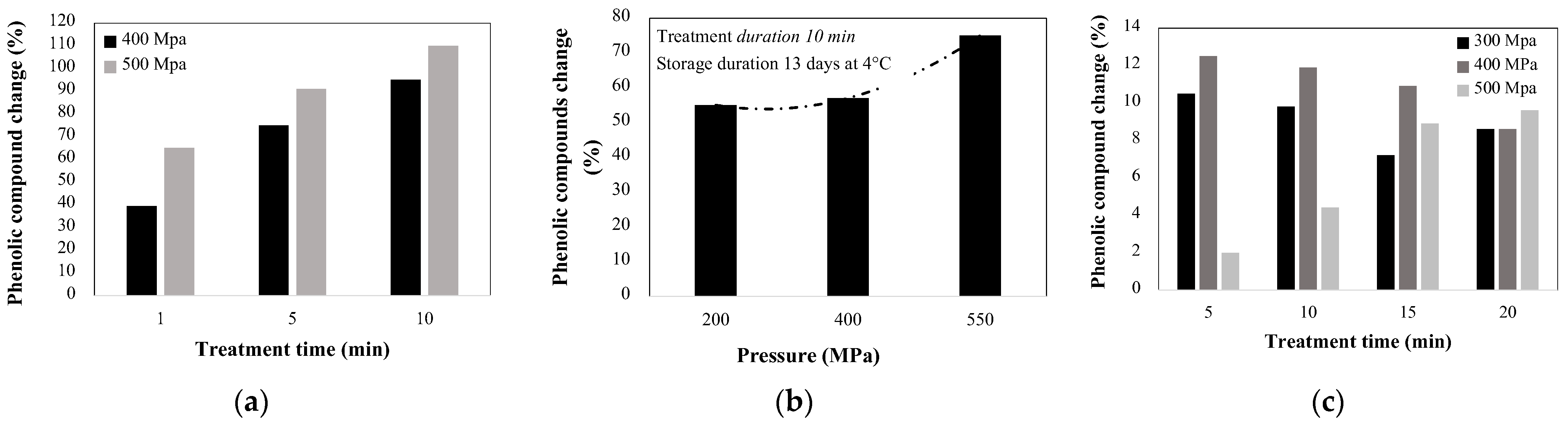
| Grape Vitis Vinifera | TPPC | 200–550 | 10 | 28.6 s–78.6 s | 20 | 4 °C until fermentation(13 days) | ↑55.0 up to ↑75.0 | [69] |
| Wild Berry Lonicera caerulea | TPC | 200–600 | 5–20 | 4–12 s | 25 | 4 °C until analysis (48 h) | ↓10.0 up to ↑14.4 | [70] |
| Anthocyanins | 200–600 | 5–20 | 4–12 s | 25 | 4 °C until analysis (48 h) | ↓6.3 up to ↑7.9 | ||
| Açai Pulp Euterpe oleracea Martius | TPC | 600 | 5 | NR | 25 and 65 | Stored for 24 h with oxygen and light barrier | ↓10.3 up to ↑11.4 | [63] |
| Cricket Acheta domesticus | TPC | 500 | 15 | NR | 30 and 40 | NR | ↑9.3 up to ↓67.3 | [71] |
| Mealworm Tenebrio molitor | TPC | 500 | 15 | NR | 30 and 40 | NR | ↓23.7 up to ↑8.6 | [71] |
| Silvetia compressa | TPPC | 400 | 15 | 2.03 | 35 | Stored in brown glass flask at 10 °C | ↓41.0 | [72] |
| 600 | 5 | 3.07 | 35 | ↓30.0 | ||||
| Ecklonia arborea | TPPC | 400 | 15 | 2.03 | 35 | Stored in brown glass flask at 10 °C | ↑46.0 | [72] |
| 600 | 5 | 3.07 | 35 | ↑20.0 | ||||
| Green tea Camellia sinensis L. | TPC | 490 | 15 | 25 | 25 | NR | ↑32.6 | [73] |
| Longan fruit pericarp Dimocarpus longan L. | TPC | 500 | 2.5 | NR | 30 | 4 °C until analysis | ↑43.8 | [59] |
| Korean barberry Berberis koreana | TPC | 500 | 5 & 15 | NR | 25 | −20 °C until analysis | ↑29.9 up to ↑33.1 | [74] |
| Grape pomace | TPC | 50–200 | 5–30 | NR | 25 | NR | ↓27.9 up to ↑18.6 | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Baez, J.E.; Martínez, L.M.; Welti-Chanes, J.; Buitimea-Cantúa, G.V.; Escobedo-Avellaneda, Z. High Hydrostatic Pressure to Increase the Biosynthesis and Extraction of Phenolic Compounds in Food: A Review. Molecules 2022, 27, 1502. https://doi.org/10.3390/molecules27051502
Navarro-Baez JE, Martínez LM, Welti-Chanes J, Buitimea-Cantúa GV, Escobedo-Avellaneda Z. High Hydrostatic Pressure to Increase the Biosynthesis and Extraction of Phenolic Compounds in Food: A Review. Molecules. 2022; 27(5):1502. https://doi.org/10.3390/molecules27051502
Chicago/Turabian StyleNavarro-Baez, Jorge E., Luz María Martínez, Jorge Welti-Chanes, Génesis V. Buitimea-Cantúa, and Zamantha Escobedo-Avellaneda. 2022. "High Hydrostatic Pressure to Increase the Biosynthesis and Extraction of Phenolic Compounds in Food: A Review" Molecules 27, no. 5: 1502. https://doi.org/10.3390/molecules27051502
APA StyleNavarro-Baez, J. E., Martínez, L. M., Welti-Chanes, J., Buitimea-Cantúa, G. V., & Escobedo-Avellaneda, Z. (2022). High Hydrostatic Pressure to Increase the Biosynthesis and Extraction of Phenolic Compounds in Food: A Review. Molecules, 27(5), 1502. https://doi.org/10.3390/molecules27051502










