Natural Deep Eutectic Solvent (NADES) Extraction Improves Polyphenol Yield and Antioxidant Activity of Wild Thyme (Thymus serpyllum L.) Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening of the Extraction Solvent
2.2. Preliminary Study
2.3. Optimization Study
2.3.1. Accuracy of Fit and Influence Analysis
2.3.2. Polyphenol Content, Antioxidant Activity and Effect of NADES Extraction Parameters
2.3.3. NMR Characterization of the Optimal NADES
2.3.4. Optimization and Validation
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals
3.3. NADES Preparation
3.4. NADES Extraction and Experimental Plan
3.5. Polyphenol Analysis
3.5.1. Total Phenols Content
3.5.2. Total Flavonoids Content
3.5.3. HPLC Analysis of Major Polyphenols
3.6. In Vitro Antioxidant Activity
3.6.1. Scavenging Capacity toward DPPH Radicals
3.6.2. Reducing Capacity of Fe3+ Ions
3.6.3. Scavenging Capacity towards ABTS+ Radicals
3.7. Nuclear Magnetic Resonance
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jarić, S.; Mitrović, M.; Pavlović, P. Review of ethnobotanical, phytochemical, and pharmacological study of Thymus serpyllum L. Evid.-based Complement. Altern. Med. 2015, 2015, 101978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boros, B.; Jakabová, S.; Dörnyei, Á.; Horváth, G.; Pluhár, Z.; Kilár, F.; Felinger, A. Determination of polyphenolic compounds by liquid chromatography-mass spectrometry in Thymus species. J. Chromatogr. A 2010, 1217, 7972–7980. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [Green Version]
- Ramić, M.; Vidović, S.; Zeković, Z.; Vladić, J.; Cvejin, A.; Pavlić, B. Modeling and optimization of ultrasound-assisted extraction of polyphenolic compounds from Aronia melanocarpa by-products from filter-tea factory. Ultrason. Sonochem. 2015, 23, 360–368. [Google Scholar] [CrossRef]
- Naffati, A.; Vladić, J.; Pavlić, B.; Radosavljević, R.; Gavarić, A.; Vidović, S. Recycling of filter tea industry by-products: Application of subcritical water extraction for recovery of bioactive compounds from A. uva-ursi herbal dust. J. Supercrit. Fluids 2017, 121, 1–9. [Google Scholar] [CrossRef]
- Rombaut, N.; Tixier, A.S.; Bily, A.; Chemat, F. Green extraction processes of natural products as tools for biorefinery. Biofuel. Bioprod. Biorefin. 2014, 8, 530–544. [Google Scholar] [CrossRef]
- Mrkonjić, Ž.; Rakić, D.; Olgun, E.O.; Canli, O.; Kaplan, M.; Teslić, N.; Zeković, Z.; Pavlić, B. Optimization of antioxidants recovery from wild thyme (Thymus serpyllum L.) by ultrasound-assisted extraction: Multi-response approach. J. Appl. Res. Med. Aromat. Plants 2021, 24, 100333. [Google Scholar] [CrossRef]
- Mrkonjić, Ž.; Rakić, D.; Kaplan, M.; Teslić, N.; Zeković, Z.; Pavlić, B. Pressurized-liquid extraction as an efficient method for valorization of Thymus serpyllum herbal dust towards sustainable production of antioxidants. Molecules 2021, 26, 2548. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Namieśnik, J.; Pena-Pereira, F. Environmental risk-based ranking of solvents using the combination of a multimedia model and multi-criteria decision analysis. Green Chem. 2017, 19, 1034–1042. [Google Scholar] [CrossRef] [Green Version]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel lactic acid-based natural deep eutectic solvents: Efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 120–127. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Which is the best food emerging solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146. [Google Scholar] [CrossRef]
- Mišan, A.; Nađpal, J.; Stupar, A.; Pojić, M.; Mandić, A.; Verpoorte, R.; Choi, Y.H. The perspectives of natural deep eutectic solvents in agri-food sector. Crit. Rev. Food Sci. Nutr. 2019, 60, 2564–2592. [Google Scholar] [CrossRef] [PubMed]
- Chanioti, S.; Tzia, C. Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Ivanović, M.; Alañón, M.E.; Arráez-Román, D.; Segura-Carretero, A. Enhanced and green extraction of bioactive compounds from Lippia citriodora by tailor-made natural deep eutectic solvents. Food Res. Int. 2018, 111, 67–76. [Google Scholar] [CrossRef]
- Miličević, N.; Panić, M.; Valinger, D.; Cvjetko Bubalo, M.; Benković, M.; Jurina, T.; Gajdoš Kljusurić, J.; Radojčić Redovniković, I.; Jurinjak Tušek, A. Development of continuously operated aqueous two-phase microextraction process using natural deep eutectic solvents. Sep. Purif. Technol. 2020, 244, 116746. [Google Scholar] [CrossRef]
- Popovic, B.M.; Micic, N.; Potkonjak, A.; Blagojevic, B.; Pavlovic, K.; Milanov, D.; Juric, T. Novel extraction of polyphenols from sour cherry pomace using natural deep eutectic solvents—Ultrafast microwave-assisted NADES preparation and extraction. Food Chem. 2022, 366, 130562. [Google Scholar] [CrossRef]
- da Silva, D.T.; Smaniotto, F.A.; Costa, I.F.; Baranzelli, J.; Muller, A.; Somacal, S.; Monteiro, C.S.A.; Vizzotto, M.; Rodrigues, E.; Barcia, M.T.; et al. Natural deep eutectic solvent (NADES): A strategy to improve the bioavailability of blueberry phenolic compounds in a ready-to-use extract. Food Chem. 2021, 364, 130370. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [Green Version]
- Shikov, A.N.; Narkevich, I.A.; Flisyuk, E.V.; Luzhanin, V.G.; Pozharitskaya, O.N. Medicinal plants from the 14th edition of the Russian Pharmacopoeia, recent updates. J. Ethnopharmacol. 2021, 268, 113685. [Google Scholar] [CrossRef]
- Fernández, M.d.l.Á.; Boiteux, J.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Natural deep eutectic solvents-mediated extractions: The way forward for sustainable analytical developments. Anal. Chim. Acta 2018, 1038, 1–10. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Butler, L.G. The specificity of proanthocyanidin-protein interactions. J. Biol. Chem. 1981, 256, 4494–4497. [Google Scholar] [CrossRef]
- Nam, M.W.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: Application to flavonoid extraction from Flos sophorae. Green Chem. 2015, 17, 1718–1727. [Google Scholar] [CrossRef]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Ultrasonic-Assisted Extraction and Natural Deep Eutectic Solvents Combination: A Green Strategy to Improve the Recovery of Phenolic Compounds from Lavandula pedunculata subsp. lusitanica (Chaytor) Franco. Antioxidants 2021, 10, 582. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, J.; Zhao, Y.; Yu, L.; He, Y.; Wan, H.; Li, C. Screening, Optimization, and Bioavailability Research of Natural Deep Eutectic Solvent Extracts from Radix Pueraria. Molecules 2021, 26, 729. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Faggian, M.; Sut, S.; Perissutti, B.; Baldan, V.; Grabnar, I.; Dall’Acqua, S. Natural Deep Eutectic Solvents (NADES) as a Tool for Bioavailability Improvement: Pharmacokinetics of Rutin Dissolved in Proline/Glycine after Oral Administration in Rats: Possible Application in Nutraceuticals. Molecules 2016, 21, 1531. [Google Scholar] [CrossRef] [Green Version]
- Guo, N.; Ping-Kou; Jiang, Y.W.; Wang, L.T.; Niu, L.J.; Liu, Z.M.; Fu, Y.J. Natural deep eutectic solvents couple with integrative extraction technique as an effective approach for mulberry anthocyanin extraction. Food Chem. 2019, 296, 78–85. [Google Scholar] [CrossRef]
- Shikov, A.N.; Kosman, V.M.; Flissyuk, E.V.; Smekhova, I.E.; Elameen, A.; Pozharitskaya, O.N. Natural Deep Eutectic Solvents for the Extraction of Phenyletanes and Phenylpropanoids of Rhodiola rosea L. Molecules 2020, 25, 1826. [Google Scholar] [CrossRef] [Green Version]
- Doldolova, K.; Bener, M.; Lalikoğlu, M.; Aşçı, Y.S.; Arat, R.; Apak, R. Optimization and modeling of microwave-assisted extraction of curcumin and antioxidant compounds from turmeric by using natural deep eutectic solvents. Food Chem. 2021, 353, 129337. [Google Scholar] [CrossRef]
- Ivanović, M.; Albreht, A.; Krajnc, P.; Vovk, I.; Razboršek, M.I. Sustainable ultrasound-assisted extraction of valuable phenolics from inflorescences of Helichrysum arenarium L. using natural deep eutectic solvents. Ind. Crops Prod. 2021, 160, 113102. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 4th ed.; Wiley & Sons Ltd.: New York, NY, USA, 2016; p. 894. [Google Scholar]
- Bosiljkov, T.; Dujmić, F.; Cvjetko Bubalo, M.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Radojčić Redovniković, I.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Alañón, M.E.; Ivanović, M.; Pimentel-Mora, S.; Borrás-Linares, I.; Arráez-Román, D.; Segura-Carretero, A. A novel sustainable approach for the extraction of value-added compounds from Hibiscus sabdariffa L. calyces by natural deep eutectic solvents. Food Res. Int. 2020, 137, 109646. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, A.A.; Djordjević, V.B.; Petrović, P.M.; Pljevljakušić, D.S.; Zdunić, G.M.; Šavikin, K.P.; Bugarski, B.M. The influence of different extraction conditions on polyphenol content, antioxidant and antimicrobial activities of wild thyme. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100328. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Đorđević, V.B.; Zdunić, G.M.; Pljevljakušić, D.S.; Šavikin, K.P.; Gođevac, D.M.; Bugarski, B.M. Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat- and ultrasound-assisted techniques. Sep. Purif. Technol. 2017, 179, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Bi, W.; Tian, M.; Row, K.H. Evaluation of alcohol-based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J. Chromatogr. A 2013, 1285, 22–30. [Google Scholar] [CrossRef]
- Katsampa, P.; Valsamedou, E.; Grigorakis, S.; Makris, D.P. A green ultrasound-assisted extraction process for the recovery of antioxidant polyphenols and pigments from onion solid wastes using Box–Behnken experimental design and kinetics. Ind. Crops Prod. 2015, 77, 535–543. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharova, L.V.; Daurtseva, A.V.; Flisyuk, E.V.; Shikov, A.N. Efficacy of Natural Deep Eutectic Solvents for Extraction of Hydrophilic and Lipophilic Compounds from Fucus vesiculosus. Molecules 2021, 26, 4198. [Google Scholar] [CrossRef]
- Teslic, N.; Santos, F.; Oliveira, F.; Stupar, A.; Pojić, M.; Mandić, A.; Pavlić, B.; Cvetanović Kljakić, A.; Duarte, A.R.C.; Paiva, A.; et al. Simultaneous Hydrolysis of Ellagitannins and Extraction of Ellagic Acid from Defatted Raspberry Seeds Using Natural Deep Eutectic Solvents (NADES). Antioxidants 2022, 11, 254. [Google Scholar] [CrossRef]
- Craveiro, R.; Aroso, I.; Flammia, V.; Carvalho, T.; Viciosa, M.T.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Properties and thermal behavior of natural deep eutectic solvents. J. Mol. Liq. 2016, 215, 534–540. [Google Scholar] [CrossRef]
- Manuela, P.; Drakula, S.; Cravotto, G.; Verpoorte, R.; Hruškar, M.; Radojčić Redovniković, I.; Radošević, K. Biological activity and sensory evaluation of cocoa by-products NADES extracts used in food fortification. Innov. Food Sci. Emerg. Technol. 2020, 66, 102514. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous Optimization of Several Response Variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Harborne, J.B. Methods of Plant Analysis; Springer: Dordrecht, The Netherlands, 1984. [Google Scholar]
- Mišan, A.Č.; Mimica-Dukić, N.M.; Mandić, A.I.; Sakač, M.B.; Milovanović, I.L.; Sedej, I.J. Development of a Rapid Resolution HPLC method for the separation and determination of 17 phenolic compounds in crude plant extracts. Cent. Eur. J. Chem. 2011, 9, 133–142. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Meneses, L.; Santos, F.; Gameiro, A.R.; Paiva, A.; Duarte, A.R.C. Preparation of Binary and Ternary Deep Eutectic Systems. J. Vis. Exp. 2019, 152, e60326. [Google Scholar] [CrossRef]
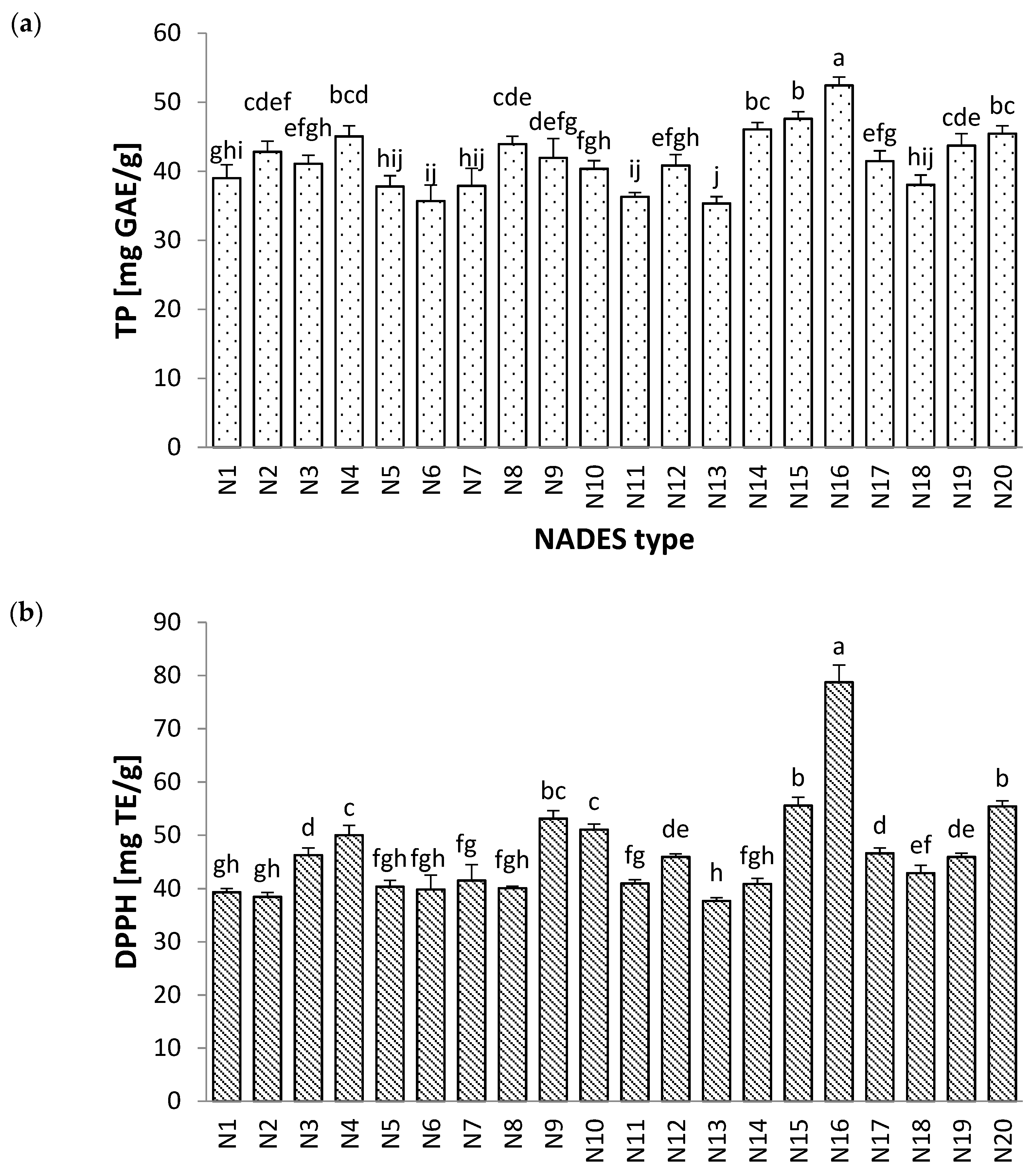
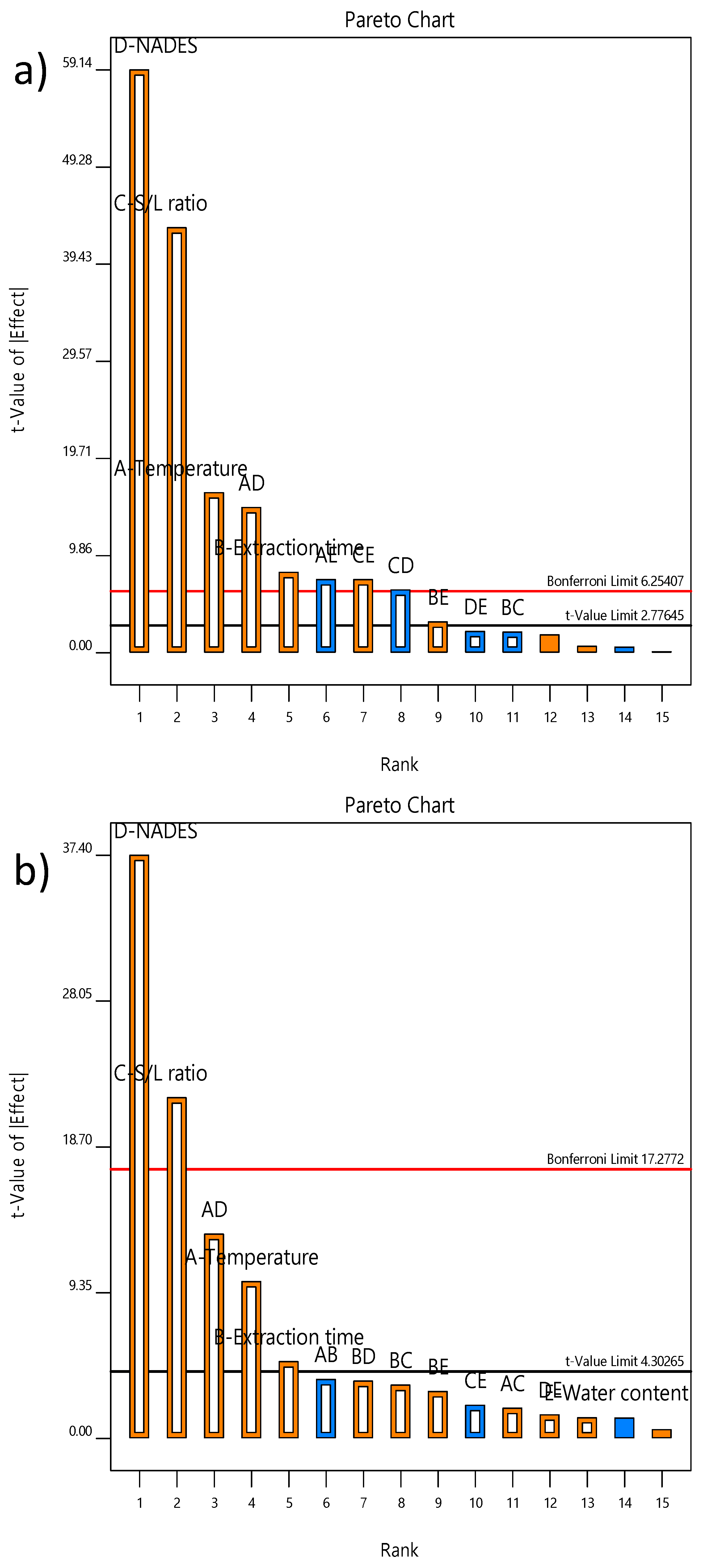
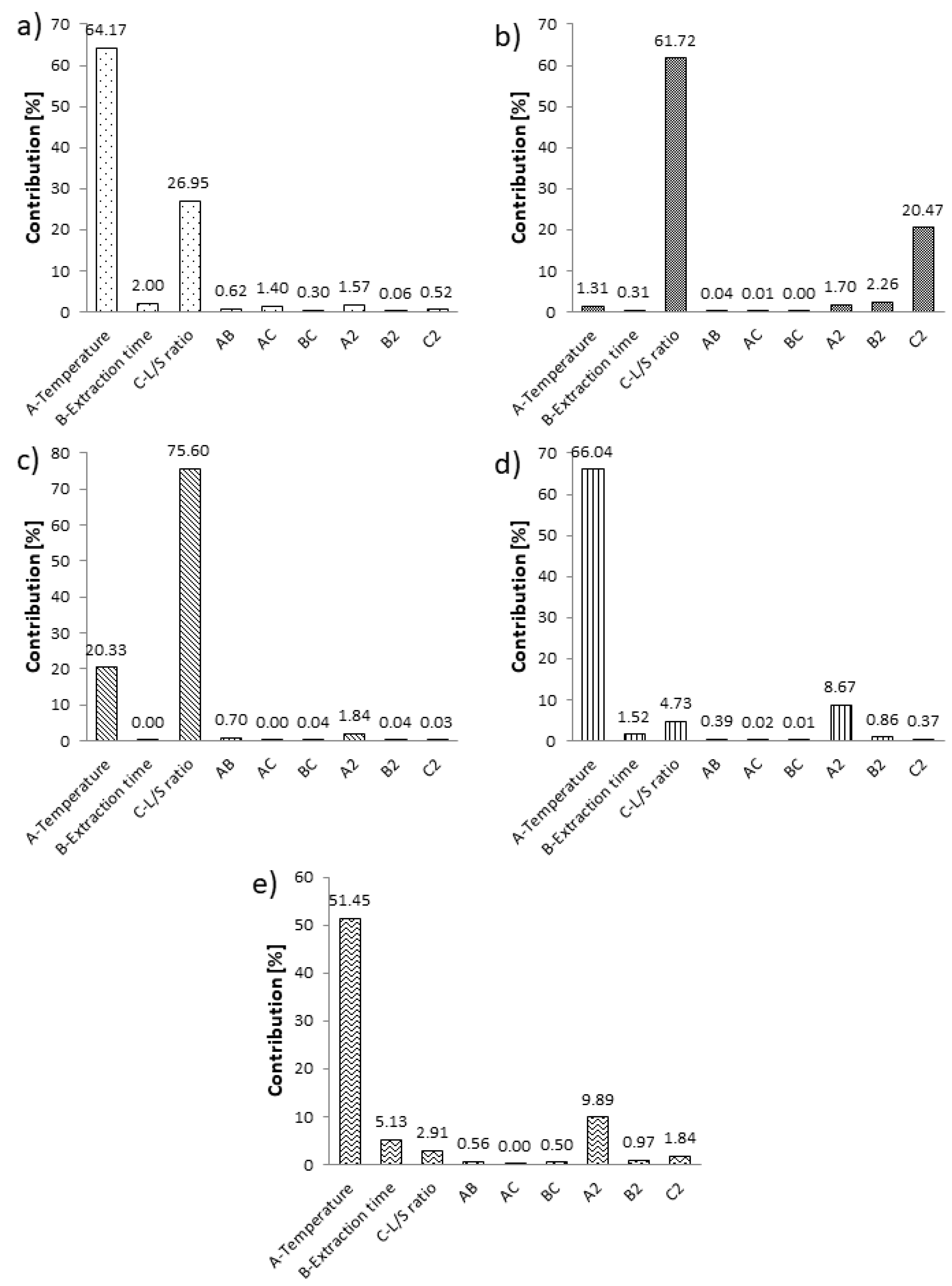

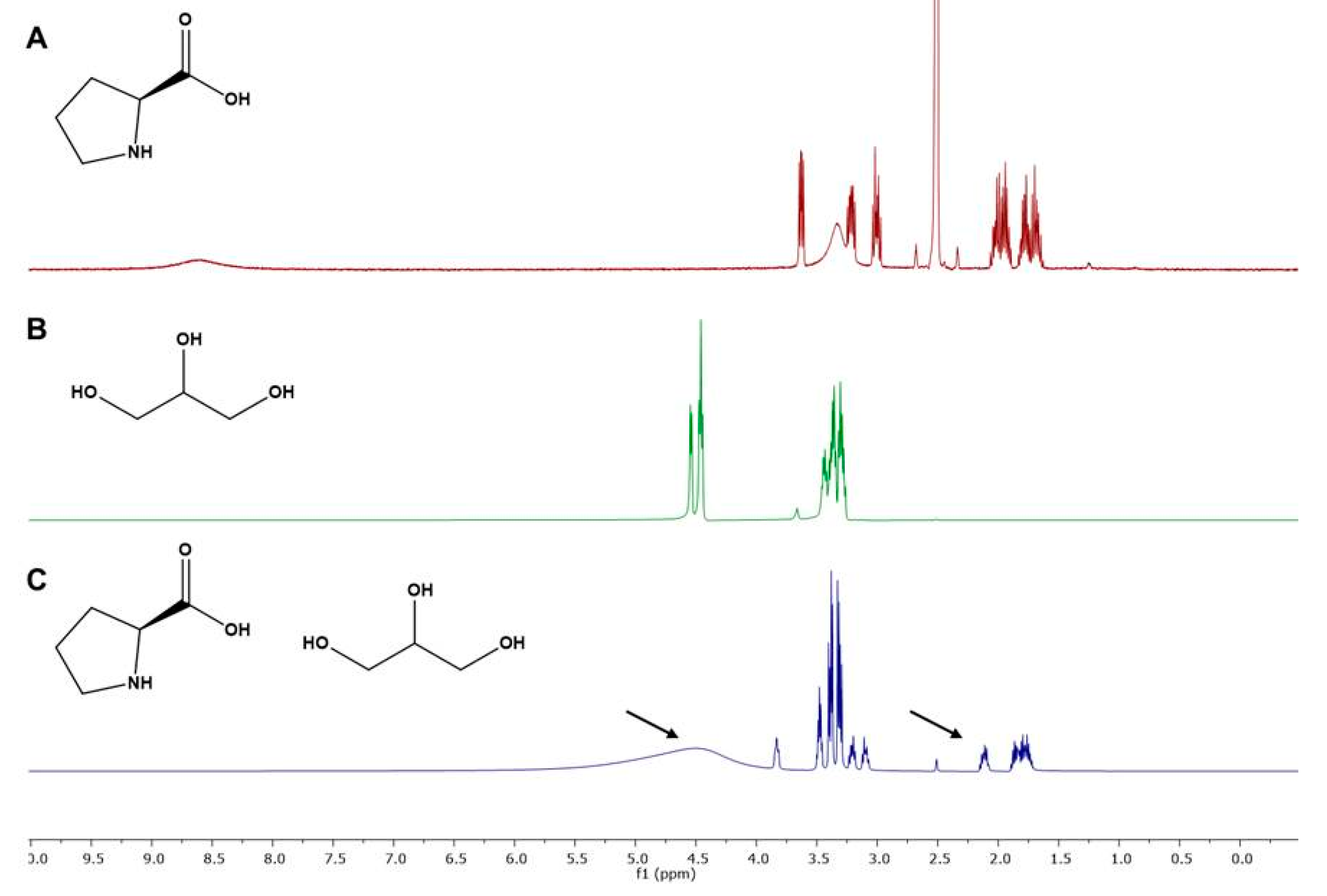
| Run | Factors | Responses | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A: Temperature [°C] | B: Extraction Time [min] | C: L/S Ratio [g NADES/g DW] | D: NADES Type * | E: Water Content [%] | TP [mg GAE/g] | DPPH [mg TE/g] | ||||||
| 1 | −1 | 50 | 1 | 120 | 1 | 20 | Level 2 | N16 | −1 | 20 | 57.79 | 74.55 |
| 2 | 1 | 60 | −1 | 60 | 1 | 20 | Level 2 | N16 | −1 | 20 | 63.41 | 84.86 |
| 3 | −1 | 50 | 1 | 120 | −1 | 10 | Level 2 | N16 | 1 | 25 | 53.57 | 63.45 |
| 4 | −1 | 50 | 1 | 120 | 1 | 20 | Level 1 | N15 | 1 | 25 | 54.19 | 57.96 |
| 5 | −1 | 50 | −1 | 60 | 1 | 20 | Level 1 | N15 | −1 | 20 | 50.62 | 54.25 |
| 6 | 1 | 60 | −1 | 60 | 1 | 20 | Level 1 | N15 | 1 | 25 | 52.11 | 52.50 |
| 7 | 1 | 60 | 1 | 120 | −1 | 10 | Level 1 | N15 | 1 | 25 | 44.13 | 37.59 |
| 8 | 1 | 60 | 1 | 120 | −1 | 10 | Level 2 | N16 | −1 | 20 | 59.52 | 69.75 |
| 9 | −1 | 50 | −1 | 60 | −1 | 10 | Level 2 | N16 | −1 | 20 | 51.87 | 54.60 |
| 10 | 1 | 60 | 1 | 120 | 1 | 20 | Level 1 | N15 | −1 | 20 | 52.37 | 54.25 |
| 11 | −1 | 50 | −1 | 60 | 1 | 20 | Level 2 | N16 | 1 | 25 | 58.47 | 64.19 |
| 12 | 1 | 60 | 1 | 120 | 1 | 20 | Level 2 | N16 | 1 | 25 | 64.24 | 91.16 |
| 13 | −1 | 50 | −1 | 60 | −1 | 10 | Level 1 | N15 | 1 | 25 | 43.31 | 39.97 |
| 14 | 1 | 60 | −1 | 60 | −1 | 10 | Level 2 | N16 | 1 | 25 | 54.84 | 72.47 |
| 15 | −1 | 50 | 1 | 120 | −1 | 10 | Level 1 | N15 | −1 | 20 | 44.45 | 40.13 |
| 16 | 1 | 60 | −1 | 60 | −1 | 10 | Level 1 | N15 | −1 | 20 | 44.93 | 39.88 |
| Run | Factor 1 | Factor 2 | Factor 3 | Response 1 | Response 2 | Response 3 | Response 4 | Response 5 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A: Temperature [°C] | B: Extraction Time [min] | C: L/S Ratio [g NADES/g DW] | TP [mg GAE/g] | TF [mg CE/g] | DPPH [mg TE/g] | FRAP [mg Fe2+/g] | ABTS [mg TE/g] | ||||
| 1 | 1 | 70 | 1 | 180 | −1 | 10 | 59.69 | 4.82 | 110.59 | 37.63 | 125.42 |
| 2 | −1 | 40 | −1 | 60 | −1 | 10 | 49.13 | 10.29 | 78.63 | 19.85 | 70.81 |
| 3 | 0 | 55 | 0 | 120 | 0 | 20 | 61.64 | 23.48 | 143.49 | 42.03 | 140.84 |
| 4 | 0 | 55 | 1 | 180 | 0 | 20 | 65.69 | 22.29 | 145.81 | 33.75 | 120.62 |
| 5 | 0 | 55 | 0 | 120 | 0 | 20 | 62.53 | 23.54 | 144.10 | 33.29 | 116.87 |
| 6 | 0 | 55 | 0 | 120 | 0 | 20 | 59.14 | 23.48 | 145.61 | 33.19 | 115.34 |
| 7 | 0 | 55 | 0 | 120 | 1 | 30 | 66.74 | 20.55 | 174.14 | 35.56 | 113.90 |
| 8 | 0 | 55 | 0 | 120 | 0 | 20 | 59.34 | 23.02 | 147.25 | 42.61 | 139.94 |
| 9 | −1 | 40 | 1 | 180 | 1 | 30 | 54.86 | 24.00 | 156.07 | 25.62 | 84.75 |
| 10 | 0 | 55 | 0 | 120 | 0 | 20 | 59.61 | 23.22 | 152.76 | 44.70 | 147.87 |
| 11 | −1 | 40 | 1 | 180 | −1 | 10 | 47.38 | 10.37 | 89.46 | 21.56 | 75.29 |
| 12 | 1 | 70 | 0 | 120 | 0 | 20 | 70.01 | 22.70 | 155.44 | 37.51 | 118.08 |
| 13 | 1 | 70 | −1 | 60 | 1 | 30 | 67.74 | 20.33 | 188.08 | 38.12 | 119.57 |
| 14 | −1 | 40 | −1 | 60 | 1 | 30 | 53.82 | 24.66 | 153.19 | 24.09 | 77.97 |
| 15 | 1 | 70 | −1 | 60 | −1 | 10 | 57.65 | 6.01 | 125.61 | 33.17 | 100.35 |
| 16 | 0 | 55 | 0 | 120 | −1 | 10 | 53.40 | 5.20 | 120.33 | 33.38 | 100.96 |
| 17 | 1 | 70 | 1 | 180 | 1 | 30 | 71.43 | 19.34 | 188.01 | 42.00 | 124.55 |
| 18 | −1 | 40 | 0 | 120 | 0 | 20 | 48.27 | 14.37 | 116.73 | 23.29 | 77.56 |
| 19 | 0 | 55 | −1 | 60 | 0 | 20 | 57.82 | 24.65 | 143.44 | 34.08 | 98.24 |
| Source | Sum of | df * | Mean | F-Value | p-Value |
|---|---|---|---|---|---|
| TP | |||||
| Model | 831.71 | 9 | 92.41 | 11.7975 | 0.00055 |
| Residual | 70.50 | 9 | 7.83 | ||
| Lack of Fit | 61.07 | 5 | 12.21 | 5.1803 | 0.06809 |
| Pure Error | 9.43 | 4 | 2.36 | ||
| Cor Total | 902.21 | 18 | |||
| R2 | 0.922 | ||||
| CV [%] | 4.72 | ||||
| TF | |||||
| Model | 844.22 | 9 | 93.80 | 9.4944 | 0.00127 |
| Residual | 88.92 | 9 | 9.88 | ||
| Lack of Fit | 88.72 | 5 | 17.74 | 359.7893 | <0.0001 |
| Pure Error | 0.20 | 4 | 0.05 | ||
| Cor Total | 933.14 | 18 | |||
| R2 | 0.905 | ||||
| CV [%] | 17.24 | ||||
| DPPH | |||||
| Model | 14,833.37 | 9 | 1648.15 | 61.0444 | <0.0001 |
| Residual | 242.99 | 9 | 27.00 | ||
| Lack of Fit | 187.76 | 5 | 37.55 | 2.71975 | 0.17690 |
| Pure Error | 55.23 | 4 | 13.81 | ||
| Cor Total | 15,076.36 | 18 | |||
| R2 | 0.984 | ||||
| CV [%] | 3.69 | ||||
| FRAP | |||||
| Model | 829.49 | 9 | 92.17 | 4.9684 | 0.01280 |
| Residual | 166.95 | 9 | 18.55 | ||
| Lack of Fit | 45.98 | 5 | 9.20 | 0.3041 | 0.88809 |
| Pure Error | 120.97 | 4 | 30.24 | ||
| Cor Total | 996.45 | 18 | |||
| R2 | 0.832 | ||||
| CV [%] | 12.88 | ||||
| ABTS | |||||
| Model | 7899.47 | 9 | 877.72 | 4.0763 | 0.02406 |
| Residual | 1937.89 | 9 | 215.32 | ||
| Lack of Fit | 1038.55 | 5 | 207.71 | 0.9238 | 0.54581 |
| Pure Error | 899.34 | 4 | 224.84 | ||
| Cor Total | 9837.36 | 18 | |||
| R2 | 0.803 | ||||
| CV [%] | 13.48 |
| Input and Output Parameters | Goal | Lower Limit | Upper Limit | Predicted Values | Experimental Values |
|---|---|---|---|---|---|
| Optimal Conditions | Optimal Conditions | ||||
| Temperature [°C] | is in range | 40 | 70 | 65 | 65 |
| Extraction time [min] | is in range | 60 | 180 | 180 | 180 |
| L/S ratio [g NADES/g DW] | is in range | 10 | 30 | 28 | 28 |
| TP [mg GAE/g] | maximize | 43.38 | 71.43 | 71.43 | 71.43 ± 1.17 |
| TF [mg CE/g] | maximize | 4.82 | 24.66 | 22.81 | 19.43 ± 0.20 |
| DPPH [mg TE/g] | maximize | 78.63 | 188.08 | 179.52 | 188.01 ± 11.19 |
| FRAP [mg Fe2+/g] | maximize | 19.85 | 44.70 | 41.09 | 42.00 ± 0.28 |
| ABTS [mg TE/g] | maximize | 70.80 | 147.87 | 130.06 | 124.55 ± 3.25 |
| No. | Compound | Content [mg/100 g] |
|---|---|---|
| 1. | Gallic acid | 20.61 |
| 2. | Caffeic acid | 25.83 |
| 3. | Epicatechin | 21.06 |
| 4. | Rosmarinic acid | 524.18 |
| 5. | Luteolin | 28.27 |
| 6. | Quercetin | 42.27 |
| Code | Content | Molar Ratio | Water Content [%] |
|---|---|---|---|
| N1 | Citric acid (CA)–glucose (Glu) | 1:1 | - |
| N2 | Citric acid (CA)–sucrose (Suc) | 1:1 | - |
| N3 | Citric acid (CA)–betaine (Bet)–water (H2O) | 1:1:1 | 5.50 |
| N4 | Choline chloride (ChCl)–glucose (Glu) | 1:1 | - |
| N5 | Glycerin (Gly)–betaine (BET) | 2:1 | - |
| N6 | Betaine (Bet)–glycerine (Gly)–water (H2O) | 1:2:1 | 5.64 |
| N7 | Betaine (Bet)–glucose (Glu) | 1:1 | - |
| N8 | Glycerin (Gly)–fructose (Fru) | 4:1 | - |
| N9 | Choline chloride (ChCl)–glycerin (Gly) | 1:2 | - |
| N10 | Choline chloride (ChCl)–glycerin (Gly)–water (H2O) | 2:1:1 | 5.27 |
| N11 | Lactic acid (LA)–glucos –water (H2O) | 5:1:3 | 7.89 |
| N12 | Choline chloride (ChCl)–lactic acid (LA) | 1:4 | 11.23 |
| N13 | Glucose (Glu)–tartaric acid (TA) | 1:1 | - |
| N14 | Lactic acid (LA)–fructose (Fru) | 5:1 | 11.16 |
| N15 | l-proline (Pro)–lactic acid (LA) | 1:2 | 9.69 |
| N16 | l-proline (Pro)–glycerin (Gly)–water (H2O) | 1:2:1 | 5.68 |
| N17 | Malic acid (MA)–betaine (Bet)–water (H2O) | 2:1:5 | 18.95 |
| N18 | Tartaric acid (TA)–betaine (Bet)–water (H2O) | 2:1:5 | 17.75 |
| N19 | Choline chloride (ChCl)–citric acid (CA) | 1:1 | - |
| N20 | 1,2-Propanediol (PD)–choline chloride (ChCl)–water (H2O) | 1:1:1 | 7.71 |
| I Step—Screening of the Extraction Solvent | |||
| Approach | Constant Parameters | Factors | Responses * |
| OFAT 1 | Sample to solvent ratio: 1:20 m/mTemperature: 50 °C Extraction time: 60 min Stirring speed: 700 rpm Water content: 20% | NADES: N1–N20 | TP 2 DPPH 3 |
| II Step—Preliminary Study | |||
| Approach | Constant Parameters | Factors | Responses |
| 25−1 fractional factorial design | Stirring speed: 700 rpm | Temperature: 50 and 60 °C Extraction time: 60 and 120 min Sample to solvent ratio: 1:10 and 1:20 m/m NADES: N15 and N16 Water content: 20 and 25% | TP DPPH |
| III Step—Optimization | |||
| Approach | Constant Parameters | Factors | Responses |
| RSM | NADES: N16 Water content: 20% Stirring speed: 700 rpm | Temperature: 40, 55 and 70 °C Extraction time: 60, 120 and 180 min Sample to solvent ratio: 1:10, 1:20 and 1:30 m/m | TP TF 4 DPPH FRAP 5 ABTS 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlić, B.; Mrkonjić, Ž.; Teslić, N.; Kljakić, A.C.; Pojić, M.; Mandić, A.; Stupar, A.; Santos, F.; Duarte, A.R.C.; Mišan, A. Natural Deep Eutectic Solvent (NADES) Extraction Improves Polyphenol Yield and Antioxidant Activity of Wild Thyme (Thymus serpyllum L.) Extracts. Molecules 2022, 27, 1508. https://doi.org/10.3390/molecules27051508
Pavlić B, Mrkonjić Ž, Teslić N, Kljakić AC, Pojić M, Mandić A, Stupar A, Santos F, Duarte ARC, Mišan A. Natural Deep Eutectic Solvent (NADES) Extraction Improves Polyphenol Yield and Antioxidant Activity of Wild Thyme (Thymus serpyllum L.) Extracts. Molecules. 2022; 27(5):1508. https://doi.org/10.3390/molecules27051508
Chicago/Turabian StylePavlić, Branimir, Živan Mrkonjić, Nemanja Teslić, Aleksandra Cvetanović Kljakić, Milica Pojić, Anamarija Mandić, Alena Stupar, Filipa Santos, Ana Rita C. Duarte, and Aleksandra Mišan. 2022. "Natural Deep Eutectic Solvent (NADES) Extraction Improves Polyphenol Yield and Antioxidant Activity of Wild Thyme (Thymus serpyllum L.) Extracts" Molecules 27, no. 5: 1508. https://doi.org/10.3390/molecules27051508
APA StylePavlić, B., Mrkonjić, Ž., Teslić, N., Kljakić, A. C., Pojić, M., Mandić, A., Stupar, A., Santos, F., Duarte, A. R. C., & Mišan, A. (2022). Natural Deep Eutectic Solvent (NADES) Extraction Improves Polyphenol Yield and Antioxidant Activity of Wild Thyme (Thymus serpyllum L.) Extracts. Molecules, 27(5), 1508. https://doi.org/10.3390/molecules27051508











