A Dual pH/O2 Sensing Film Based on Functionalized Electrospun Nanofibers for Real-Time Monitoring of Cellular Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
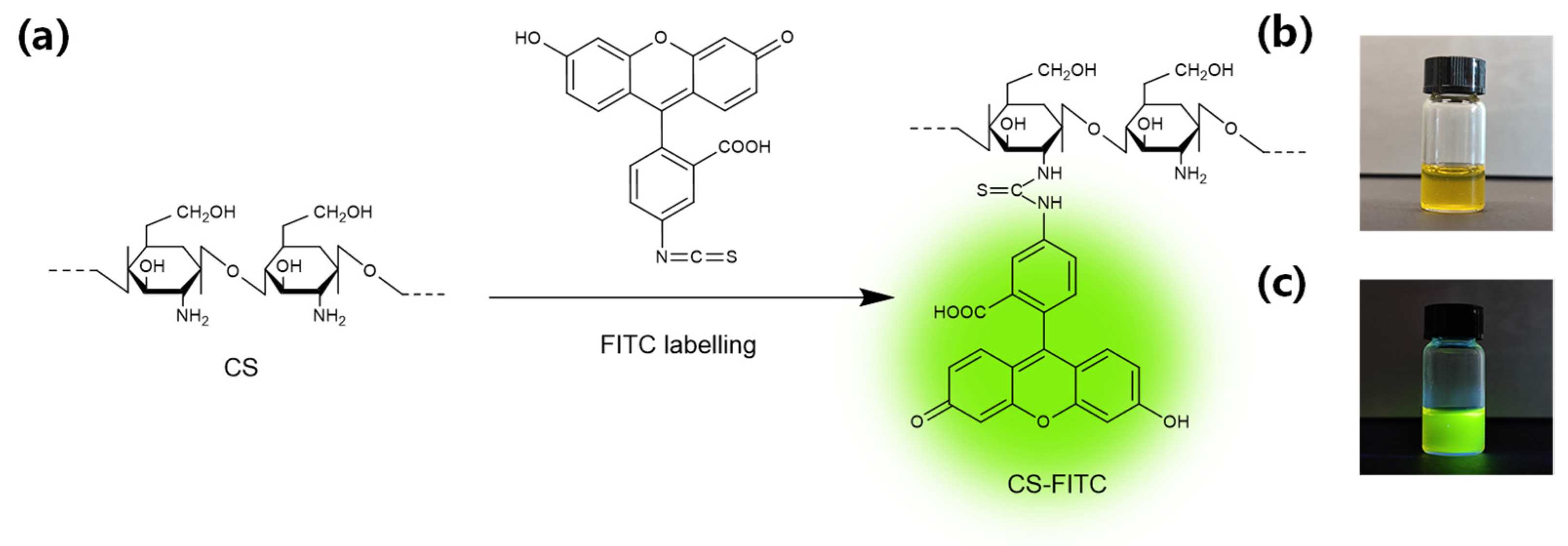
2.2. Synthesis of CS-FITC
2.3. Preparation of Electrospun Nanofibrous Matrix
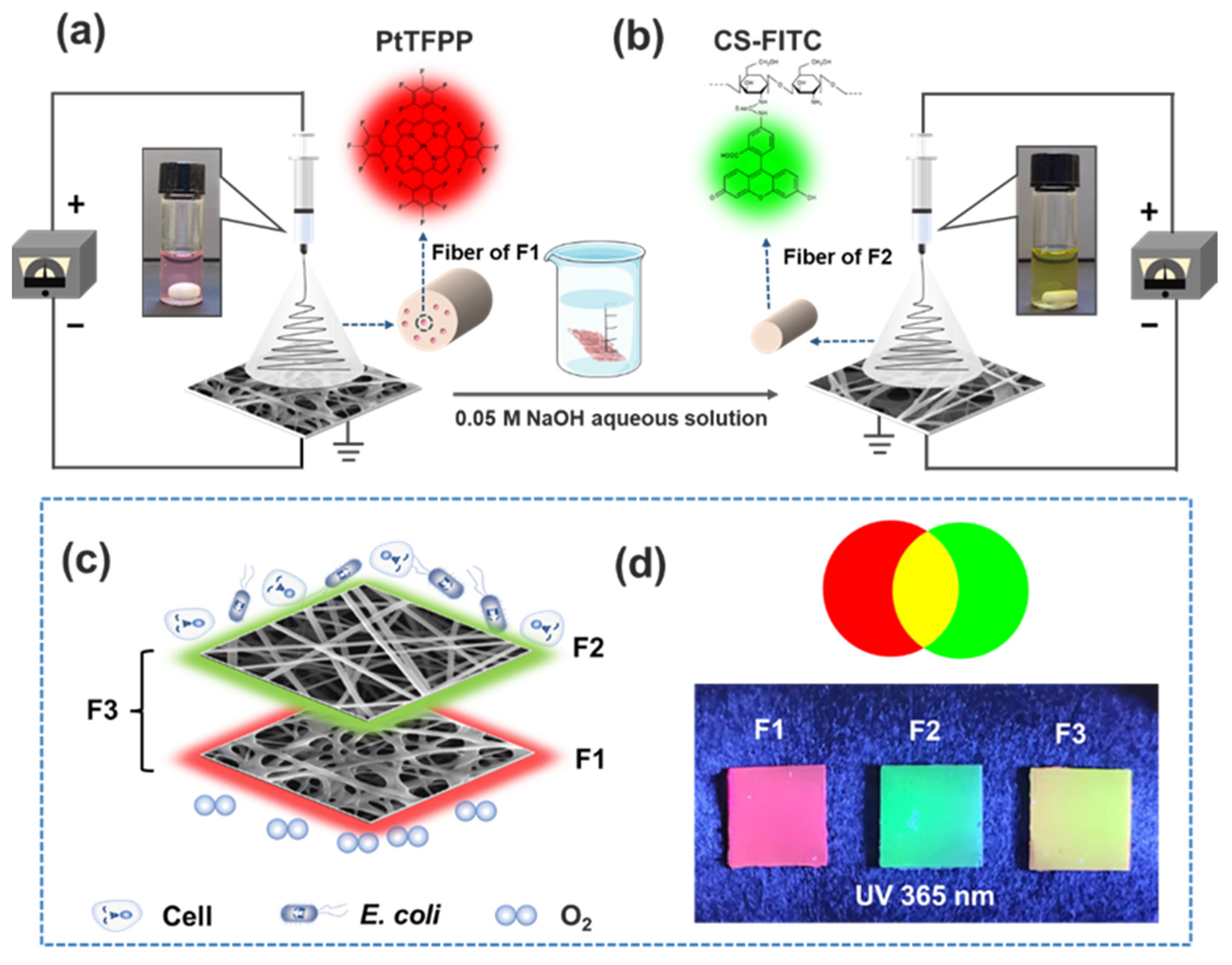
2.3.1. Preparation of CA&PCL Electrospun Nanofibrous Matrix (F1) for Oxygen Sensing
2.3.2. Preparation of CS Electrospun Nanofibrous Matrix for pH Sensing (F2)
2.3.3. Preparation of CS Electrospun Nanofibrous Matrix on the Top of F1 for Dual Senor F3
2.3.4. Preparation of Nanofibrous CA (F4) or PCL (F5) as Matrices for Comparison with F1
2.4. Characterization of Nanofibrous Matrices
2.5. pH and DO Sensing Performances
2.5.1. Response to pH and DO
2.5.2. Reversibility of the Sensing Response
2.5.3. Real-Time Monitoring of the Cell Respiration Process
2.6. In Vitro Biocompatibility Test
2.6.1. MTT Assay
2.6.2. Cell Proliferation and Spreading
3. Results and Discussion
3.1. Synthesis of CS-FITC Conjugates
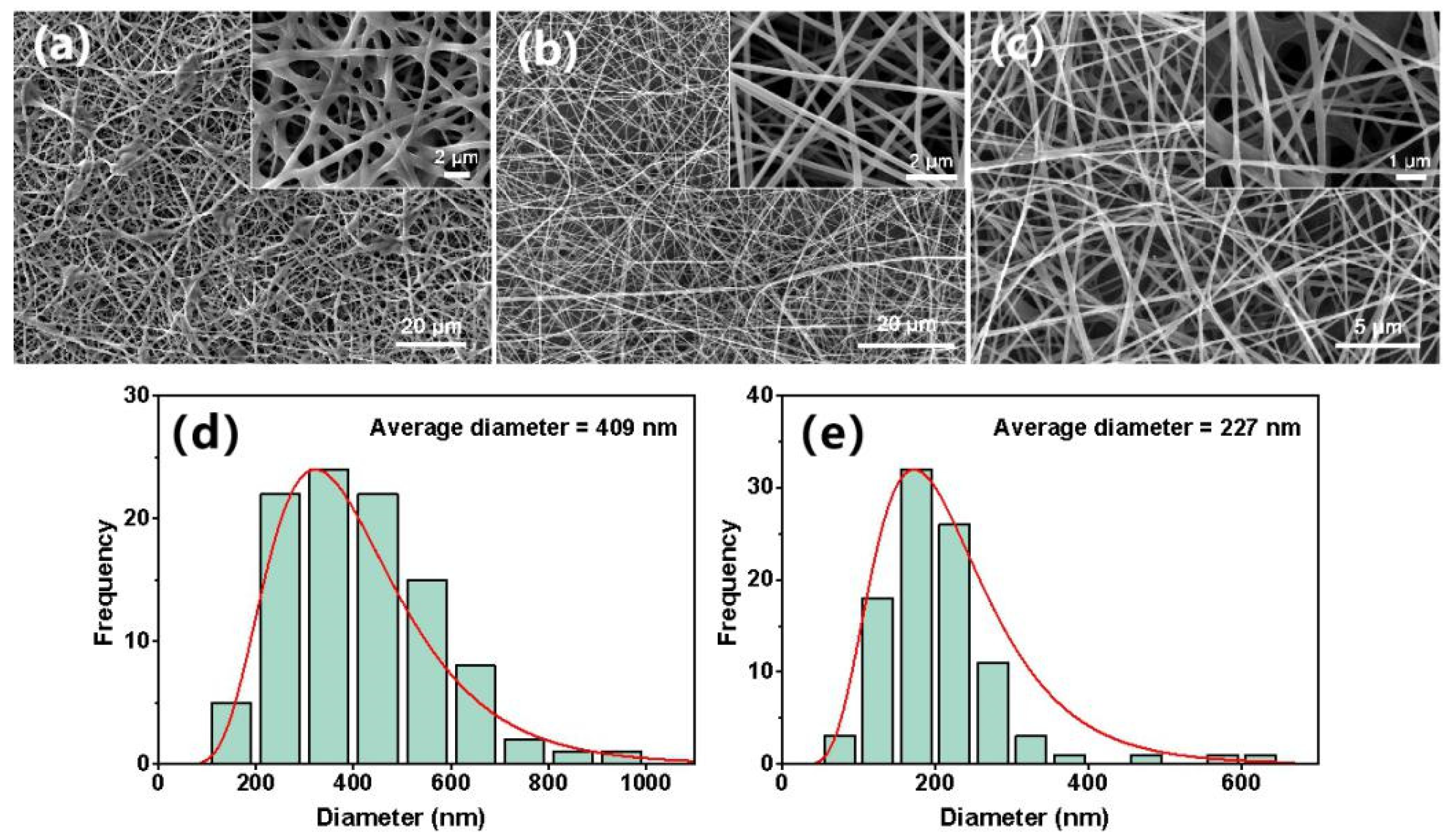
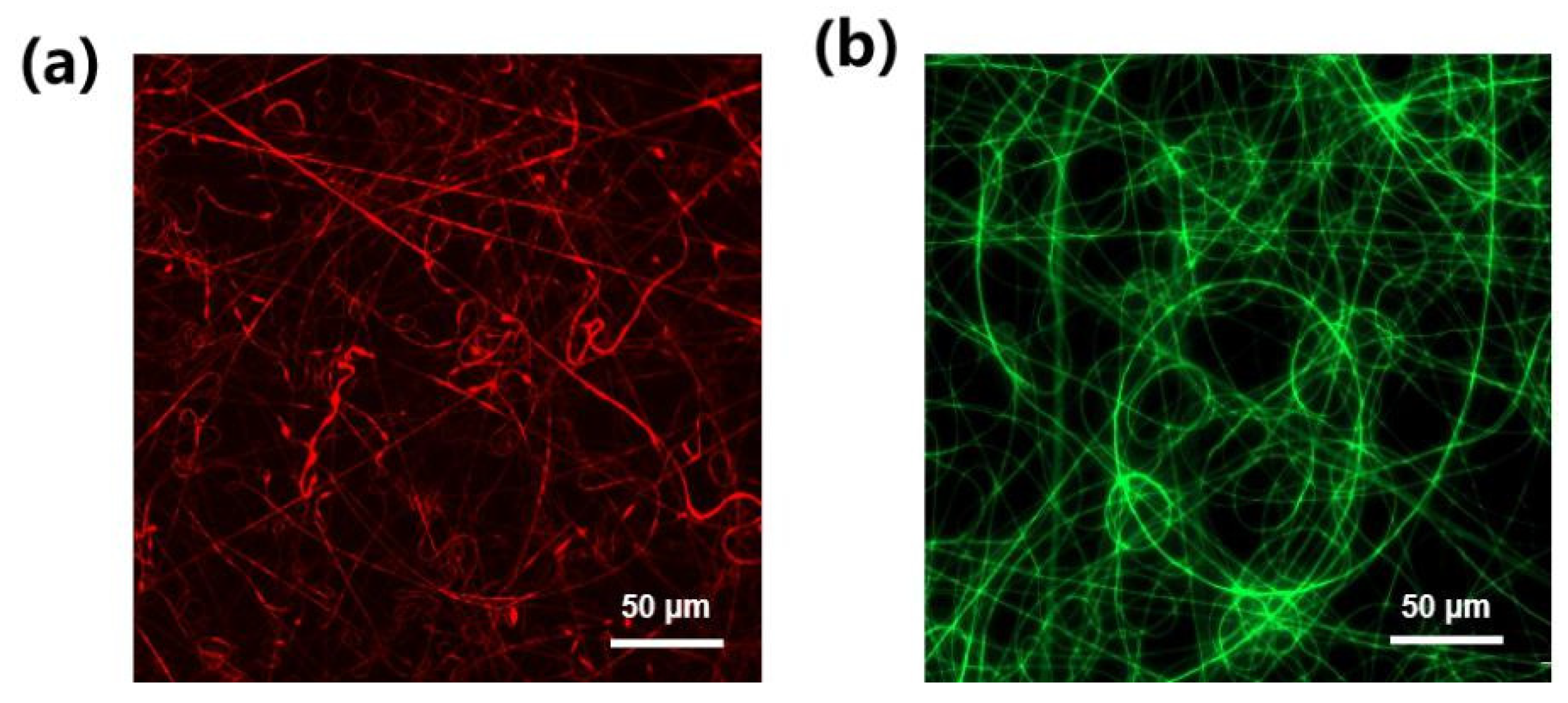
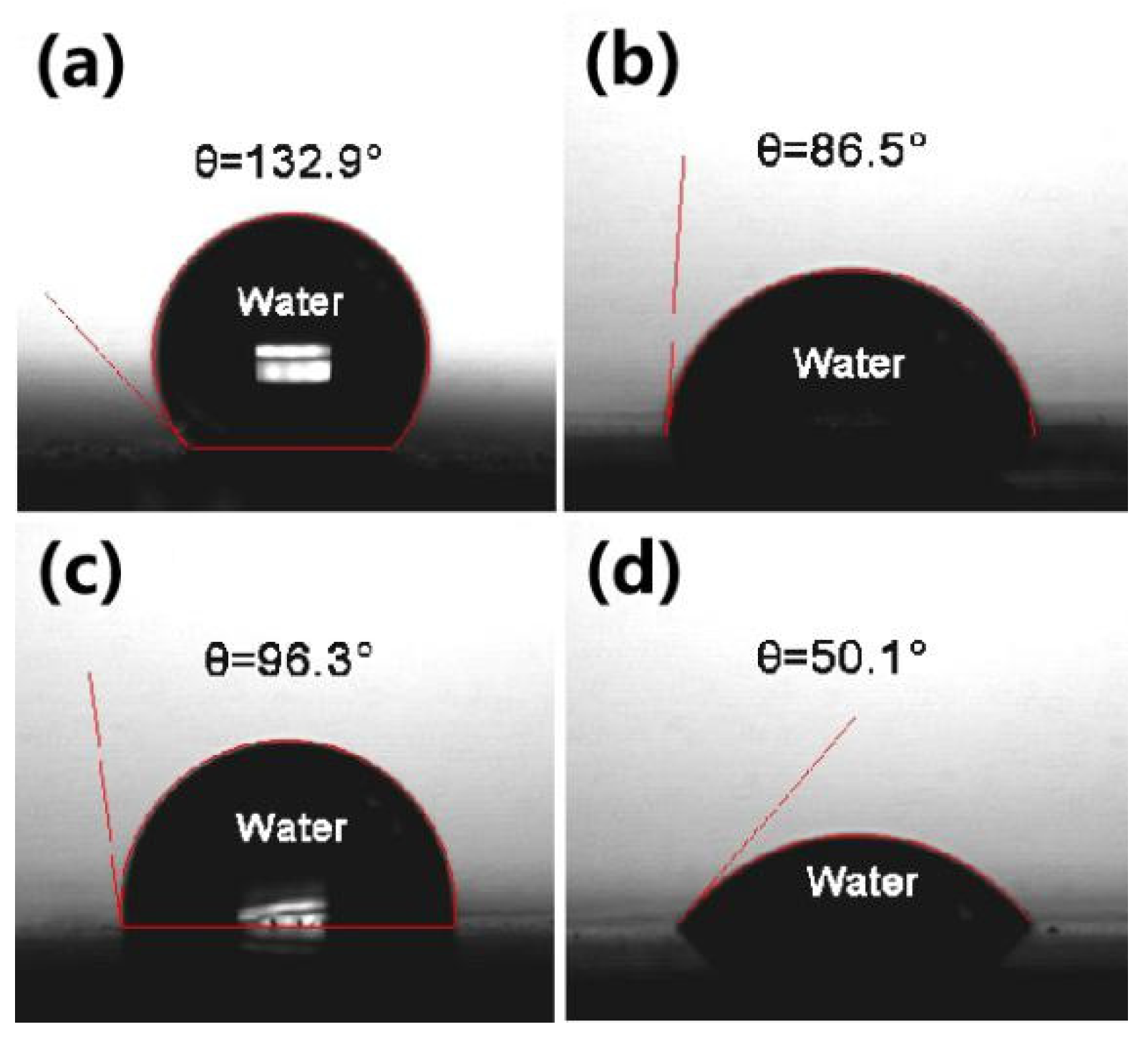
3.2. Membrane Characterization
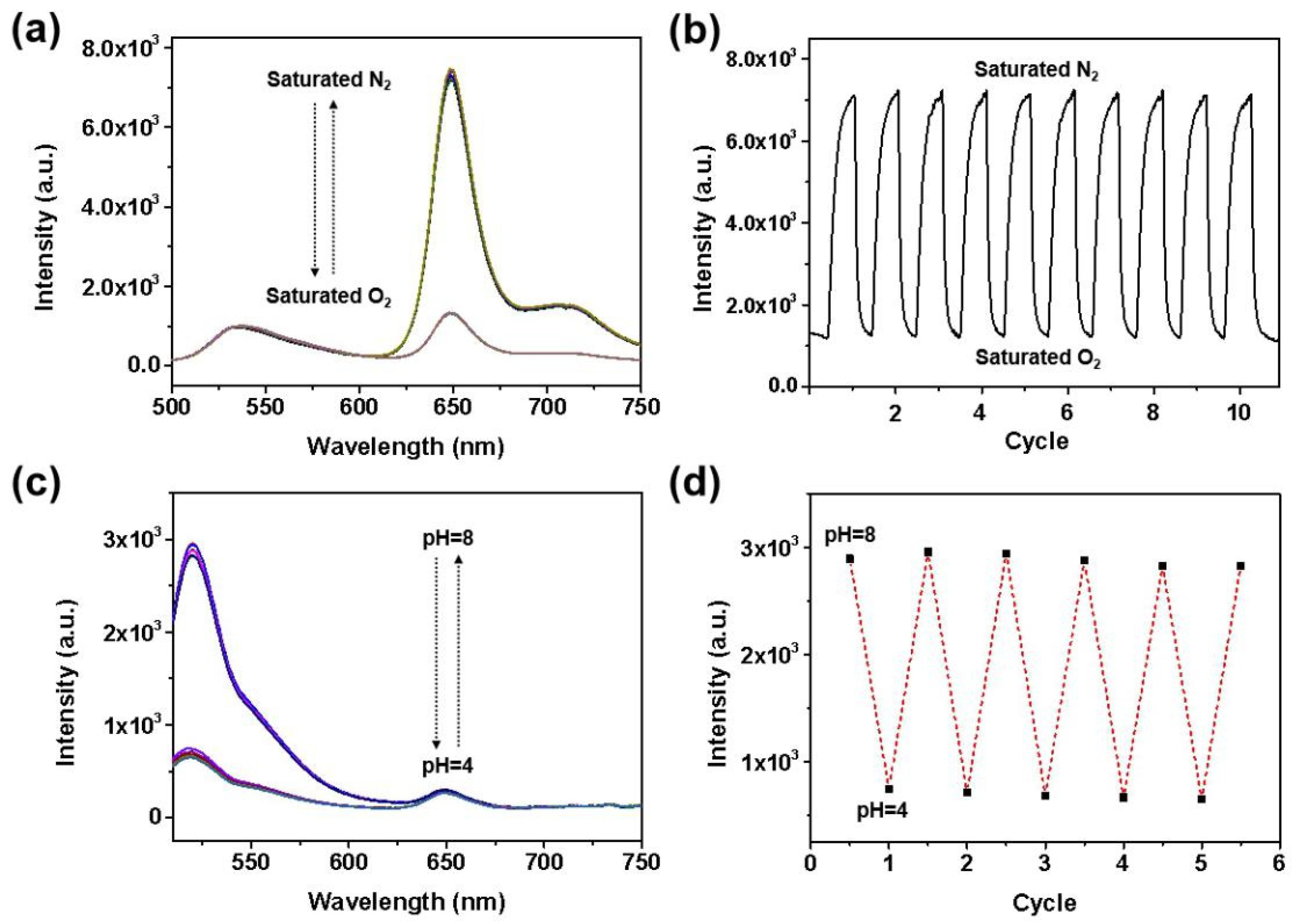
3.3. Oxygen Sensing Performance
3.4. pH Sensing Performance
3.5. Reversibility of the Sensing Film F3
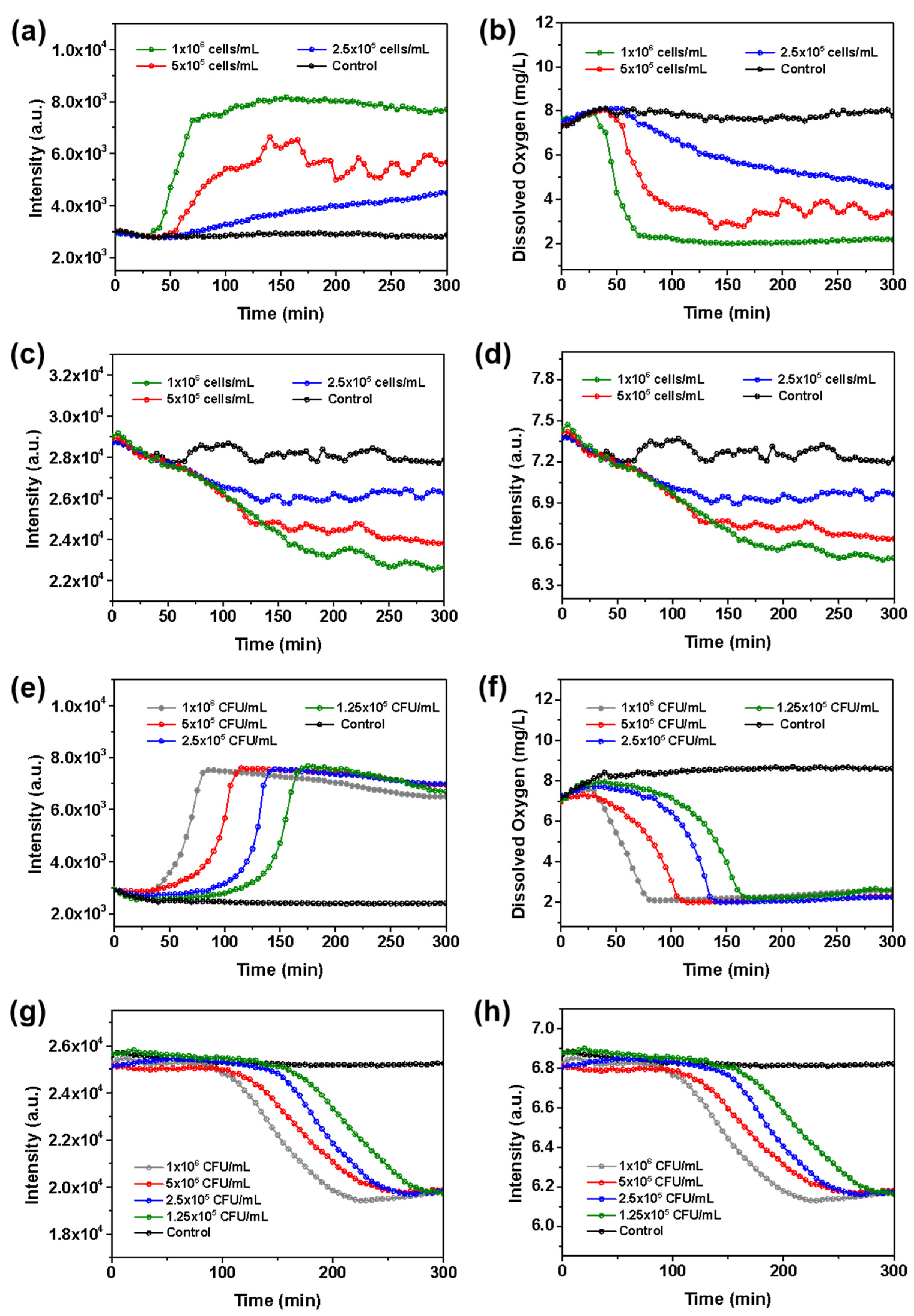
3.6. Real-Time Monitoring of DO and pH during Cellular Respiration Process
3.7. In Vitro Biocompatibility Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kang, M.; Jo, Y.; Mun, C.; Yeom, J.; Park, J.S.; Jung, H.S.; Kim, D.H.; Park, S.G.; Yoo, S.M. Nanoconfined 3D redox capacitor-based electrochemical sensor for ultrasensitive monitoring of metabolites in bacterial communication. Sens. Actuators B Chem. 2021, 345, 130427. [Google Scholar] [CrossRef]
- Wang, X.D.; Meier, R.J.; Wolfbeis, O.S. Fluorescent pH-sensitive nanoparticles in an agarose matrix for imaging of bacterial growth and metabolism. Angew. Chem. Int. Ed. 2013, 52, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Ibarlucea, B.; Rim, T.; Baek, C.K.; Visser, J.D.; Baraban, L.; Cuniberti, G. Nanowire sensors monitor bacterial growth kinetics and response to antibiotics. Lab Chip 2017, 1, 810. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Pan, T.; Jiang, J.; Li, G.; Song, C.; Sun, R.; Yang, Z.; Sun, D.; Hou, C.; Chen, M.; et al. Poly(ε-caprolactone)-containing graft copolymers for ratiometric extracellular oxygen sensing. Sens. Actuators B Chem. 2017, 248, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Grist, S.M.; Chrostowski, L.; Cheung, K.C.J.S. Optical Oxygen Sensors for Applications in Microfluidic Cell Culture. Sensors 2010, 10, 9286–9316. [Google Scholar] [CrossRef] [Green Version]
- Deng, M.; Qiao, Y.; Liu, C.; Wang, Z.; Shi, J.; Pan, T.; Mao, Y.; Mei, Z.; Huang, F.; Tian, Y. Tricolor core/shell polymeric ratiometric nanosensors for intracellular glucose and oxygen dual sensing. Sens. Actuators B Chem. 2019, 286, 437–444. [Google Scholar] [CrossRef]
- Yang, X.; Peng, L.; Yuan, L.; Teng, P.; Luo, S. Oxygen gas optrode based on microstructured polymer optical fiber segment. Opt. Commun. 2011, 284, 3462–3466. [Google Scholar] [CrossRef]
- Eteshola, E.; Pandian, R.P.; Lee, S.C.; Kuppusamy, P. Polymer coating of paramagnetic particulates for in vivo oxygen-sensing applications. Biomed. Microdevices 2009, 11, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Mills, A.; Williams, F.C. Chemical influences on the luminescence of ruthenium diimine complexes and its response to oxygen. Thin. Solid. Film. 1997, 306, 163–170. [Google Scholar] [CrossRef]
- Hess, S.; Becker, A.; Baluschev, S.; Yakutkin, V.; Wegner, G. A Comparative Study of Oxygen Permeabilities of Film-Forming Polymers by Quenching of Platinum Porphyrin Phosphorescence. Macromol. Chem. Phy. 2007, 208, 2173–2188. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Z.Z. CA/silica composite film for the determination of dissolved oxygen. Chin. J. Anal. Lab. 2015, 24, 369–372. [Google Scholar]
- Logith Kumar, R.; Keshav Narayan, A.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A review of chitosan and its derivatives in bone tissue engineering. Carbohydr. Polym. 2016, 151, 172–188. [Google Scholar] [CrossRef]
- Jiang, Z.; Yu, X.; Hao, Y. Design and Fabrication of a Ratiometric Planar Optode for Simultaneous Imaging of pH and Oxygen. Sensors 2017, 17, 1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Shumway, B.R.; Youngbull, A.C.; Li, Y.; Jen, A.K.; Johnson, R.H.; Meldrum, D.R. Dually Fluorescent Sensing of pH and Dissolved Oxygen Using a Membrane Made from Polymerizable Sensing Monomers. Sens. Actuators B Chem. 2010, 147, 714–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocincová, A.; Nagl, S.; Arain, S.; Krause, C.; Borisov, S.M.; Arnold, M.; Wolfbeis, O.S. Multiplex bacterial growth monitoring in 24-well microplates using a dual optical sensor for dissolved oxygen and pH. Biotechnol. Bioeng. 2010, 100, 430–438. [Google Scholar] [CrossRef]
- Chu, C.S.; Jhih-Jheng, S. Optical sensor for dual sensing of oxygen and carbon dioxide based on sensing films coated on filter paper. Appl. Opt. 2017, 56, 1225. [Google Scholar] [CrossRef]
- Borisov, S.M.; Krause, C.; Arain, S.; Wolfbeis, O. Composite Material for Simultaneous and Contactless Luminescent Sensing and Imaging of Oxygen and Carbon Dioxide. Adv. Mater. 2006, 18, 1511–1516. [Google Scholar] [CrossRef]
- Muhammad, I.; Nunzio, M.; Mahnaz, S. Electrospun one-dimensional nanostructures: A new horizon for gas sensing materials. Beilstein J. Nanotechnol. 2018, 9, 2128–2170. [Google Scholar]
- Kim, H.C.; Park, W.H. Fluorescent property of glycol chitosan-fluorescein isothiocyanate conjugate for bio-imaging material. Int. J. Biol. Macromol. 2019, 135, 1217–1221. [Google Scholar] [CrossRef]
- Xue, R.; Behera, P.; Viapiano, M.S.; Lannutti, J.J. Rapid response oxygen-sensing nanofibers. Mater. Sci. Eng. C 2013, 33, 3450–3457. [Google Scholar] [CrossRef] [Green Version]
- Mao, Y.; Liu, Z.; Liang, L.; Zhou, Y.; Qiao, Y.; Mei, Z.; Zhou, B.; Tian, Y. Silver Nanowire-Induced Sensitivity Enhancement of Optical Oxygen Sensors Based on AgNWs-Palladium Octaethylporphine-Poly(methyl methacrylate) Microfiber Mats Prepared by Electrospinning. ACS Omega 2018, 3, 5669–5677. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Drew, C.; Lee, S.H.; Senecal, K.J.; Kumar, J.; Samuelson, L.A. Electrospun Nanofibrous Membranes for Highly Sensitive Optical Sensors. ACS Omega 2002, 2, 1273–1275. [Google Scholar] [CrossRef]
- Tian, Y.; Fuller, E.; Klug, S.; Lee, F.; Su, F.; Zhang, L.; Chao, S.H.; Meldrum, D.R. A fluorescent colorimetric pH sensor and the influences of matrices on sensing performances. Sens. Actuators B Chem. 2013, 188, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulukutla, B.C.; Khan, S.; Lange, A.; Hu, W.S. Glucose metabolism in mammalian cell culture: New insights for tweaking vintage pathways. Trends Biotechnol. 2010, 28, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.R.; Michelsen, O. Carbon and energy metabolism of atp mutants of Escherichia coli. J. Bacteriol. 1992, 174, 7635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herst, P.M.; Berridge, M.V. Cell surface oxygen consumption: A major contributor to cellular oxygen consumption in glycolytic cancer cell lines. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 170–177. [Google Scholar]
- Angert, E. Alternatives to binary fission in bacteria. Nat. Rev. Microbiol. 2005, 3, 214–224. [Google Scholar] [CrossRef]
- Wu, S.; Wu, S.; Yi, Z.; Zeng, F.; Wu, W.; Qiao, Y.; Zhao, X.; Cheng, X.; Tian, Y. Hydrogel-Based Fluorescent Dual pH and Oxygen Sensors Loaded in 96-Well Plates for High-Throughput Cell Metabolism Studies. Sensors 2018, 18, 564. [Google Scholar] [CrossRef] [Green Version]


| Films | Compositions | Probe Molecules | Response | Cross-Linking |
|---|---|---|---|---|
| F1 | CA:PCL = 5:4 (weight ratio) | PtTFPP | DO | - |
| F2 | CS | FITC | pH | GA vapor |
| F3 | F1 + F2 | PtTFPP and FITC | DO and pH | GA vapor |
| F4 | CA | PtTFPP | DO | - |
| F5 | PCL | PtTFPP | DO | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, D.; Liu, H.; Ning, J.; Cao, G.; Zhang, H.; Deng, M.; Tian, Y. A Dual pH/O2 Sensing Film Based on Functionalized Electrospun Nanofibers for Real-Time Monitoring of Cellular Metabolism. Molecules 2022, 27, 1586. https://doi.org/10.3390/molecules27051586
Zhou D, Liu H, Ning J, Cao G, Zhang H, Deng M, Tian Y. A Dual pH/O2 Sensing Film Based on Functionalized Electrospun Nanofibers for Real-Time Monitoring of Cellular Metabolism. Molecules. 2022; 27(5):1586. https://doi.org/10.3390/molecules27051586
Chicago/Turabian StyleZhou, Dongyan, Hongtian Liu, Juewei Ning, Ge Cao, He Zhang, Mengyu Deng, and Yanqing Tian. 2022. "A Dual pH/O2 Sensing Film Based on Functionalized Electrospun Nanofibers for Real-Time Monitoring of Cellular Metabolism" Molecules 27, no. 5: 1586. https://doi.org/10.3390/molecules27051586






