Chemical Activation of Lignocellulosic Precursors and Residues: What Else to Consider?
Abstract
:1. Introduction
2. Preparation of Activated Carbons
2.1. Precursors
- The activating agent. It covers H2SO4, ZnCl2, H3PO4, KOH, and NaOH, etc., showing from neutral to acid or basic character. It reacts in a controlled manner with carbon, consuming some of it, and creating porosity [4,9,11,21,25]. Literature states that, as a general trend, ZnCl2 and H3PO4 are the most suitable activating agents for low-ordered precursors (including lignocellulosic precursors and low-rank coals), whereas alkaline hydroxides are more suitable for the activation of highly ordered ones (i.e., high-rank coals, such as anthracites or carbon nanotubes) [10,39].
- Heating treatment. Either pyrolysis followed by activation (if two-step chemical activation-procedure is used) and/or a single step chemical activation are required for preparing activated carbons, being the heating rate, the activation temperature, and the holding time at the activation temperature among the most influencing experimental parameters [25,37]. In any case, conventional furnaces based on standard electrical resistances are commonly used, and the heat transfer occurs from the gas to the sample to be activated, either in slow or in flash conditions [9,40]. In conventional furnaces, heat is transferred from the external surface to the internal part of the sample to be activated. In other cases, the heat transfer can also occur via microwave heating [19,25]; heat is transferred from the internal parts of the particles (i.e., lignocellulosic and activating agent) towards their surface. More recently, hydrothermal treatments have been implemented for chemical activation, either for the carbonization (pyrolysis) step or for the direct activation [41,42,43,44]. In these conditions, water media (containing the activating agent dissolved if activation) acts as a “reactant”, favoring and accelerating the desired processes [41].
- Atmosphere. The heating occurs in presence of a controlled gaseous atmosphere, commonly flowing gas [11,25], which removes the evolved gaseous products (favoring the activation reaction [21]) and facilities the heat transmission to the solid sample. Different atmospheres, mainly nitrogen and steam, can be used [12,13,18,19,25,37,45], being the flow rate used an important parameter influencing the process.
| Precursor | Ultimate Analysis (wt %) | Lignocellulosic Composition (wt %) | Ash (wt %) | |||||
|---|---|---|---|---|---|---|---|---|
| C | H | O | N | Cellulose | Hemicellulose | Lignin | ||
| Almond shell | 49.5 | 6.3 | 44.0 | 0.2 | 32 | 26 | 25 | 2.2 |
| Coconut shell | 48.7 | 6.3 | 43.4 | 1.5 | 41 | 27 | 29 | 4.0 |
| Palm shell | 47.8 | 6.0 | 45.3 | 0.9 | 30 | 17 | 53 | 4.2 |
| Hazelnut shell | 47.0 | 6.5 | 46.0 | 1.0 | 25 | 28 | 42 | 1.4 |
| Peanut shell | 41.5 | 5.6 | 2.2 | 2.1 | 45 | 8 | 33 | 4.3 |
| Palm kernel shell | 43.6 | 4.9 | 51.6 | 0.5 | 30 | 21 | 47 | 2.4 |
| Peach stone | 45.9 | 6.1 | 47.4 | 0.6 | 46 | 14 | 33 | 1.5 |
| Olive stone | 45.0 | 5.8 | 48.3 | 0.2 | 32 | 33 | 30 | 2.1 |
| Date pits | 45.6 | 7.1 | 46.5 | 0.7 | 24 | 27 | 22 | 1.0 |
| Orange peel | 46.6 | 6.1 | 47.1 | 0.2 | 65 | 5 | 20 | 1.0 |
| Tomato waste | 59.0 | 8.2 | 29.8 | 0.3 | 33 | 24 | 35 | 1.6 |
| Tobaco stalk | 46.2 | 6.1 | 43.4 | 2.4 | 42 | 28 | 27 | 2.4 |
| Cotton stalk | 41.2 | 5.0 | 34.0 | 2.6 | 39 | 17 | 29 | 5.0 |
| Corn stalk | 45.5 | 6.2 | 41.1 | 0.8 | 23 | 43 | 16 | 7.5 |
| Corn cob | 46.3 | 5.6 | 42.2 | 0.6 | 43 | 37 | 15 | 3.5 |
| Olive tree pruning | 49.9 | 6.0 | 43.4 | 0.7 | 29 | 21 | 27 | 5.0 |
| Vineyward pruning | 47.6 | 5.6 | 41.1 | 1.8 | 38 | 34 | 27 | 3.5 |
| Peach tree pruning | 53.0 | 5.9 | 39.1 | 0.3 | 31 | 28 | 28 | 3.7 |
| Oats straw | 46.0 | 5.9 | 43.5 | 1.1 | 35 | 37 | 18 | 8.7 |
| Sunflower straw | 52.9 | 6.6 | 35.9 | 1.4 | 32 | 19 | 22 | 9.0 |
| Barley straw | 46.2 | 5.8 | 41.9 | 0.6 | 38 | 35 | 16 | 7.0 |
| Rice straw | 49.5 | 6.1 | 44.3 | 0.2 | 38 | 32 | 12 | 20.0 |
| Wheat straw | 42.7 | 5.6 | 39.7 | 0.3 | 33 | 20 | 15 | 3.7 |
2.2. Processes: Pyrolysis and Activation
2.2.1. Carbonization or Pyrolysis
2.2.2. Chemical Activation
2.3. AC Structure
3. Further Properties (Different from Porosity) of the ACs Prepared by Chemical Activation That Merit to Be Analyzed
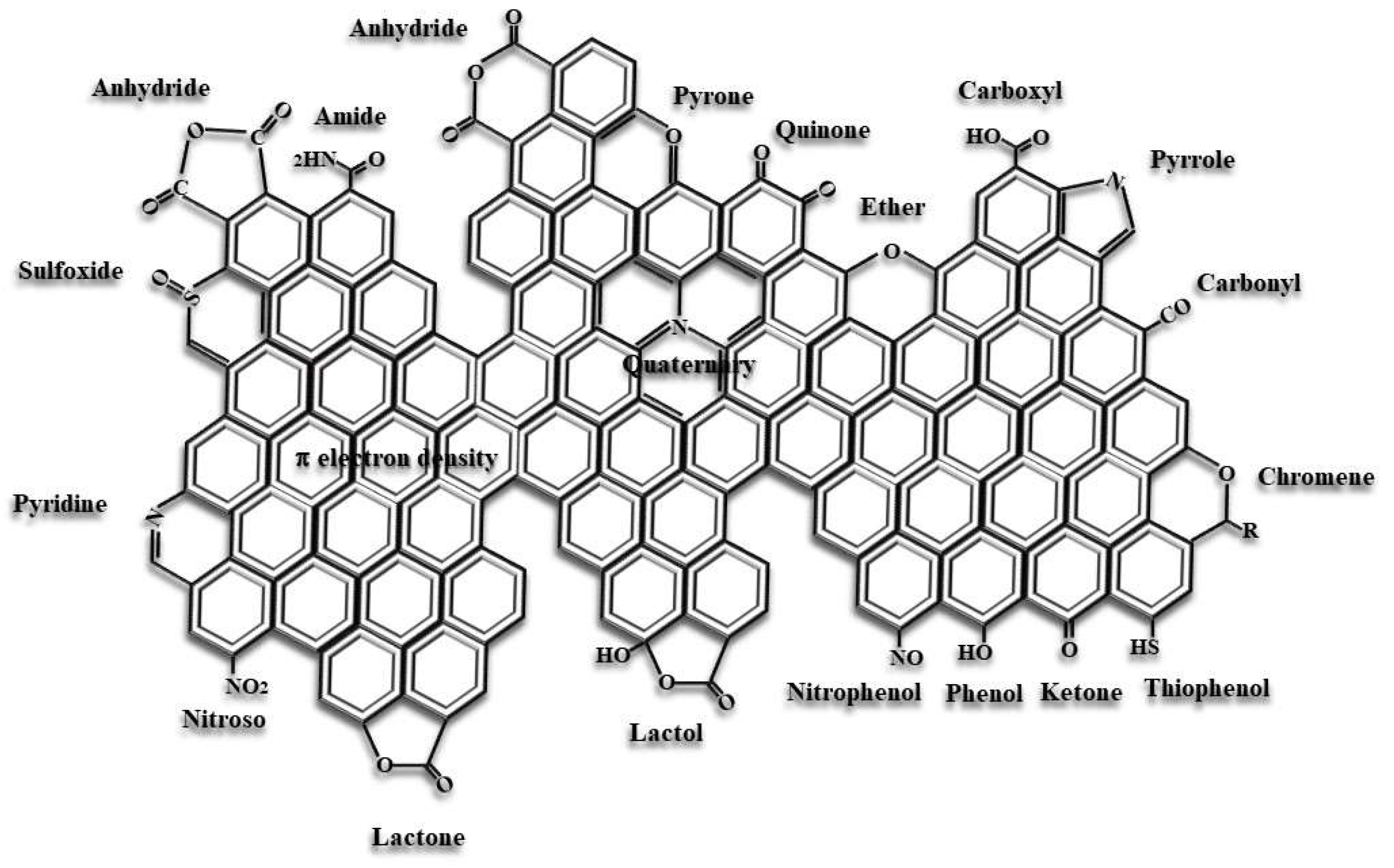
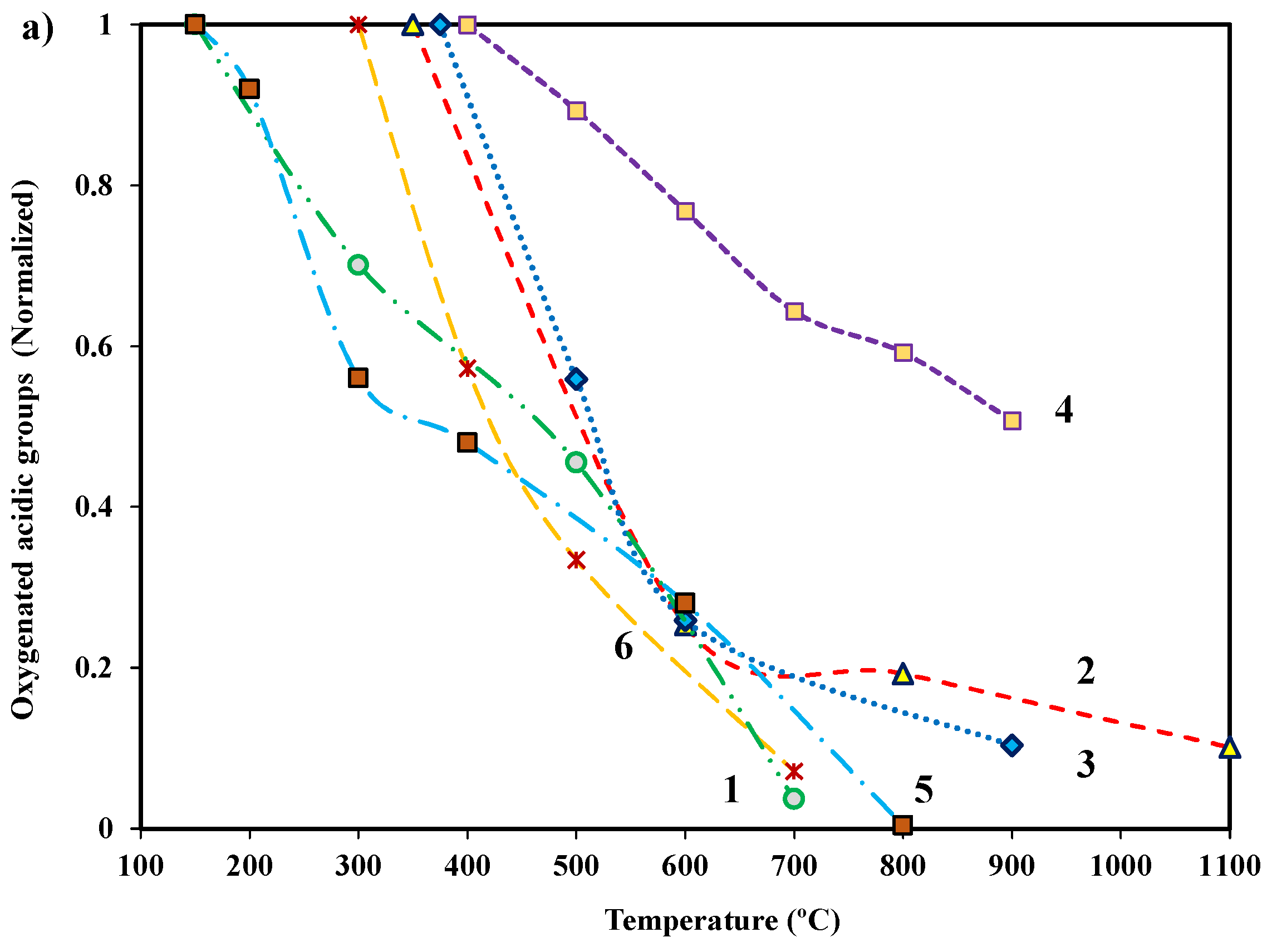
3.1. Surface Chemistry

3.2. Shape/Morphology
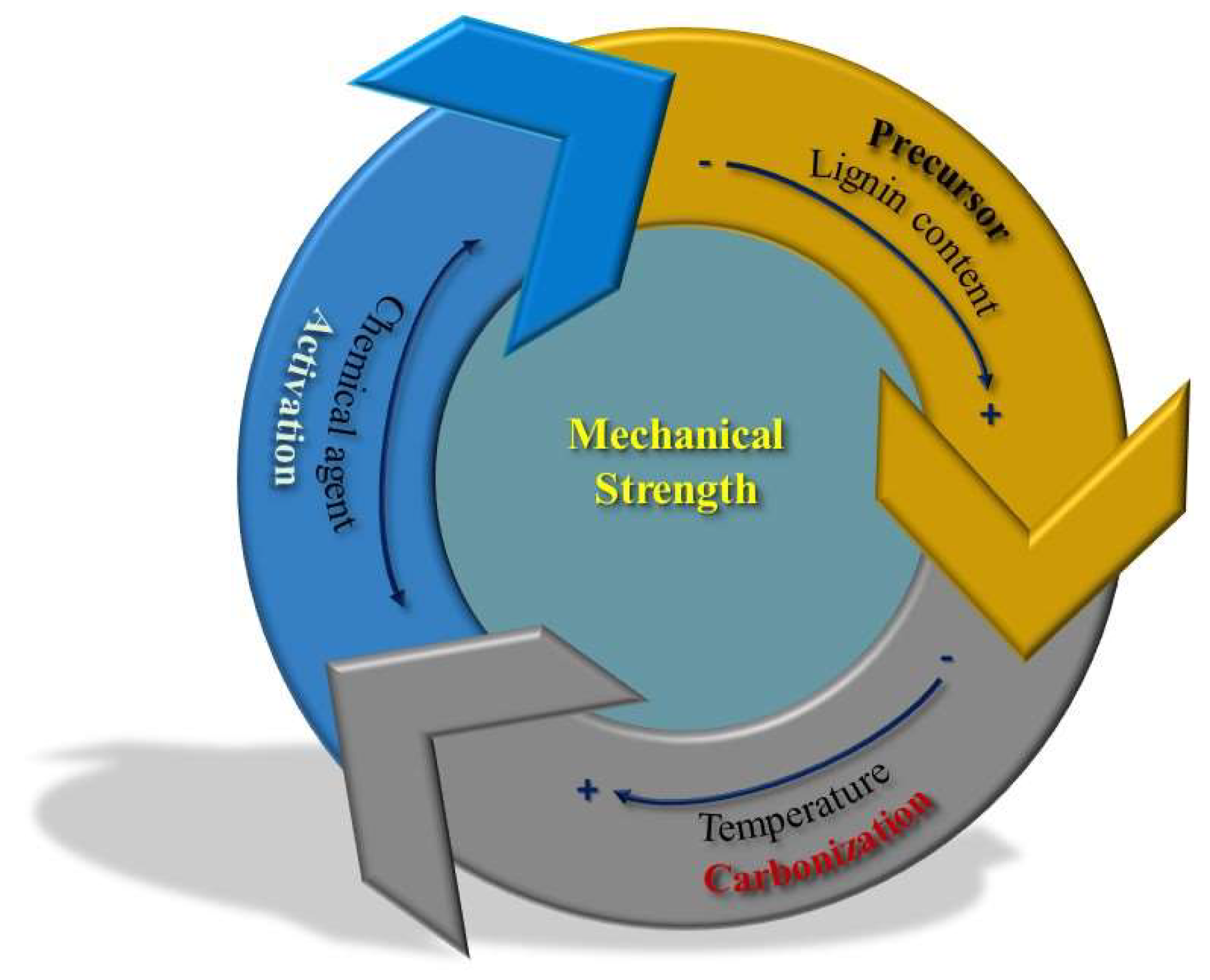
3.3. Mechanical Properties
4. Aspects to Be Taken into Account for the Large-Scale Application of Chemical Activation for the Production of ACs
4.1. Scaling up of the Published Procedures and Economic Issues
4.2. Environmental Concerns
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dumas, P.; Hanson, C.; Ranganathan, J.; Searchinger, T.; Waite, R. Creating a Sustainable Food Future; World Resources Institute: Washington, DC, USA, 2019; ISBN 9781569739631. [Google Scholar]
- UN General Assembly. United Nations Millennium Declaration, Resolution Adopted by the General Assembly. 18 September 2000. A/RES/55/2. Available online: https://www.refworld.org/docid/3b00f4ea3.html (accessed on 30 December 2021).
- Bansal, R.C.; Goyal, M. Activated Carbon Adsorption; CRC Press: Boca Ratón, FL, USA, 2005; ISBN 9781420028812. [Google Scholar]
- Bansal, R.C.; Donnet, J.B.; Stoeckli, F. Active Carbon; Marcel Dekker: New York, NY, USA, 1988; ISBN 9780824778422. [Google Scholar]
- Environmental Protection Agency. Federal Register/Rules and Regulations; No. 79; Environmental Protection Agency: Washington, DC, USA, 2013; Volume 78, pp. 24073–24094.
- The Freedonia Group. World Activated Carbon: Industry Study with Forecasts for 2016 & 2021; The Freedonia Group Inc.: Cleveland, OH, USA, 2012; Available online: https://www.freedoniagroup.com/brochure/28xx/2878smwe.pdf (accessed on 30 December 2021).
- The Business Research Company. Activated Carbon Global Market Report 2021: COVID-19 Impact And Recovery; The Business Research Company: London, UK, 2021. [Google Scholar]
- Ostrejko, R. Available online: https://speicyte.wixsite.com/raphael-von-ostrejko (accessed on 30 December 2021).
- Marsh, H.; Rodríguez-Reinoso, F. Activated Carbon; Elsevier Ltd.: Amsterdam, The Netherlands, 2006; ISBN 9780080444635. [Google Scholar]
- Linares-Solano, A.; Lozano-Castelló, D.; Lillo-Ródenas, M.A.; Cazorla-Amorós, D. Carbon activation by alkaline hydroxides preparation and reactions, porosity and performance. In Chemistry and Physics of Carbon; Radovic, L.R., Ed.; CRC Press: New York, NY, USA, 2008; Volume 30, pp. 1–62. ISBN 9780429143915. [Google Scholar]
- Molina-Sabio, M.; Rodríguez-Reinoso, F. Role of chemical activation in the development of carbon porosity. Colloids Surf. A Physicochem. Eng. Asp. 2004, 241, 15–25. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.W.Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Lozano-Castelló, D.; Cazorla-Amorós, D.; Linares-Solano, A. Preparation of activated carbons from Spanish anthracite-II. Activation by NaOH. Carbon N. Y. 2001, 39, 751–759. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Marco-Lozar, J.P.; Cazorla-Amorós, D.; Linares-Solano, A. Activated carbons prepared by pyrolysis of mixtures of carbon precursor/alkaline hydroxide. J. Anal. Appl. Pyrolysis 2007, 80, 166–174. [Google Scholar] [CrossRef]
- González, J.F.; Román, S.; Encinar, J.M.; Martínez, G. Pyrolysis of various biomass residues and char utilization for the production of activated carbons. J. Anal. Appl. Pyrolysis 2009, 85, 134–141. [Google Scholar] [CrossRef]
- Derbyshire, F.; Jagtoyen, M.; Thwaites, M. Porosity in Carbons: Characterization and Applications; Edward Arnold: London, UK, 1995; ISBN 9780470234549. [Google Scholar]
- Panwar, N.L.; Pawar, A. Influence of activation conditions on the physicochemical properties of activated biochar: A review. Biomass Convers. Biorefinery 2022, 12, 925–948. [Google Scholar] [CrossRef]
- Ukanwa, K.; Patchigolla, K.; Sakrabani, R.; Anthony, E.; Mandavgane, S. A Review of Chemicals to Produce Activated Carbon from Agricultural Waste Biomass. Sustainability 2019, 11, 6204. [Google Scholar] [CrossRef] [Green Version]
- Otowa, T.; Tanibata, R.; Itoh, M. Production and adsorption characteristics of MAXSORB: High-surface-area active carbon. Gas. Sep. Purif. 1993, 7, 241–245. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Understanding chemical reactions between carbons and NaOH and KOH: An insight into the chemical activation mechanism. Carbon N. Y. 2003, 41, 267–275. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Juan-Juan, J.; Cazorla-Amorós, D.; Linares-Solano, A. About reactions occurring during chemical activation with hydroxides. Carbon N. Y. 2004, 42, 1371–1375. [Google Scholar] [CrossRef]
- Jagtoyen, M.; Derbyshire, F. Activated carbons from yellow poplar and white oak by H3PO4 activation. Carbon N. Y. 1998, 36, 1085–1097. [Google Scholar] [CrossRef]
- Hu, Z.; Srinivasan, M.P.; Ni, Y. Novel activation process for preparing highly microporous and mesoporous activated carbons. Carbon N. Y. 2001, 39, 877–886. [Google Scholar] [CrossRef]
- González-García, P. Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications. Renew. Sustain. Energy Rev. 2018, 82, 1393–1414. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.C.M.; Almeida, M.F.; Rivera-Utrilla, J.; Sánchez-Polo, M. Waste materials for activated carbon preparation and its use in aqueous-phase treatment: A review. J. Environ. Manag. 2007, 85, 833–846. [Google Scholar] [CrossRef]
- Biochar; Ok, Y.S.; Uchimiya, S.M.; Chang, S.X.; Bolan, N. (Eds.) CRC Press: Boca Ratón, FL, USA; Miami, FL, USA, 2015; ISBN 9780429161070. [Google Scholar]
- Hadjittofi, L.; Prodromou, M.; Pashalidis, I. Activated biochar derived from cactus fibres—Preparation, characterization and application on Cu(II) removal from aqueous solutions. Bioresour. Technol. 2014, 159, 460–464. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Phuriragpitikhon, J.; Choma, J.; Jaroniec, M. Recent advances in the development and applications of biomass-derived carbons with uniform porosity. J. Mater. Chem. A 2020, 8, 18464–18491. [Google Scholar] [CrossRef]
- Gao, F.; Zang, Y.; Wang, Y.; Guan, C.; Qu, J.; Wu, M. A review of the synthesis of carbon materials for energy storage from biomass and coal/heavy oil waste. New Carbon Mater. 2021, 36, 34–48. [Google Scholar] [CrossRef]
- Thompson, M.; Xia, Q.; Hu, Z.; Zhao, X.S. A review on biomass-derived hard carbon materials for sodium-ion batteries. Mater. Adv. 2021, 2, 5881–5905. [Google Scholar] [CrossRef]
- Pramanik, P.; Patel, H.; Charola, S.; Neogi, S.; Maiti, S. High surface area porous carbon from cotton stalk agro-residue for CO2 adsorption and study of techno-economic viability of commercial production. J. CO2 Util. 2021, 45, 101450. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T. A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew. Sustain. Energy Rev. 2018, 87, 1–21. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B.; Gupta, V.; Sharma, P. Chemically activated carbon from lignocellulosic wastes for heavy metal wastewater remediation: Effect of activation conditions. J. Colloid Interface Sci. 2017, 493, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.S. Biomass-Derived Renewable Carbonaceous Materials for Sustainable Chemical and Environmental Applications. ACS Sustain. Chem. Eng. 2019, 7, 6458–6470. [Google Scholar] [CrossRef]
- Mironova, M.; Makarov, I.; Golova, L.; Vinogradov, M.; Shandryuk, G.; Levin, I. Improvement in Carbonization Efficiency of Cellulosic Fibres Using Silylated Acetylene and Alkoxysilanes. Fibers 2019, 7, 84. [Google Scholar] [CrossRef] [Green Version]
- Ioannidou, O.; Zabaniotou, A. Agricultural residues as precursors for activated carbon production—A review. Renew. Sustain. Energy Rev. 2007, 11, 1966–2005. [Google Scholar] [CrossRef]
- ASTM D388(2011); Classification of Coals by Rank. Annual Book of ASTM Standards, Section 05.05. American Society for Testing and Materials: West Conshohocken, PA, USA, 2011.
- Raymundo-Piñero, E.; Azaïs, P.; Cacciaguerra, T.; Cazorla-Amorós, D.; Linares-Solano, A.; Béguin, F. KOH and NaOH activation mechanisms of multiwalled carbon nanotubes with different structural organisation. Carbon N. Y. 2005, 43, 786–795. [Google Scholar] [CrossRef]
- González, J.F.; Ramiro, A.; González-García, C.M.; Gañán, J.; Encinar, J.M.; Sabio, E.; Rubiales, J. Pyrolysis of Almond Shells. Energy Applications of Fractions. Ind. Eng. Chem. Res. 2005, 44, 3003–3012. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.H.; Antonietti, M.; Titirici, M.M. Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef]
- Titirici, M.M.; White, R.J.; Falco, C.; Sevilla, M. Black perspectives for a green future: Hydrothermal carbons for environment protection and energy storage. Energy Environ. Sci. 2012, 5, 6796–6822. [Google Scholar] [CrossRef]
- Quesada-Plata, F.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amorós, D. Activated Carbons Prepared through H3PO4-Assisted Hydrothermal Carbonisation from Biomass Wastes: Porous Texture and Electrochemical Performance. Chempluschem 2016, 81, 1349–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Castilla, C.; Carrasco-Marín, F.; Maldonado-Hódar, F.J.; Rivera-Utrilla, J. Effects of non-oxidant and oxidant acid treatments on the surface properties of an activated carbon with very low ash content. Carbon N. Y. 1998, 36, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.R.; Mohammadi, M.; Darzi, G.N. Preparation of carbon molecular sieve from lignocellulosic biomass: A review. Renew. Sustain. Energy Rev. 2010, 14, 1591–1599. [Google Scholar] [CrossRef]
- Devi, M.; Rawat, S.; Sharma, S. A comprehensive review of the pyrolysis process: From carbon nanomaterial synthesis to waste treatment. Oxford Open Mater. Sci. 2020, 1, itab014. [Google Scholar] [CrossRef]
- Fitzer, E.; Kochling, K.-H.; Boehm, H.P.; Marsh, H. Recommended terminology for the description of carbon as a solid (IUPAC Recommendations 1995). Pure Appl. Chem. 1995, 67, 473–506. [Google Scholar] [CrossRef]
- Walker, P.L. Carbon: An old but new material revisited. Carbon N. Y. 1990, 28, 261–279. [Google Scholar] [CrossRef]
- Mackay, D.M.; Roberts, P.V. The dependence of char and carbon yield on lignocellulosic precursor composition. Carbon N. Y. 1982, 20, 87–94. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood—Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany; New York, NY, USA, 1989; ISBN 9783110120593. [Google Scholar]
- Higuchi, T. Lignin biochemistry: Biosynthesis and biodegradation. Wood Sci. Technol. 1990, 24, 23–63. [Google Scholar] [CrossRef]
- Lv, G.; Wu, S.; Yang, G.; Chen, J.; Liu, Y.; Kong, F. Comparative study of pyrolysis behaviors of corn stalk and its three components. J. Anal. Appl. Pyrolysis 2013, 104, 185–193. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S.; Bridgwater, A.V. The thermal performance of the polysaccharides extracted from hardwood: Cellulose and hemicellulose. Carbohydr. Polym. 2010, 82, 39–45. [Google Scholar] [CrossRef]
- Lou, R.; Wu, S. Products properties from fast pyrolysis of enzymatic/mild acidolysis lignin. Appl. Energy 2011, 88, 316–322. [Google Scholar] [CrossRef]
- Oberlin, A. Carbonization and graphitization. Carbon N. Y. 1984, 22, 521–541. [Google Scholar] [CrossRef]
- Lewis, L.C.; Singer, L.S. Further electron spin resonance studies of the pyrolysis of aromatic hydrocarbons. Carbon N. Y. 1967, 5, 373–381. [Google Scholar] [CrossRef]
- Brooks, J.D.; Taylor, G.H. The formation of graphitizing carbons from the liquid phase. Carbon N. Y. 1965, 3, 187–193. [Google Scholar] [CrossRef]
- Martínez De Yuso, A.; Rubio, B.; Izquierdo, M.T. Influence of activation atmosphere used in the chemical activation of almond shell on the characteristics and adsorption performance of activated carbons. Fuel Process. Technol. 2014, 119, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Puziy, A.M.; Poddubnaya, O.I.; Martínez-Alonso, A.; Castro-Muñiz, A.; Suárez-García, F.; Tascón, J.M.D. Oxygen and phosphorus enriched carbons from lignocellulosic material. Carbon N. Y. 2007, 45, 1941–1950. [Google Scholar] [CrossRef]
- Benaddi, H.; Legras, D.; Rouzaud, J.N.; Beguin, F. Influence of the atmosphere in the chemical activation of wood by phosphoric acid. Carbon N. Y. 1998, 36, 1941–1950. [Google Scholar] [CrossRef]
- Gao, Y.; Yue, Q.; Gao, B.; Li, A. Insight into activated carbon from different kinds of chemical activating agents: A review. Sci. Total Environ. 2020, 746, 141094. [Google Scholar] [CrossRef]
- Reza, M.S.; Yun, C.S.; Afroze, S.; Radenahmad, N.; Bakar, M.S.A.; Saidur, R.; Taweekun, J.; Azad, A.K. Preparation of activated carbon from biomass and its’ applications in water and gas purification, a review. Arab J. Basic Appl. Sci. 2020, 27, 208–238. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosiy; Academic Press: London, UK, 1982. [Google Scholar]
- Alcañiz-Monge, J.; Cazorla-Amorós, D.; Linares-Solano, A. Production of activated carbons: Use of CO2 versus H2O as activating agent. A reply to a letter from P. L. Walker Jr. Carbon N. Y. 1997, 35, 1665–1668. [Google Scholar] [CrossRef]
- Alcañiz-Monge, J.; Illán-Gómez, M.J. Insight into hydroxides-activated coals: Chemical or physical activation? J. Colloid Interface Sci. 2008, 318, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Palmer, J.C.; Gubbins, K.E. Atomistic models for disordered nanoporous carbons using reactive force fields. Microporous Mesoporous Mater. 2012, 154, 24–37. [Google Scholar] [CrossRef]
- Porosity in Carbons; Patrick, J.W. (Ed.) Wiley: New York, NY, USA, 1995; ISBN 9780470234549. [Google Scholar]
- Harris, P.J.F.; Liu, Z.; Suenaga, K. Imaging the atomic structure of activated carbon. J. Phys. Condens. Matter 2008, 20, 362201. [Google Scholar] [CrossRef]
- Terzyk, A.P.; Furmaniak, S.; Gauden, P.A.; Harris, P.J.F.; Włoch, J.; Kowalczyk, P. Hyper-parallel tempering Monte Carlo simulations of Ar adsorption in new models of microporous non-graphitizing activated carbon: Effect of microporosity. J. Phys. Condens. Matter 2007, 19, 406208. [Google Scholar] [CrossRef] [PubMed]
- Rideal, E.K.; Wright, W.M. CLXXXIV.—Low temperature oxidation at charcoal surfaces. Part, I. the behaviour of charcoal in the absence of promoters. J. Chem. Soc. Trans. 1925, 127, 1347–1357. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Ania, C.O. Chapter 4 Surface chemistry of activated carbons and its characterization. Interface Sci. Technol. 2006, 7, 159–229. [Google Scholar] [CrossRef]
- Boehm, H.P. Chemical Identification of Surface Groups. Adv. Catal. 1966, 16, 179–274. [Google Scholar] [CrossRef]
- ASTM D3838-80(2011); Standard Test Method for pH of Activated Carbon. Annual Book of ASTM Standards, Section 15.01. American Society for Testing and Materials: West Conshohocken, PA, USA, 2011.
- Corapcioglu, M.O.; Huang, C.P. The surface acidity and characterization of some commercial activated carbons. Carbon N. Y. 1987, 25, 569–578. [Google Scholar] [CrossRef]
- Fanning, P.E.; Vannice, M.A. A DRIFTS study of the formation of surface groups on carbon by oxidation. Carbon N. Y. 1993, 31, 721–730. [Google Scholar] [CrossRef]
- Kapteijn, F.; Moulijn, J.A.; Matzner, S.; Boehm, H.-P. The development of nitrogen functionality in model chars during gasification in CO2 and O2. Carbon N. Y. 1999, 37, 1143–1150. [Google Scholar] [CrossRef]
- Kinoshita, K. Carbon: Electrochemical and Physicochemical Properties; Wiley-Interscience Publication: New York, NY, USA, 1988; ISBN 978-0-471-84802-8. [Google Scholar]
- Otake, Y.; Jenkins, R.G. Characterization of oxygen-containing surface complexes created on a microporous carbon by air and nitric acid treatment. Carbon N. Y. 1993, 31, 109–121. [Google Scholar] [CrossRef]
- Lopez-Ramon, M.V.; Stoeckli, F.; Moreno-Castilla, C.; Carrasco-Marin, F. On the characterization of acidic and basic surface sites on carbons by various techniques. Carbon N. Y. 1999, 37, 1215–1221. [Google Scholar] [CrossRef]
- Hennig, G.R. Electron Microscopy of Reactivity Changes near Lattice Defects in Graphite. In Chemistry and Physics of Carbons; Walker, P.L., Jr., Ed.; Marcel Dekker: New York, NY, USA, 1966; Volume 2, pp. 1–49. [Google Scholar]
- Radović, L.R.; Walker, P.L.; Jenkins, R.G. Importance of carbon active sites in the gasification of coal chars. Fuel 1983, 62, 849–856. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Leon y Leon, C.A.; Radovic, L.R. Interfacial Chemistry and Electrochemistry of Carbon Surfaces. In Chemistry and Physics of Carbon; Thrower, P.A., Ed.; Dekker: New York, NY, USA, 1994; Volume 24, pp. 213–310. [Google Scholar]
- Puziy, A.M.; Poddubnaya, O.I.; Martínez-Alonso, A.; Suárez-García, F.; Tascón, J.M.D. Synthetic carbons activated with phosphoric—Acid, I. Surface chemistry and ion binding properties. Carbon N. Y. 2002, 40, 1493–1505. [Google Scholar] [CrossRef]
- Elmouwahidi, A.; Castelo-Quibén, J.; Vivo-Vilches, J.F.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J.; Carrasco-Marín, F. Activated carbons from agricultural waste solvothermally doped with sulphur as electrodes for supercapacitors. Chem. Eng. J. 2018, 334, 1835–1841. [Google Scholar] [CrossRef]
- Guo, J.; Lua, A.C. Surface functional groups on oil-palm-shell adsorbents prepared by H3PO4 and KOH activation and their effects on adsorptive capacity. Chem. Eng. Res. Des. 2003, 81, 585–590. [Google Scholar] [CrossRef]
- Danish, M.; Hashim, R.; Ibrahim, M.N.M.; Sulaiman, O. Effect of acidic activating agents on surface area and surface functional groups of activated carbons produced from Acacia mangium wood. J. Anal. Appl. Pyrolysis 2013, 104, 418–425. [Google Scholar] [CrossRef]
- Bansal, R.C.; Dhami, T.L.; Parkash, S. Surface characteristics and surface behavior of polymer carbons—I: Associated oxygen and hydrogen. Carbon N. Y. 1977, 15, 157–160. [Google Scholar] [CrossRef]
- Arslanoğlu, H. Direct and facile synthesis of highly porous low cost carbon from potassium-rich wine stone and their application for high-performance removal. J. Hazard. Mater. 2019, 374, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Puri, B.R. Surface Complexes on Carbons. In Chemistry and. Physics of Carbons; Walker, P.L., Jr., Ed.; Marcel Dekker: New York, NY, USA, 1970; Volume 6, pp. 191–282. [Google Scholar]
- Leon y Leon, C.A.; Solar, J.M.; Calemma, V.; Radovic, L.R. Evidence for the protonation of basal plane sites on carbon. Carbon N. Y. 1992, 30, 797–811. [Google Scholar] [CrossRef]
- Moreno-Castilla, C. Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon N. Y. 2004, 42, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Nowrouzi, M.; Behin, J.; Younesi, H.; Bahramifar, N.; Charpentier, P.A.; Rohani, S. An enhanced counter-current approach towards activated carbon from waste tissue with zero liquid discharge. Chem. Eng. J. 2017, 326, 934–944. [Google Scholar] [CrossRef]
- Vivo-Vilches, J.F.; Bailón-García, E.; Pérez-Cadenas, A.F.; Carrasco-Marín, F.; Maldonado-Hódar, F.J. Tailoring the surface chemistry and porosity of activated carbons: Evidence of reorganization and mobility of oxygenated surface groups. Carbon N. Y. 2014, 68, 520–530. [Google Scholar] [CrossRef]
- Gonzalez-Serrano, E.; Cordero, T.; Rodriguez-Mirasol, J.; Cotoruelo, L.; Rodriguez, J.J. Removal of water pollutants with activated carbons prepared from H3PO4 activation of lignin from kraft black liquors. Water Res. 2004, 38, 3043–3050. [Google Scholar] [CrossRef]
- Figueiredo, J.; Pereira, M.F.; Freitas, M.M.; Órfão, J.J. Modification of the surface chemistry of activated carbons. Carbon N. Y. 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Li, L.; Yao, X.; Li, H.; Liu, Z.; Ma, W.; Liang, X. Thermal Stability of Oxygen-Containing Functional Groups on Activated Carbon Surfaces in a Thermal Oxidative Environment. J. Chem. Eng. Jpn. 2014, 47, 21–27. [Google Scholar] [CrossRef]
- Mahajan, O.P.; Moreno-castilla, C.; Walker, P.L. Surface-Treated Activated Carbon for Removal of Phenol from Water. Sep. Sci. Technol. 1980, 15, 1733–1752. [Google Scholar] [CrossRef]
- Puri, B.; Singh, D.D.; Nath, J.; Sharma, L. Chemisorption of Oxygen on Activated Charcoal and Sorption of Acids and Bases. Ind. Eng. Chem. 1958, 50, 1071–1074. [Google Scholar] [CrossRef]
- Kyotani, T.; Zhang, Z.G.; Hayashi, S.; Tomita, A. TPD Study on H2O-Gasified and O2-Chemisorbed Coal Chars. Energy Fuels 1988, 2, 136–141. [Google Scholar] [CrossRef]
- Takaesu, H.; Matsui, Y.; Nishimura, Y.; Matsushita, T.; Shirasaki, N. Micro-milling super-fine powdered activated carbon decreases adsorption capacity by introducing oxygen/hydrogen-containing functional groups on carbon surface from water. Water Res. 2019, 155, 66–75. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Bouafif, H.; Neculita, C.M.; Koubaa, A. Influence of Pyro-Gasification and Activation Conditions on the Porosity of Activated Biochars: A Literature Review. Waste Biomass Valorization 2020, 11, 5079–5098. [Google Scholar] [CrossRef]
- Mariana, M.; Abdul, A.K.; Mistar, E.M.; Yahya, E.B.; Alfatah, T.; Danish, M.; Amayreh, M. Recent advances in activated carbon modification techniques for enhanced heavy metal adsorption. J. Water Process. Eng. 2021, 43, 102221. [Google Scholar] [CrossRef]
- Pradhan, B.K.; Sandle, N.K. Effect of different oxidizing agent treatments on the surface properties of activated carbons. Carbon N. Y. 1999, 37, 1323–1332. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Gómez-Serrano, V.; Álvarez, P.M.; Alvim-Ferraz, M.C.M.; Dias, J.M. Activated carbon modifications to enhance its water treatment applications. An overview. J. Hazard. Mater. 2011, 187, 1–23. [Google Scholar] [CrossRef]
- Demiral, İ.; Samdan, C.; Demiral, H. Enrichment of the surface functional groups of activated carbon by modification method. Surf. Interfaces 2021, 22, 100873. [Google Scholar] [CrossRef]
- Stöhr, B.; Boehm, H.P.; Schlögl, R. Enhancement of the catalytic activity of activated carbons in oxidation reactions by thermal treatment with ammonia or hydrogen cyanide and observation of a superoxide species as a possible intermediate. Carbon N. Y. 1991, 29, 707–720. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Chin, H.C. Preparation and characterization of carbon-sulfur surface compounds. Carbon N. Y. 1981, 19, 175–186. [Google Scholar] [CrossRef]
- Xia, Y.; Zhu, Y.; Tang, Y. Preparation of sulfur-doped microporous carbons for the storage of hydrogen and carbon dioxide. Carbon N. Y. 2012, 50, 5543–5553. [Google Scholar] [CrossRef]
- El-Shafey, E.I.; Ali, S.N.F.; Al-Busafi, S.; Al-Lawati, H.A.J. Preparation and characterization of surface functionalized activated carbons from date palm leaflets and application for methylene blue removal. J. Environ. Chem. Eng. 2016, 4, 2713–2724. [Google Scholar] [CrossRef]
- Lv, D.; Liu, Y.; Zhou, J.; Yang, K.; Lou, Z.; Baig, S.A.; Xu, X. Application of EDTA-functionalized bamboo activated carbon (BAC) for Pb(II) and Cu(II) removal from aqueous solutions. Appl. Surf. Sci. 2018, 428, 648–658. [Google Scholar] [CrossRef]
- Xie, X.; Gao, H.; Luo, X.; Su, T.; Zhang, Y.; Qin, Z. Polyethyleneimine modified activated carbon for adsorption of Cd(II) in aqueous solution. J. Environ. Chem. Eng. 2019, 7, 103183. [Google Scholar] [CrossRef]
- Boudou, J.P.; Martinez-Alonzo, A.; Tascon, J.M.D. Introduction of acidic groups at the surface of activated carbon by microwave-induced oxygen plasma at low pressure. Carbon N. Y. 2000, 38, 1021–1029. [Google Scholar] [CrossRef]
- Bagheri, M.; Jafari, S.M.; Eikani, M.H. Ultrasonic-assisted production of zero-valent iron-decorated graphene oxide/activated carbon nanocomposites: Chemical transformation and structural evolution. Mater. Sci. Eng. C 2021, 118, 111362. [Google Scholar] [CrossRef]
- Fu, Y.; Ding, X.; Zhao, J.; Zheng, Z. Study on the effect of oxidation-ultrasound treatment on the electrochemical properties of activated carbon materials. Ultrason. Sonochem. 2020, 69, 104921. [Google Scholar] [CrossRef]
- Liu, Y.; Sajjadi, B.; Chen, W.Y.; Chatterjee, R. Ultrasound-assisted amine functionalized graphene oxide for enhanced CO2 adsorption. Fuel 2019, 247, 10–18. [Google Scholar] [CrossRef]
- Yi, H.; Zhao, S.; Tang, X.; Song, C.; Gao, F.; Zhang, B.; Wang, Z.; Zuo, Y. Low-temperature hydrolysis of carbon disulfide using the FeCu/AC catalyst modified by non-thermal plasma. Fuel 2014, 128, 268–273. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, P.; Qiu, Y.; Yu, Q.; Ma, J.; Wu, H.; Luo, G.; Xu, M.; Yao, H. Increasing oxygen functional groups of activated carbon with non-thermal plasma to enhance mercury removal efficiency for flue gases. Chem. Eng. J. 2015, 263, 1–8. [Google Scholar] [CrossRef]
- Kodama, S.; Sekiguchi, H. Estimation of point of zero charge for activated carbon treated with atmospheric pressure non-thermal oxygen plasmas. Thin Solid Films 2006, 506–507, 327–330. [Google Scholar] [CrossRef]
- Inagaki, N.; Narushima, K.; Hashimoto, H.; Tamura, K. Implantation of amino functionality into amorphous carbon sheet surfaces by NH3 plasma. Carbon N. Y. 2007, 45, 797–804. [Google Scholar] [CrossRef]
- Li, K.; Liu, G.; Wang, C.; Li, K.; Sun, X.; Song, X.; Ning, P. Acidic and basic groups introducing on the surface of activated carbon during the plasma-surface modification for changing of COS catalytic hydrolysis activity. Catal. Commun. 2020, 144, 106093. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon N. Y. 2009, 47, 2281–2289. [Google Scholar] [CrossRef] [Green Version]
- Tran, H.N.; You, S.J.; Chao, H.P. Fast and efficient adsorption of methylene green 5 on activated carbon prepared from new chemical activation method. J. Environ. Manag. 2017, 188, 322–336. [Google Scholar] [CrossRef]
- Carbon Materials for Advanced Technologies; Burchell., T.D. (Ed.) Pergamon: Amsterdam, The Netherlands, 1999; ISBN 978-0080426839. [Google Scholar]
- Wang, Q.; Li, H.; Chen, L.; Huang, X. Monodispersed hard carbon spherules with uniform nanopores. Carbon N. Y. 2001, 39, 2211–2214. [Google Scholar] [CrossRef]
- Ryu, J.; Suh, Y.W.; Suh, D.J.; Ahn, D.J. Hydrothermal preparation of carbon microspheres from mono-saccharides and phenolic compounds. Carbon N. Y. 2010, 48, 1990–1998. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, C.-Y.; Chen, M.-M.; Sun, J.-H. Mechanism for the preparation of carbon spheres from potato starch treated by NH4Cl. Carbon N. Y. 2009, 47, 331–333. [Google Scholar] [CrossRef]
- Alcañiz-Monge, J.; Cazorla-Amorós, D.; Linares-Solano, A.; Yoshida, S.; Oya, A. Effect of the activating gas on tensile strength and pore structure of pitch-based carbon fibres. Carbon N. Y. 1994, 32, 1277–1283. [Google Scholar] [CrossRef]
- Cao, L.; Kruk, M. A family of ordered mesoporous carbons derived from mesophase pitch using ordered mesoporous silicas as templates. Adsorption 2010, 16, 465–472. [Google Scholar] [CrossRef]
- Rosas, J.M.; Bedia, J.; Rodríguez-Mirasol, J.; Cordero, T. Preparation of Hemp-Derived Activated Carbon Monoliths. Adsorption of Water Vapor. Ind. Eng. Chem. Res. 2008, 47, 1288–1296. [Google Scholar] [CrossRef]
- Chiang, P.C.; Wu, J.S. Evaluation of chemical and thermal regeneration of activated carbon. Water Sci. Technol. 1989, 21, 1697–1700. [Google Scholar] [CrossRef]
- Larasati, A.; Fowler, G.D.; Graham, N.J.D. Chemical regeneration of granular activated carbon: Preliminary evaluation of alternative regenerant solutions. Environ. Sci. Water Res. Technol. 2020, 6, 2043–2056. [Google Scholar] [CrossRef]
- Cevallos Toledo, R.B.; Aragón-Tobar, C.F.; Gámez, S.; de la Torre, E. Reactivation process of activated carbons: Effect on the mechanical and adsorptive properties. Molecules 2020, 25, 1681. [Google Scholar] [CrossRef] [Green Version]
- Yamada, T.; Tsumuki, K. Adsorbent Body Including Activated Carbon and Inorganic Binder for Bonding Together Activated Carbon Grains, and Method of Producing the Adsorbent Body. U.S. Patent 5,685,986, 11 November 1997. [Google Scholar]
- Guymont, F.J. The effect of capital and operating costs on GAC adsorption system design. In Activated Carbon Adsorption of Organics from the Aqueous Phase; Mcguire, M.J., Suffet, I.H., Eds.; Ann Arbor Science: Ann Arbor, MI, USA, 1980; pp. 531–538. ISBN 978-0250402694. [Google Scholar]
- Hernández, A.M.; Labady, M.; Laine, J. Granular Activated Carbon from Wood Originated from Tropical Virgin Forest. Open, J. For. 2014, 4, 44658. [Google Scholar] [CrossRef] [Green Version]
- Heschel, W.; Klose, E. On the suitability of agricultural by-products for the manufacture of granular activated carbon. Fuel 1995, 74, 1786–1791. [Google Scholar] [CrossRef]
- Abe, I.; Fukuhara, T.; Iwasaki, S.; Yasuda, K.; Nakagawa, K.; Iwata, Y.; Kominami, H.; Kera, Y. Development of a high density carbonaceous adsorbent from compressed wood. Carbon N. Y. 2001, 39, 1485–1490. [Google Scholar] [CrossRef]
- Amorós-Pérez, A.; Cano-Casanova, L.; Ouzzine, M.; Rufete-Beneite, M.; Romero-Anaya, A.J.; Lillo-Ródenas, M.Á.; Linares-Solano, Á. Spherical activated carbons with high mechanical strength directly prepared from selected spherical seeds. Materials 2018, 11, 770. [Google Scholar] [CrossRef] [Green Version]
- Plaza-Recobert, M.; Trautwein, G.; Pérez-Cadenas, M.; Alcañiz-Monge, J. Preparation of binderless activated carbon monoliths from cocoa bean husk. Microporous Mesoporous Mater. 2017, 243, 28–38. [Google Scholar] [CrossRef]
- Ravichandran, P.; Sugumaran, P.; Seshadri, S.; Basta, A.H. Optimizing the route for production of activated carbon from Casuarina equisetifolia fruit waste. R. Soc. Open Sci. 2018, 5, 171578. [Google Scholar] [CrossRef] [Green Version]
- Khadiran, T.; Hussein, M.Z.; Zainal, Z.; Rusli, R. Textural and chemical properties of activated carbon prepared from tropical peat soil by chemical activation method. BioResources 2015, 10, 986–1007. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, Y.; Molina-Sabio, M.; Rodríguez-Reinoso, F. Modification of the porous structure along the preparation of activated carbon monoliths with H3PO4 and ZnCl2. Microporous Mesoporous Mater. 2007, 103, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Qiu, G.; Guo, M. Quality of poultry litter-derived granular activated carbon. Bioresour. Technol. 2010, 101, 379–386. [Google Scholar] [CrossRef]
- Ahmedna, M.; Marshall, W.; Rao, R. Production of granular activated carbons from select agricultural by-products and evaluation of their physical, chemical and adsorption properties1Louisiana Agricultural Experiment Station manuscript 99-21-0066.1. Bioresour. Technol. 2000, 71, 113–123. [Google Scholar] [CrossRef]
- Lozano-Castelló, D.; Cazorla-Amorós, D.; Linares-Solano, A.; Quinn, D.F. Activated carbon monoliths for methane storage: Influence of binder. Carbon N. Y. 2002, 40, 2817–2825. [Google Scholar] [CrossRef]
- Ubago-Pérez, R.; Carrasco-Marín, F.; Fairén-Jiménez, D.; Moreno-Castilla, C. Granular and monolithic activated carbons from KOH-activation of olive stones. Microporous Mesoporous Mater. 2006, 92, 64–70. [Google Scholar] [CrossRef]
- Dolah, B.N.M.; Deraman, M.; Othman, M.A.R.; Farma, R.; Taer, E.; Awitdrus; Basri, N.H.; Talib, I.A.; Omar, R.; Nor, N.S.M. A method to produce binderless supercapacitor electrode monoliths from biomass carbon and carbon nanotubes. Mater. Res. Bull. 2014, 60, 10–19. [Google Scholar] [CrossRef]
- Fortune Business Insights. Activated Carbon Market Size, Share & COVID19 Impact Analysis by Type (Powdered, Granular and Others), by Application (Water Treatment, Air & Gas Purification, Food and Beverage, Others) and Regional Forecast, 2020–2028. Report ID FBI102175. 2021, p. 300. Available online: https://www.fortunebusinessinsights.com/activated-carbon-market-102175 (accessed on 30 December 2021).
- Stavropoulos, G.G.; Zabaniotou, A.A. Minimizing activated carbons production cost. Fuel Process. Technol. 2009, 90, 952–957. [Google Scholar] [CrossRef]
- Asadi-Sangachini, Z.; Galangash, M.M.; Younesi, H.; Nowrouzi, M. The feasibility of cost-effective manufacturing activated carbon derived from walnut shells for large-scale CO2 capture. Environ. Sci. Pollut. Res. 2019, 26, 26542–26552. [Google Scholar] [CrossRef]
- Namazi, A.B.; Allen, D.G.; Jia, C.Q. Effect of chemical components in white liquor on carbon activation. Can. J. Chem. Eng. 2015, 93, 1705–1712. [Google Scholar] [CrossRef]
- Yuan, M.; Kim, Y.; Jia, C.Q. Feasibility of recycling KOH in chemical activation of oil-sands petroleum coke. Can. J. Chem. Eng. 2012, 90, 1472–1478. [Google Scholar] [CrossRef]
- Głowniak, S.; Szczęśniak, B.; Choma, J.; Jaroniec, M. Highly Porous Carbons Synthesized from Tannic Acid via a Combined Mechanochemical Salt-Templating and Mild Activation Strategy. Molecules 2021, 26, 1826. [Google Scholar] [CrossRef] [PubMed]


| Wastes from the Agri-Food Industry | |
| Fruit pits | Olive, avocado, apricot, cherry, plum, date, mango, peach |
| Nut shells | Almond, hazelnut, coconut, walnut, pistachio |
| Soft shells | Avocado, pomegranate, orange, banana, yucca, corn, watermelon |
| Seeds | Orange, guava, palm, rapeseed |
| Seed husk | Rice, wheat, oat, peanut, coffee, cocoa |
| Processing paste | Flaxseed, vinegar must, apple pulp, oil, coffee |
| Fibers | Coconut, palm, banana, jute |
| Wastes from the Agricultural and Wood Sector | |
| Stems and leaves for pruning and harvesting | Cereal straw (wheat), sunflower, cotton, hemp, esparto, bamboo, cane bagasse, corn, tobacco, vine, kenaf, jute, tea |
| Wood | Tree bark and/or sawdust (eucalyptus, fir, pine, holm oak, olive, acacia, palm …) |
| Industrial and Municipal Wastes | |
| Waste materials from organic compounds | Plastics (PVC, PET), tires, paper, cardboard, wastes from the pulp industry, from the pickling of skins, textile industry. |
| Inorganic wastes | Sewage sludge, steel industry sludge. |
| Fossil Fuels and their Wastes | |
| Coal | Peat, lignite, subituminous, anthracite, fly ash, coal tar |
| Petroleum/oil | Pitch, coke |
| Precursor | Ultimate Analysis (% w/w), dmmf * Basis | ||||
|---|---|---|---|---|---|
| C | H | O | N | S | |
| Peat | 50–60 | 6.0–6.5 | 30–35 | 1.5 | 1.0 |
| Lignite | 65–70 | 5.0–5.5 | 22–26 | 1.0 | 1.5 |
| Subbituminous | 70–76 | 5.0 | 15–22 | 1.0 | 3.0 |
| Bituminous | 76–87 | 4.0–5.0 | 10–15 | 2.0 | 4.0 |
| Anthracite | 90–95 | 2.0–3.0 | 1–3 | 1.0 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcañiz-Monge, J.; Román-Martínez, M.d.C.; Lillo-Ródenas, M.Á. Chemical Activation of Lignocellulosic Precursors and Residues: What Else to Consider? Molecules 2022, 27, 1630. https://doi.org/10.3390/molecules27051630
Alcañiz-Monge J, Román-Martínez MdC, Lillo-Ródenas MÁ. Chemical Activation of Lignocellulosic Precursors and Residues: What Else to Consider? Molecules. 2022; 27(5):1630. https://doi.org/10.3390/molecules27051630
Chicago/Turabian StyleAlcañiz-Monge, Juan, María del Carmen Román-Martínez, and María Ángeles Lillo-Ródenas. 2022. "Chemical Activation of Lignocellulosic Precursors and Residues: What Else to Consider?" Molecules 27, no. 5: 1630. https://doi.org/10.3390/molecules27051630
APA StyleAlcañiz-Monge, J., Román-Martínez, M. d. C., & Lillo-Ródenas, M. Á. (2022). Chemical Activation of Lignocellulosic Precursors and Residues: What Else to Consider? Molecules, 27(5), 1630. https://doi.org/10.3390/molecules27051630