Investigation of Structural and Optical Properties of Some [1,4]Dithiine-porphyrazine Dyes
Abstract
1. Introduction
2. Results and Discussion
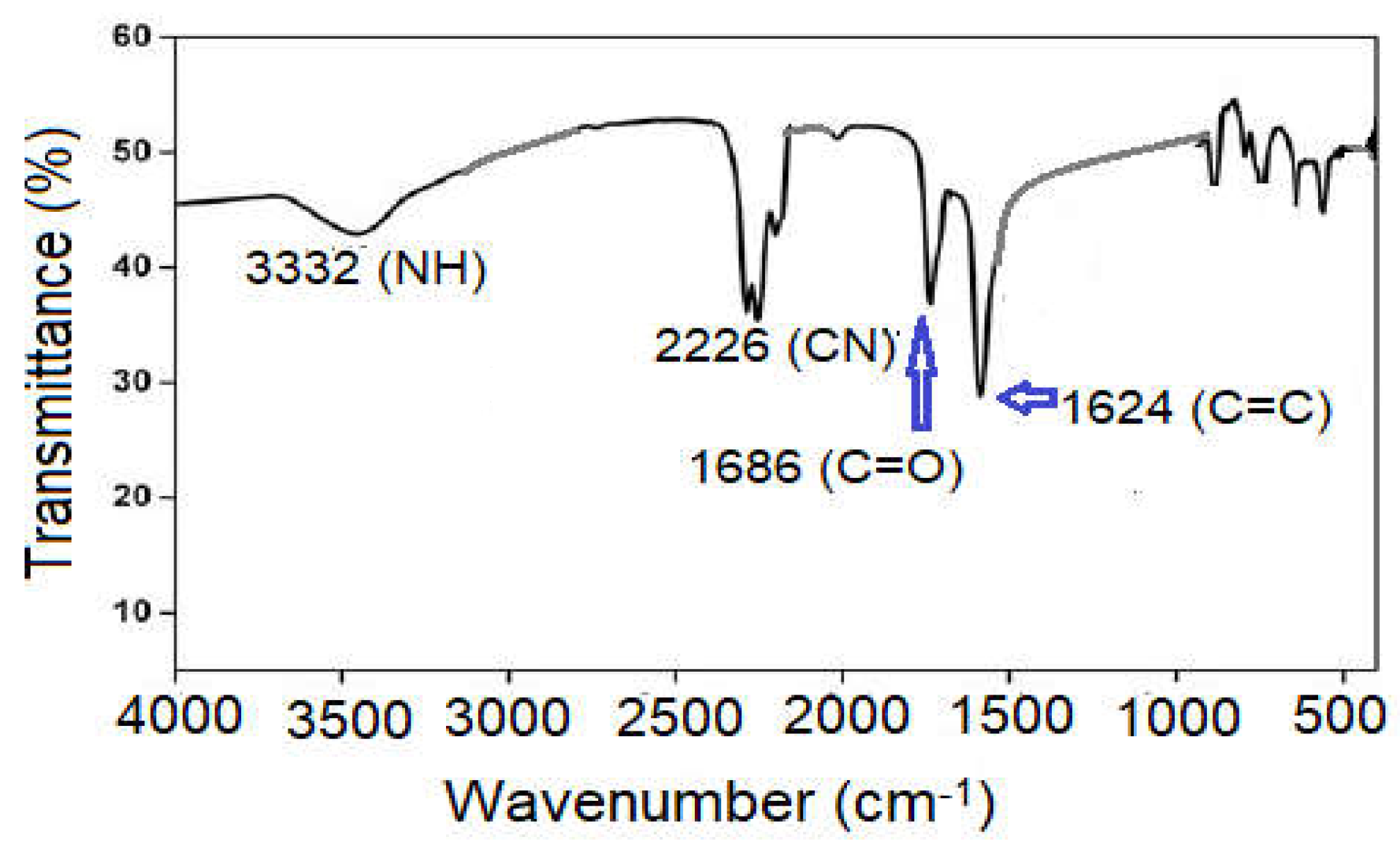
2.1. Infrared Spectra
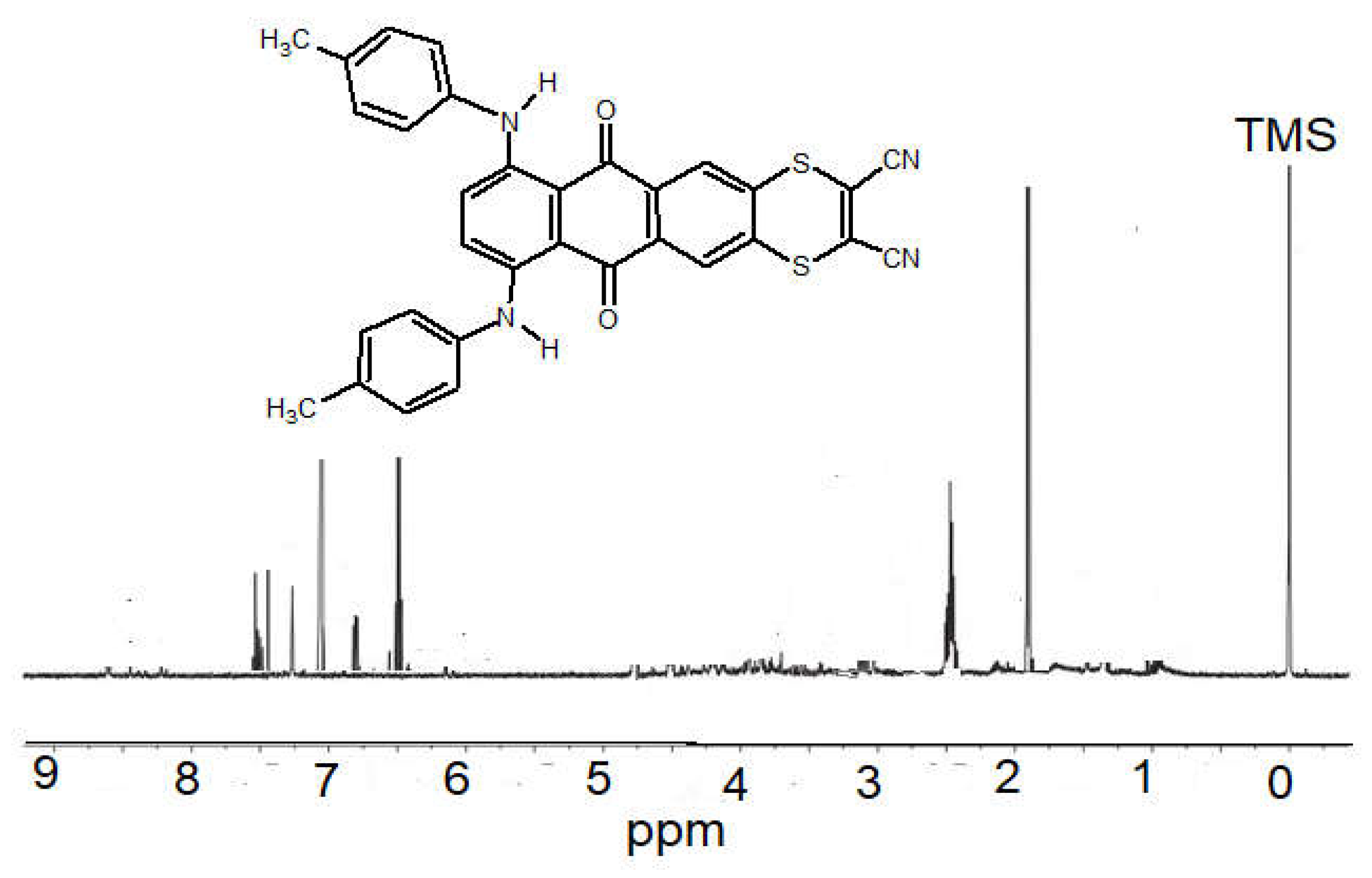
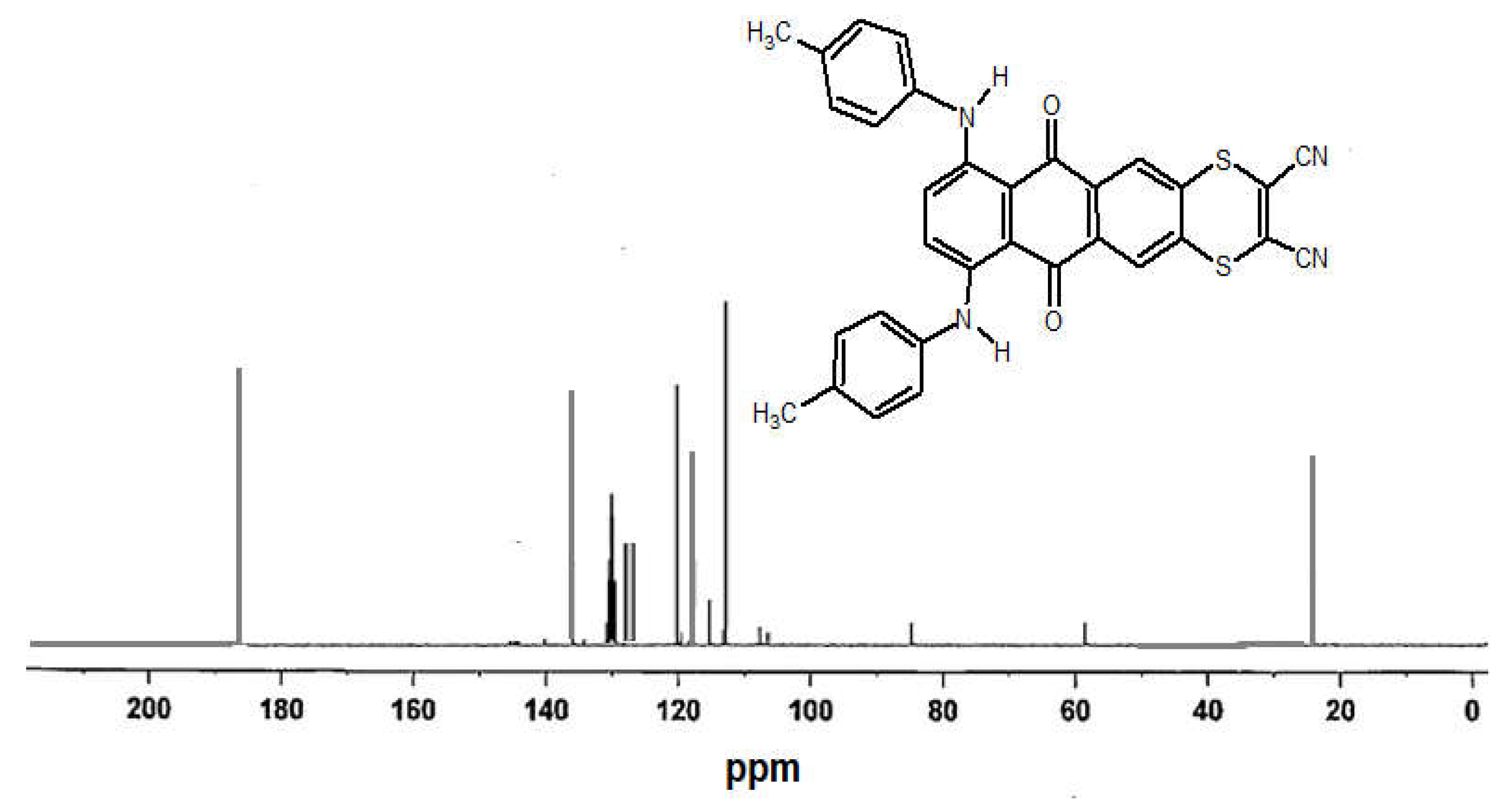
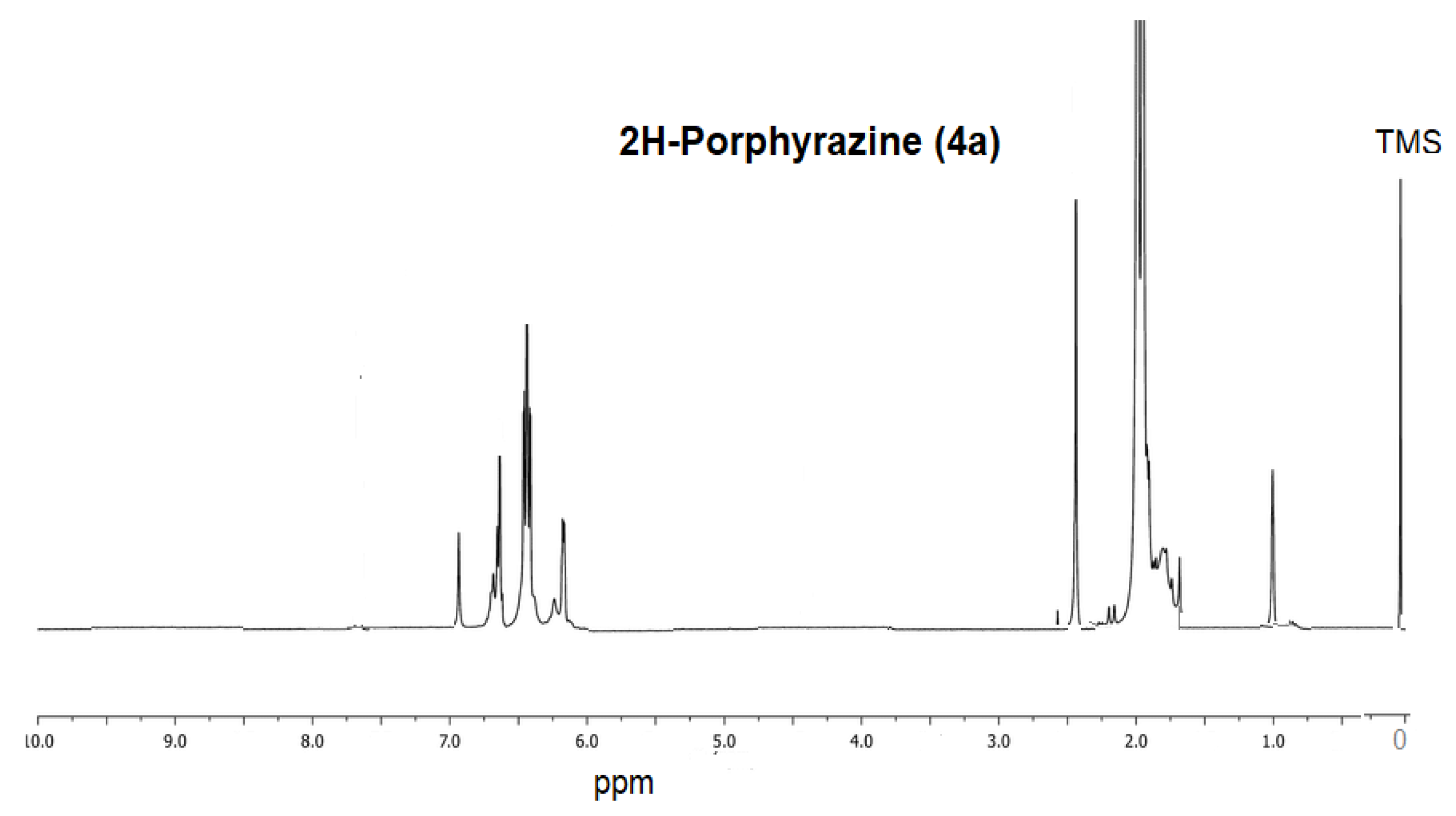
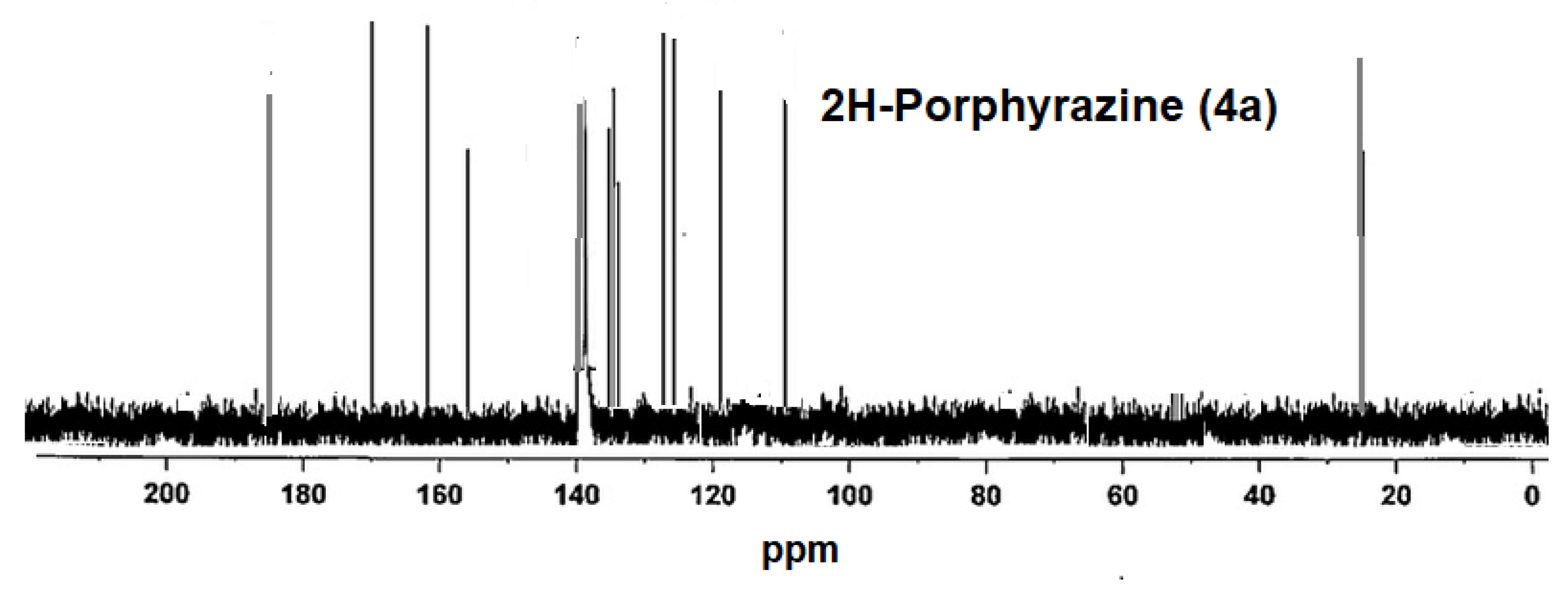
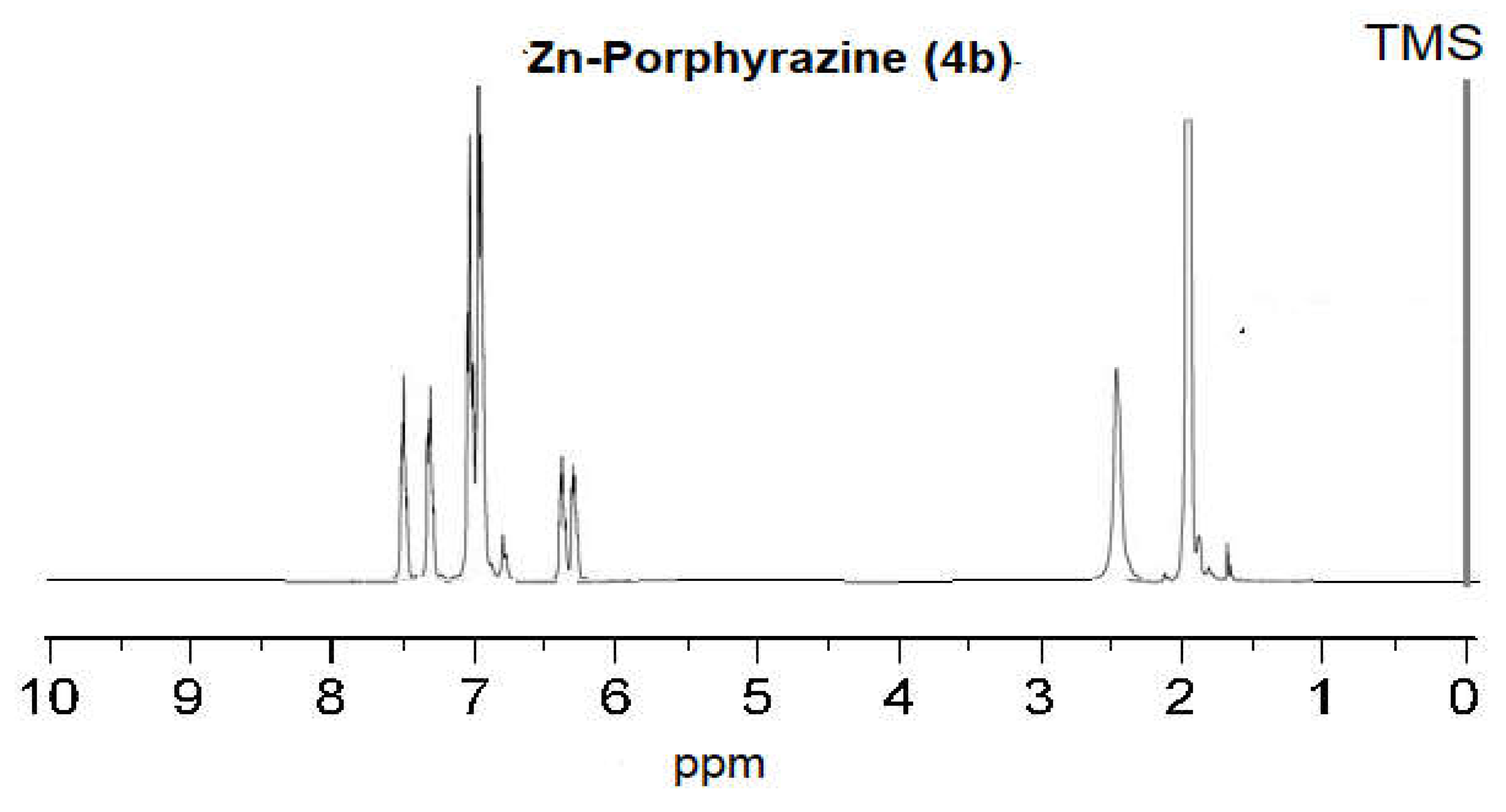
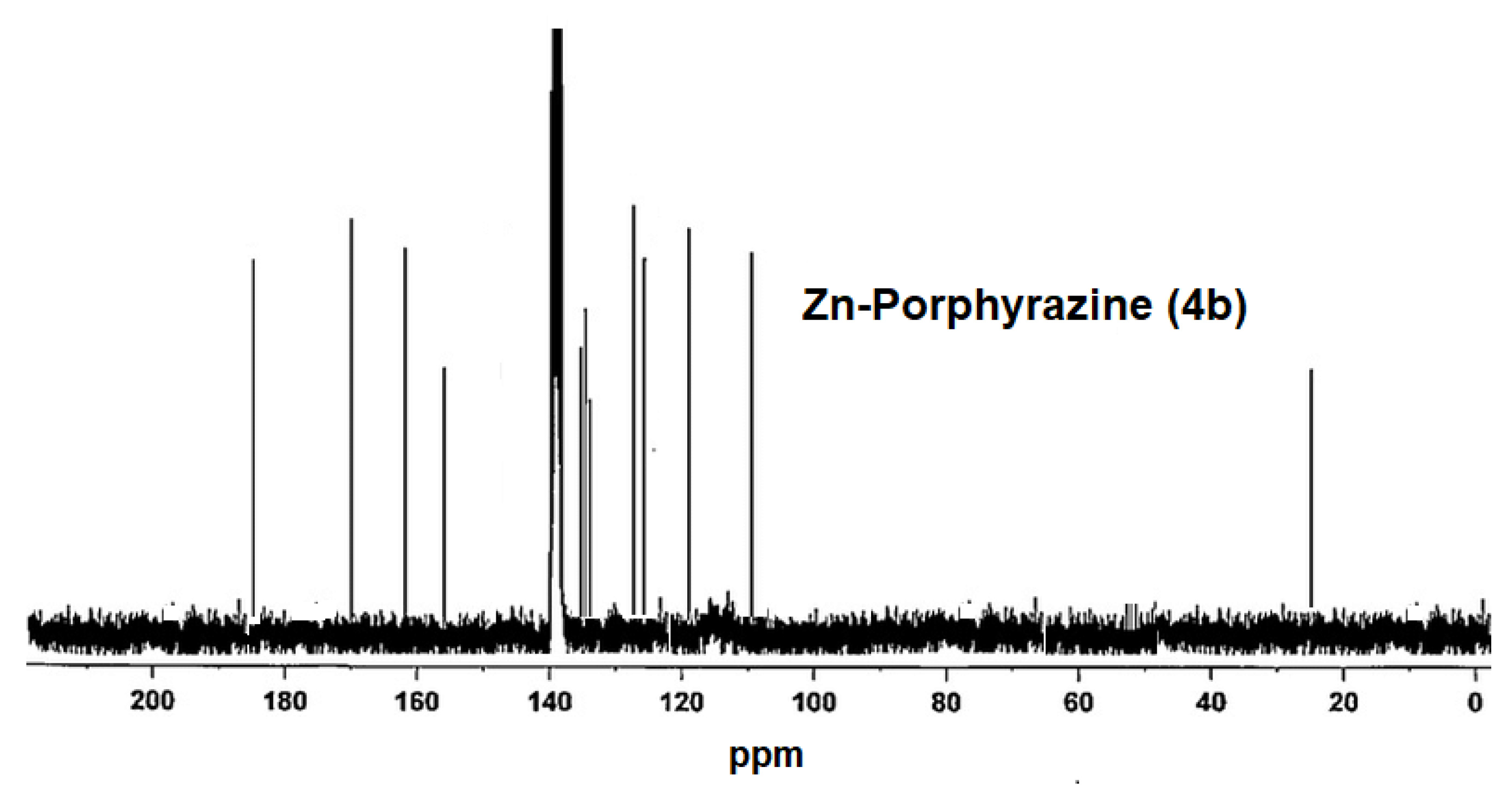
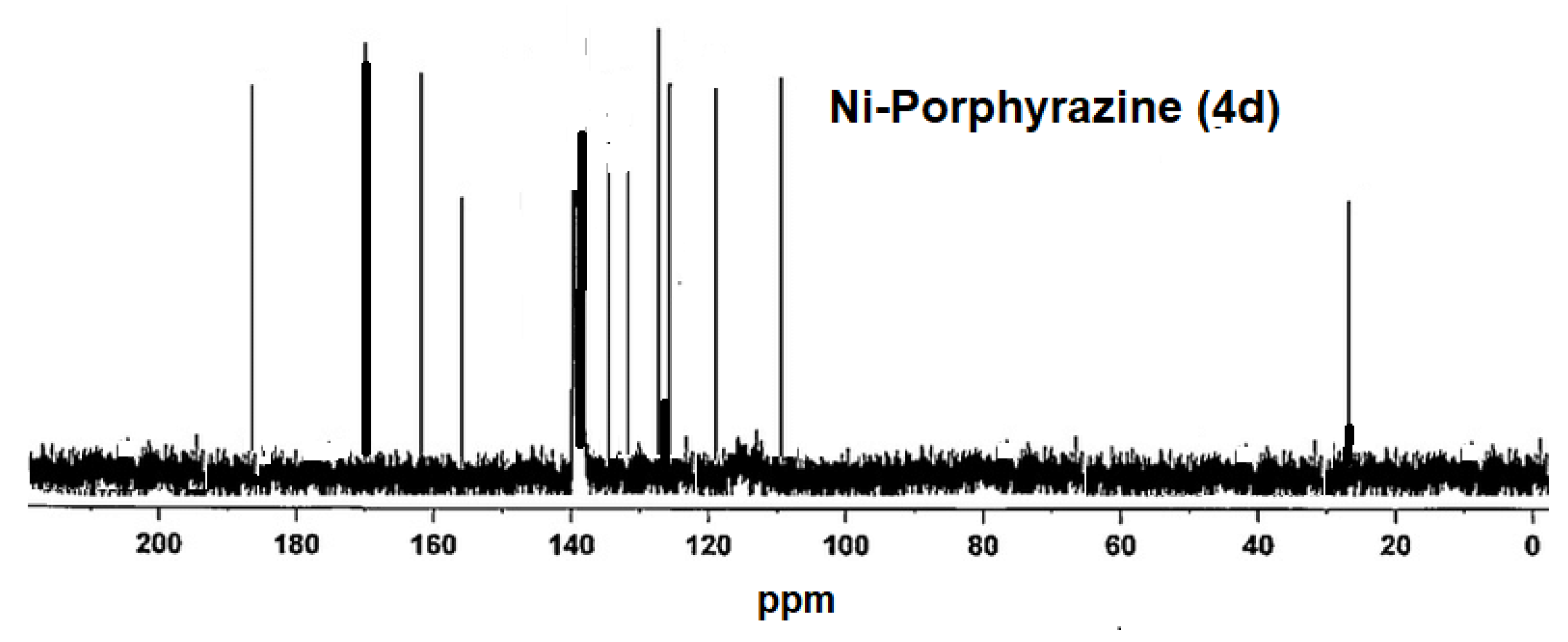
2.2. Measurements of NMR and Molecular Mass
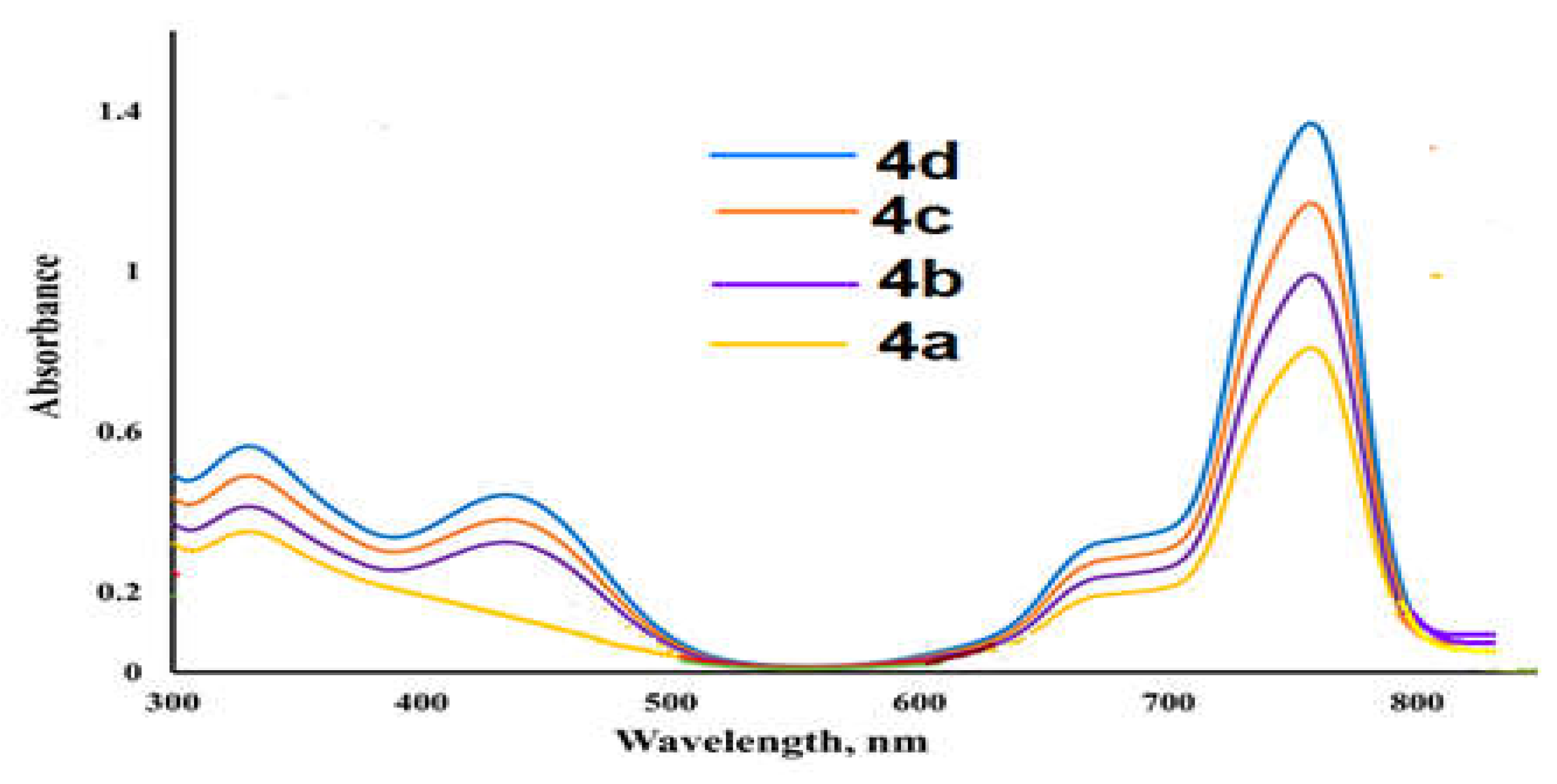
2.3. Ultraviolet-Visible Spectra
3. Materials and Methods
3.1. Characterization
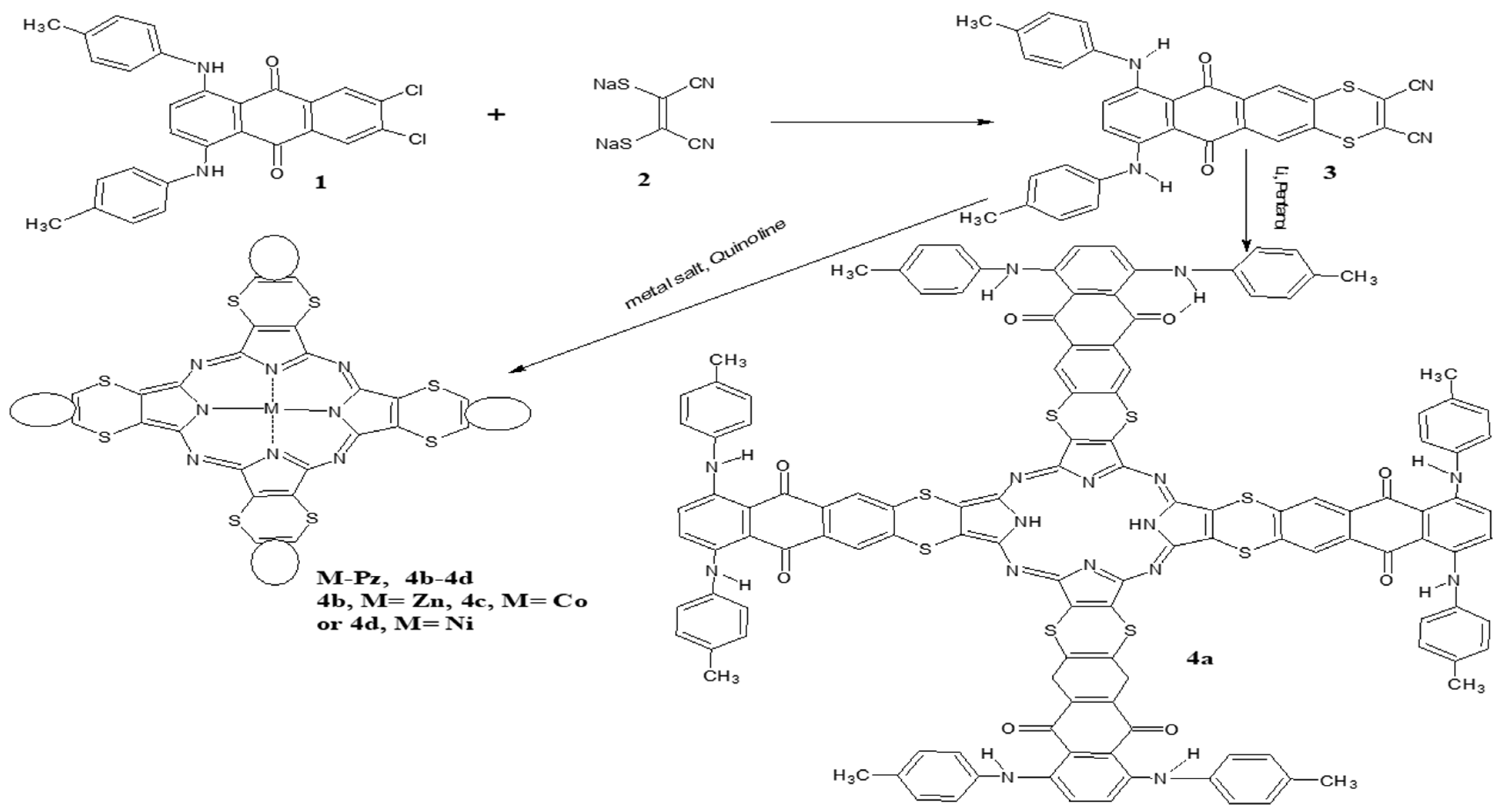
3.2. Synthesis of Dicarbonitrile Derivative 3
3.3. Synthesis of the Dye Porphyrazine Derivative (2H-Pz) 4a
3.4. Synthesis of the Dye Porphyrazinato-Metal II Derivatives (M-Pz) 4b–4d
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sudarsan, V. Functional Materials, 8—Optical Materials: Fundamentals and Applications; Banerjee, S., Tyagi, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 285–322. [Google Scholar]
- Jorg, K.; Subramanian, L.R.; Michael, H.J. Synthesis of silicon tetrapyrazinoporphyrazines. J. Porphyr. Phthalocyanines 2000, 4, 498–504. [Google Scholar]
- Turubanova, V.D.; Mishchenko, T.A.; Balalaeva, I.V.; Efimova, I.; Peskova, N.N.; Klapshina, L.G.; Lermontova, S.A.; Bachert, C.; Krysko, O.; Vedunova, M.V.; et al. Novel porphyrazine-based photodynamic anti-cancer therapy induces immunogenic cell death. Sci. Rep. 2021, 11, 7205. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Chen, L.; Sun, J.; Lv, K.; Deng, K. Synthesis and properties of iron(II) tetra(1,4-dithiin)porphyrazine bearing peripheral long-chain alkyl group of active end-bromine. Inorg. Chem. Commun. 2010, 13, 236–239. [Google Scholar] [CrossRef]
- Deng, K.; Huang, F.; Wang, D.; Peng, Z.; Zhou, Y. A Novel Catalyst Iron(II) Tetra(1,4-dithin)porphyrazine for Oxygenating Degradation of Organic Pollutants in Aqueous Solutions. Chem. Lett. 2004, 33, 34–35. [Google Scholar] [CrossRef]
- Faust, R.; Weber, C.J. Three-step synthesis and absorption and emission properties of peripherally peralkynylated tetrapyrazinoporphyrazines. Org. Chem. 1999, 64, 2571–2573. [Google Scholar] [CrossRef]
- Yang, C.; Sun, J.; Deng, K.; Wang, D. Synthesis and photocatalytic properties of iron(II)tetramethyl-tetra(1,4-dithiin)porphyrazine. Catal. Commun. 2008, 9, 321–326. [Google Scholar] [CrossRef]
- Abdel-Razik, H.H.; Abbo, M.; Almahy, H.A.; Kenawy, E. Synthesis, characterization and spectroscopic investigation of novel tetra-(1,4-dithiin)porphyrazine network polymers derived from dithianone monomer. Macromol. Indian J. (MMAIJ) 2013, 9, 45–50. [Google Scholar]
- Jin, J.; Yang, C.; Hang, B.Z.; Deng, K. Selective oxidation of amines using O2 catalyzed by cobalt thioporphyrazine under visible light. J. Catal. 2018, 361, 33–39. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, S.; Yang, C.; Zhang, B.; Li, Z.; Deng, K. Modulation of peripheral substituents of cobalt thioporphyrazines and their photocatalytic activity. Appl. Catal. B Environ. 2016, 192, 108–115. [Google Scholar] [CrossRef]
- Lu, G.; Chu, F.; Huang, X.; Li, Y.; Liang, K.; Wang, G. Recent advances in Metal-Organic Frameworks-based materials for photocatalytic selective oxidation. Coord. Chem. Rev. 2022, 450, 214240. [Google Scholar] [CrossRef]
- Zervaki, G.E.; Tsaka, V.; Vatikioti, A.; Georgakaki, I.; Nikolaou, V.; Sharma, G.D.; Coutsolelos, A.G. A triazine di(carboxy)porphyrin dyad versus a triazine di(carboxy)porphyrin triad for sensitizers in DSSCs. Dalton Trans. 2015, 44, 13550–13564. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wagner, P.; Salm, H.; Clarke, T.M.; Gordon, K.C.; Mori, S.; Mozer, A.J. Dichromophoric Zinc Porphyrins: Filling the Absorption Gap between the Soret and Q Bands. J. Phys. Chem. C 2015, 119, 5350–5363. [Google Scholar] [CrossRef]
- Tuncer, S.; Koca, A.; Gül, A.; Avcıata, U. Synthesis, characterization, electrochemistry and spectroelectrochemistry of novel soluble porphyrazines bearing unsaturated functional groups. Dyes Pigm. 2011, 92, 610–618. [Google Scholar] [CrossRef]
- Yella, A.; Lee, H.W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.G.; Yeh, C.Y.; Zakeeruddin, S.M.; Gratzel, M. Porphyrin-Sensitized Solar Cells with Cobalt (II/III)-Based Redox Electrolyte Exceed 12% Efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef]
- Bessho, T.; Zakeeruddin, S.M.; Yeh, C.Y.; Diau, E.W.G.; Gratzel, M. Highly Efficient Mesoscopic Dye-Sensitized Solar Cells Based on Donor-Acceptor-Substituted Porphyrins. Angew. Chem. 2010, 122, 6796–6799. [Google Scholar] [CrossRef]
- Wu, S.L.; Lu, H.P.; Yu, H.T.; Chuang, S.H.; Chiu, C.L.; Lee, C.W.; Diau, E.W.G.; Yeh, C.Y. Design and Characterization of Porphyrin Sensitizers with a Push-Pull Framework for Highly Efficient Dye-Sensitized Solar Cells. Energy Environ. Sci. 2010, 3, 949–955. [Google Scholar] [CrossRef]
- Keskin, B.; Denktaş, C.; Altındal, A.; Avcıata, U.; Gül, A. Synthesis of Ni(II) porphyrazine peripherally octa-substituted with the 4-tert-butylbenzylthio moiety and electronic properties of the Al/Ni(II)Pz/p-Si Schottky barrier diode. Polyhedron 2012, 38, 121–125. [Google Scholar] [CrossRef]
- Stuzhin, P.A.; Bauer, E.M.; Ercolani, C. Syntheses and properties of tetrakis(thiadiazole)porphyrazine and its magnesium and copper derivatives. Inorg. Chem. 1998, 37, 1533–1539. [Google Scholar] [CrossRef]
- Ekaterina, N.T.; Hamdoush, M.; Eroshin, A.V.; Ryzhov, I.V.; Zhabanov, Y.A.; Stuzhin, P.A. Tetra(1,2,5-thiadiazolo) porphyrazines. 10. Synthesis, spectral characterization and DFT study of complexes with yttrium(III) and lutetium(III). Polyhedron 2021, 193, 114877. [Google Scholar]
- Stuzhin, P.; Ivanova, S.; Hamdoush, M.; Kirakosyan, G.; Kiselev, A.; Popov, A.; Sliznev, V.; Ercolani, C. Tetrakis(1,2,5-thiadiazolo)porphyrazines. 9. Synthesis and spectral and theoretical studies of the lithium(i) complex and its unusual behaviour in aprotic solvents in the presence of acids. Dalton Trans. 2019, 48, 14049–14061. [Google Scholar] [CrossRef]
- Zhabanov, Y.A.; Ryzhov, I.V.; Kuzmin, I.A.; Eroshin, A.V.; Stuzhin, P.A. DFT Study of Molecular and Electronic Structure of Y, La and Lu Complexes with Porphyrazine and Tetrakis(1,2,5-thiadiazole)porphyrazine. Molecules 2021, 26, 113. [Google Scholar] [CrossRef]
- Yahya, M.; Bouziani, A.; Ocak, C.; Seferoğlu, Z.; Sillanpää, M. Organic/metal-organic photosensitizers for dye-sensitized solar cells (DSSC): Recent developments, new trends, and future perceptions. Dyes Pigm. 2021, 192, 109227. [Google Scholar] [CrossRef]
- Kim, J.; Jaung, J.Y.; Ahn, H. Tetrapyrazinoindoloporphyrazine Langmuir-Blodgett films. Macromol. Res. 2008, 16, 367. [Google Scholar] [CrossRef]
- Jaung, J.Y.; Matsuoka, M.; Fukunishi, K. Syntheses and characterization of push-pull tetrapyrazino[2,3-b] indoloporphyrazines. Synthesis 1998, 1998, 1347–1351. [Google Scholar] [CrossRef]
- Puigdollers, J.; Voz, C.; Fonrodona, M.; Cheylan, S.; Stella, M.; Andreu, J.; Vetter, M.; Alcubilla, R. Copper phthalocyanine thin-film transistors with polymeric gate dielectric. J. Non-Cryst. Solids 2006, 352, 1778. [Google Scholar] [CrossRef]
- Wizel, S.; Margel, S.; Gedanken, A.; Rojas, T.C.; Fernandez, A.; Prozorov, R. The preparation of metal–polymer composite materials using ultrasound radiation: Part II. Differences in physical properties of cobalt–polymer and iron–polymer. J. Mater. Res. 1999, 14, 3913. [Google Scholar] [CrossRef][Green Version]
- Bruder, I.; Schöneboom, J.; Dinnebier, R.; Ojala, A.; Schäfer, S.; Sens, R.; Erk, P.; Weis, J. What determines the performance of metal phthalocyanines (MPc, M = Zn, Cu, Ni, Fe) in organic heterojunction solar cells? A combined experimental and theoretical investigation. Org. Electron. 2010, 11, 377–387. [Google Scholar] [CrossRef]
- Cory, M.G.; Zerner, M.C. Metal-ligand exchange coupling in transition-metal complexes. Chem. Rev. 1991, 91, 813–822. [Google Scholar] [CrossRef]
- Chen, Q.; Gu, D.; Gan, F. Ellipsometric spectra of cobalt phthalocyanine films. Phys. B 1995, 212, 189. [Google Scholar] [CrossRef]
- Gouterman, M. Study of the Effects of Substitution on the Absorption Spectra of Porphyrin. J. Chem. Phys. 1959, 30, 1139–1161. [Google Scholar] [CrossRef]
- Gouterman, M. Spectra of Porphyrins. J. Mol. Spectrosc. 1961, 6, 138–163. [Google Scholar] [CrossRef]
- Baerends, E.; Ricciardi, G. A DFT/TDDFT Interpretation of the Ground and Excited States of Porphyrin and Porphyrazine Complexes. Coord. Chem. Rev. 2002, 230, 5–7. [Google Scholar] [CrossRef]
- Cook, P.L.; Yang, W.; Liu, X.; García-Lastra, J.M.; Rubio, A.; Himpsel, F.J. Unoccupied States in Cu and Zn Octaethyl-Porphyrin and Phthalocyanine. J. Chem. Phys. 2011, 134, 204707. [Google Scholar] [CrossRef] [PubMed]
- Katoh, R.; Furube, A. Electron injection efficiency in dye-sensitized solar cells. J. Photochem. Photobiol. C Photochem. Rev. 2014, 20, 1–16. [Google Scholar] [CrossRef]
- García-Lastra, J.M.; Cook, P.L.; Himpsel, F.J.; Rubio, A. Communication: Systematic shifts of the lowest unoccupied molecular orbital peak in X-ray absorption for a series of 3d metal porphyrins. J. Chem. Phys. 2010, 133, 151103. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, M.; Said, N.; Aziz, B.; Shujahadeen, B.; Hisham, S.; Shah, S.; Bakar, A.; Abidin, Z.; Tajuddin, H.; Sulaiman, L.; et al. Electrochemical Characteristics of Phthaloyl Chitosan Based Gel Polymer Electrolyte for Dye Sensitized Solar Cell Application. Int. J. Electrochem. Sci. 2020, 15, 7434–7447. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, O.A.A.; Abdel-Razik, H.H.; Abualnaja, M.; Fayad, E. Investigation of Structural and Optical Properties of Some [1,4]Dithiine-porphyrazine Dyes. Molecules 2022, 27, 1651. https://doi.org/10.3390/molecules27051651
Ali OAA, Abdel-Razik HH, Abualnaja M, Fayad E. Investigation of Structural and Optical Properties of Some [1,4]Dithiine-porphyrazine Dyes. Molecules. 2022; 27(5):1651. https://doi.org/10.3390/molecules27051651
Chicago/Turabian StyleAli, Ola A. Abu, Hamada H. Abdel-Razik, Matokah Abualnaja, and Eman Fayad. 2022. "Investigation of Structural and Optical Properties of Some [1,4]Dithiine-porphyrazine Dyes" Molecules 27, no. 5: 1651. https://doi.org/10.3390/molecules27051651
APA StyleAli, O. A. A., Abdel-Razik, H. H., Abualnaja, M., & Fayad, E. (2022). Investigation of Structural and Optical Properties of Some [1,4]Dithiine-porphyrazine Dyes. Molecules, 27(5), 1651. https://doi.org/10.3390/molecules27051651





