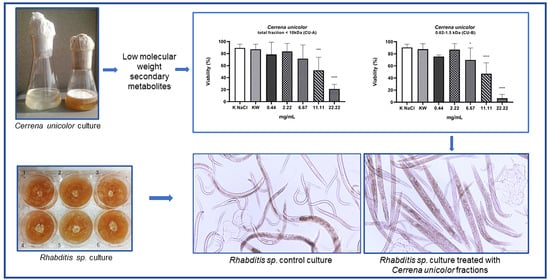
Low-Molecular-Weight Secondary Metabolites from Fungi: Cerrena unicolor as a New Proposal of an Effective Preparation against Rhabditis Nematodes
Abstract
:1. Introduction
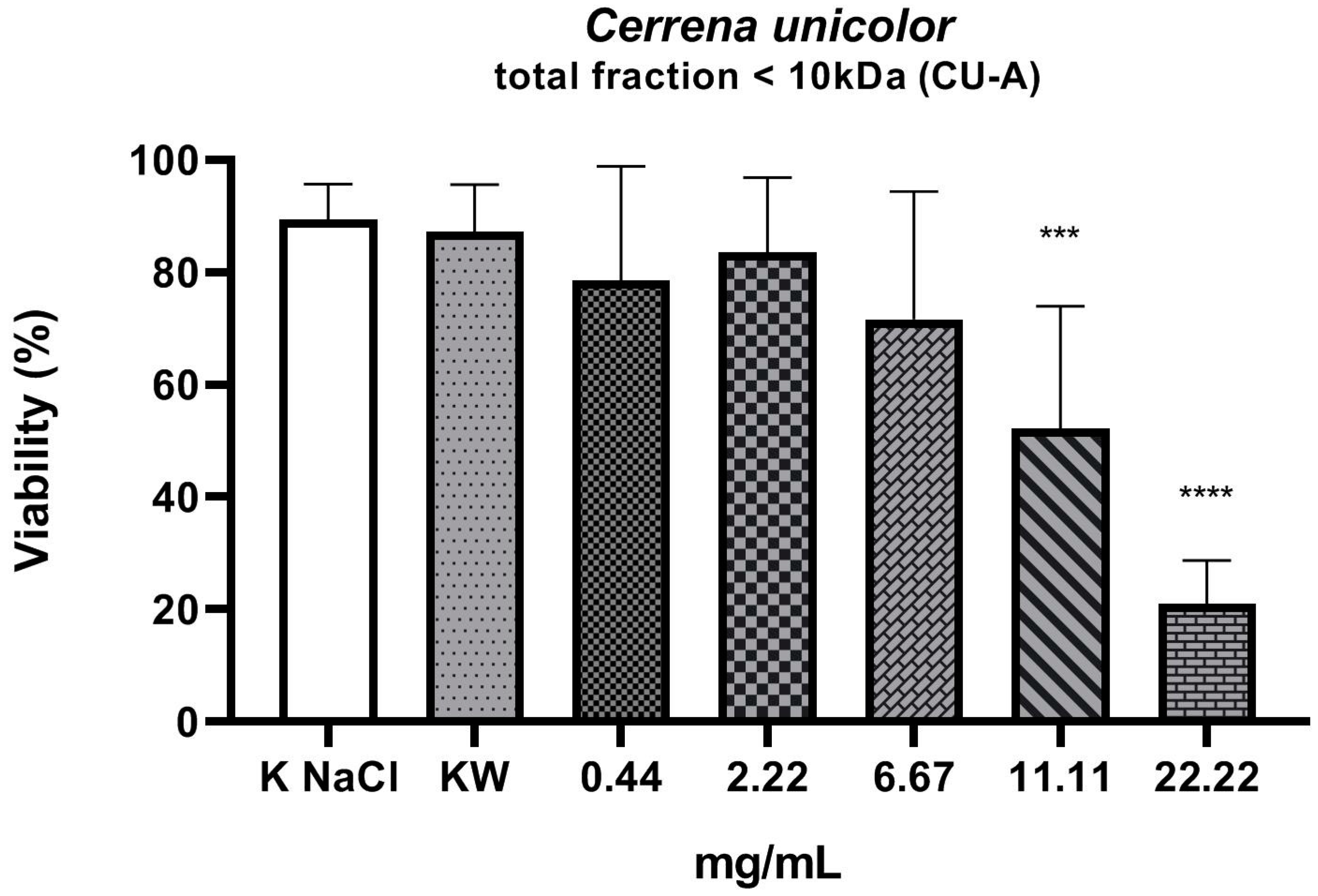
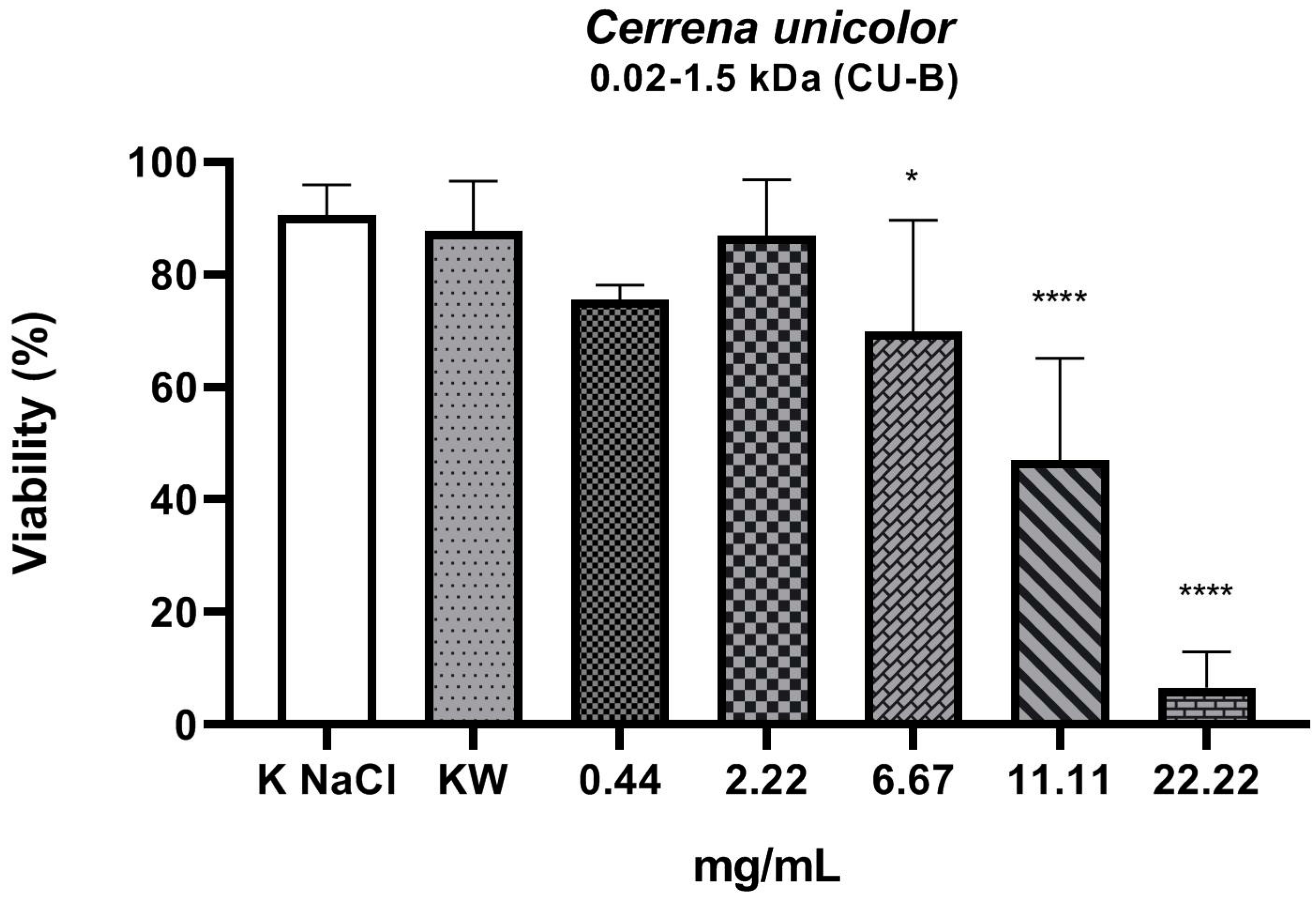
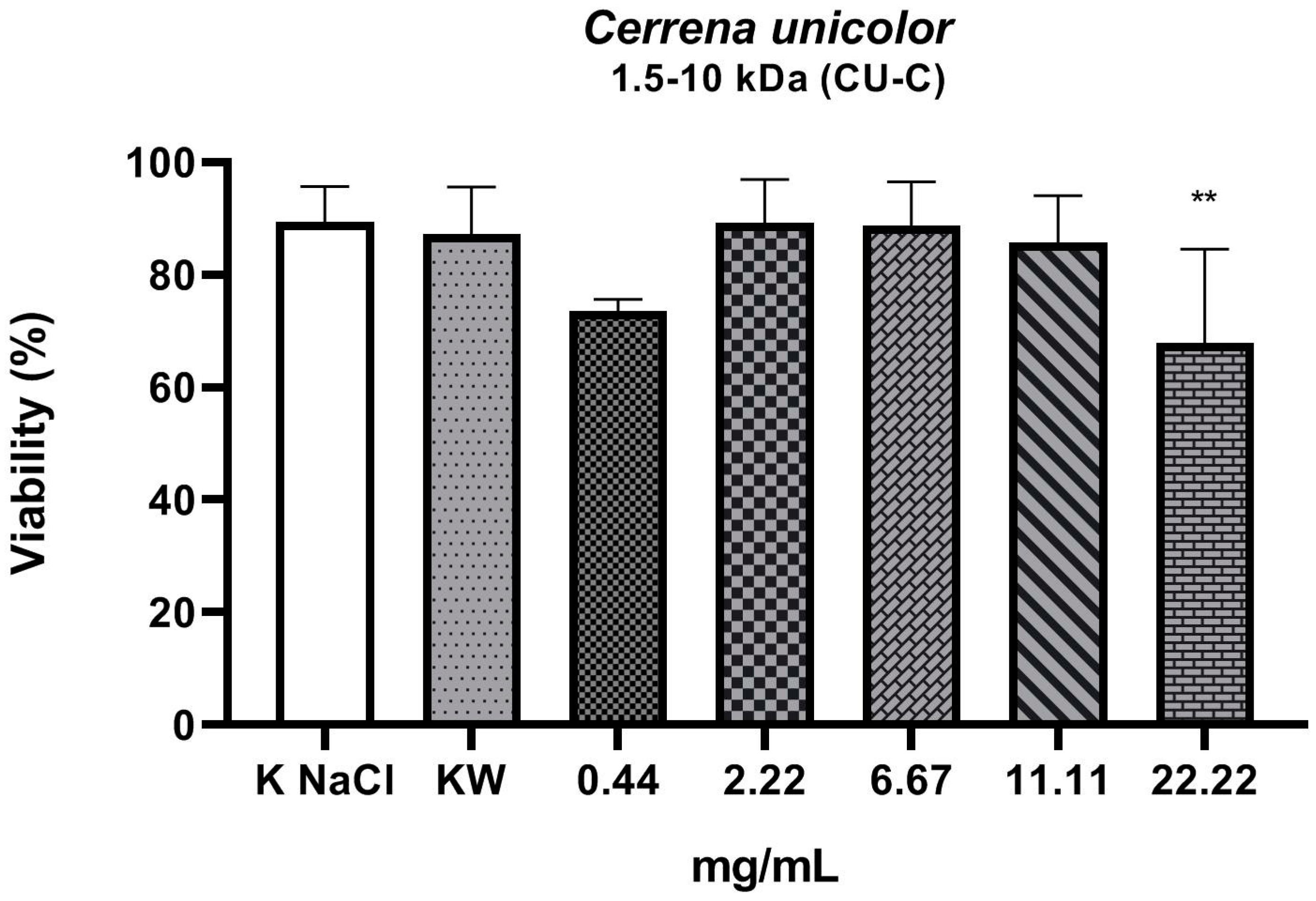
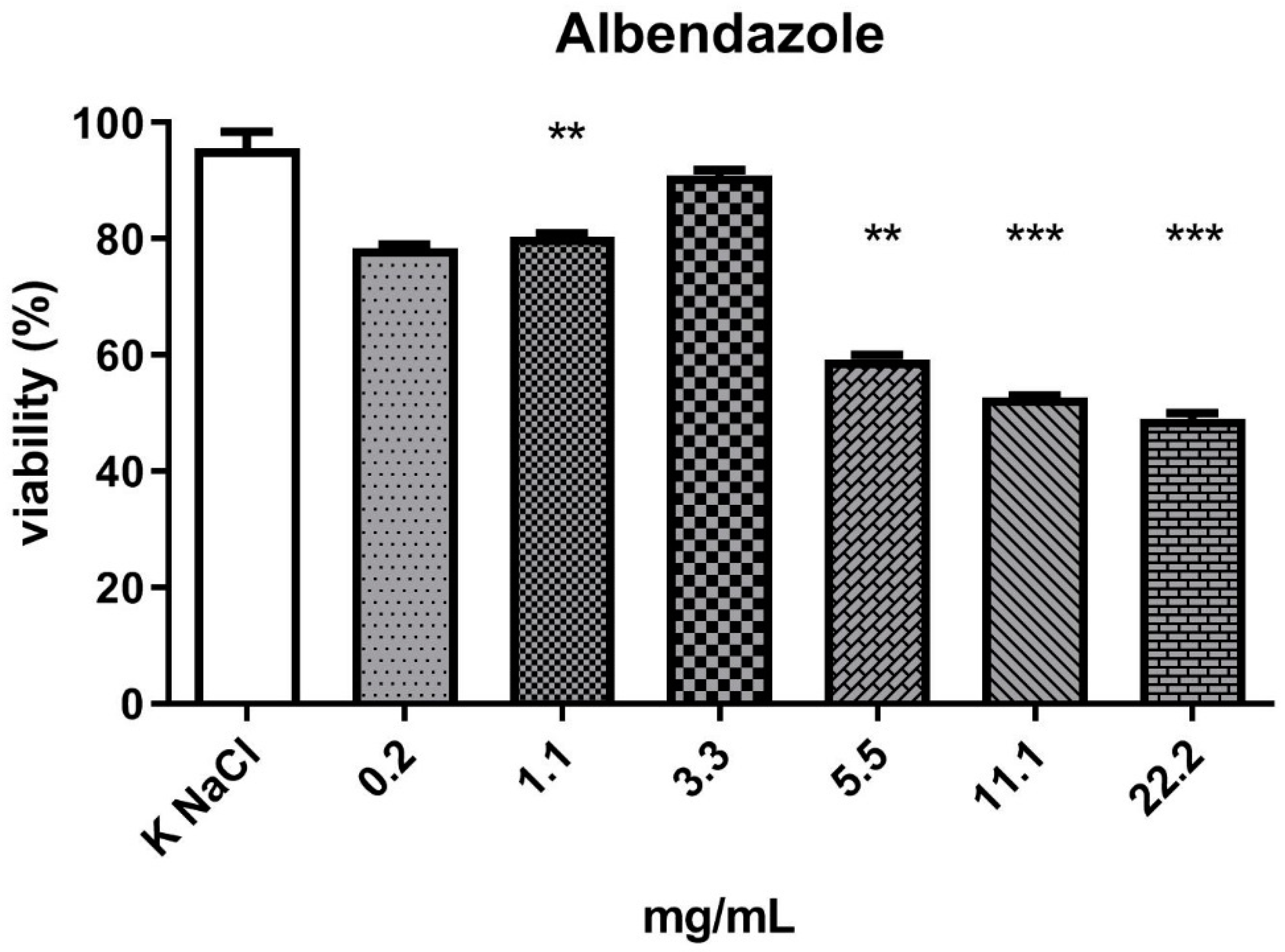
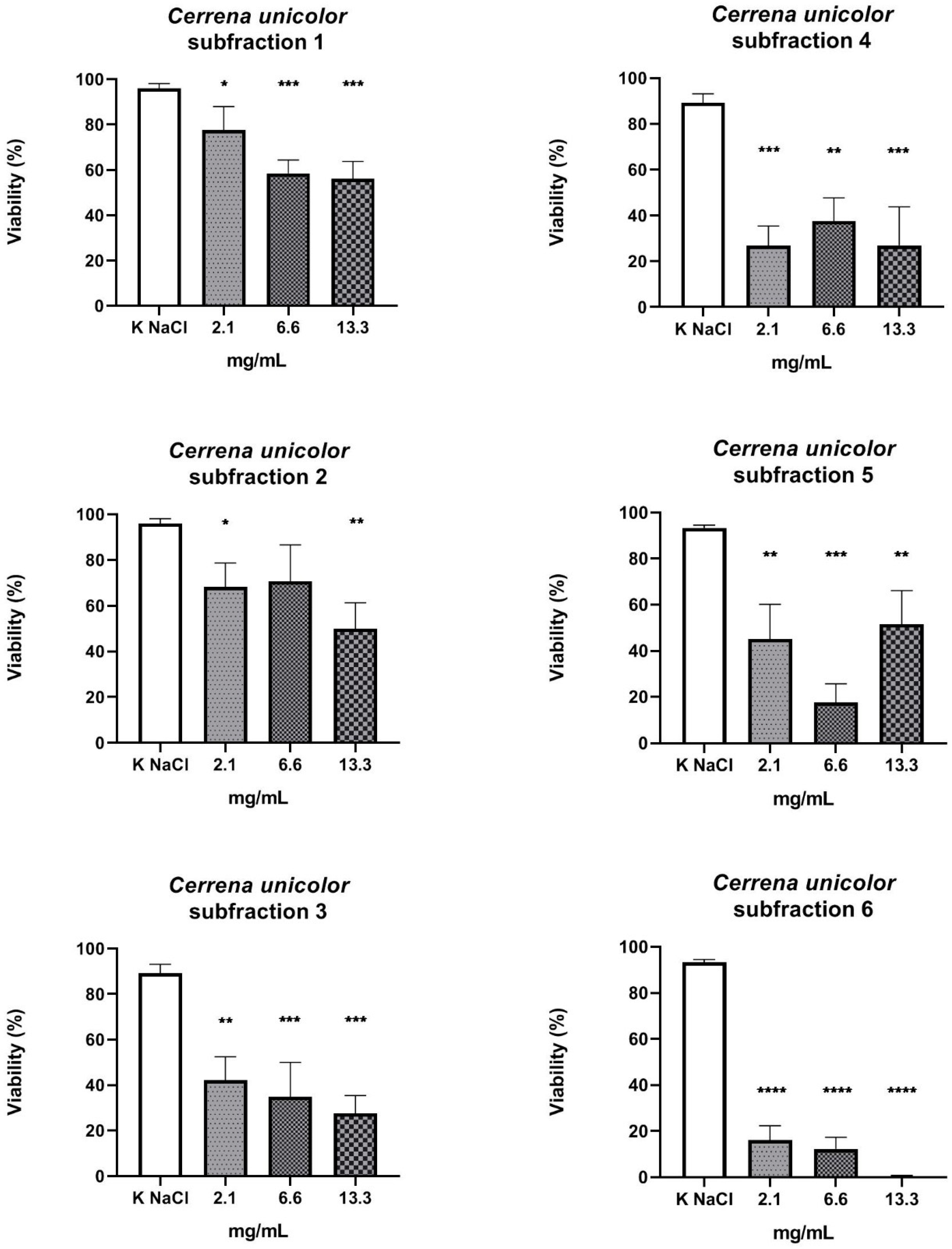
2. Results
3. Discussion
4. Materials and Methods
4.1. Strain, Medium, Growth Processing
4.2. Preparation of Fungal Fractions
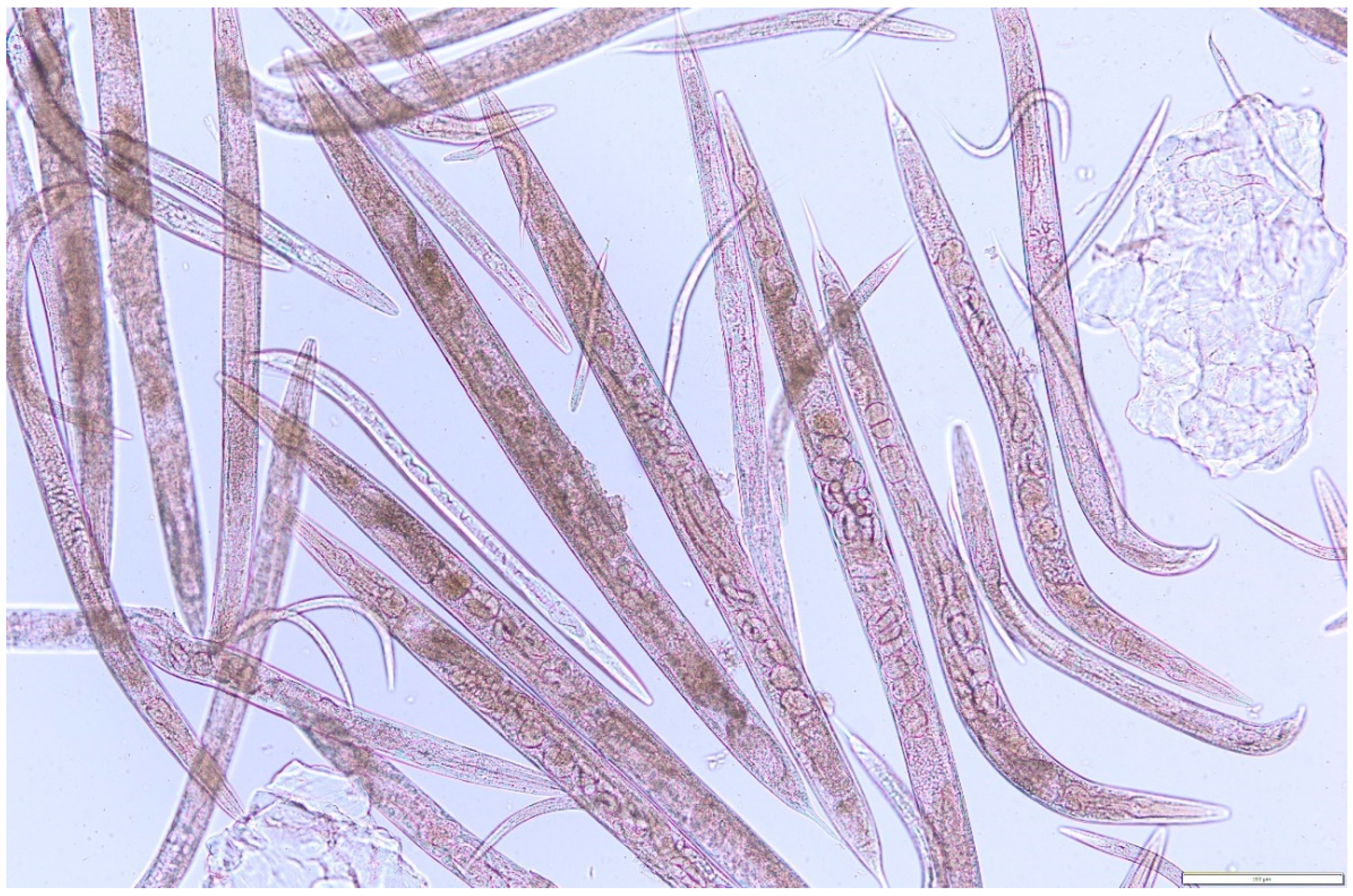
4.3. Nematicidal Activity
4.4. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, A.; Tak, H.; Nazir, R.; Lone, B.A.; Parray, J.A. In vitro anthelmintic and antimicrobial activities of methanolic extracts of Fumaria indica. Clin. Microbiol. 2014, 3, 161. [Google Scholar] [CrossRef]
- Satou, T.; Kaneko, K.; Li, W.; Koike, K. The toxin produced by Pleurotus ostreatus reduces the head size of nematodes. Biol. Pharm. Bull. 2008, 31, 574–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization Soil-Transmitted Helminth Infections. Available online: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (accessed on 19 February 2022).
- Garcia-Bustos, J.F.; Sleebs, B.E.; Gasser, R.B. An appraisal of natural products active against parasitic nematodes of animals. Parasites Vectors 2019, 12, 306. [Google Scholar] [CrossRef] [Green Version]
- Lustigman, S.; Prichard, R.K.; Gazzinelli, A.; Grant, W.N.; Boatin, B.A.; McCarthy, J.S.; Basáñez, M.G. A research agenda for helminth diseases of humans: The problem of helminthiases. PLoS Negl. Trop. Dis. 2012, 6, e1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besier, R.B.; Kahn, L.P.; Sargison, N.D.; Van Wyk, J.A. The pathophysiology, ecology and epidemiology of Haemonchus contortus infection in small ruminants. Adv. Parasitol. 2016, 93, 95–143. [Google Scholar] [CrossRef]
- Arsenopoulos, K.V.; Fthenakis, G.C.; Katsarou, E.I.; Papadopoulos, E. Haemonchosis: A challenging parasitic infection of sheep and goats. Animals 2021, 11, 363. [Google Scholar] [CrossRef]
- Wimmer, B.; Craig, B.H.; Pilkington, J.G.; Pemberton, J.M. Non-invasive assessment of parasitic nematode species diversity in wild Soay sheep using molecular markers. Int. J. Parasitol. 2004, 34, 625–631. [Google Scholar] [CrossRef]
- Stepek, G.; Buttle, D.J.; Duce, I.R.; Behnke, J.M. Human gastrointestinal nematode infections: Are new control methods required? Int. J. Exp. Pathol. 2006, 87, 325–341. [Google Scholar] [CrossRef]
- Whittaker, J.H.; Carlson, S.A.; Jones, D.E.; Brewer, M.T. Molecular mechanisms for anthelmintic resistance in strongyle nematode parasites of veterinary importance. J. Vet. Pharmacol. Ther. 2017, 40, 105–115. [Google Scholar] [CrossRef]
- Holden-Dye, L.; Walker, R.J. Anthelmintic drugs. WormBook 2007, 2, 1–29. [Google Scholar] [CrossRef]
- Soares, A.M.D.S.; Wanderley, L.F.; Costa Junior, L.M. The potential of plant and fungal proteins in the control of gastrointestinal nematodes from animals. Rev. Bras. Parasitol. Vet. 2019, 28, 339–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, T.M.; Fonseca, L.D.; Bastos, G.A.; de Oliveira Vasconcelos, V.; Silva, M.L.F.; Morais-Costa, F.; de Paiva Ferreira, A.V.; Oliveira, N.J.F.; Duarte, E.R. Control of Haemonchus contortus in sheep using basidiocarps of Agaricus blazei Murril. Vet. Res. Commun. 2017, 41, 99–106. [Google Scholar] [CrossRef]
- Matuszewska, A.; Jaszek, M.; Stefaniuk, D.; Ciszewski, T.; Matuszewski, Ł. Anticancer, antioxidant, and antibacterial activities of low molecular weight bioactive subfractions isolated from cultures of wood degrading fungus Cerrena unicolor. PLoS ONE 2018, 13, e0197044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizerska-Dudka, M.; Jaszek, M.; Błachowicz, A.; Rejczak, T.P.; Matuszewska, A.; Osińska-Jaroszuk, M.; Stefaniuk, D.; Janusz, G.; Sulej, J.; Kandefer-Szerszeń, M. Fungus cerrena unicolor as an effective source of new antiviral, immunomodulatory, and anticancer compounds. Int. J. Biol. Macromol. 2015, 79, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Ziaja-Sołtys, M.; Radzki, W.; Nowak, J.; Topolska, J.; Jabłońska-Ryś, E.; Sławińska, A.; Skrzypczak, K.; Kuczumow, A.; Bogucka-Kocka, A. Processed fruiting bodies of Lentinus edodes as a source of biologically active polysaccharides. Appl. Sci. 2020, 10, 470. [Google Scholar] [CrossRef] [Green Version]
- Basnet, B.B.; Liu, L.; Bao, L.; Liu, H. Current and future perspective on antimicrobial and anti-parasitic activities of Ganoderma sp.: An update. Mycology 2017, 8, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Cör, D.; Knez, Ž.; Knez Hrnčič, M. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma lucidum terpenoids and polysaccharides: A review. Molecules 2018, 23, 649. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.; Goyal, A. Recent developments in mushrooms as anti-cancer therapeutics: A review. 3 Biotech. 2012, 2, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Muszyńska, B.; Sułkowska-Ziaja, K.; Łojewski, M.; Opoka, W.; Zając, M.; Rojowski, J. Edible mushrooms in prophylaxis and treatment of human diseases. Med. Int. Rev. 2013, 25, 170–183. [Google Scholar]
- Statkiewicz, M.; Matuszewska, A.; Jaszek, M.; Janusz, G.; Osińska, M.; Sulej, J.; Stefaniuk, D.; Mikula, M.; Ostrowski, J. Antimelanomic effects of high- and low-molecular weight bioactive subfractions isolated from the Mossy maze mushroom, Cerrena unicolor (Agaricomycetes). Int. J. Med. Mushrooms 2017, 19, 619–628. [Google Scholar] [CrossRef]
- Duru, M.E.; Çayan, G.T. Biologically active terpenoids from mushroom origin: A review. Rec. Nat. Prod. 2015, 9, 456–483. [Google Scholar]
- Kayser, O.; Kiderlen, A.F.; Croft, S.L. Natural products as antiparasitic drugs. Parasitol. Res. 2003, 90, S55–S62. [Google Scholar] [CrossRef]
- Jaszek, M.; Osińska-Jaroszuk, M.; Janusz, G.; Matuszewska, A.; Stefaniuk, D.; Sulej, J.; Polak, J.; Ruminowicz, M.; Grzywnowicz, K.; Jarosz-Wilkołazka, A. New bioactive fungal molecules with high antioxidant and antimicrobial capacity isolated from Cerrena unicolor idiophasic cultures. Biomed. Res. Int. 2013, 2013, 497492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matuszewska, A.; Stefaniuk, D.; Jaszek, M.; Pięt, M.; Zając, A.; Matuszewski, Ł.; Cios, I.; Grąz, M.; Paduch, R.; Bancerz, R. Antitumor potential of new low molecular weight antioxidative preparations from the white rot Fungus Cerrena unicolor against human colon cancer cells. Sci. Rep. 2019, 9, 1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefaniuk, D.; Misztal, T.; Pięt, M.; Zając, A.; Kopycińska, M.; Matuszewska, A.; Ruminowicz-Stefaniuk, M.; Matuszewski, Ł.; Marcińczyk, N.; Belcarz, A.; et al. Thromboelastometric analysis of anticancer Cerrena unicolor subfractions reveal their potential as fibrin glue drug carrier enhancers. Biomolecules 2021, 11, 1263. [Google Scholar] [CrossRef] [PubMed]
- Schulenburg, H.; Félix, M.A. The natural biotic environment of Caenorhabditis elegans. Genetics 2017, 206, 55–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teschner, M.; Würfel, W.; Sedlacek, L.; Suerbaum, S.; Tappe, D.; Hornef, M.W. Outer ear canal infection with Rhabditis sp. nematodes in a human. J. Clin. Microbiol. 2014, 52, 1793–1795. [Google Scholar] [CrossRef] [Green Version]
- Goldsmid, J. Rhabditis (Rhabditella) axei in the urine of an African in rhodesia. J. Helminthol. 1967, 41, 305–308. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Shi, D.; Cui, L.; Qiao, R.; Li, S. Polyparasitism of Rhabditis axei and Enterobius vermicularis in a child from Beijing, China. Clin. Lab. 2018, 1, 1773–1776. [Google Scholar] [CrossRef]
- Thompson, D.P.; Klein, R.D.; Geary, T.G. Prospects for rational approaches to anthelmintic discovery. Parasitology 1996, 113, 217–238. [Google Scholar] [CrossRef]
- Yang, J.; Liang, L.; Li, J.; Zhang, K.Q. Nematicidal enzymes from microorganisms and their applications. Appl. Microbiol. Biotechnol. 2013, 97, 7081–7095. [Google Scholar] [CrossRef] [PubMed]
- Elhusseiny, S.M.; El-Mahdy, T.S.; Awad, M.F.; Elleboudy, N.S.; Farag, M.M.S.; Aboshanab, K.M.; Yassien, M.A. Antiviral, cytotoxic, and antioxidant activities of three edible agaricomycetes mushrooms: Pleurotus columbinus, Pleurotus sajor-caju, and Agaricus bisporus. J. Fungi 2021, 7, 645. [Google Scholar] [CrossRef] [PubMed]
- Gründemanna, C.; Reinhardt, J.K.; Lindequist, U. European medicinal mushrooms: Do they have potential for modern medicine?—An update. Phytomedicine 2020, 66, 153131. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.S.; Martins, A.; Barros, L.; Ferreira, I.C. Antioxidant properties and phenolic profile of the most widely appreciated cultivated mushrooms: A comparative study between in vivo and in vitro samples. Food Chem. Toxicol. 2012, 50, 1201–1207. [Google Scholar] [CrossRef]
- Chang, S.T.; Wasser, S.P. Current and future research trends in agricultural and biomedical applications of medicinal mushrooms and mushroom products (review). Int. J. Med. Mushrooms 2018, 20, 1121–1133. [Google Scholar] [CrossRef]
- Chang, S.T.; Wasser, S.P. The role of culinary-medicinal mushrooms on human welfare with a pyramid model for human health. Int. J. Med. Mushrooms 2012, 14, 95–134. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, V.P.; Singh, N.K. A review on phytochemistry and pharmacology of medicinal as well as poisonous mushrooms. Mini-Rev. Med. Chem. 2018, 18, 1095–1109. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, F.; Guo, W.; Zhang, J.; Xiao, L.; Li, H.; Jia, W.; Huang, Z. Caenorhabditis elegans in Chinese medicinal studies: Making the case for aging and neurodegeneration. Rejuvenation Res. 2014, 17, 205–208. [Google Scholar] [CrossRef] [Green Version]
- González-Cortázar, M.; Sánchez, J.E.; Huicochea-Medina, M.; Hernández-Velázquez, V.M.; Mendoza-de-Gives, P.; Zamilpa, A.; López-Arellano, M.E.; Pineda-Alegría, J.A.; Aguilar-Marcelino, L. In vitro and in vivo nematicide effect of extract fractions of Pleurotus djamor against Haemonchus contortus. J. Med. Food 2021, 24, 310–318. [Google Scholar] [CrossRef]
- Li, C. The ever-expanding neuropeptide gene families in the nematode Caenorhabditis elegans. Parasitology 2005, 131, 109–127. [Google Scholar] [CrossRef]
- Kadri, D.; Crater, A.K.; Lee, H.; Solomon, V.R.; Ananvoranich, S. The potential of quinoline derivatives for the treatment of Toxoplasma gondii infection. Exp. Parasitol. 2014, 145, 135–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lolli, G.; Cozza, G.; Mazzorana, M.; Tibaldi, E.; Cesaro, L.; Donella-Deana, A.; Meggio, F.; Venerando, A.; Franchin, C.; Sarno, S.; et al. Inhibition of protein kinase CK2 by flavonoids and thyropstins. A structural insight. Biochemistry 2012, 51, 6097–6107. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Guo, Y.X.; Liu, Q.H.; Wang, H.X.; Ng, T.B. Lectins but not antifungal proteins exhibit anti-nematode activity. Environ. Toxicol. Pharmacol. 2009, 28, 265–268. [Google Scholar] [CrossRef]
- Sabotič, J.; Kos, J. CNL—Clitocybe nebularis lectin—The fungal GalNAcβ1-4GlcNAc-binding lectin. Molecules 2019, 24, 4204. [Google Scholar] [CrossRef] [Green Version]
- Carvajal, A.E.S.S.; Koehnlein, E.A.; Soares, A.A.; Eler, G.J.; Nakashima, A.T.A.; Bracht, A.; Peralta, R.M. Bioactives of fruiting bodies and submerged culture mycelia of Agaricus brasiliensis (A. blazei) and their antioxidant properties. LWT—Food Sci. Technol. 2012, 493, e499. [Google Scholar] [CrossRef] [Green Version]
- Ayers, S.; Zink, D.L.; Mohn, K.; Powell, J.S.; Brown, C.M.; Bills, G.; Grund, A.; Thompson, D.; Singh, S.B. Anthelmintic constituents of Clonostachys candelabrum. J. Antibiot. 2010, 63, 119–122. [Google Scholar] [CrossRef] [Green Version]
- Abugri, D.A.; Ayariga, J.A.; Tiimob, B.J.; Yedjou, C.G.; Mrema, F.; Witola, W.H. Medicinal mushrooms as novel sources for new antiparasitic drug development. In Medicinal Mushrooms; Agrawal, D., Dhanasekaran, M., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Janusz, G.; Rogalski, J.; Szczodrak, J. Increased production of laccase by Cerrena unicolor in submerged liquid cultures. World J. Microbiol. Biotechnol. 2007, 23, 1459–1464. [Google Scholar] [CrossRef]
- Jennings, D.H.; Lysek, G. Fungal Biology: Understanding the Fungal Lifestyle; Bios Scientific Publishers LTD: Oxford, UK, 1999. [Google Scholar]
- Dziduch, K.; Kołodziej, P.; Paneth, A.; Bogucka-Kocka, A.; Wujec, M. Synthesis and anthelmintic activity of new thiosemicarbazide derivatives—A preliminary study. Molecules 2020, 25, 2770. [Google Scholar] [CrossRef]

| Protein (μg/mL) | Total Sugars (μg/mL) | Phenolic Compounds (μg/mL) | Antioxidant Properties (mM Trolox/g) | |||
|---|---|---|---|---|---|---|
| ABTS | DPPH | |||||
| 1.5–10 kDa (CU-C) | 4.3 ± 0.3 | 329.0 ± 38.6 | 64.9 ± 0.9 | 250.2 ± 0.5 | 52.0 ± 0.7 | Unpublished data |
| 0.02–1.5 kDa (CU-B) | 10.9 ± 1.8 | 220.2 ± 2.8 | 55.4 ± 1.3 | 176.2 ± 0.4 | 24.8 ± 0.9 | Matuszewska, 2019 [25] |
| S1 | 8.0 ± 0.4 | 253.8 ± 31.4 | 64.7 ± 0.2 | 308.8 ± 1.1 | 117.7 ± 7.2 | |
| S2 | 1.9 ± 0.1 | 287.2 ± 44.1 | 50.5 ± 1.5 | 243.1 ± 0.1 | 119.5 ± 5.3 | |
| S3 | 0 ± 0.0 | 215.5 ± 11.1 | 57.9 ± 1.2 | 169.0 ± 0.1 | 0.0 ± 0.2 | |
| S4 | 2.3 ± 0.1 | 404.4 ± 30.5 | 61.5 ± 2.1 | 239.3 ± 1.0 | 91.5 ± 5.1 | |
| S5 | 3.7 ± 0.2 | 328.7 ± 33.6 | 57.8 ± 1.1 | 303.0 ± 0.6 | 119.6 ± 4.3 | |
| S6 | 31.8 ± 0.5 | 190.4 ± 44.2 | 73.5 ± 1.1 | 569.8 ± 3.8 | 168.4 ± 3.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziaja-Sołtys, M.; Kołodziej, P.; Stefaniuk, D.; Matuszewska, A.; Jaszek, M.; Bogucka-Kocka, A. Low-Molecular-Weight Secondary Metabolites from Fungi: Cerrena unicolor as a New Proposal of an Effective Preparation against Rhabditis Nematodes. Molecules 2022, 27, 1660. https://doi.org/10.3390/molecules27051660
Ziaja-Sołtys M, Kołodziej P, Stefaniuk D, Matuszewska A, Jaszek M, Bogucka-Kocka A. Low-Molecular-Weight Secondary Metabolites from Fungi: Cerrena unicolor as a New Proposal of an Effective Preparation against Rhabditis Nematodes. Molecules. 2022; 27(5):1660. https://doi.org/10.3390/molecules27051660
Chicago/Turabian StyleZiaja-Sołtys, Marta, Przemysław Kołodziej, Dawid Stefaniuk, Anna Matuszewska, Magdalena Jaszek, and Anna Bogucka-Kocka. 2022. "Low-Molecular-Weight Secondary Metabolites from Fungi: Cerrena unicolor as a New Proposal of an Effective Preparation against Rhabditis Nematodes" Molecules 27, no. 5: 1660. https://doi.org/10.3390/molecules27051660