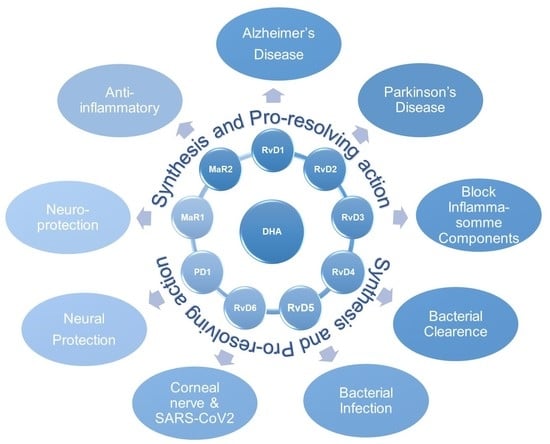
Resolvins, Protectins, and Maresins: DHA-Derived Specialized Pro-Resolving Mediators, Biosynthetic Pathways, Synthetic Approaches, and Their Role in Inflammation
Abstract
:1. Introduction
2. From DHA to SPMs and Their Functional Role
3. Resolvins from DHA
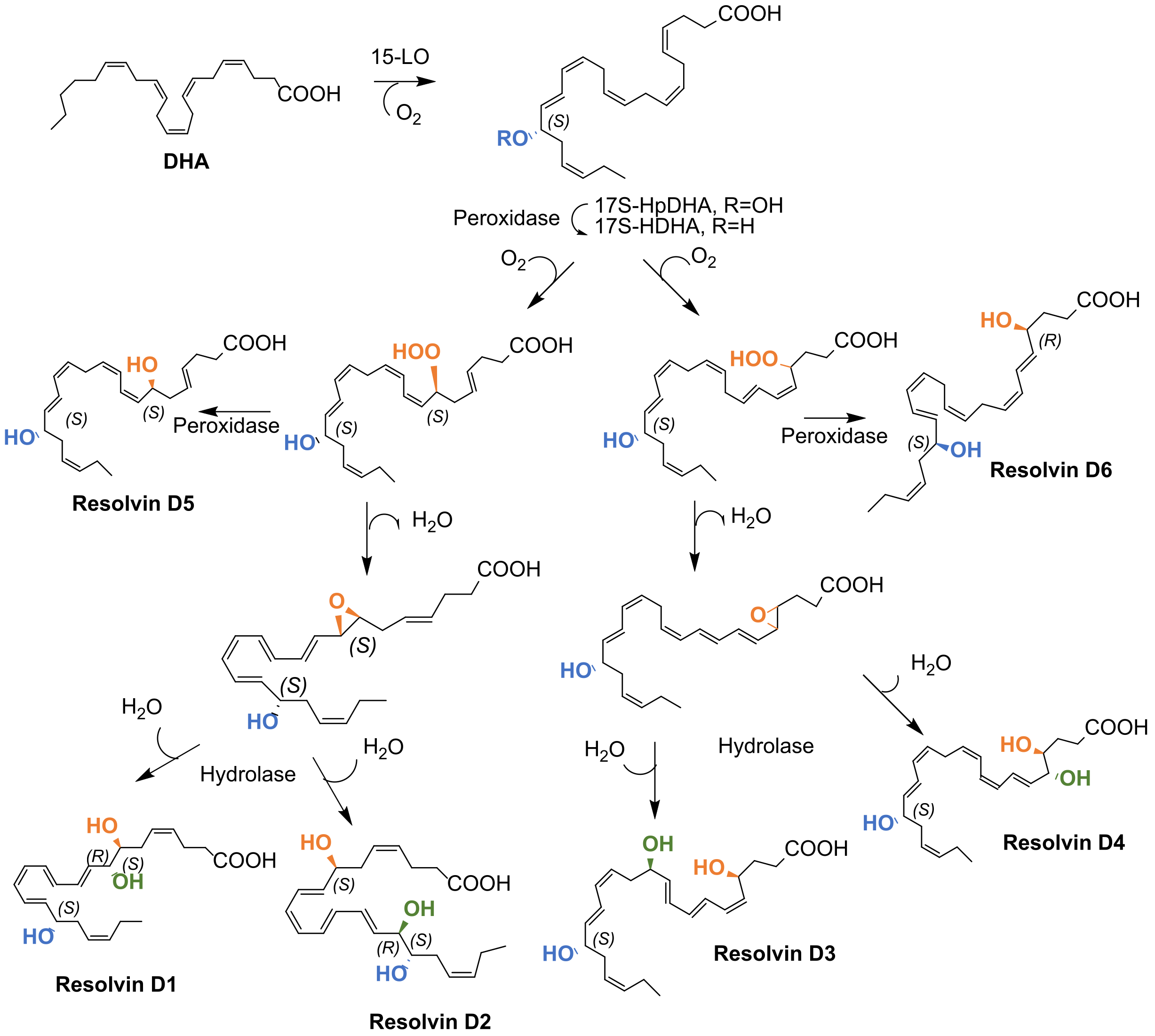
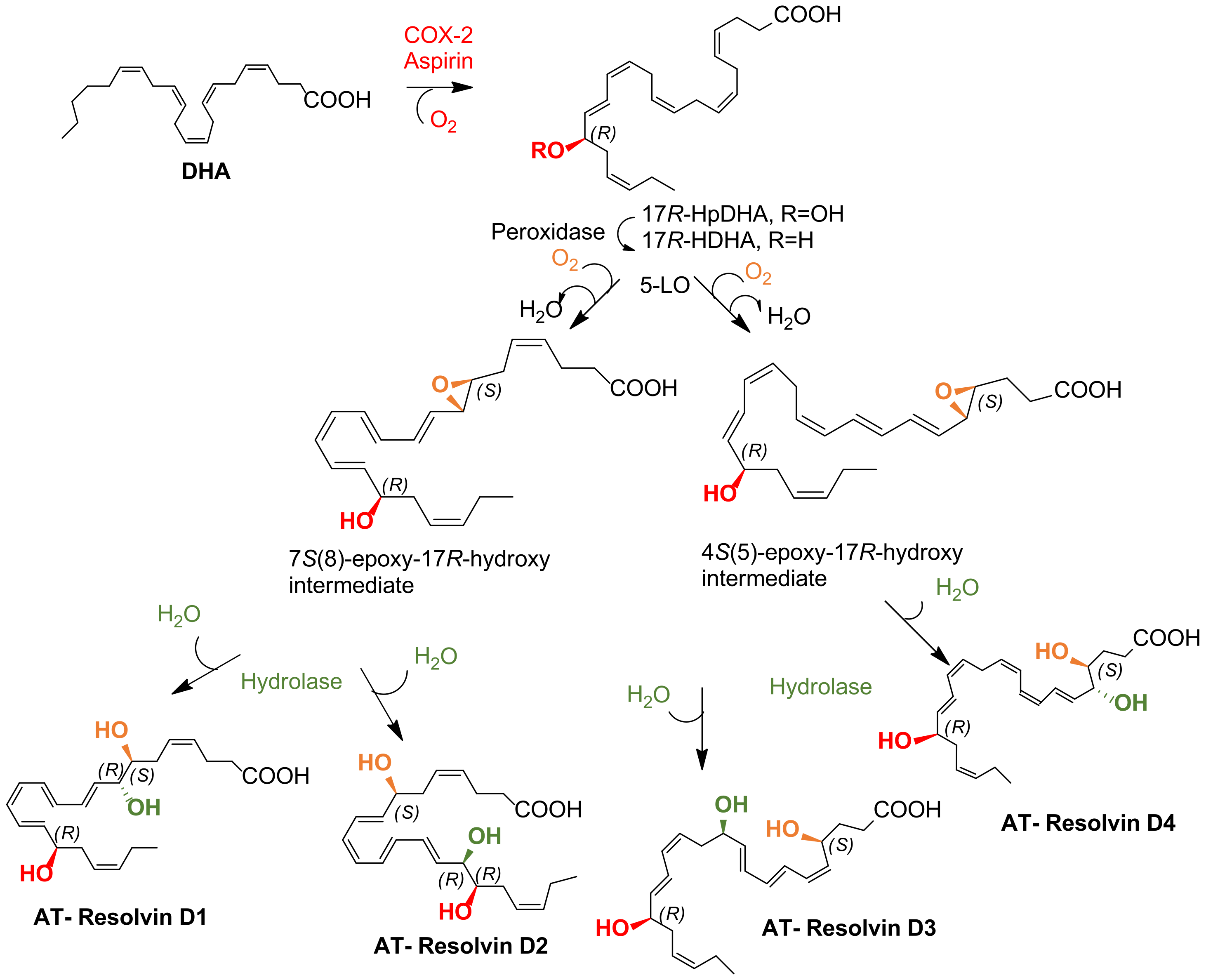
3.1. Biosynthesis of D-Series and Aspirin-Dependent D-Series Resolvins
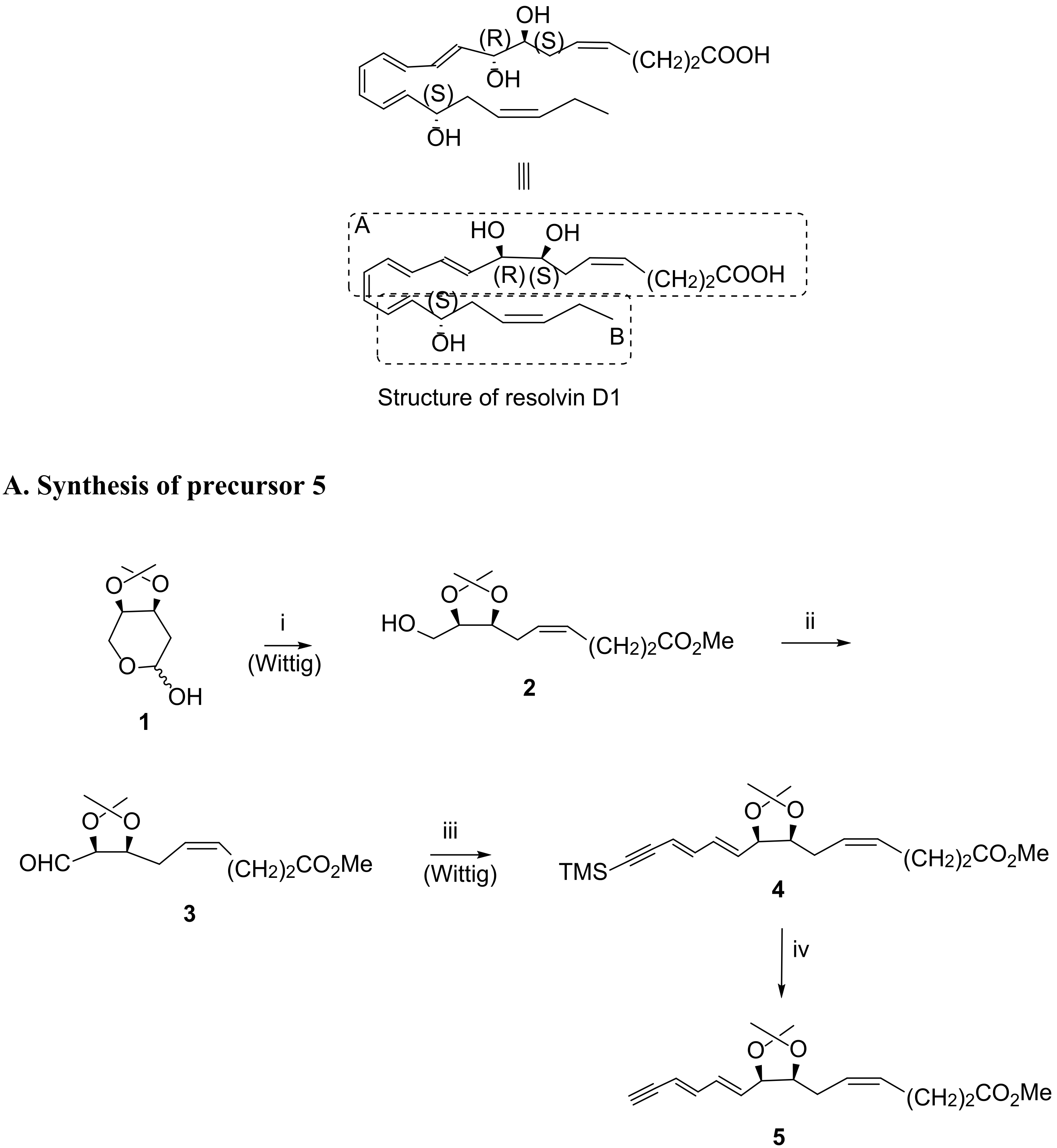
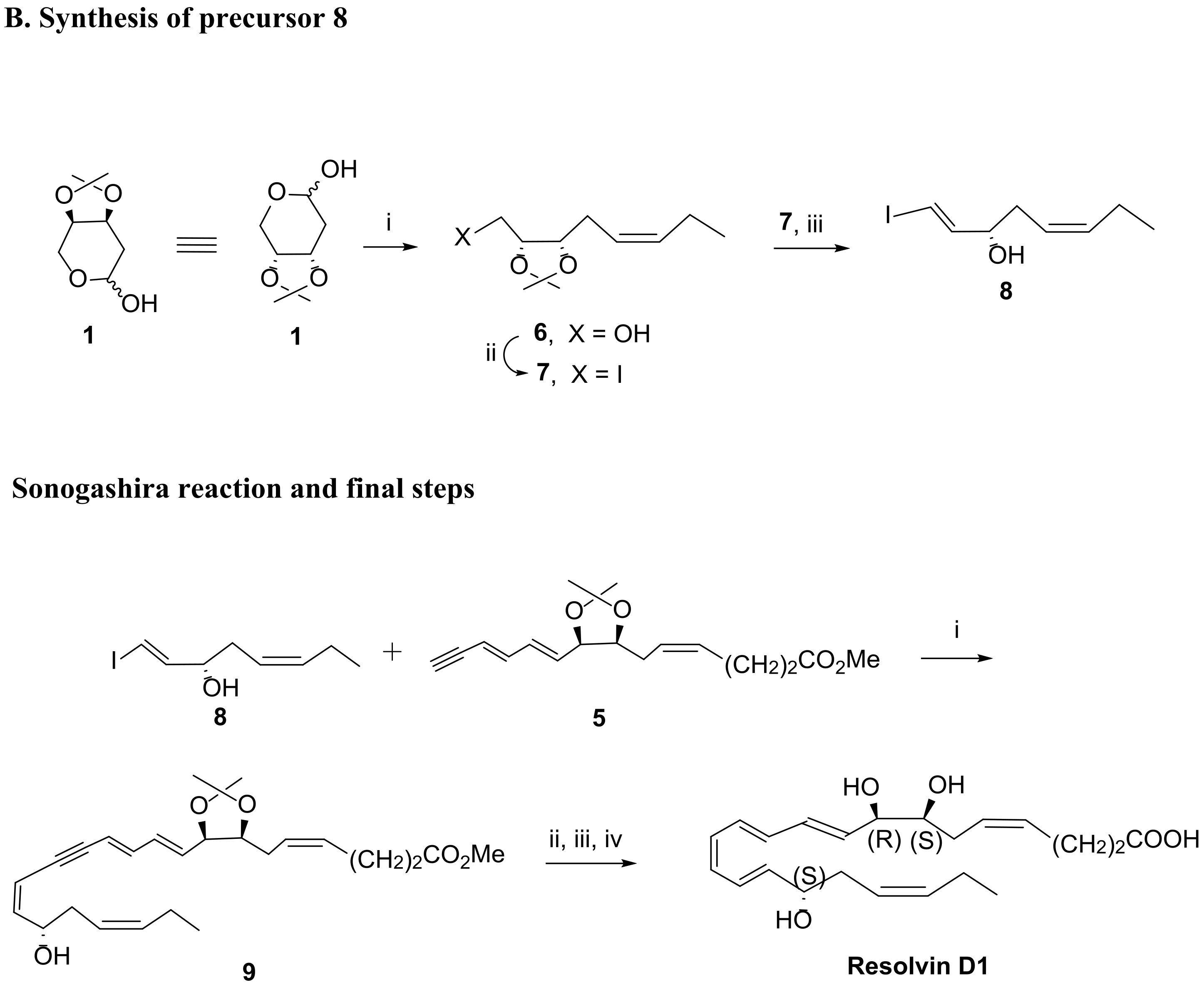
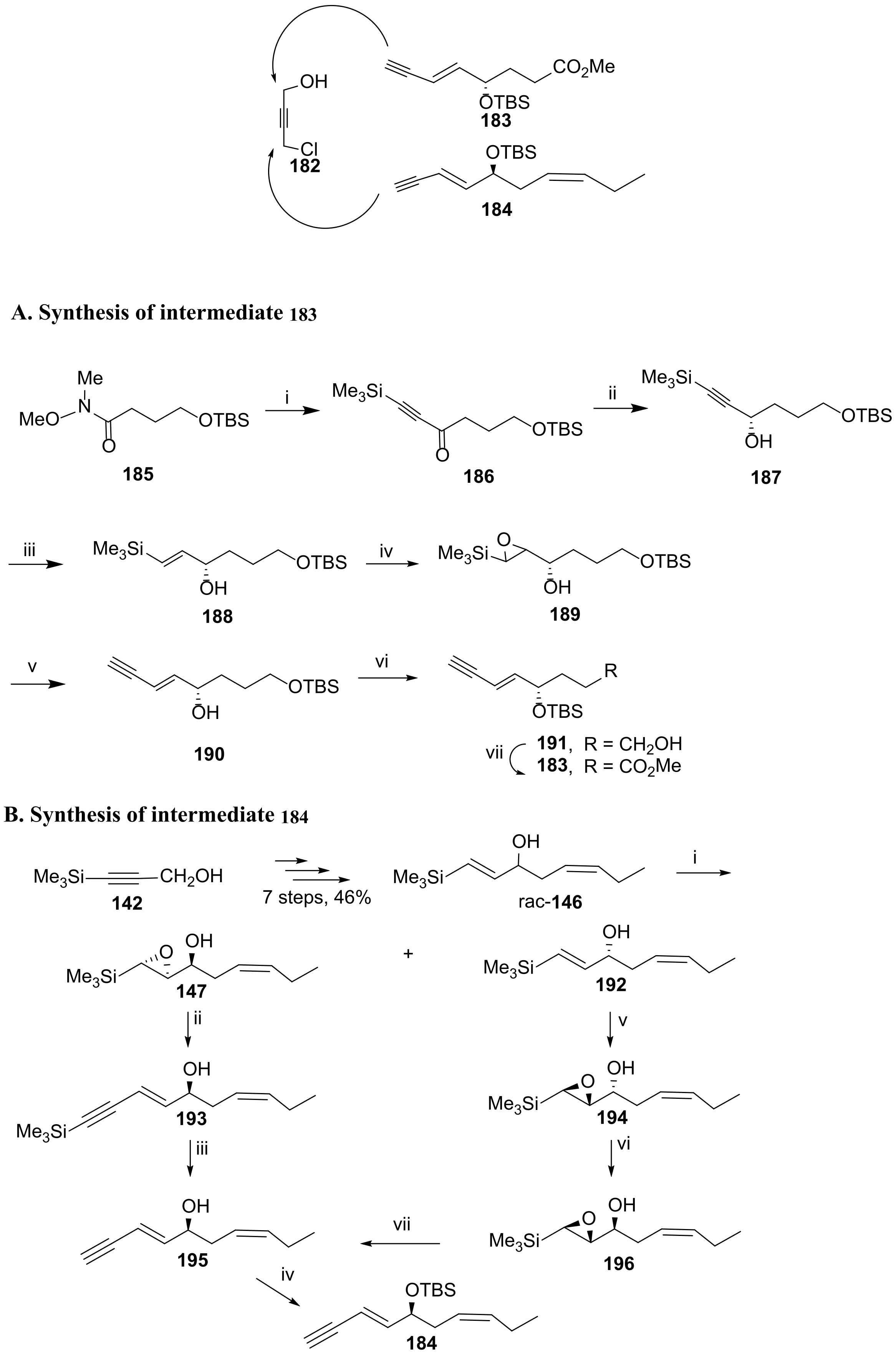
3.2. Total Synthesis of D-Series Resolvins
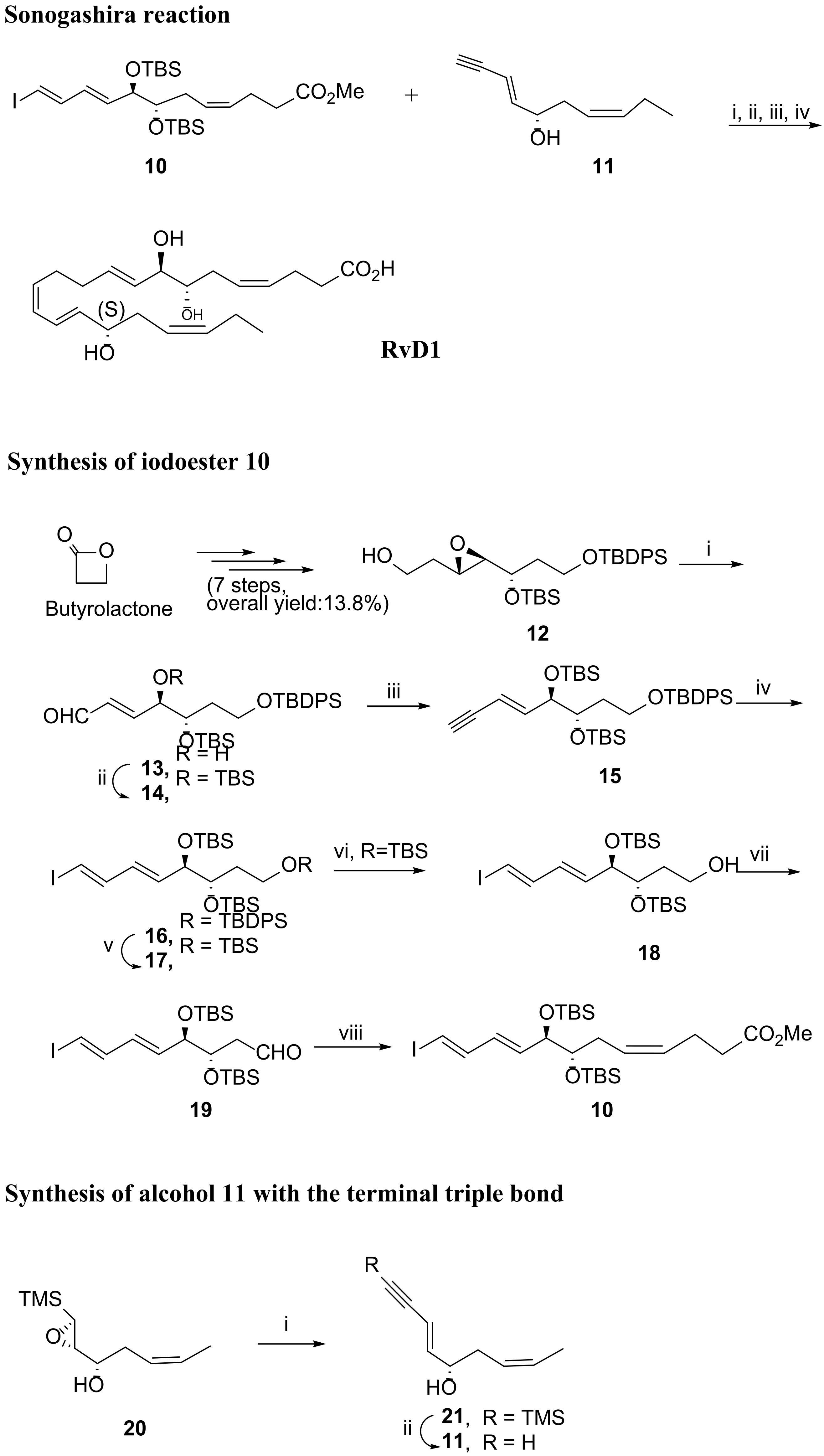
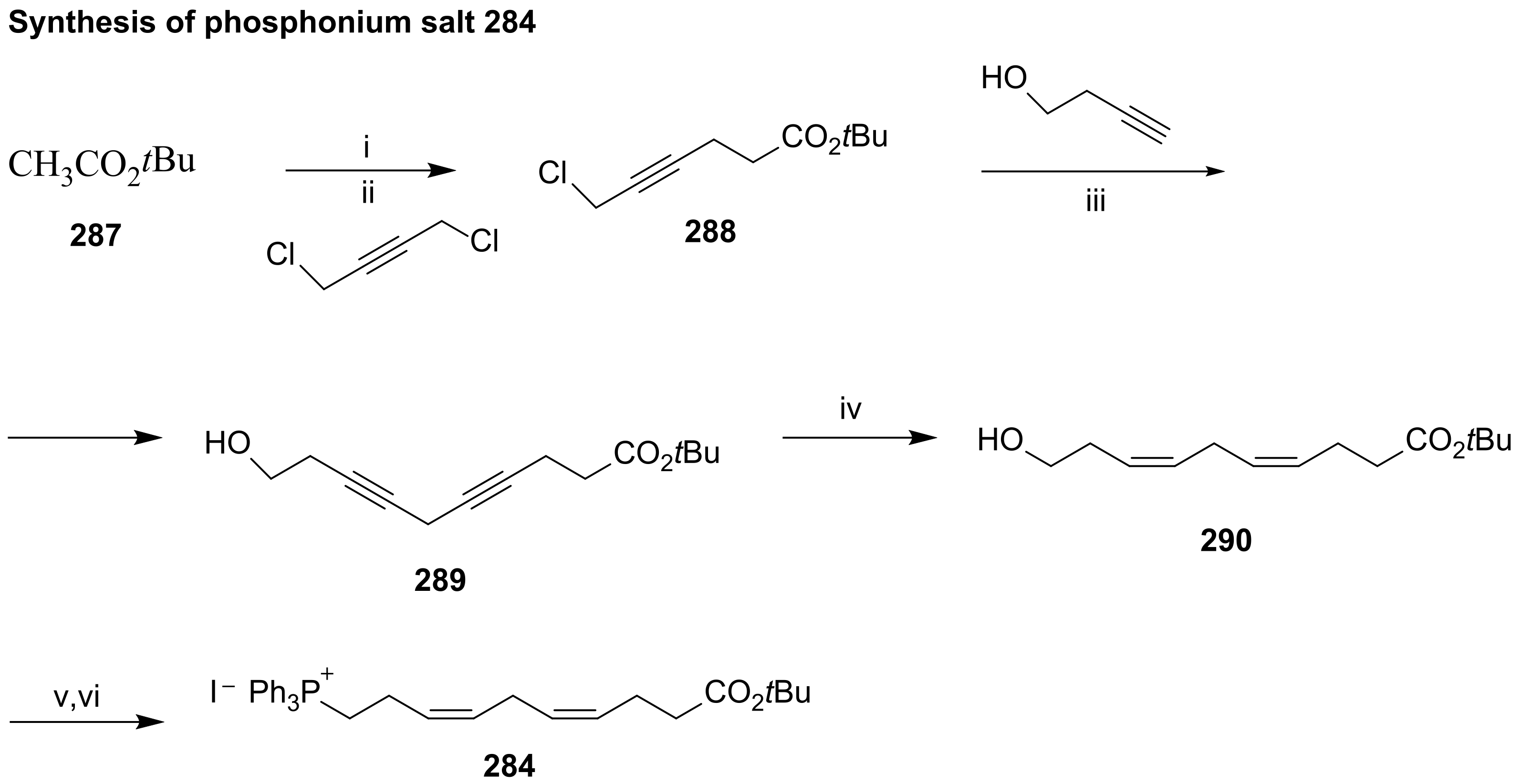
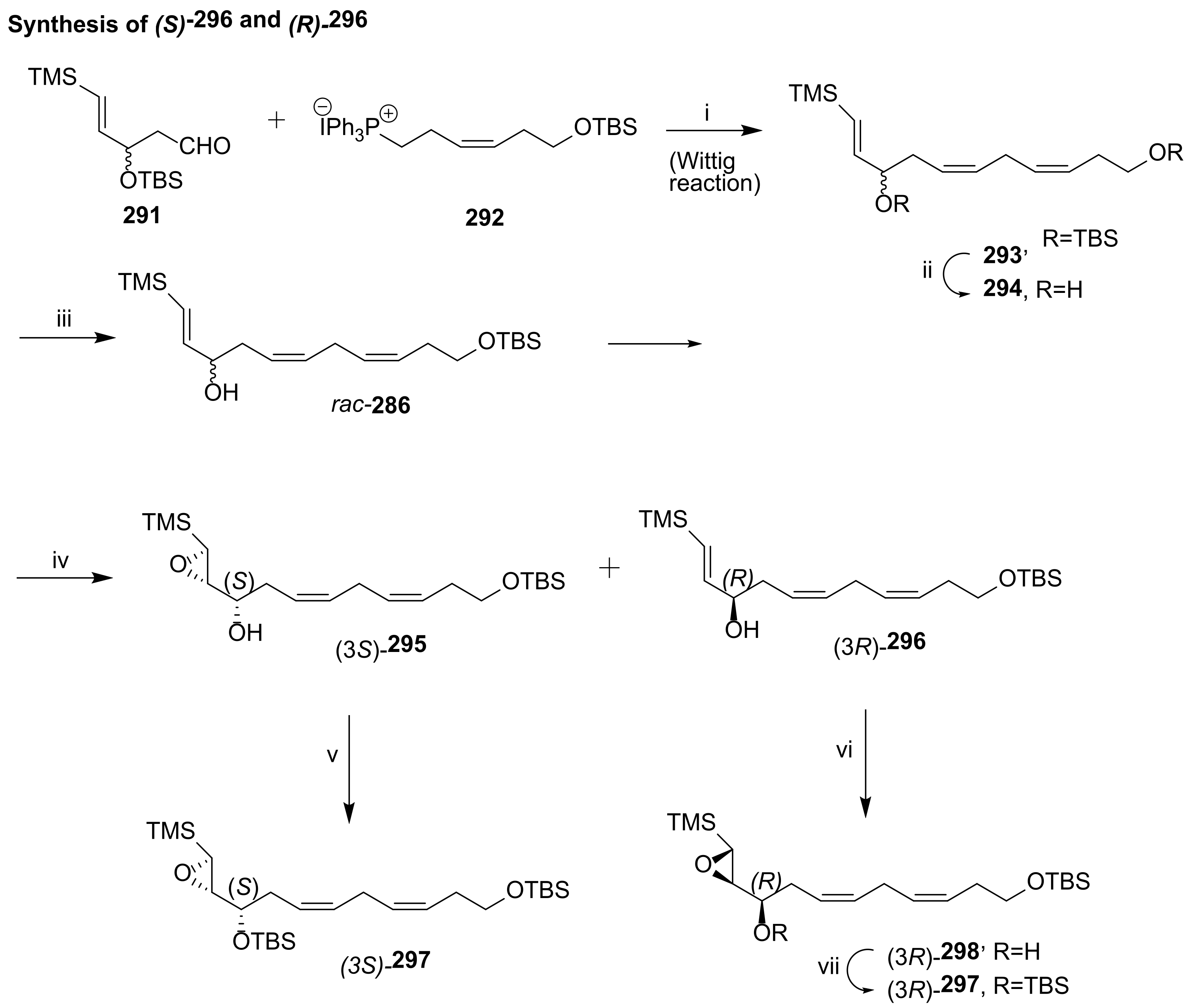
3.2.1. Resolvin D1
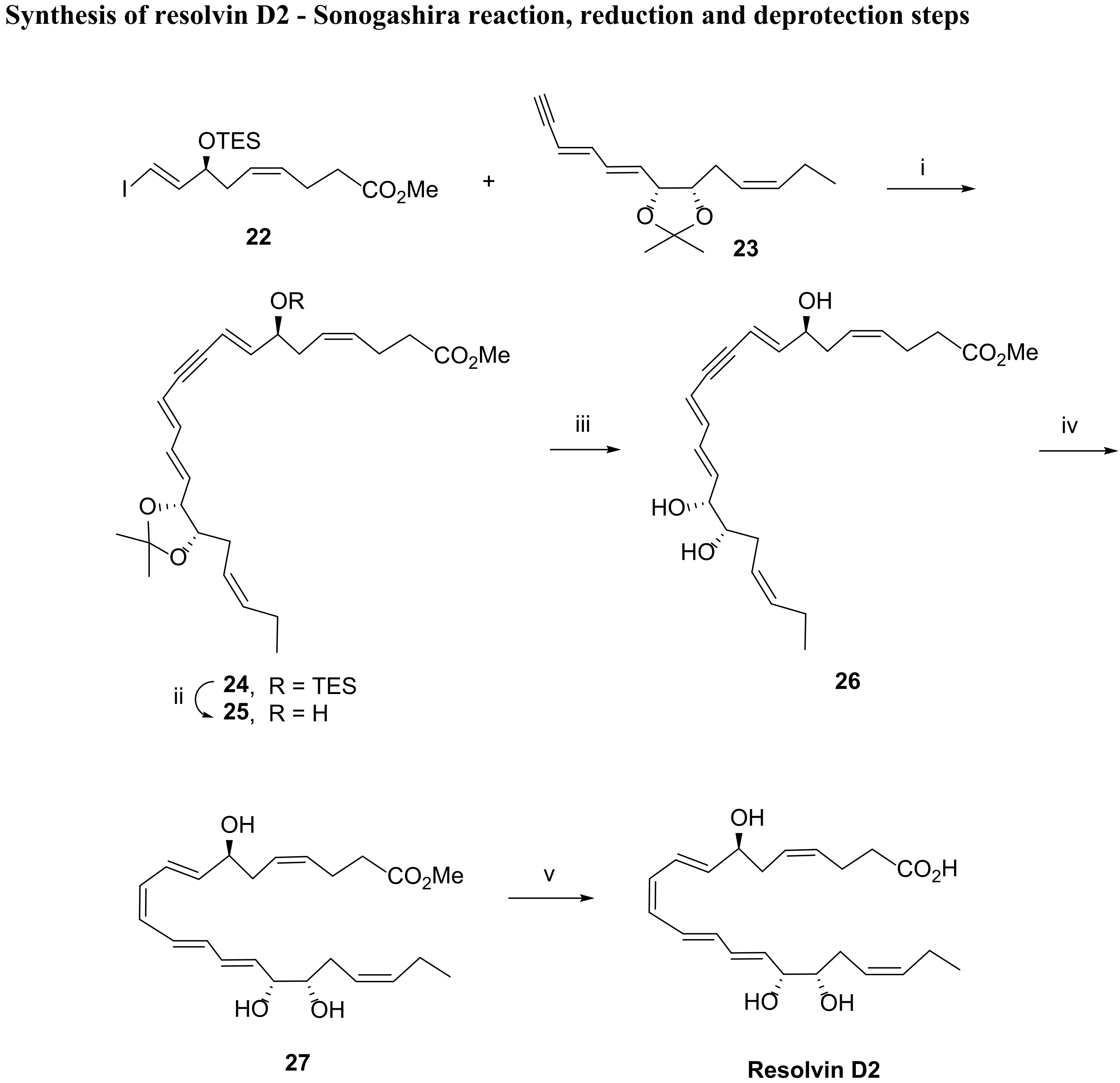
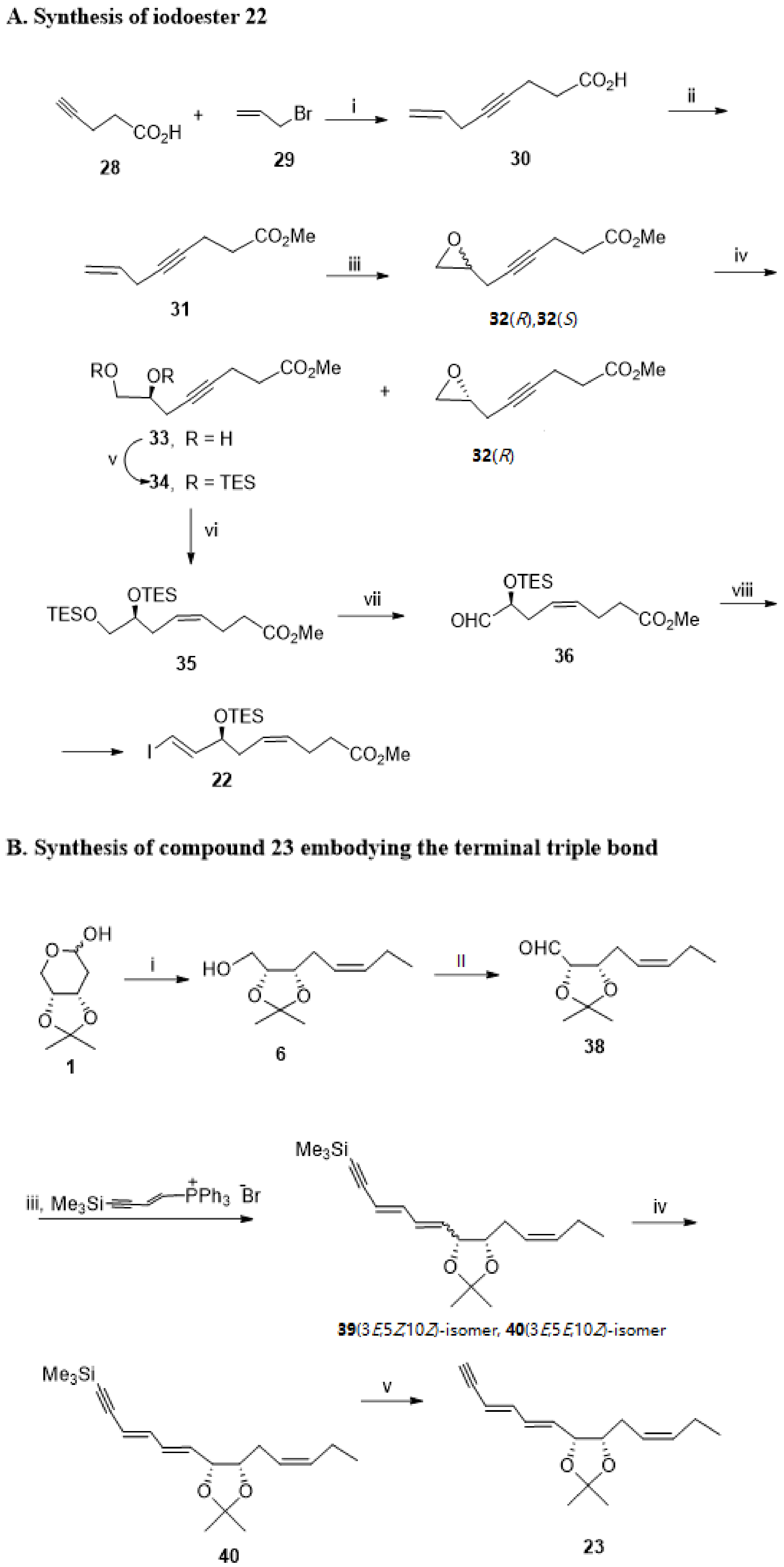
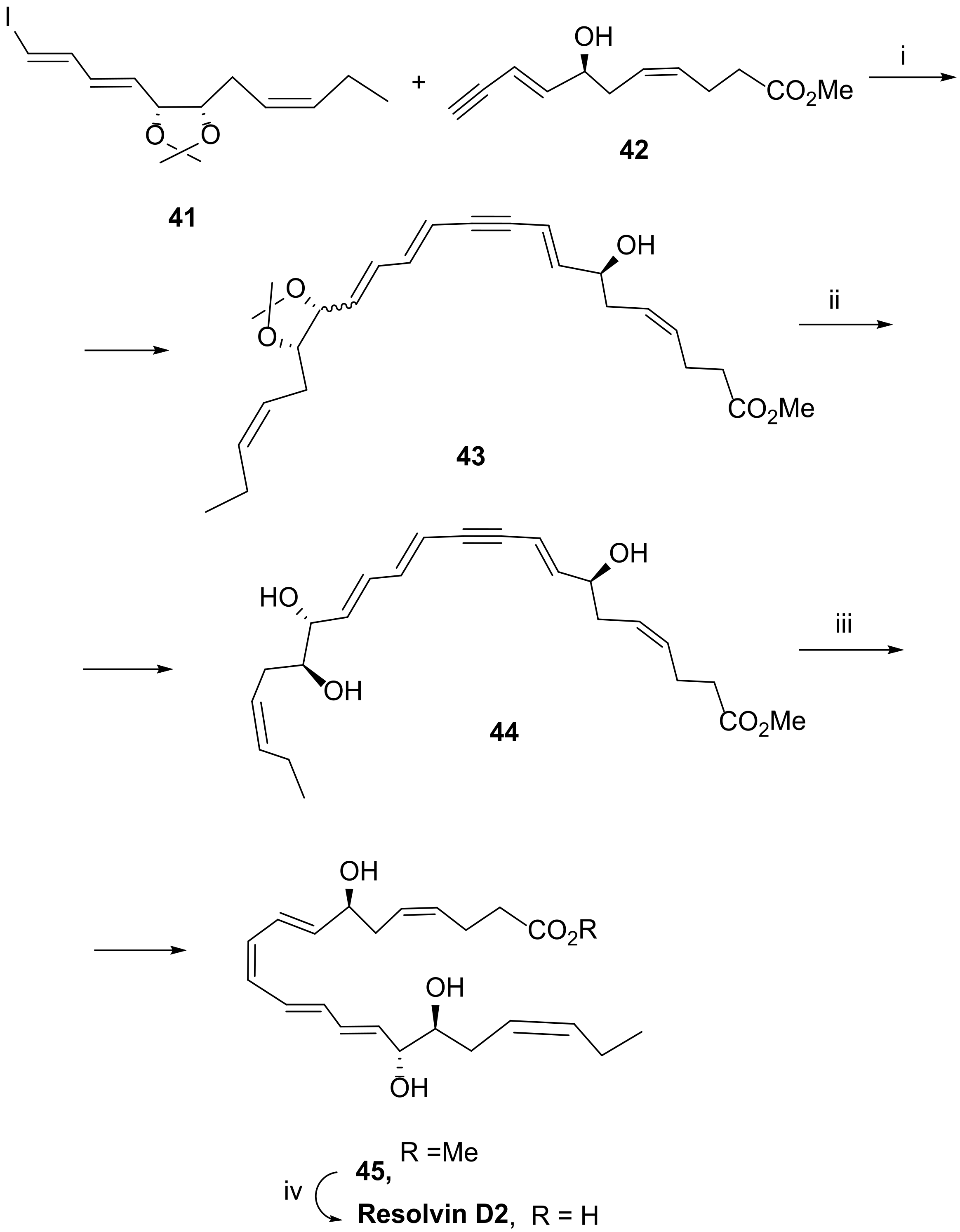
3.2.2. Resolvin D2
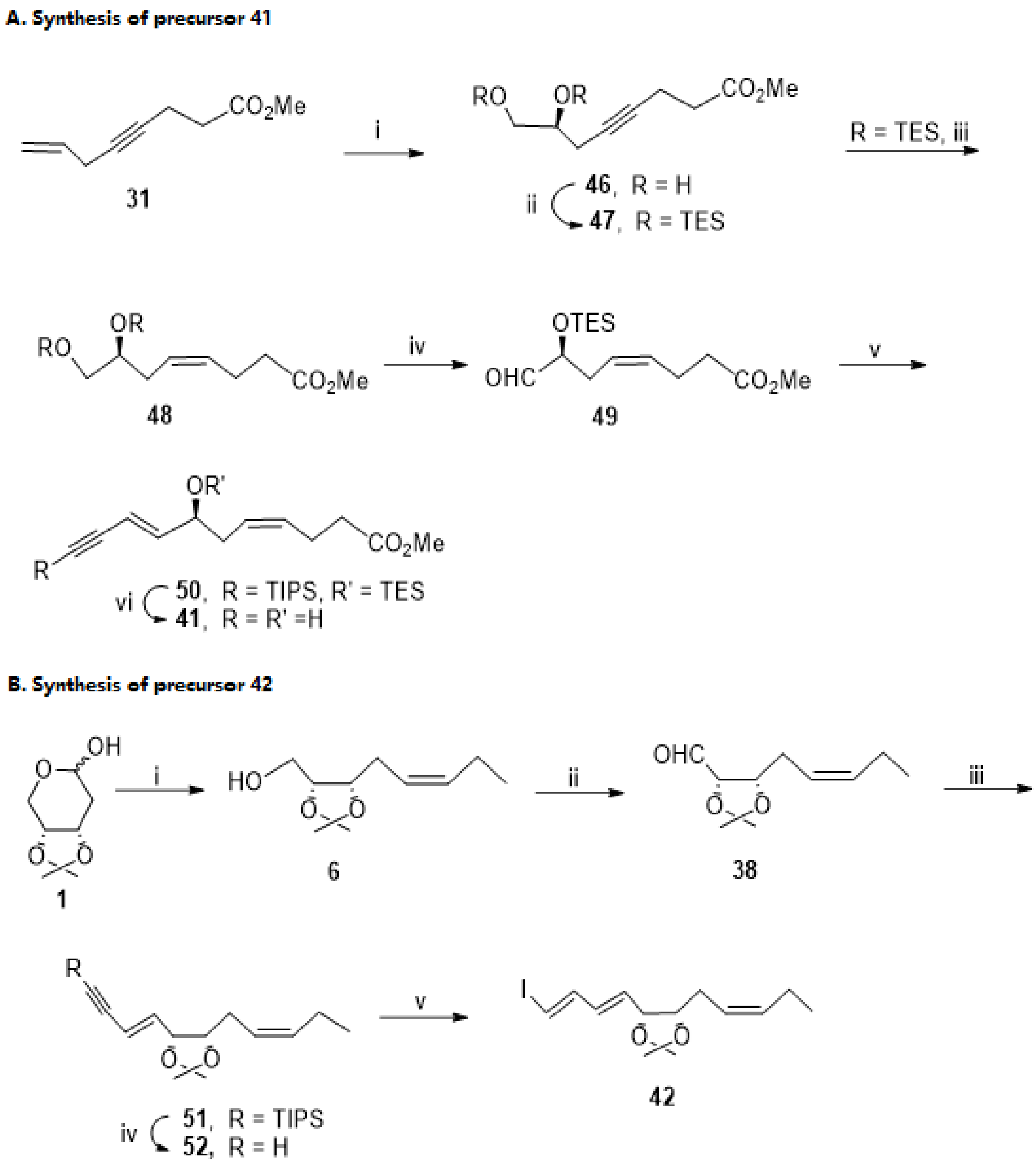
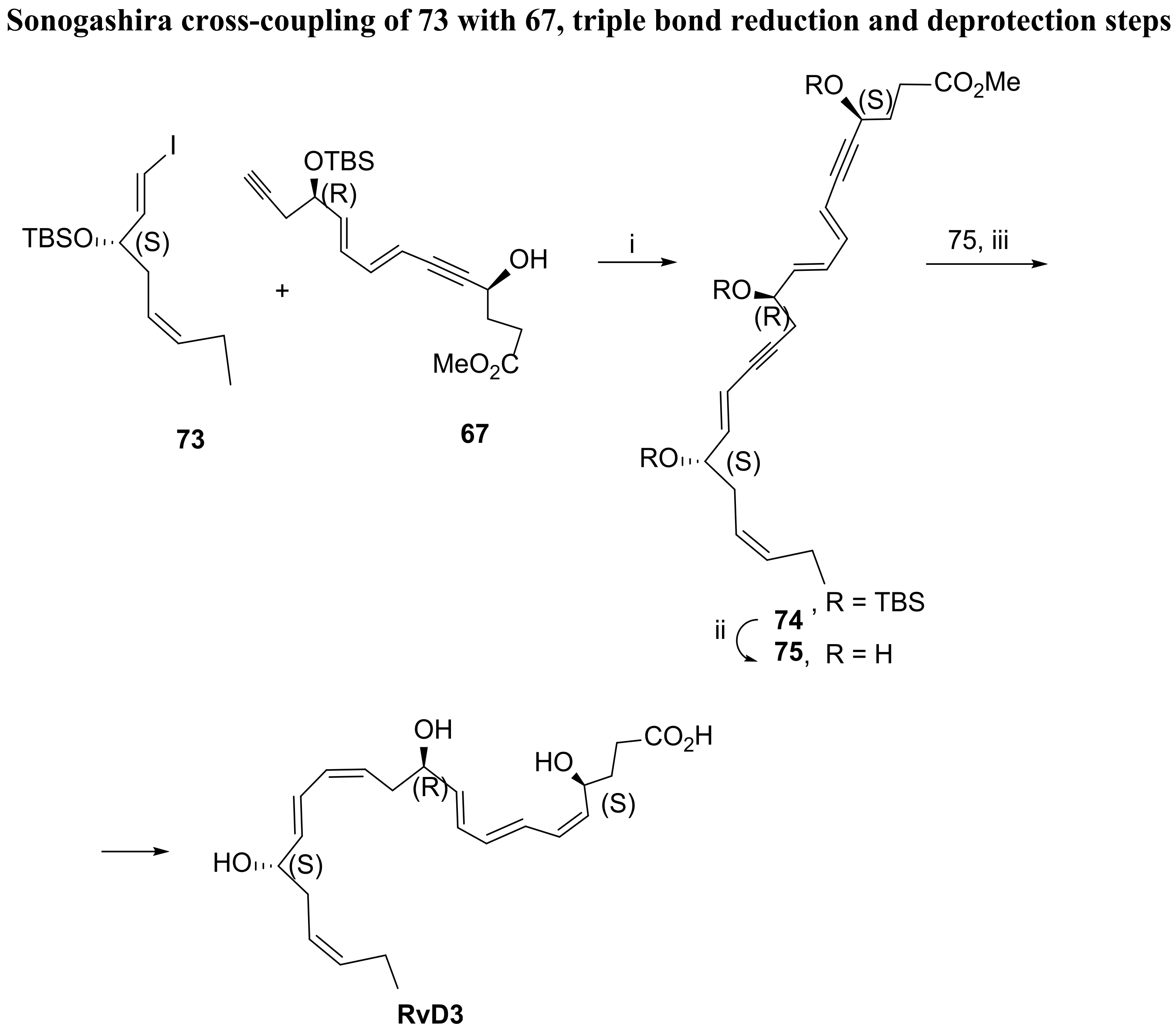
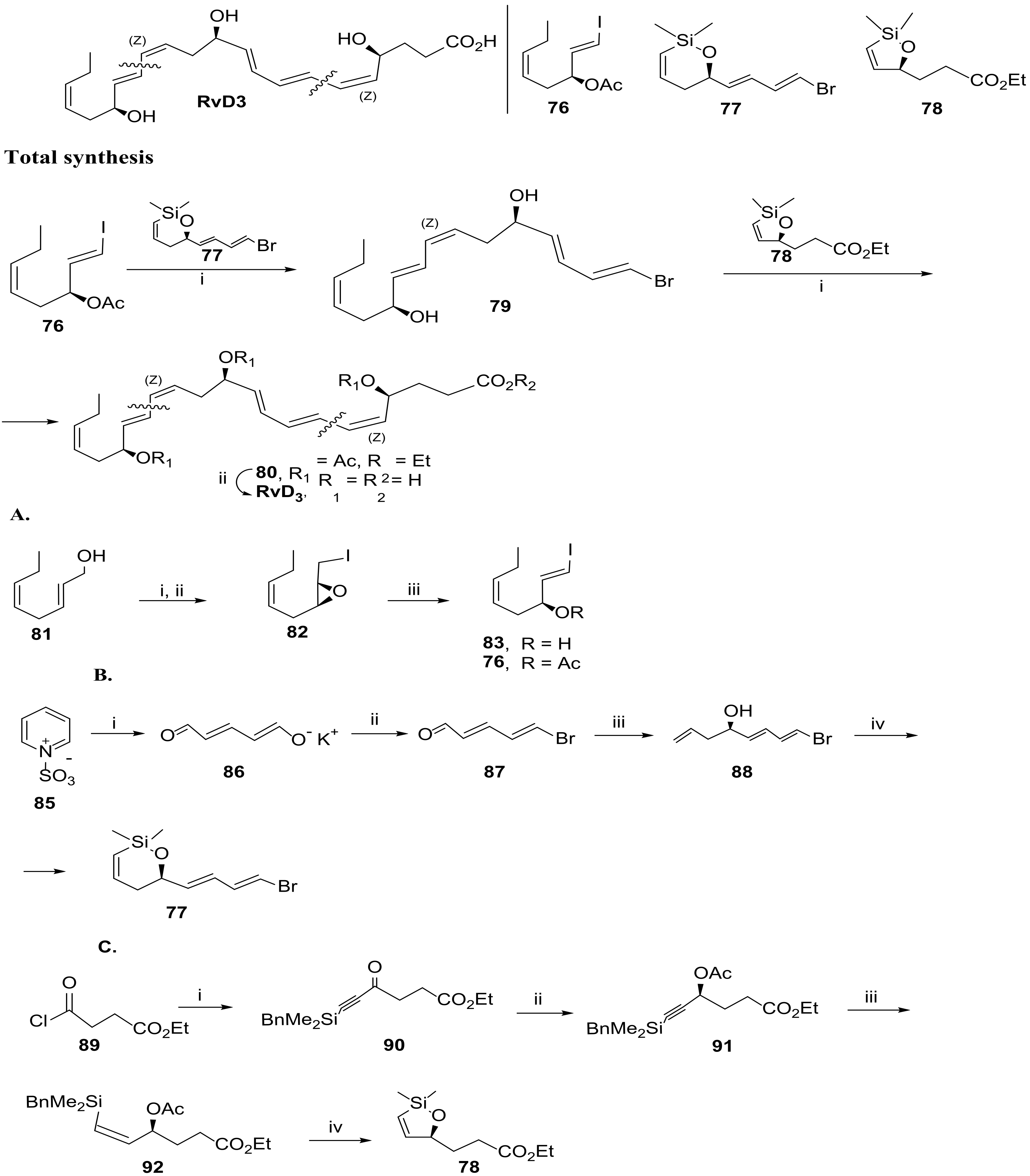
3.2.3. Resolvin D3
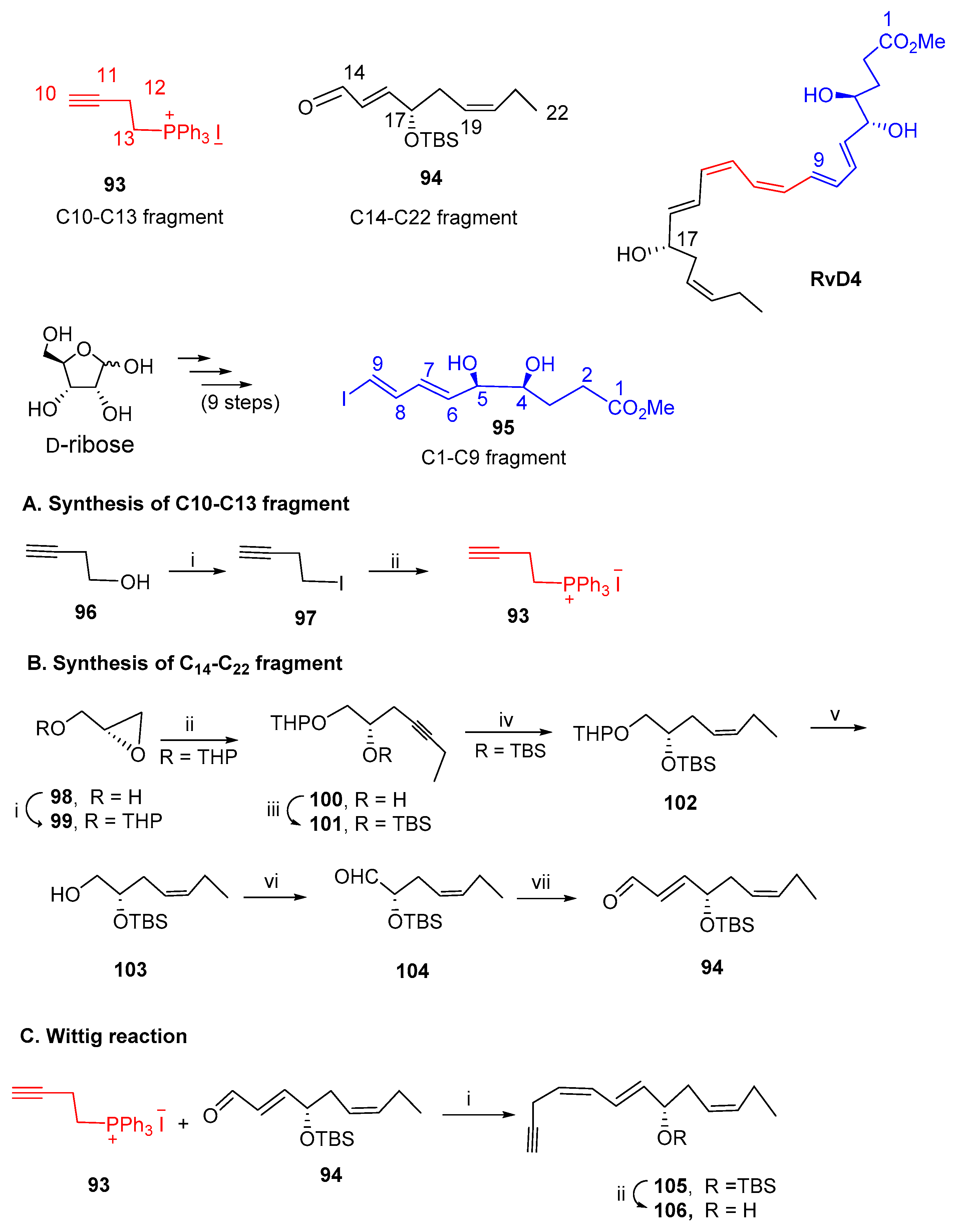
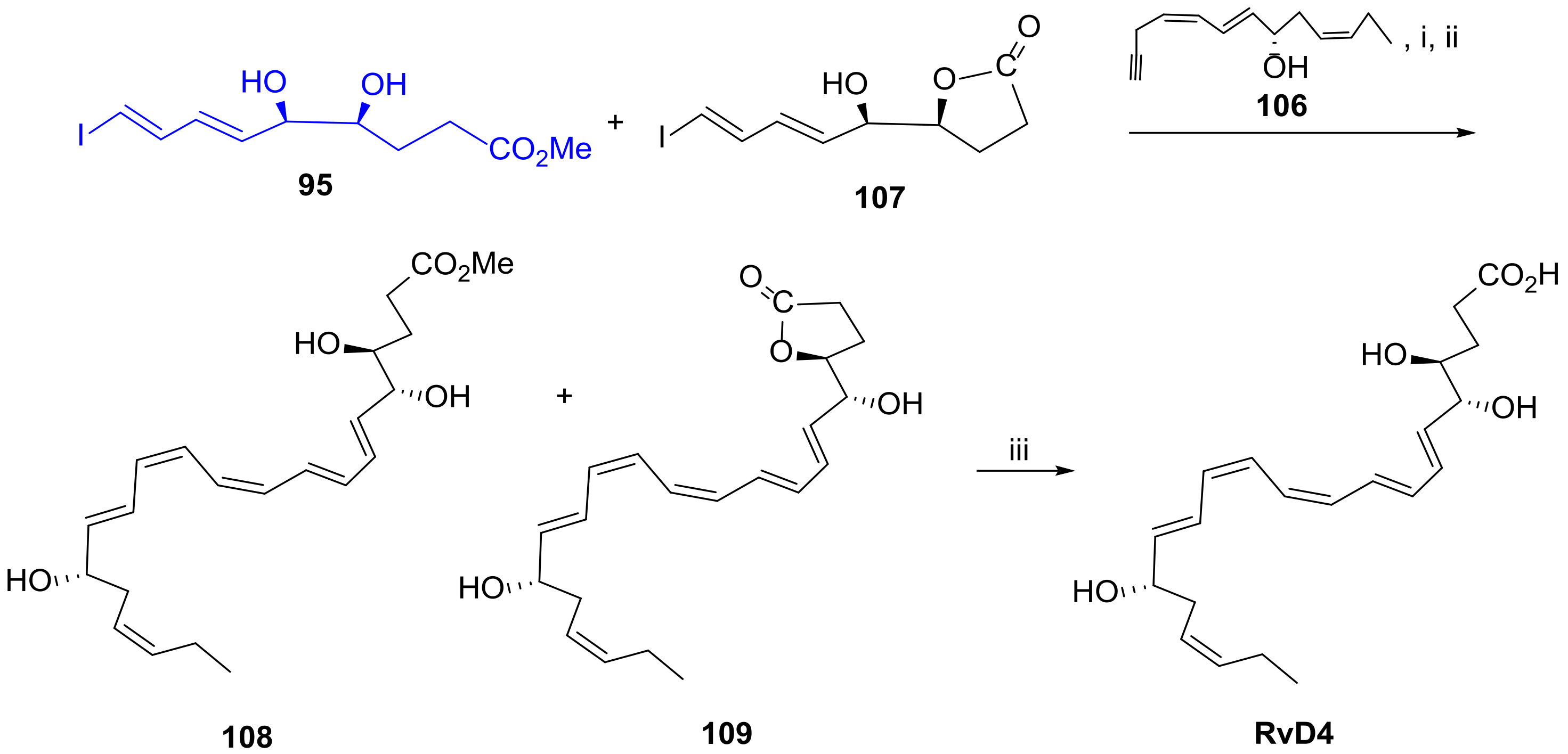
3.2.4. Resolvin D4
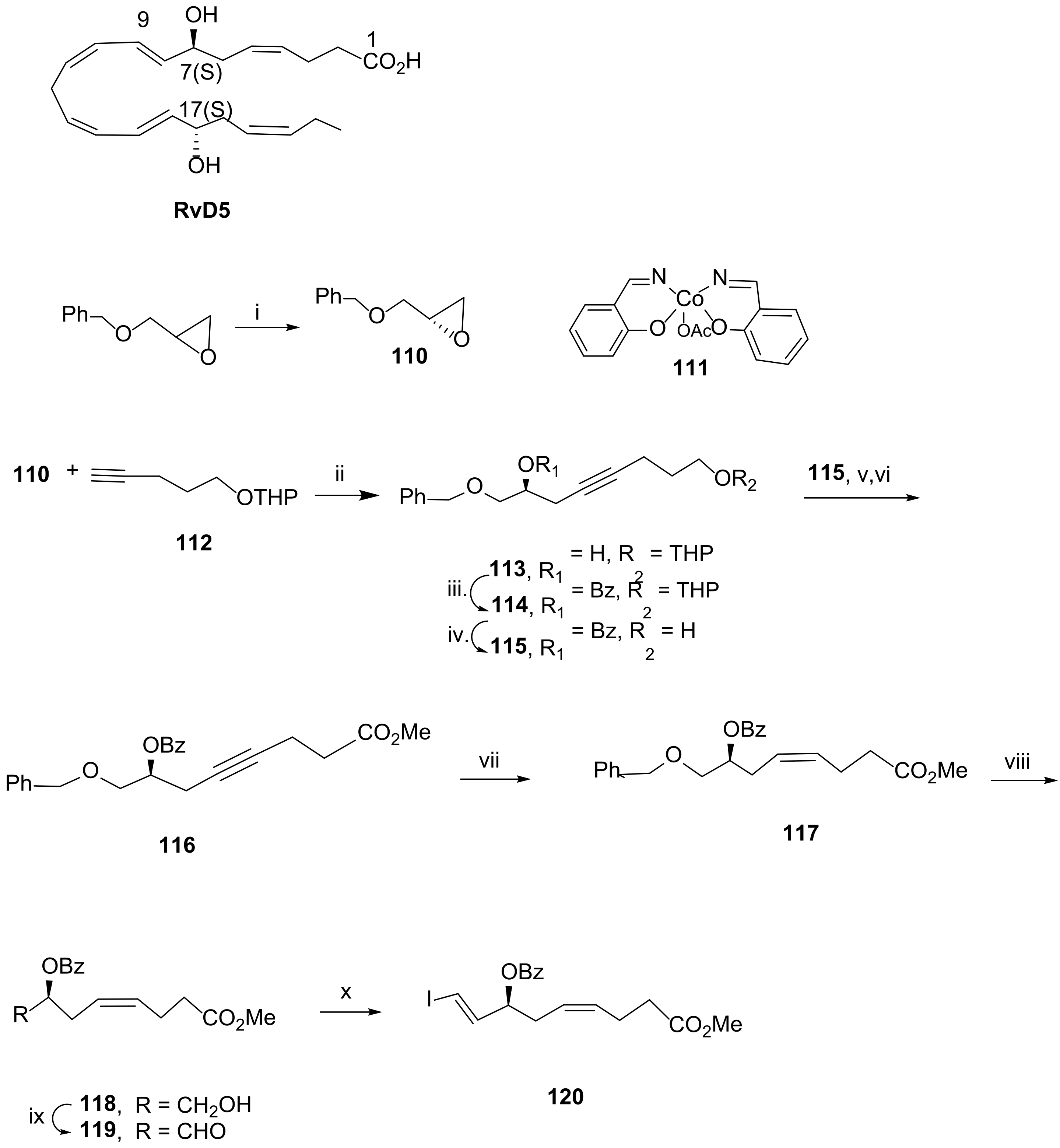
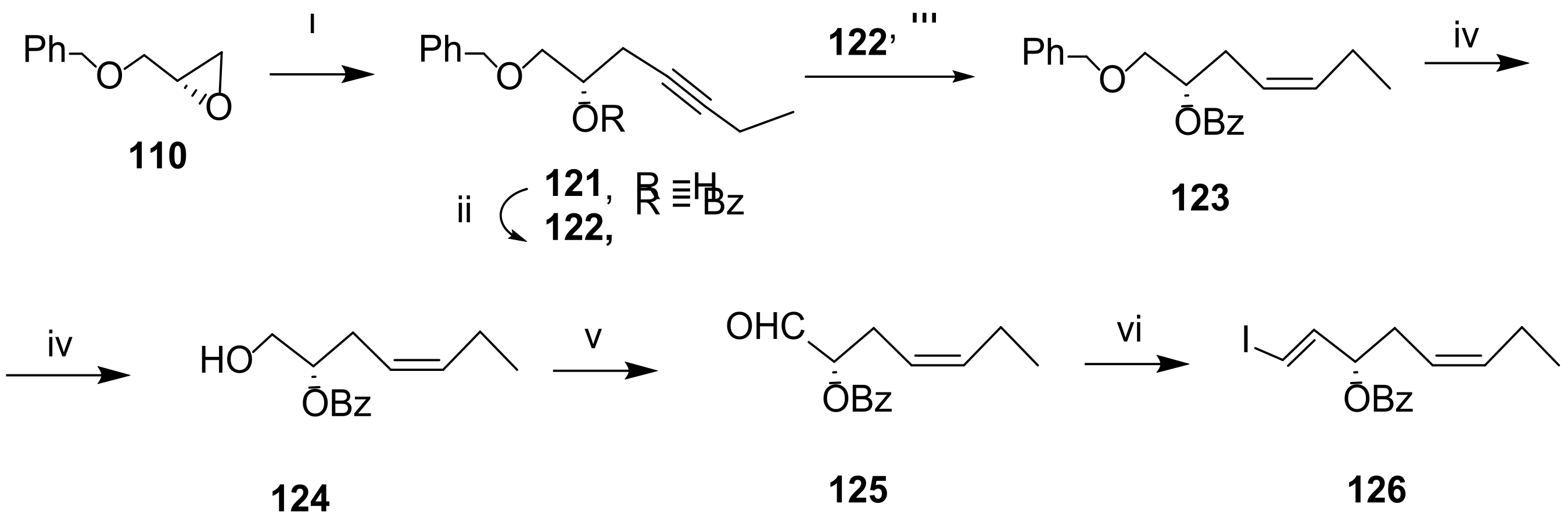
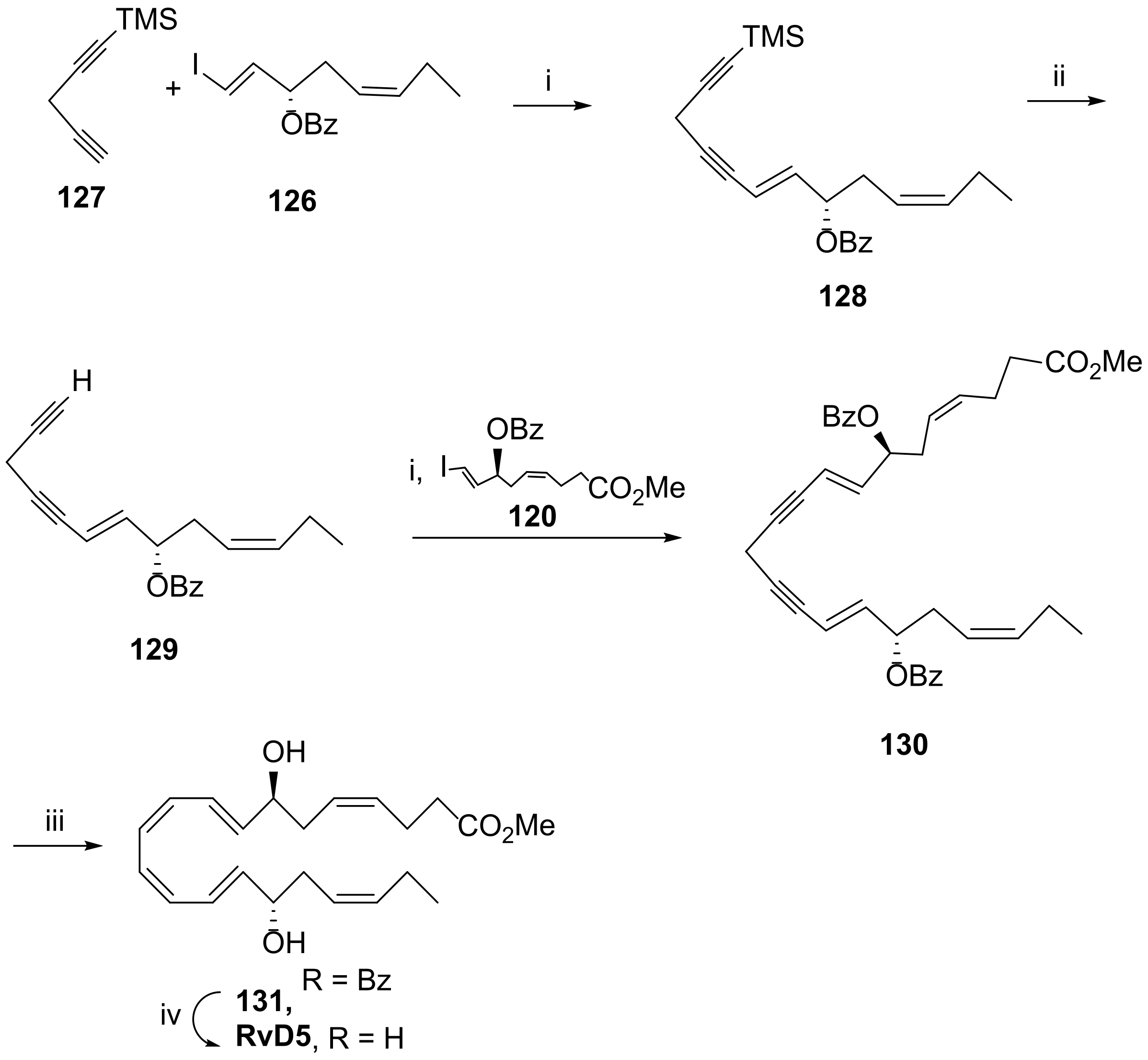
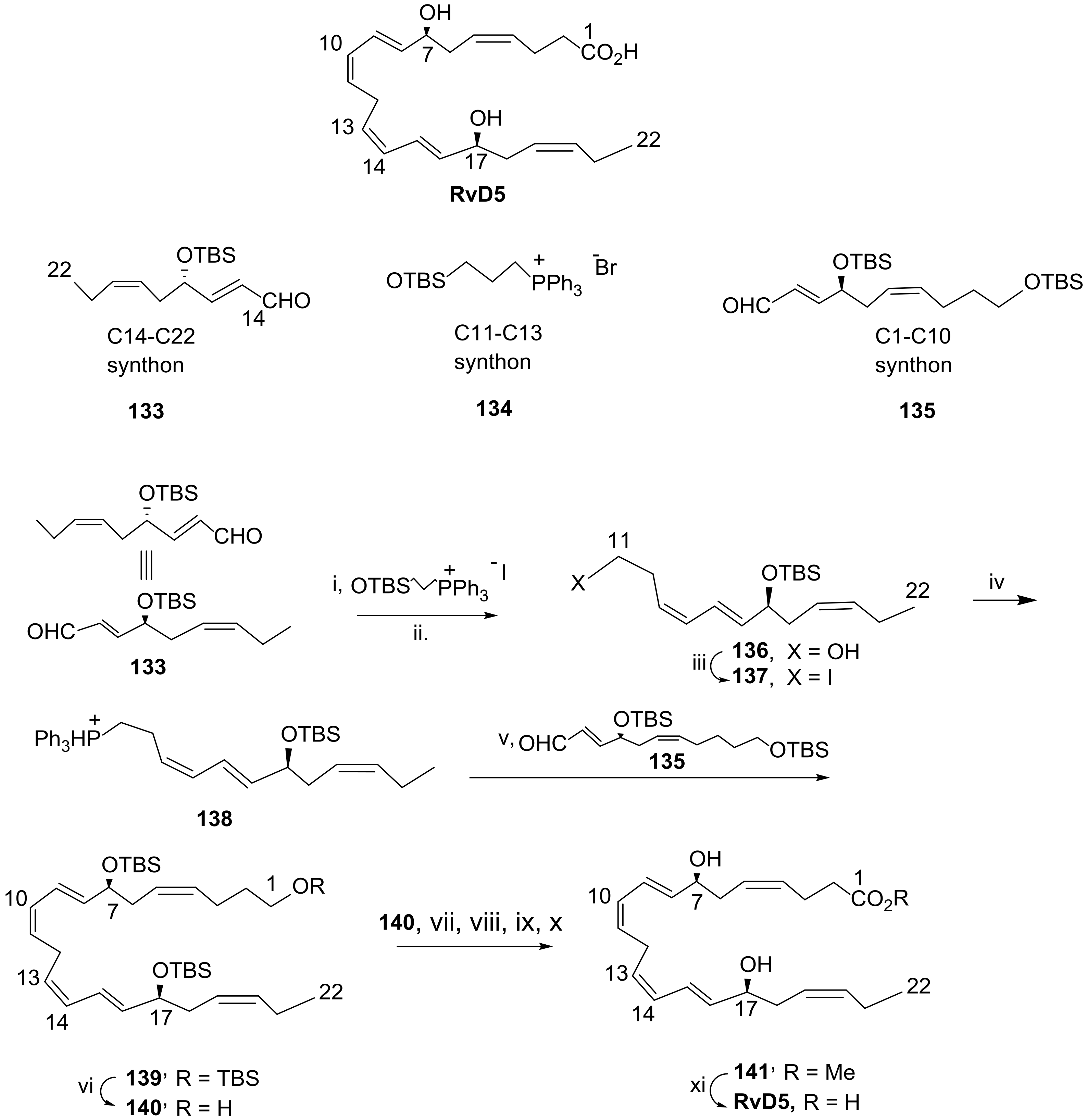
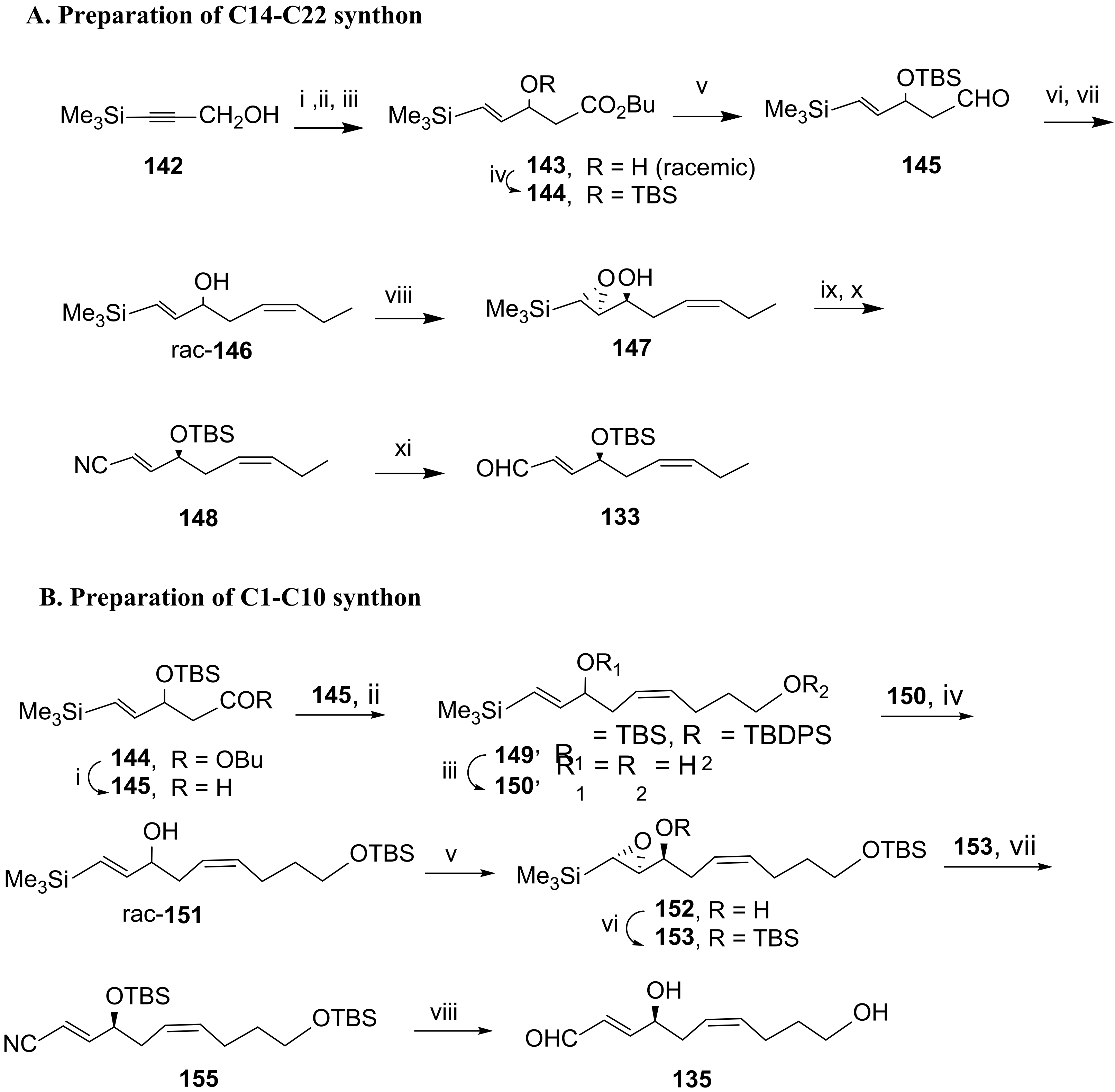
3.2.5. Resolvin D5
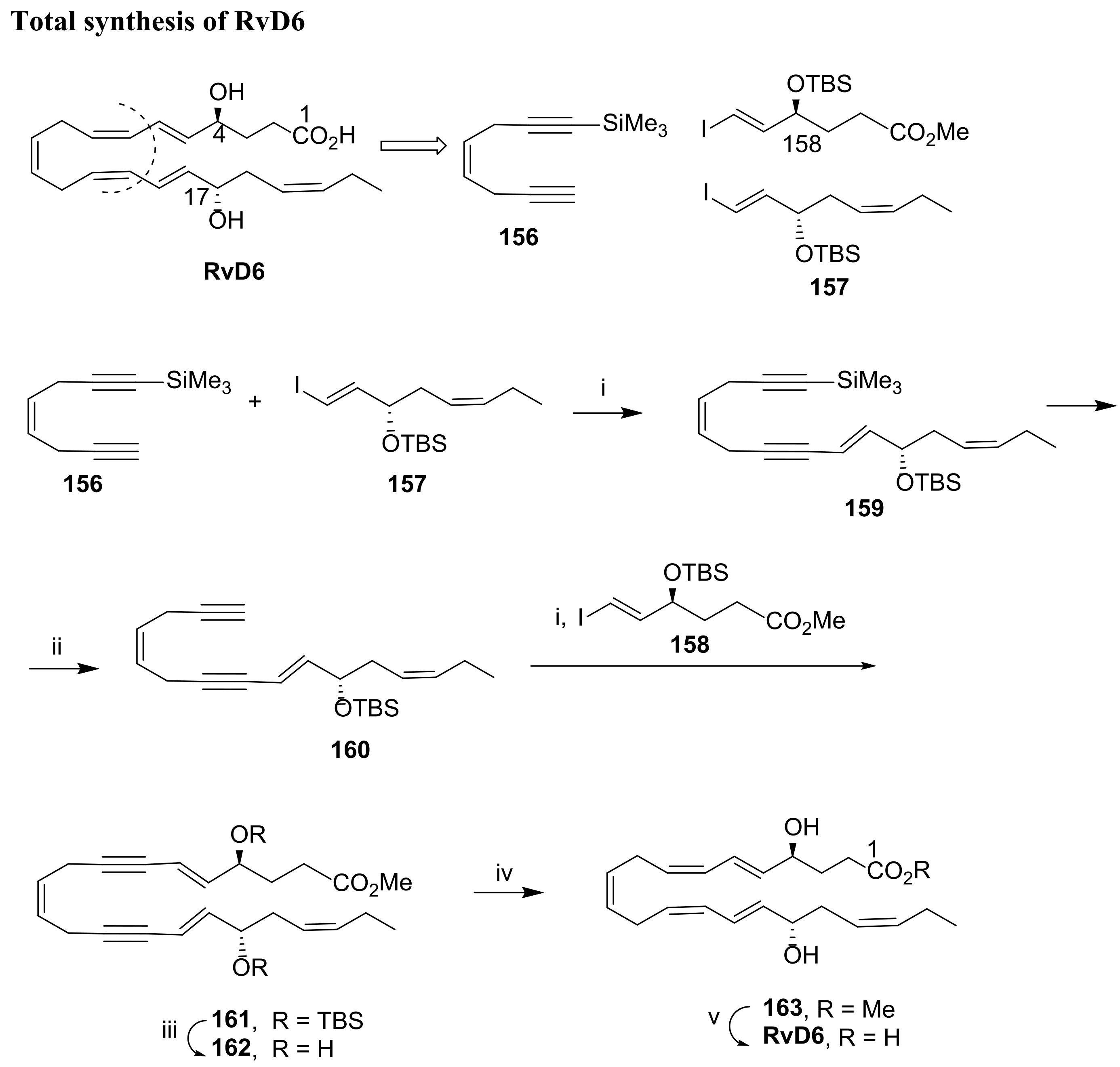
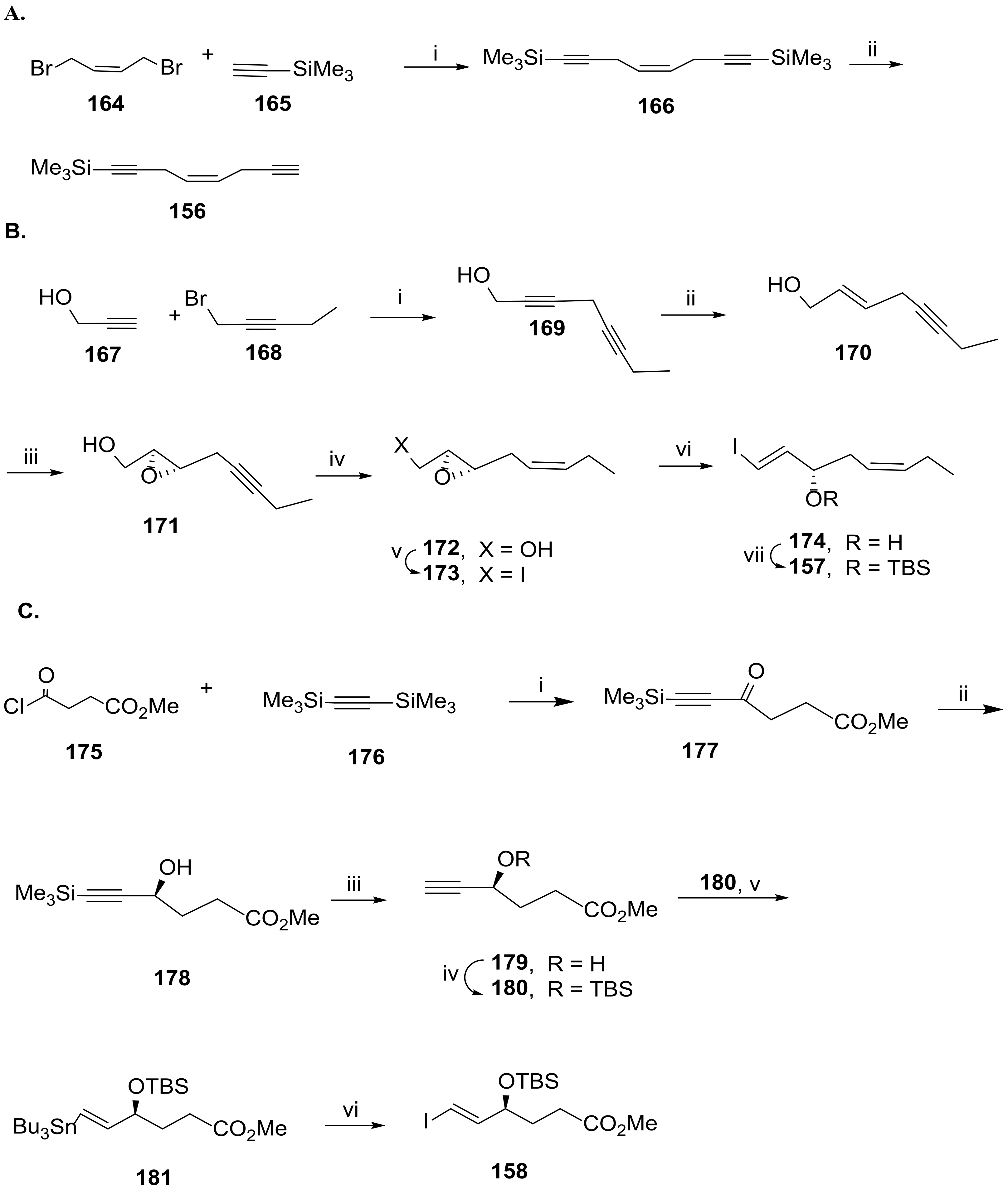
3.2.6. Resolvin D6
4. Protectins from DHA
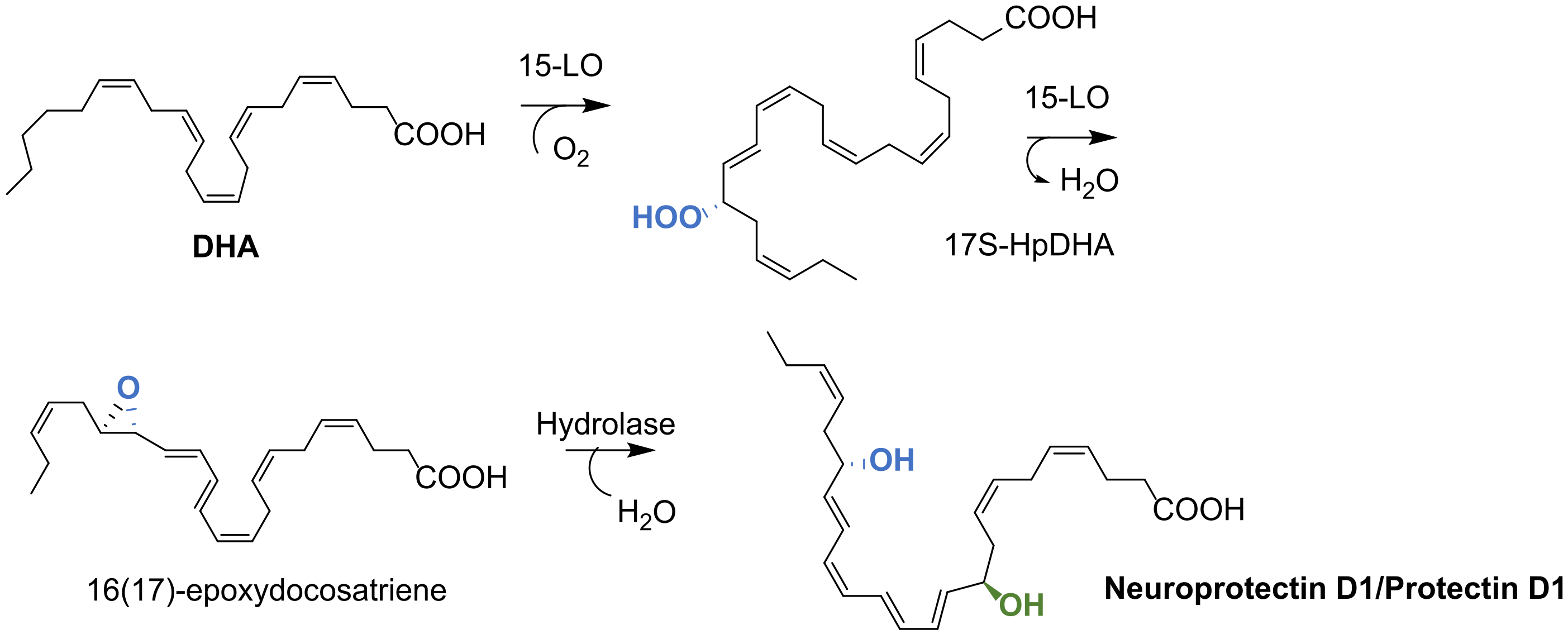
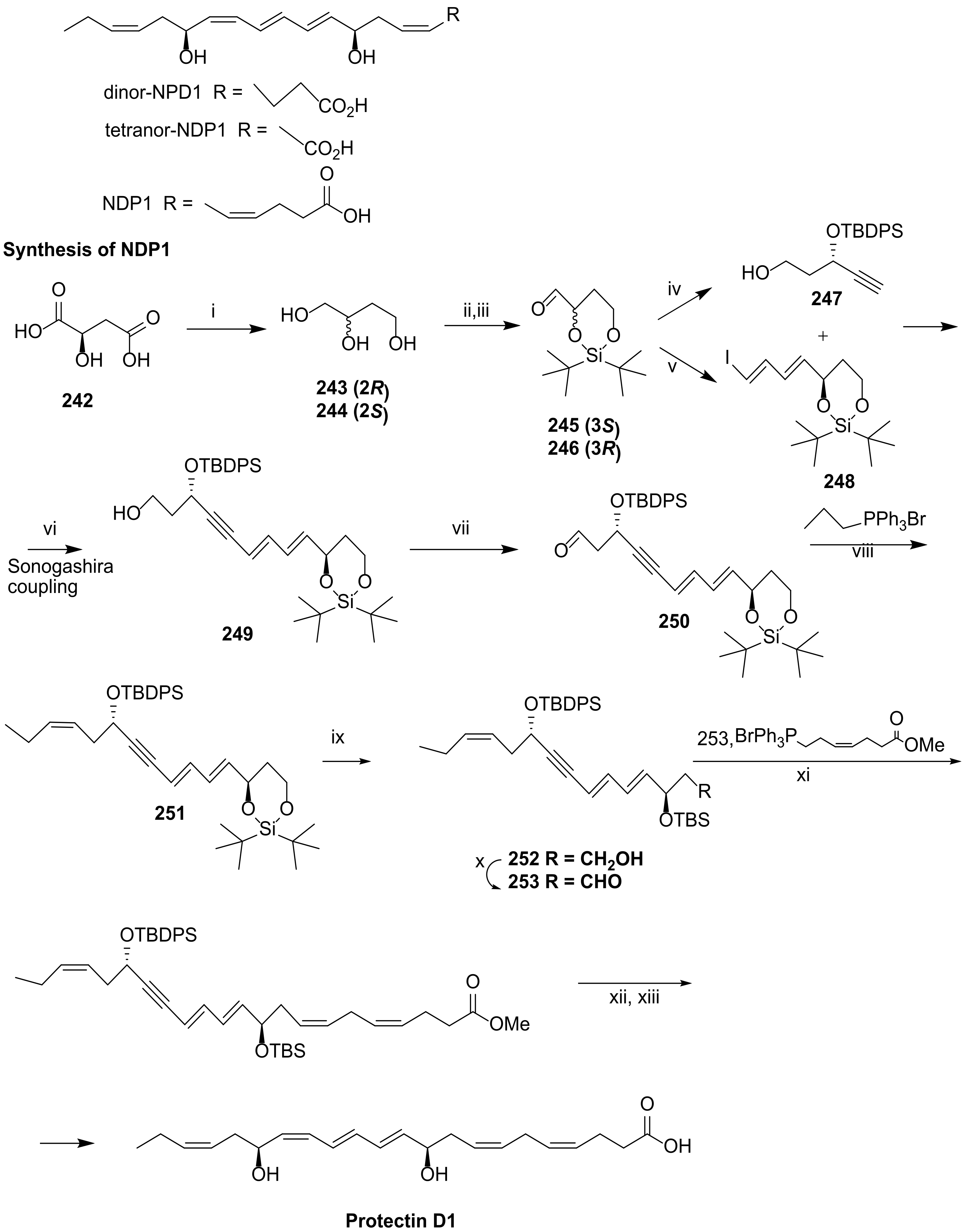
4.1. Biosynthesis of Protectin D1
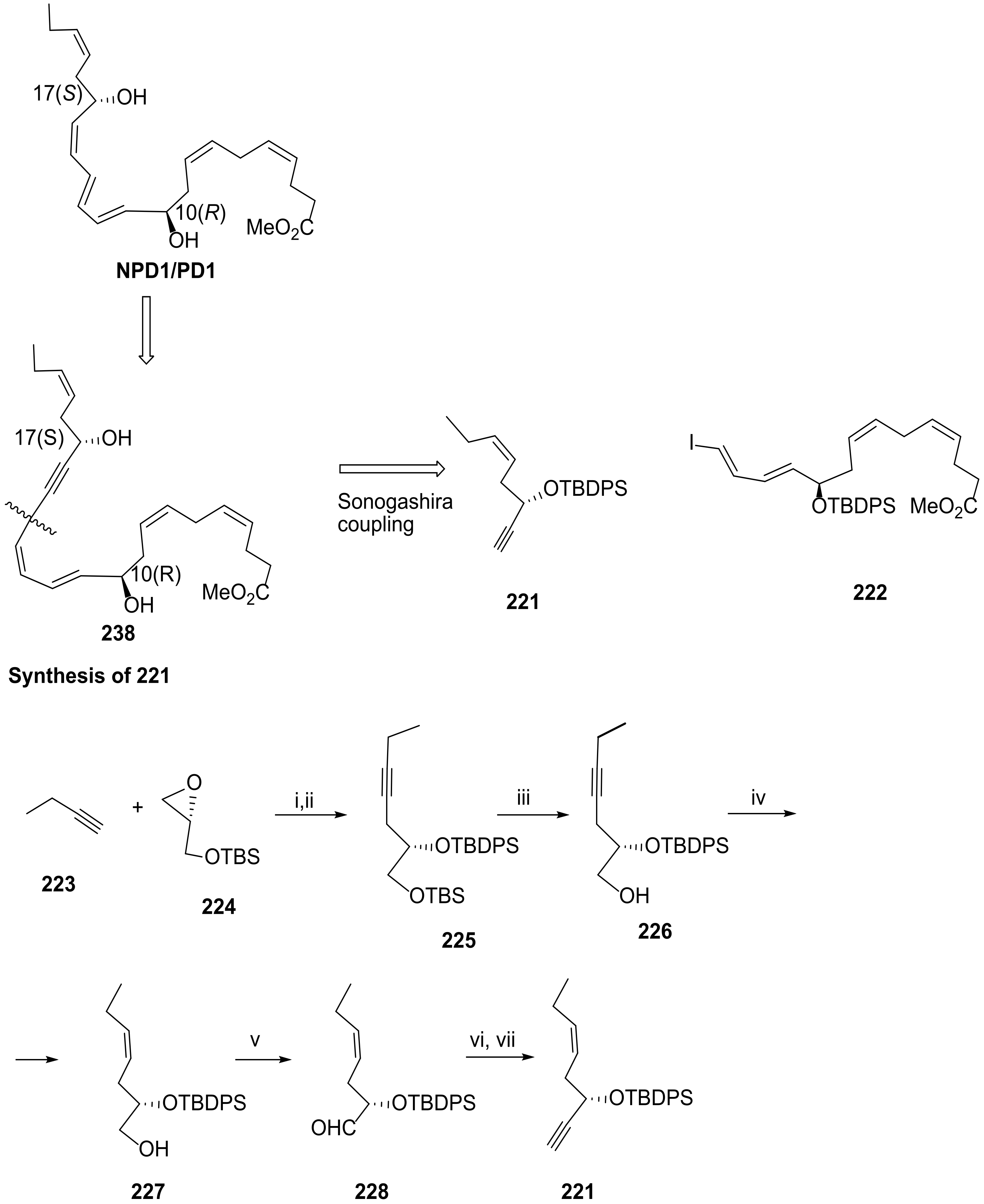
4.2. Total Synthesis of Protectin D1
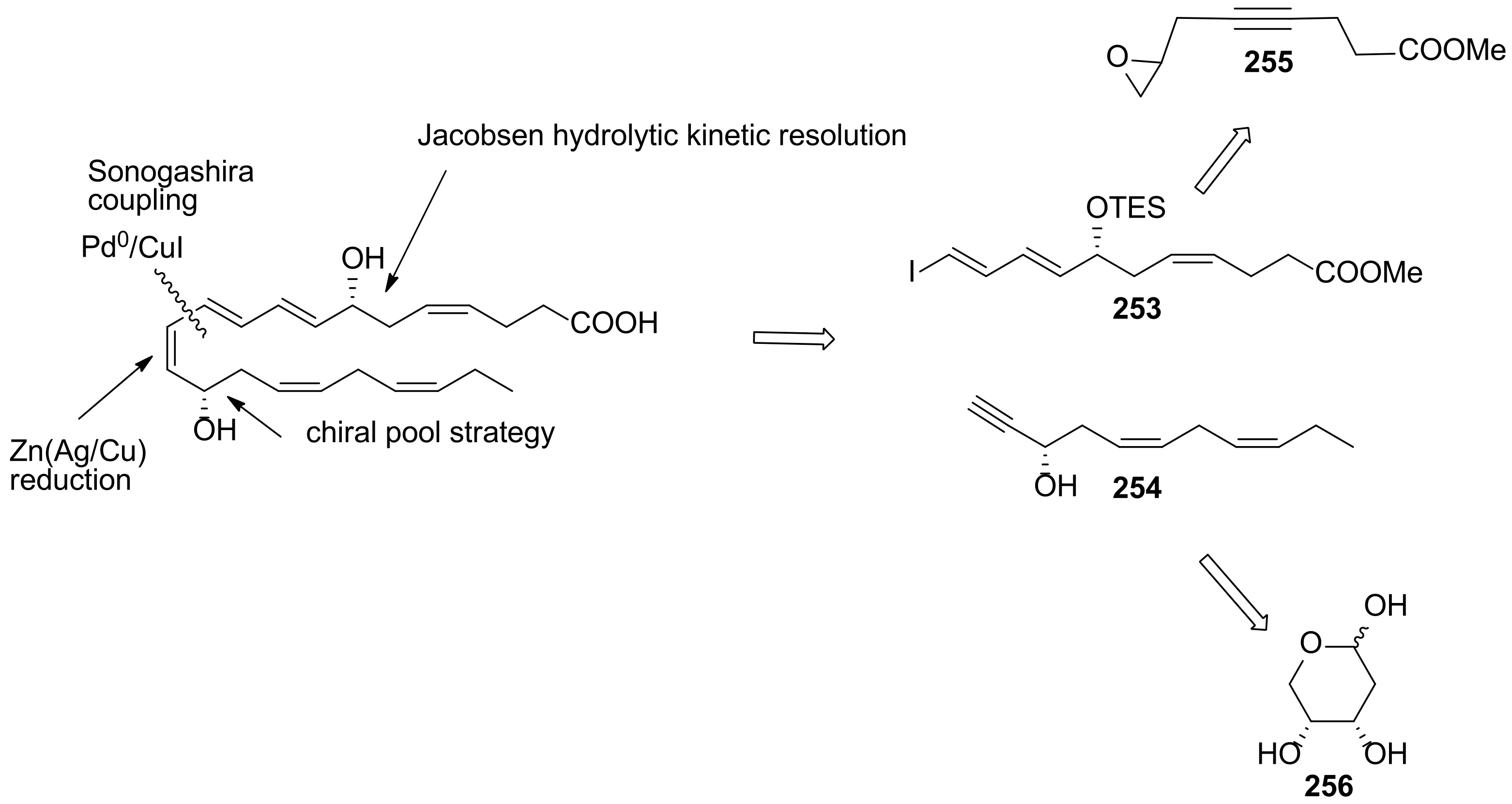
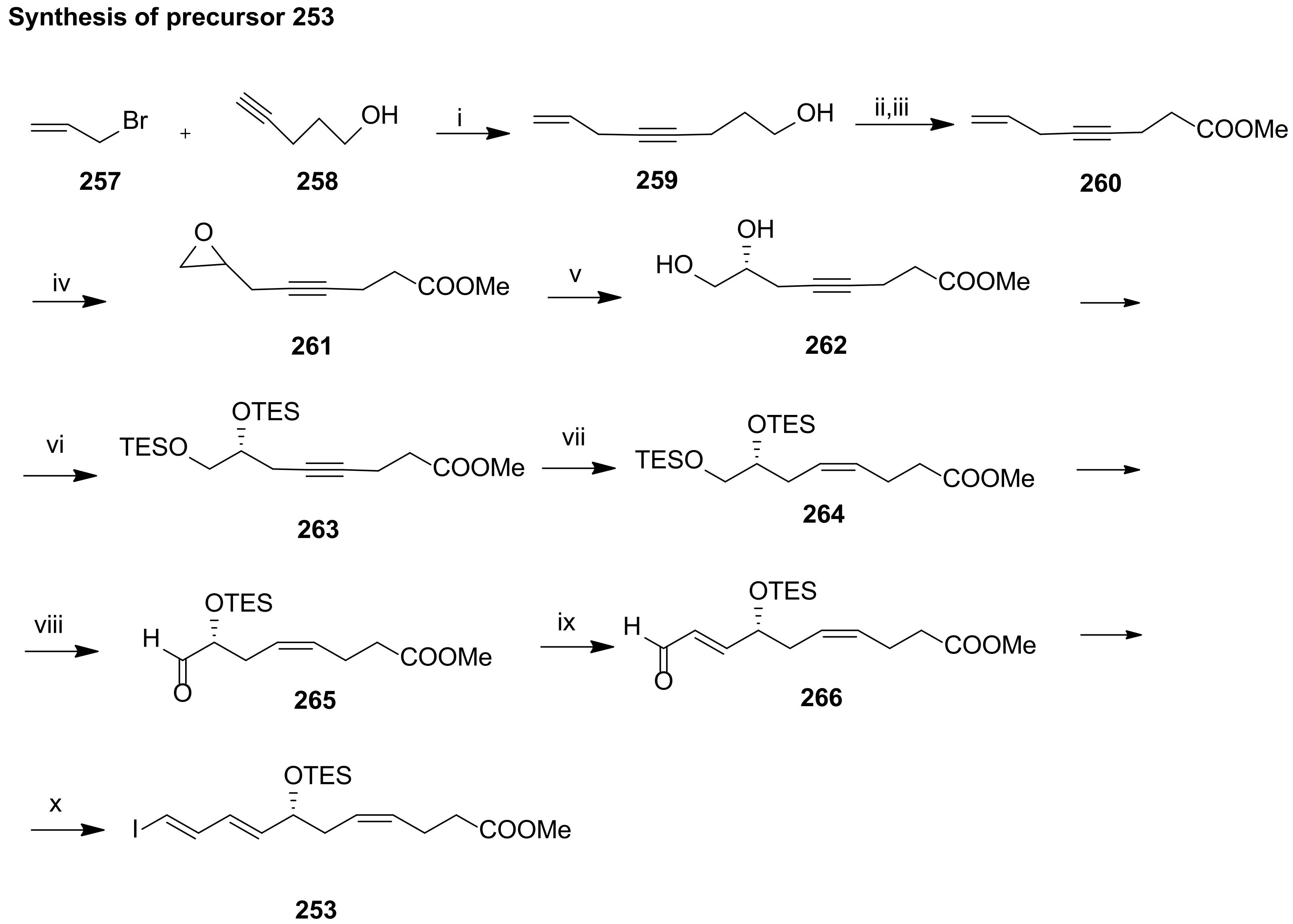
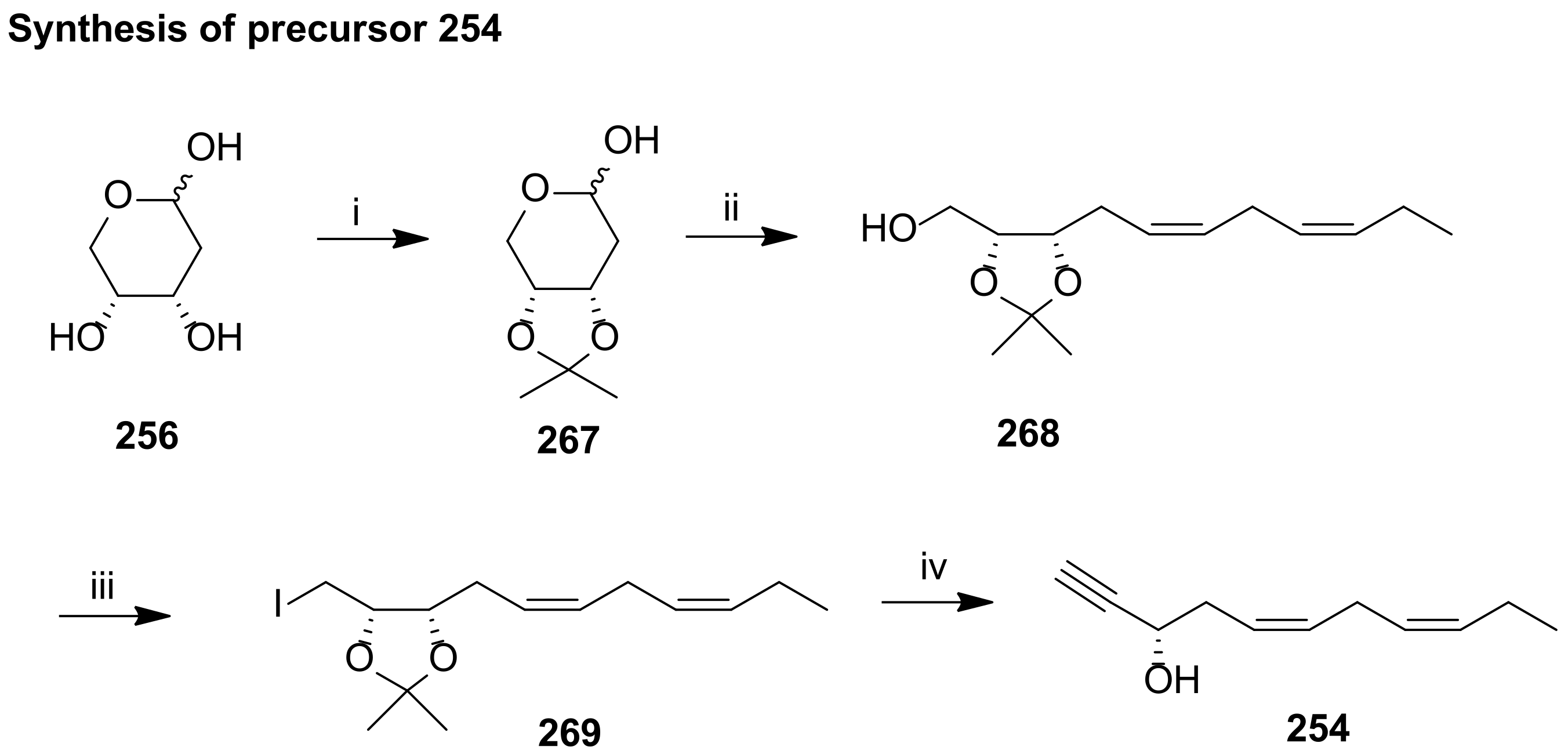
4.2.1. Ogawa and Kobayashi Approach
4.2.2. Serhan Approach
4.2.3. Approach by Balas and Sala
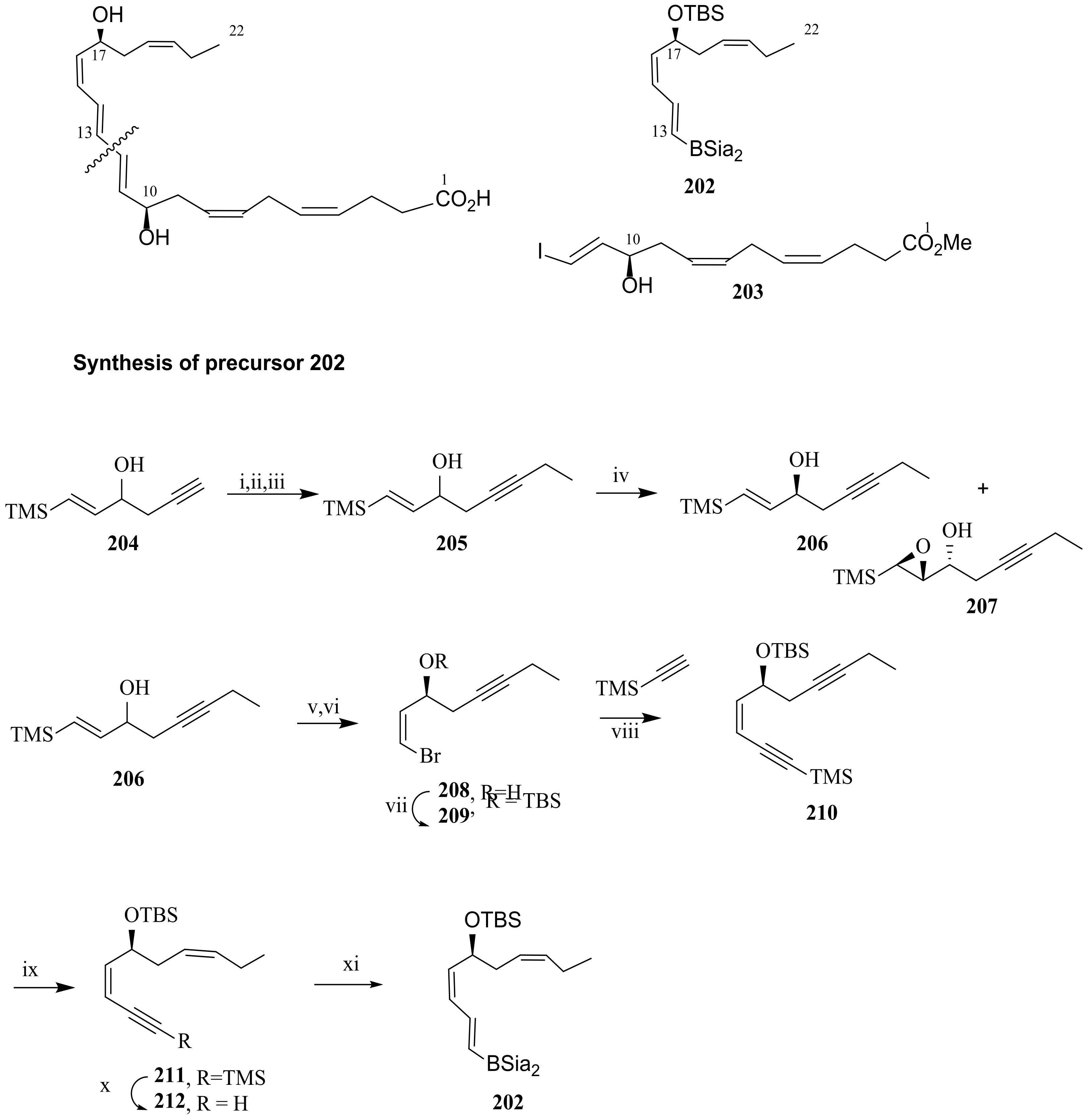
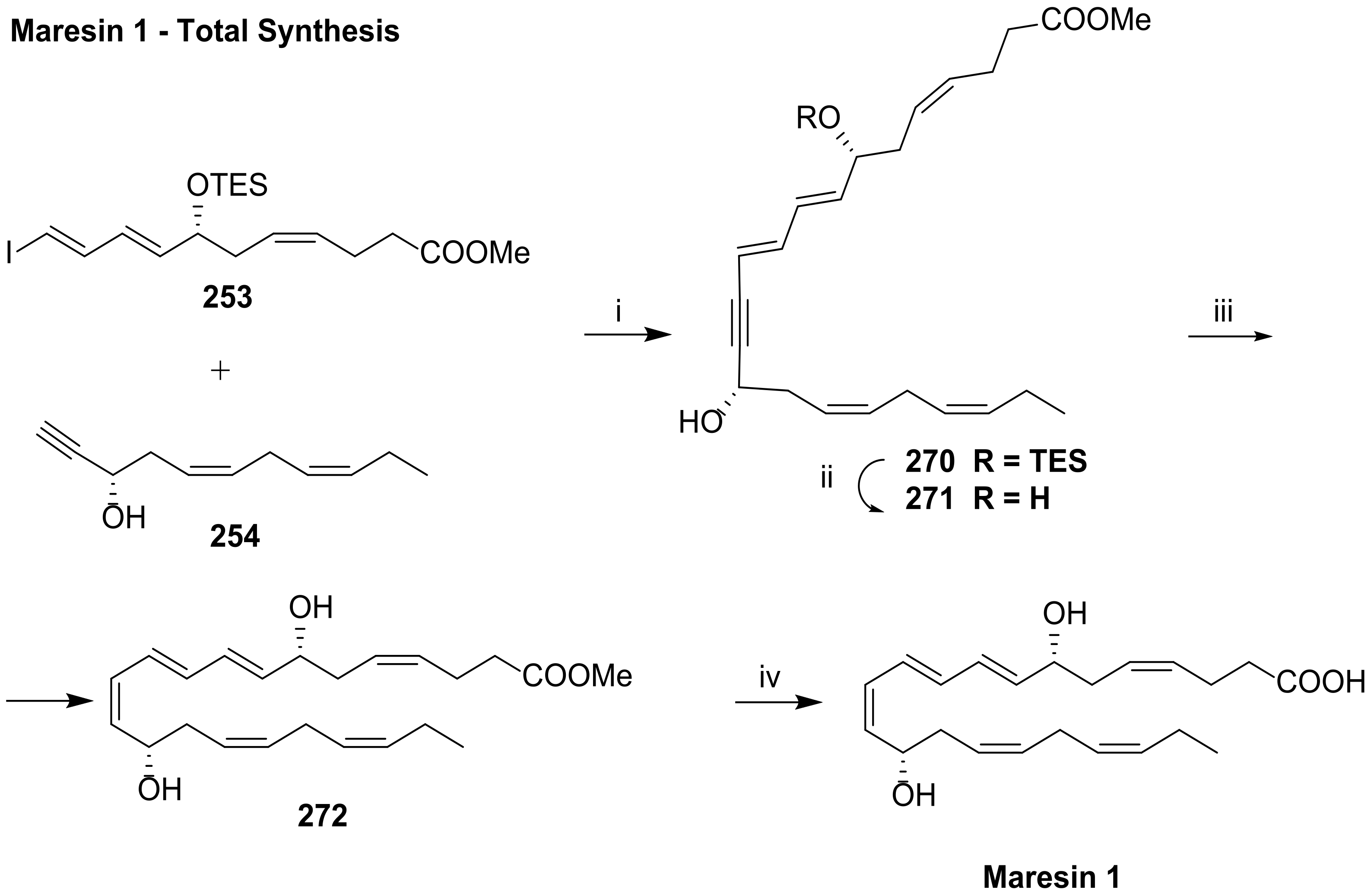
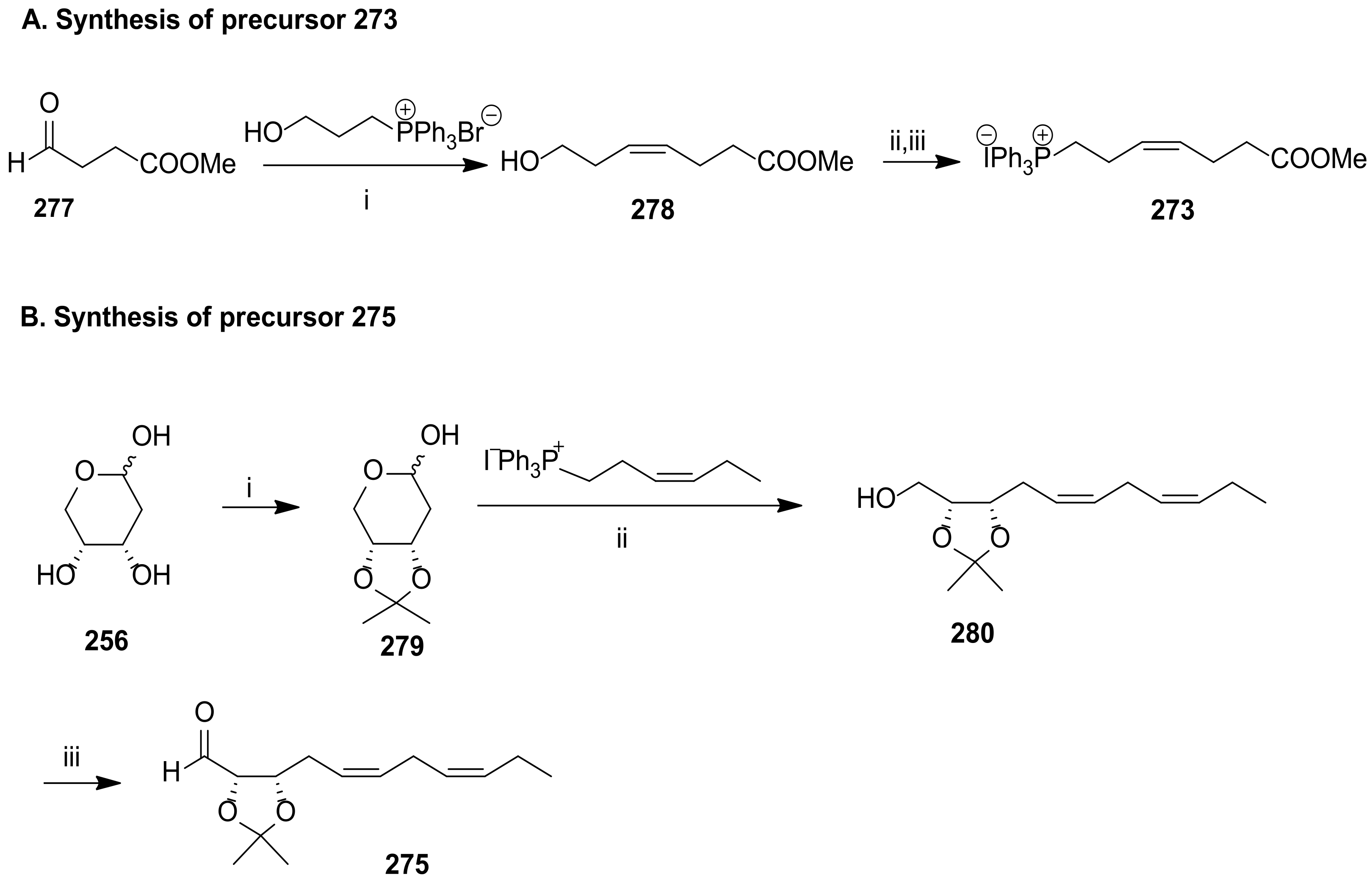
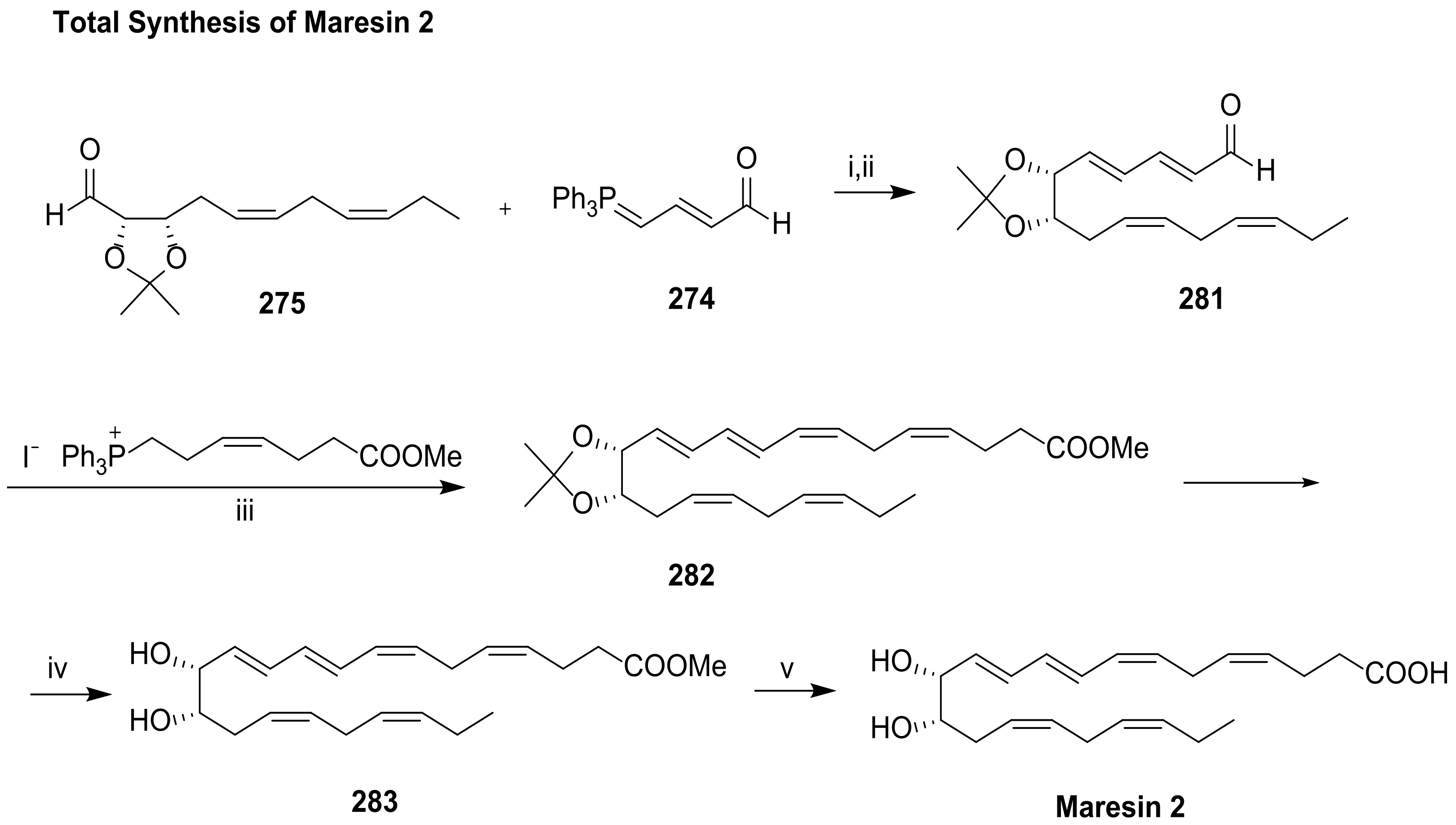
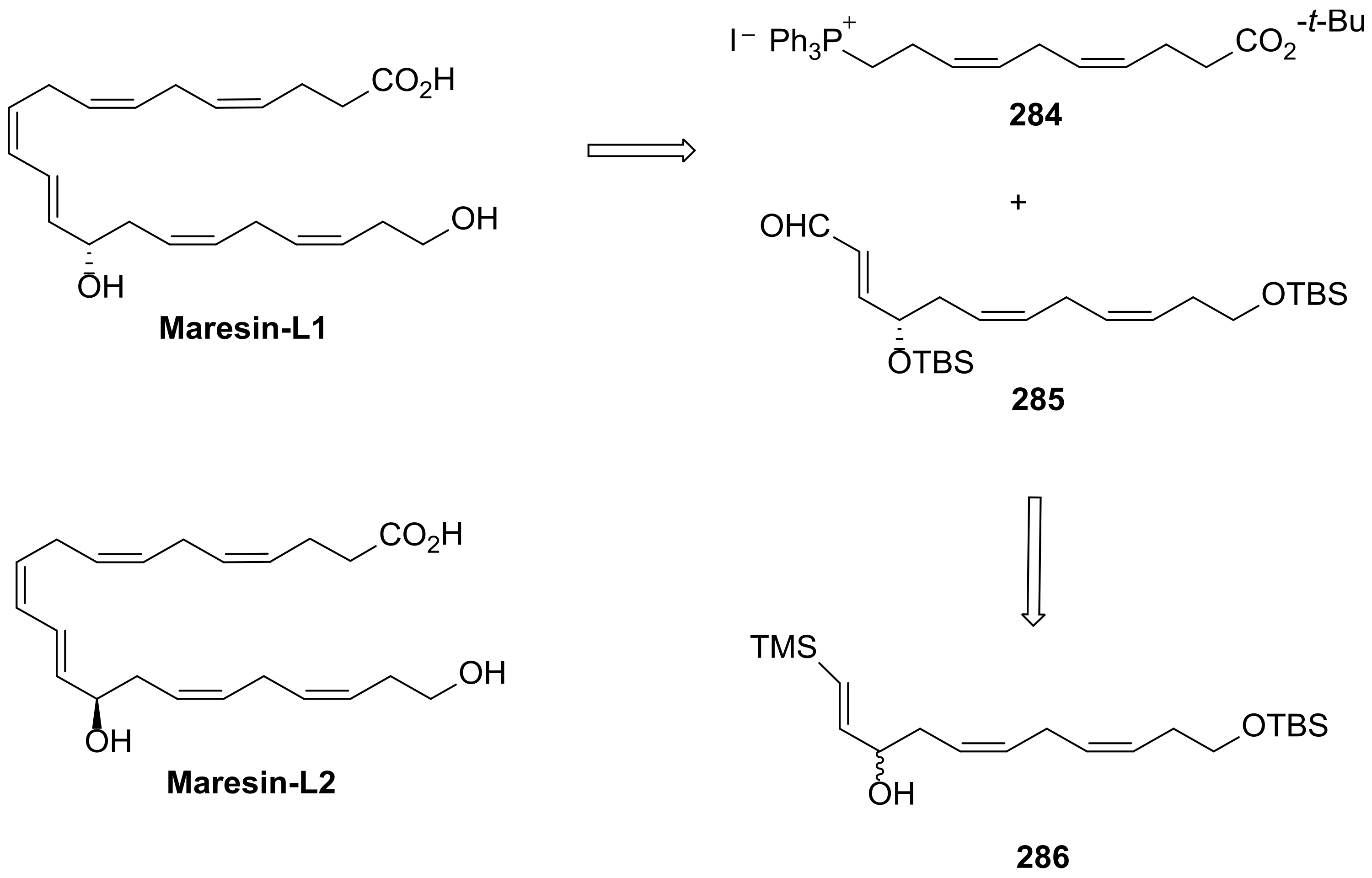
5. Maresins from DHA
5.1. Biosynthesis of Maresin 1 and Maresin 2
5.2. Total Synthesis of Maresin 1 and Maresin 2
5.3. Maresin-Like Lipid Mediators
6. The New Coronavirus (SARS-CoV-2) and DHA-Derived SPMs
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aneiros, A.; Garateix, A. Bioactive peptides from marine sources: Pharmacological properties and isolation procedures. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 803, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.-E.; Kellermann, M.Y.; Schupp, P.J. Secondary Metabolites of Marine Microbes: From Natural Products Chemistry to Chemical Ecology. YOUMARES 2020, 9, 159–180. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallimore, W. Marine Metabolites: Oceans of Opportunity. Pharmacogn. Fundam. Appl. Strategy 2017, 377–400. [Google Scholar] [CrossRef]
- Barzkar, N.; Jahromi, S.T.; Poorsaheli, H.B.; Vianello, F. Metabolites from marine microorganisms, micro, and macroalgae: Immense scope for pharmacology. Mar. Drugs 2019, 17, 464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine Bioactive Compounds and Their Health Benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Zhang, G.; Panigrahy, D.; Mahakian, L.M.; Yang, J.; Liu, J.Y.; Lee, K.S.; Wettersten, H.I.; Ulu, A.; Hu, X.; Tam, S.; et al. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc. Natl. Acad. Sci. USA 2013, 110, 6530–6535. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.A.; Beilin, L.J. Omega-3 fatty acids and inflammation. Curr. Atheroscler. Rep. 2004, 6, 461–467. [Google Scholar] [CrossRef]
- Oleñik, A.; Mahillo-Fernández, I.; Alejandre-Alba, N.; Fernández-Sanz, G.; Alarcón Pérez, M.; Luxan, S.; Quintana, S.; Martínez de Carneros Llorente, A.; García-Sandoval, B.; Jiménez-Alfaro, I. Benefits of omega-3 fatty acid dietary supplementation on health-related quality of life in patients with meibomian gland dysfunction. Clin. Ophthalmol. 2014, 8, 831–836. [Google Scholar] [CrossRef] [Green Version]
- Innes, J.K.; Calder, P.C. Marine omega-3 (N-3) fatty acids for cardiovascular health: An update for 2020. Int. J. Mol. Sci. 2020, 21, 1362. [Google Scholar] [CrossRef] [Green Version]
- Avallone, R.; Vitale, G.; Bertolotti, M. Omega-3 fatty acids and neurodegenerative diseases: New evidence in clinical trials. Int. J. Mol. Sci. 2019, 20, 4256. [Google Scholar] [CrossRef] [Green Version]
- Tur, J.A.; Bibiloni, M.M.; Sureda, A.; Pons, A. Dietary sources of omega 3 fatty acids: Public health risks and benefits. Br. J. Nutr. 2012, 107, S23–S52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, C.; Afonso, C.; Bandarra, N.M. Dietary DHA, bioaccessibility, and neurobehavioral development in children. Crit. Rev. Food Sci. Nutr. 2018, 58, 2617–2631. [Google Scholar] [CrossRef] [PubMed]
- Bandarra, N.M.; Batista, I.; Nunes, M.L.; Empis, J.M.; Christie, W.W. Seasonal changes in lipid composition of sardine (Sardina pilchardus). J. Food Sci. 1997, 62, 40–42. [Google Scholar] [CrossRef]
- Ferreira, I.; Gomes-Bispo, A.; Lourenço, H.; Matos, J.; Afonso, C.; Cardoso, C.; Castanheira, I.; Motta, C.; Prates, J.A.M.; Bandarra, N.M. The chemical composition and lipid profile of the chub mackerel (Scomber colias) show a strong seasonal dependence: Contribution to a nutritional evaluation. Biochimie 2020, 178, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Welch, A.A.; Shakya-Shrestha, S.; Lentjes MA, H.; Wareham, N.J.; Khaw, K.T. Dietary intake and status of n-3 polyunsaturated fatty acids in a population of fish-eating and non-fish-eating meat-eaters, vegetarians, and vegans and the precursor-product ratio of α-linolenic acid to long-chain n-3 polyunsaturated fatty acids: Results. Am. J. Clin. Nutr. 2010, 92, 1040–1051. [Google Scholar] [CrossRef]
- Cardoso, C.; Afonso, C.; Bandarra, N.M. Dietary DHA and health: Cognitive function ageing. Nutr. Res. Rev. 2016, 29, 281–294. [Google Scholar] [CrossRef]
- Calder, P.C. Docosahexaenoic acid. Ann. Nutr. Metab. 2016, 69, 8–21. [Google Scholar] [CrossRef]
- Kuratko, C.N.; Barrett, E.C.; Nelson, E.B.; Salem, N. The relationship of docosahexaenoic acid (DHA) with learning and behavior in healthy children: A review. Nutrients 2013, 5, 2777–2810. [Google Scholar] [CrossRef] [Green Version]
- Martins, B.P.; Bandarra, N.M.; Figueiredo-Braga, M. The role of marine omega-3 in human neurodevelopment, including Autism Spectrum Disorders and Attention-Deficit/Hyperactivity Disorder–a review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1431–1446. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Malau-Aduli, B.S.; John, C.; Nichols, P.D.; Malau-Aduli, E.O. Enhancing Omega-3 Long-Chain Polyunsaturated Human Consumption. Nutrients 2019, 743, 1–23. [Google Scholar]
- Kwon, Y. Immuno-Resolving Ability of Resolvins, Protectins, and Maresins Derived from Omega-3 Fatty Acids in Metabolic Syndrome. Mol. Nutr. Food Res. 2020, 64, 1–12. [Google Scholar] [CrossRef]
- Kuda, O. Bioactive metabolites of docosahexaenoic acid. Biochimie 2017, 136, 12–20. [Google Scholar] [CrossRef]
- Jaén, R.I.; Sánchez-García, S.; Fernández-Velasco, M.; Boscá, L.; Prieto, P. Resolution-Based Therapies: The Potential of Lipoxins to Treat Human Diseases. Front. Immunol. 2021, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Duvall, M.G.; Levy, B.D. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur. J. Pharmacol. 2016, 785, 144–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Tungen, J.E.; Aursnes, M.; Hansen, T.V. Stereoselective synthesis of maresin 1. Tetrahedron Lett. 2015, 56, 1843–1846. [Google Scholar] [CrossRef]
- Serhan, C.N.; Petasis, N.A. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef] [Green Version]
- Malawista, S.E.; Chevance AD, B.; Van Damme, J.; Serhan, C.N. Tonic inhibition of chemotaxis in human plasma. Proc. Natl. Acad. Sci. USA 2008, 105, 17949–17954. [Google Scholar] [CrossRef] [Green Version]
- Gordon, S. Phagocytosis: An Immunobiologic Process. Immunity 2016, 44, 463–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serhan, C.N.; Chiang, N. Resolution phase lipid mediators of inflammation: Agonists of resolution. Curr. Opin. Pharmacol. 2013, 13, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serhan, C.N. Treating inflammation and infection in the 21st century: New hints from decoding resolution mediators and mechanisms. FASEB J. 2017, 31, 1273–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Wu, X.; Liu, S.; Shen, D.; Zhu, J.; Liu, K. Role of Resolvins in the Inflammatory Resolution of Neurological Diseases. Front. Pharmacol. 2020, 11, 612. [Google Scholar] [CrossRef]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.-L. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serhan, C.N.; Dalli, J.; Karamnov, S.; Choi, A.; Park, C.; Xu, Z.; Ji, R.; Zhu, M.; Petasis, N.A. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012, 26, 1755–1765. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.K.; Marcheselli, V.L.; Serhan, C.N.; Bazan, N.G. Neuroprotectin D1: A docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. USA 2004, 101, 8491–8496. [Google Scholar] [CrossRef] [Green Version]
- Chiang, N.; Serha, C.N. Specialized pro-resolving mediator network: An update on production and actions. Essays Biochem. 2020, 64, 443–462. [Google Scholar]
- Han, Y.H.; Lee, K.; Saha, A.; Han, J.; Choi, H.; Noh, M.; Lee, Y.H.; Lee, M.O. Specialized proresolving mediators for therapeutic interventions targeting metabolic and inflammatory disorders. Biomol. Ther. 2021, 29, 455–464. [Google Scholar] [CrossRef]
- Ariyoshi, T.; Hagihara, M.; Eguchi, S.; Fukuda, A.; Iwasaki, K.; Oka, K.; Takahashi, M.; Yamagishi, Y.; Mikamo, H. Clostridium butyricum MIYAIRI 588-Induced Protectin D1 Has an Anti-inflammatory Effect on Antibiotic-Induced Intestinal Disorder. Front. Microbiol. 2020, 11, 2789. [Google Scholar] [CrossRef] [PubMed]
- Duffield, J.S.; Hong, S.; Vaidya, V.S.; Lu, Y.; Fredman, G.; Serhan, C.N.; Bonventre, J.V. Resolvin D Series and Protectin D1 Mitigate Acute Kidney Injury. J. Immunol. 2006, 177, 5902–5911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohli, P.; Levy, B.D. Resolvins and protectins: Mediating solutions to inflammation. Br. J. Pharmacol. 2009, 158, 960–971. [Google Scholar] [CrossRef] [Green Version]
- Miyahara, T.; Runge, S.; Chatterjee, A.; Chen, M.; Mottola, G.; Fitzgerald, J.M.; Serhan, C.N.; Conte, M.S. D-series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB J. 2013, 27, 2220–2232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizwicki, M.T.; Liu, G.; Fiala, M.; Magpantay, L.; Sayre, J.; Siani, A.; Mahanian, M.; Weitzman, R.; Hayden, E.Y.; Rosenthal, M.J.; et al. 1α,25-dihydroxyvitamin D3 and resolvin D1 retune the balance between amyloid-β phagocytosis and inflammation in Alzheimer’s disease patients. J. Alzheimer’s Dis. 2013, 34, 155–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.Z.; Zhang, L.; Liu, T.; Park, J.Y.; Berta, T.; Yang, R.; Serhan, C.N.; Ji, R.R. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med. 2010, 16, 592–597. [Google Scholar] [CrossRef] [Green Version]
- Bang, S.; Yoo, S.; Yang, T.J.; Cho, H.; Hwang, S. 17(R)-resolvin D1 specifically inhibits transient receptor potential ion channel vanilloid 3 leading to peripheral antinociception. Br. J. Pharmacol. 2012, 165, 683–692. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Chiang, N.; Dalli, J.; Levy, B.D.; Tracey, K.J.; Serhan, C.N.; Chiang, N.; Dalli, J.; De Zoete, M.R.; Palm, N.W.; et al. Lipid mediators in the resolution of inflammation. Cold Spring Harb. Perspect. Biol. 2014, 7, a016311. [Google Scholar] [CrossRef] [Green Version]
- Lima-Garcia, J.F.; Dutra, R.C.; Silva, D.A.; Motta, E.M.; Campos, M.M.; Calixto, J.B. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br. J. Pharmacol. 2011, 164, 278–293. [Google Scholar] [CrossRef] [Green Version]
- Klein, C.P.; Sperotto ND, M.; Maciel, I.S.; Leite, C.E.; Souza, A.H.; Campos, M.M. Effects of D-series resolvins on behavioral and neurochemical changes in a fibromyalgia-like model in mice. Neuropharmacology 2014, 86, 57–66. [Google Scholar] [CrossRef]
- Terrando, N.; Gómez-Galán, M.; Yang, T.; Carlström, M.; Gustavsson, D.; Harding, R.E.; Lindskog, M.; Eriksson, L.I. Aspirin-triggered resolvin D1 prevents surgery-induced cognitive decline. FASEB J. 2013, 27, 3564–3571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcheselli, V.L.; Hong, S.; Lukiw, W.J.; Tian, X.H.; Gronert, K.; Musto, A.; Hardy, M.; Gimenez, J.M.; Chiang, N.; Serhan, C.N.; et al. Novel Docosanoids Inhibit Brain Ischemia-Reperfusion-mediated Leukocyte Infiltration and Pro-inflammatory Gene Expression. J. Biol. Chem. 2003, 278, 43807–43817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariel, A.; Fredman, G.; Sun, Y.P.; Kantarci, A.; Dyke TE, V.a.n.; Luster, A.D.; Serhan, C.N. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat. Immunol. 2006, 7, 1209–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwab, J.M.; Chiang, N.; Arita, M.; Serhan, C.N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 2007, 447, 869–874. [Google Scholar] [CrossRef] [Green Version]
- Ariel, A.; Li, P.L.; Wang, W.; Tang, W.X.; Fredman, G.; Hong, S.; Gotlinger, K.H.; Serhan, C.N. The docosatriene protectin D1 is produced by TH2 skewing promotes human T cell via lipid raft clustering. J. Biol. Chem. 2005, 280, 43079–43086. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Gronert, K.; Devchand, P.R.; Moussignac, R.L.; Serhan, C.N. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells: Autacoids in anti-inflammation. J. Biol. Chem. 2003, 278, 14677–14687. [Google Scholar] [CrossRef] [Green Version]
- Levy, B.D.; Kohli, P.; Gotlinger, K.; Haworth, O.; Hong, S.; Kazani, S.; Israel, E.; Haley, K.J.; Serhan, C.N. Protectin D1 Is Generated in Asthma and Dampens Airway Inflammation and Hyperresponsiveness. J. Immunol. 2007, 178, 496–502. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Zhang, Y.; Zhang, R.; Qiao, S.; Fan, J. Resolvin D2 recovers neural injury by suppressing inflammatory mediators expression in lipopolysaccharide-induced Parkinson’s disease rat model. Biochem. Biophys. Res. Commun. 2015, 460, 799–805. [Google Scholar] [CrossRef]
- Dalli, J.; Winkler, J.W.; Colas, R.A.; Arnardottir, H.; Cheng CY, C.; Chiang, N.; Petasis, N.A.; Serhan, C.N. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem. Biol. 2013, 20, 188–201. [Google Scholar] [CrossRef] [Green Version]
- Winkler, J.W.; Orr, S.K.; Dalli, J.; Cheng, C.Y.; Sanger, J.M.; Chiang, N.; Petasis, N.A.; Serhan, C.N. Resolvin D4 stereoassignment and its novel actions in host protection and bacterial clearance. Sci. Rep. 2016, 6, 18972. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.L.; Kakazu, A.H.; He, J.; Nshimiyimana, R.; Petasis, N.A.; Jun, B.; Bazan, N.G.; Bazan HE, P. Elucidating the structure and functions of Resolvin D6 isomers on nerve regeneration with a distinctive trigeminal transcriptome. FASEB J. 2021, 35, e21775. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.M.; Chung, G.; Kim, Y.H.; Park, C.K. The role of maresins in inflammatory pain: Function of macrophages in wound regeneration. Int. J. Mol. Sci. 2019, 20, 5849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francos-Quijorna, I.; Santos-Nogueira, E.; Gronert, K.; Sullivan, A.B.; Kopp, M.A.; Brommer, B.; David, S.; Schwab, J.M.; López-Vales, R. Maresin 1 promotes inflammatory resolution, neuroprotection, and functional neurological recovery after spinal cord injury. J. Neurosci. 2017, 37, 11731–11743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, A.R.; Spur, B.W. First total synthesis of the macrophage derived anti-inflammatory and pro-resolving lipid mediator Maresin 2. Tetrahedron Lett. 2015, 56, 256–259. [Google Scholar] [CrossRef]
- Dangi, B.; Obeng, M.; Nauroth, J.M.; Teymourlouei, M.; Needham, M.; Raman, K.; Arterburn, L.M. Biogenic synthesis, purification, and chemical characterization of anti-inflammatory resolvins derived from docosapentaenoic acid (DPAn-6). J. Biol. Chem. 2009, 284, 14744–14759. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.F.; Vickery, T.; Schmidt, B.A.; Serhan, C.N.; Chiang, N.; Fredman, G.; Ba, F. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 2012, 484, 524–529. [Google Scholar]
- Oh, S.F.; Pillai, P.S.; Recchiuti, A.; Yang, R.; Serhan, C.N. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J. Clin. Investig. 2011, 121, 569–581. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Lu, Y.; Yang, R.; Gotlinger, K.H.; Petasis, N.A.; Serhan, C.N. Resolvin D1, Protectin D1, and related docosahexaenoic acid-derived products: Analysis via electrospray/low energy tandem mass spectrometry based on spectra and fragmentation mechanisms. J. Am. Soc. Mass Spectrom. 2007, 18, 128–144. [Google Scholar] [CrossRef] [Green Version]
- Bazan, N.G. Neuroprotectin D1 (NPD1): A DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol. 2005, 15, 159–166. [Google Scholar] [CrossRef]
- Chiang, N.; Serhan, C.N. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol. Asp. Med. 2017, 58, 114–129. [Google Scholar] [CrossRef]
- Brennan, E.; Kantharidis, P.; Cooper, M.E. Pro-resolving lipid mediators: Regulators of inflammation, metabolism and kidney function. Nat. Rev. Nephrol. 2021, 17, 725–739. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shetty, S.; Zhang, P.; Gao, R.; Hu, Y.; Wang, S.; Li, Z.; Fu, J. Aspirin-triggered resolvin D1 down-regulates inflammatory responses and protects against endotoxin-induced acute kidney injury. Toxicol. Appl. Pharmacol. 2014, 277, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Wang, C.; Sun, J.; Zhao, L.; Li, L.; Zhou, B.; Shao, S.; Shen, X.; Xu, Y. Resolvin D1 Protects Against Ischemia/Reperfusion-Induced Acute Kidney Injury by Increasing Treg Percentages via the ALX/FPR2 Pathway. Front. Physiol. 2020, 11, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, X.; Zhang, X.; Yao, J.; Song, J.; Nikolic-Paterson, D.J.; Li, J. Resolvins E1 and D1 inhibit interstitial fibrosis in the obstructed kidney via inhibition of local fibroblast proliferation. J. Pathol. 2012, 228, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Sulciner, M.L.; Serhan, C.N.; Gilligan, M.M.; Mudge, D.K.; Chang, J.; Gartung, A.; Lehner, K.A.; Bielenberg, D.R.; Schmidt, B.; Dalli, J.; et al. Resolvins suppress tumor growth and enhance cancer therapy. J. Exp. Med. 2018, 215, 115–140. [Google Scholar] [CrossRef]
- Videla, L.A.; Vargas, R.; Valenzuela, R.; Muñoz, P.; Corbari, A.; Hernandez-Rodas, M.C. Combined administration of docosahexaenoic acid and thyroid hormone synergistically enhances rat liver levels of resolvins RvD1 and RvD2. Prostaglandins Leukot. Essent. Fat. Acids 2019, 140, 42–46. [Google Scholar] [CrossRef]
- Hansen, T.V.; Vik, A.; Serhan, C.N. The protectin family of specialized pro-resolving mediators: Potent immunoresolvents enabling innovative approaches to target obesity and diabetes. Front. Pharmacol. 2019, 9, 1582. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, M.M.; Gartung, A.; Sulciner, M.L.; Norris, P.C.; Sukhatme, V.P.; Bielenberg, D.R.; Huang, S.; Kieran, M.W.; Serhan, C.N.; Panigrahy, D. Aspirin-triggered proresolving mediators stimulate resolution in cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 6292–6297. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.P.; Oh, S.F.; Uddin, J.; Yang, R.; Gotlinger, K.; Campbell, E.; Colgan, S.P.; Petasis, N.A.; Serhan, C.N. Resolvin D1 and its aspirin-triggered 17R epimer: Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J. Biol. Chem. 2007, 282, 9323–9334. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, A.R.; Spur, B.W. Total synthesis of Resolvin D1, a potent anti-inflammatory lipid mediator. Tetrahedron Lett. 2012, 53, 6990–6994. [Google Scholar] [CrossRef]
- Morita, M.; Wu, S.; Kobayashi, Y. Stereocontrolled synthesis of resolvin D1. Org. Biomol. Chem. 2019, 17, 2212–2222. [Google Scholar] [CrossRef]
- Colvin, W.; Hamill, J. One- step Conversion of Carbonyl Compounds into Acetylenes By ERNEST. Chem. Commun. 1973, 5, 151–152. [Google Scholar] [CrossRef]
- Kazuhiro Miwa, T.A.T.S. Extension of the colvin rearrangement using trimethylsilyldiazomethane. A new synthesis of alkynes. Synlett 1993, 1994, 107–108. [Google Scholar] [CrossRef]
- Huang, Z.; Negishi, E.I. A convenient and genuine equivalent to HZrCp2Cl generated in situ from ZrCp2Cl2-DIBAL-H. Org. Lett. 2006, 8, 3675–3678. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.R.; Spur, B.W. First total synthesis of 7(S),16(R),17(S)-Resolvin D2, a potent anti-inflammatory lipid mediator. Tetrahedron Lett. 2004, 45, 8717–8720. [Google Scholar] [CrossRef]
- Li, J.; Leong, M.M.; Stewart, A.; Rizzacasa, M.A. Total synthesis of the endogenous inflammation resolving lipid resolvin D2 using a common lynchpin. Beilstein J. Org. Chem. 2013, 9, 2762–2766. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, A.; Nomen, M.; Spur, B.W.; Godfroid, J.J. Selective oxidation of primary silyl ethers and its application to the synthesis of natural products. Tetrahedron Lett. 1999, 40, 5161–5164. [Google Scholar] [CrossRef]
- Urbitsch, F.; Elbert, B.L.; Llaveria, J.; Streatfeild, P.E.; Anderson, E.A. A Modular, Enantioselective Synthesis of Resolvins D3, E1, and Hybrids. Org. Lett. 2020, 22, 1510–1515. [Google Scholar] [CrossRef] [Green Version]
- Soullez, D.; Plé, G.; Duhamel, L. ω-Halogeno polyenals: Preparation and application to a one-pot synthesis of polyenals from carbonyl compounds. J. Chem. Soc. Perkin Trans. 1997, 1, 1639–1645. [Google Scholar] [CrossRef]
- Winkler, J.W.; Libreros, S.; La Rosa, X.D.e.; Sansbury, B.E.; Norris, P.C.; Chiang, N.; Fichtner, D.; Keyes, G.S.; Wourms, N.; Spite, M.; et al. Frontline Science: Structural insights into Resolvin D4 actions and further metabolites via a new total organic synthesis and validation. J. Leukoc. Biol. 2018, 103, 995–1010. [Google Scholar] [CrossRef]
- Spur, B.W.; Rodrı, A.R. First total synthesis of 7(S), 17(S)-Resolvin D5, a potent anti-inflammatory docosanoid. Tetrahedron Lett. 2005, 46, 3623–3627. [Google Scholar]
- Takai, K.; Nitta, K.; Utimoto, K. Simple and selective method for aldehydes (RCHO). fwdarw. (E)-haloalkenes (RCH:CHX) conversion by means of a haloform-chromous chloride system. J. Am. Chem. Soc. 1986, 108, 7408–7410. [Google Scholar] [CrossRef]
- Ogawa, N.; Sugiyama, T.; Morita, M.; Suganuma, Y.; Kobayashi, Y. Total Synthesis of Resolvin D5. J. Org. Chem. 2017, 82, 2032–2039. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.R.; Spur, B.W. First total synthesis of the anti-inflammatory lipid mediator Resolvin D6. Tetrahedron Lett. 2012, 53, 86–89. [Google Scholar] [CrossRef]
- Morita, M.; Tanabe, S.; Arai, T.; Kobayashi, Y. Synthesis of Resolvin D6 and the Silyl Ether of the Resolvin E2 Methyl Ester via trans -Enynyl Alcohols. Synlett 2019, 30, 1351–1355. [Google Scholar] [CrossRef] [Green Version]
- Labarre-Lainé, J.; Beniazza, R.; Desvergnes, V.; Landais, Y. Convergent access to bis-spiroacetals through a sila-Stetter-ketalization cascade. Org. Lett. 2013, 15, 4706–4709. [Google Scholar] [CrossRef]
- Matsumura, K.; Hashiguchi, S.; Ikariya, T.; Noyori, R. The first asymmetric transfer hydrogenation of acetylenic ketones using chiral Ru (II) catalysts and 2-propanol as the hydrogen donor. 9 This method allows highly selective reduction of structurally diverse acetylenic ketones to propargylic alcohols of. J. Am. Chem. Soc. 1997, 119, 8738–8739. [Google Scholar] [CrossRef]
- Aursnes, M.; Tungen, J.E.; Vik, A.; Colas, R.; Cheng, C.C.; Dalli, J.; Serhan, C.N.; Hansen, T. V Total Synthesis of the Lipid Mediator PD1. J. Nat. Prod. 2015, 77, 910–916. [Google Scholar] [CrossRef]
- Asatryan, A.; Bazan, N.G. Molecular mechanisms of signaling via the docosanoid neuroprotectin D1 for cellular homeostasis and neuroprotection. J. Biol. Chem. 2017, 292, 12390–12397. [Google Scholar] [CrossRef] [Green Version]
- Marcheselli, V.L.; Mukherjee, P.K.; Arita, M.; Hong, S.; Antony, R.; Sheets, K.; Winkler, J.W.; Petasis, N.A.; Serhan, C.N.; Bazan, N.G. Neuroprotectin D1/protectin D1 stereoselective and specific binding with human retinal pigment epithelial cells and neutrophils. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Bazan, N.G.; Calandria, J.M.; Serhan, C.N. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J. Lipid Res. 2010, 51, 2018–2031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.K.; Rao, G.N. Emerging role of 12/15-Lipoxygenase (ALOX15) in human pathologies. Prog. Lipid Res. 2019, 73, 28–45. [Google Scholar] [CrossRef] [PubMed]
- González-Périz, A.; Horrillo, R.; Ferré, N.; Gronert, K.; Dong, B.; Morán-Salvador, E.; Titos, E.; Martínez-Clemente, M.; López-Parra, M.; Arroyo, V.; et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by ω-3 fatty acids: A role for resolvins and protectins. FASEB J. 2009, 23, 1946–1957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clària, J.; López-Vicario, C.; Rius, B.; Titos, E. Pro-resolving actions of SPM in adipose tissue biology. Mol. Asp. Med. 2017, 58, 83–92. [Google Scholar] [CrossRef]
- Ogawa, N.; Kobayashi, Y. Total synthesis of the antiinflammatory and proresolving protectin D1. Tetrahedron Lett. 2011, 52, 3001–3004. [Google Scholar] [CrossRef]
- Petasis, N.A.; Yang, R.; Winkler, J.W.; Zhu, M.; Uddin, J.; Bazan, N.G.; Serhan, C.N. Stereocontrolled total synthesis of Neuroprotectin D1/Protectin D1 and its aspirin-triggered stereoisomer. Tetrahedron Lett. 2012, 53, 1695–1698. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, A.R.; Spur, B.W. Total synthesis of the potent anti-inflammatory lipid mediator Protectin D1. Tetrahedron Lett. 2014, 55, 6011–6015. [Google Scholar] [CrossRef]
- Balas, L.; Risé, P.; Gandrath, D.; Rovati, G.; Bolego, C.; Stellari, F.; Trenti, A.; Buccellati, C.; Durand, T.; Sala, A. Rapid Metabolization of Protectin D1 by β-Oxidation of Its Polar Head Chain. J. Med. Chem. 2019, 62, 9961–9975. [Google Scholar] [CrossRef]
- Müller, S.; Liepold, B.; Gerald, J.; Roth, H.J.B. An Improved One-pot Procedure for the Synthesis of Alkynes from Aldehydes. Synlett 1996, 521–522. [Google Scholar] [CrossRef]
- Dayaker, G.; Durand, T.; Balas, L. A versatile and stereocontrolled total synthesis of dihydroxylated docosatrienes containing a conjugated E,E,Z-triene. Chem. Eur. J. 2014, 20, 2879–2887. [Google Scholar] [CrossRef]
- Li, Q.F.; Hao, H.; Tu, W.S.; Guo, N.; Zhou, X.Y. Maresins: Anti-inflammatory pro-resolving mediators with therapeutic potential. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7442–7453. [Google Scholar] [PubMed]
- Sasaki, K.; Urabe, D.; Arai, H.; Arita, M.; Inoue, M. Total synthesis and bioactivities of two proposed structures of maresin. Chem. Asian J. 2011, 6, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.R.; Spur, B.W. Total synthesis of the macrophage derived anti-inflammatory lipid mediator Maresin 1. Tetrahedron Lett. 2012, 53, 4169–4172. [Google Scholar] [CrossRef]
- Dess, D.B.; Martin, J.C. A Useful 12-I-5 Triacetoxyperiodinane (the Dess-Martin Periodinane) for the Selective Oxidation of Primary or Secondary Alcohols and a Variety of Related 12-I-5 Species. J. Am. Chem. Soc. 1991, 113, 7277–7287. [Google Scholar] [CrossRef]
- Hong, S.; Lu, Y.; Tian, H.; Alapure, B.V.; Wang, Q.; Bunnell, B.A.; Laborde, J.M. Maresin-like lipid mediators are produced by leukocytes and platelets and rescue reparative function of diabetes-impaired macrophages. Chem. Biol. 2014, 21, 1318–1329. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Lu, Y.; Morita, M.; Saito, S.; Kobayashi, Y.; Jun, B.; Bazan, N.G.; Xu, X.; Wang, Y. Stereoselective Synthesis of Maresin-Like Lipid Mediators. Synlett 2019, 30, 343–347. [Google Scholar]
- Calder, P.C. Nutrition, immunity and COVID-19. BMJ Nutr. Prev. Health 2020, 3, 74–92. [Google Scholar] [CrossRef]
- Alimohamadi, Y.; Sepandi, M.; Taghdir, M.; Hosamirudsari, H. Determine the most common clinical symptoms in COVID-19 patients: A systematic review and meta-analysis. J. Prev. Med. Hyg. 2020, 61, E304–E312. [Google Scholar]
- Forni, G.; Mantovani, A.; Forni, G.; Mantovani, A.; Moretta, L.; Rappuoli, R.; Rezza, G.; Bagnasco, A.; Barsacchi, G.; Bussolati, G.; et al. COVID-19 vaccines: Where we stand and challenges ahead. Cell Death Differ. 2021, 28, 626–639. [Google Scholar] [CrossRef]
- Simopoulos, A.P.; Serhan, C.N.; Bazinet, R.P. The need for precision nutrition, genetic variation and resolution in Covid-19 patients. Mol. Asp. Med. 2021, 77, 100943. [Google Scholar] [CrossRef]
- Torrinhas, R.S.; Calder, P.C.; Lemos, G.O.; Waitzberg, D.L. Parenteral fish oil: An adjuvant pharmacotherapy for coronavirus disease 2019? Nutrition 2021, 81, 110900. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Can Bioactive Lipids Inactivate Coronavirus (COVID-19)? Arch. Med. Res. 2020, 51, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.M.; Leão, M.D.C.; Santana, T.M.; Pimentel, M.V.D.M.B. Potential benefits and risks of omega-3 fatty acids supplementation to patients with COVID-19. Free Radic. Biol. Med. J. 2020, 156, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, A.V.; Karathanasis, S.K.; Yang, Z.H.; Freeman, L.; Kotani, K.; Remaley, A.T. COVID-19—Associated dyslipidemia: Implications for mechanism of impaired resolution and novel therapeutic approaches. FASEB J. 2020, 34, 9843–9853. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.L.; He, J.; Kakazu, A.H.; Calandria, J.; Do, K.V.; Nshimiyimana, R.; Lam, T.F.; Petasis, N.A.; Bazan HE, P.; Bazan, N.G. ELV-N32 and RvD6 isomer decrease pro-inflammatory cytokines, senescence programming, ACE2 and SARS-CoV-2-spike protein RBD binding in injured cornea. Sci. Rep. 2021, 11, 12787. [Google Scholar] [CrossRef]
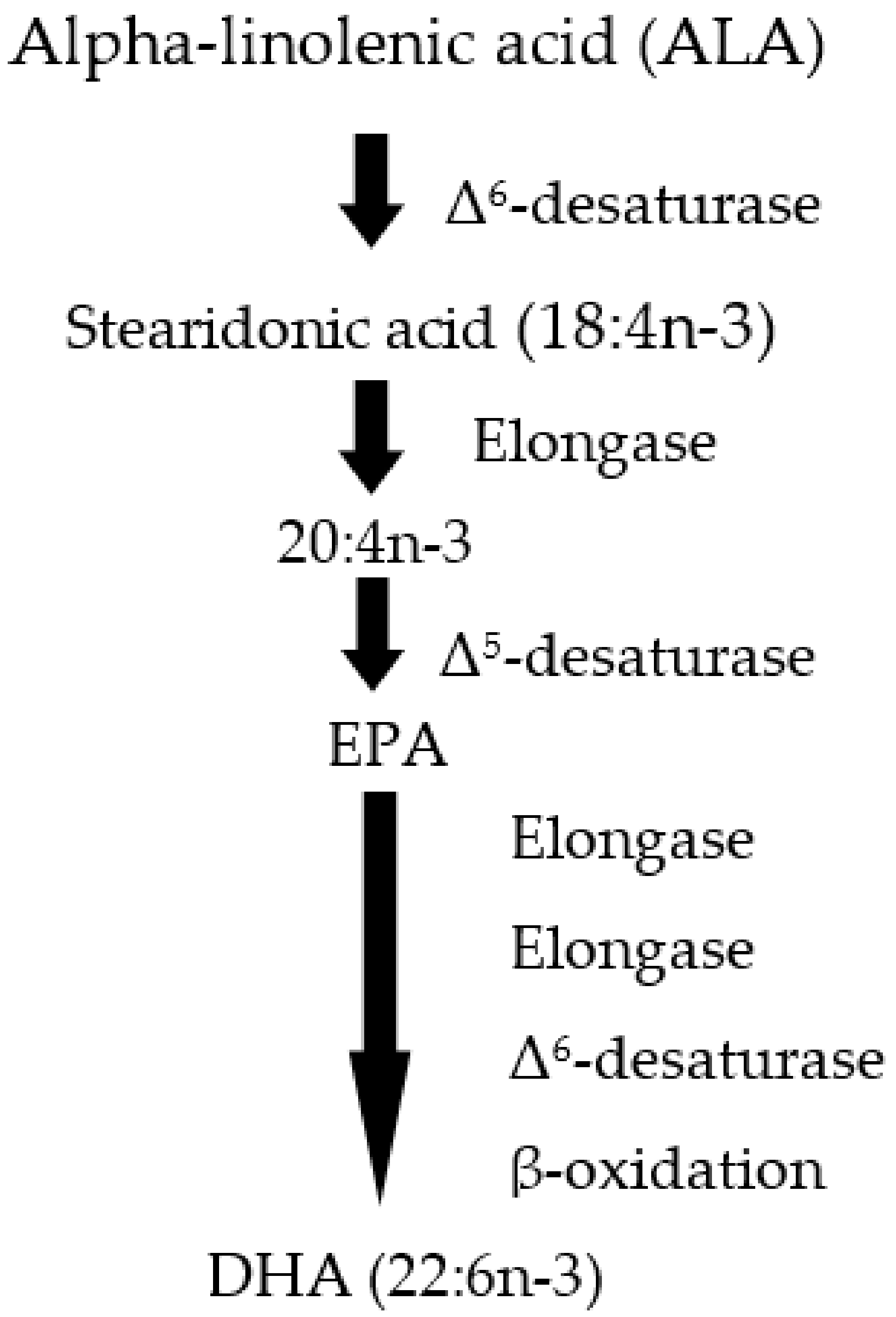
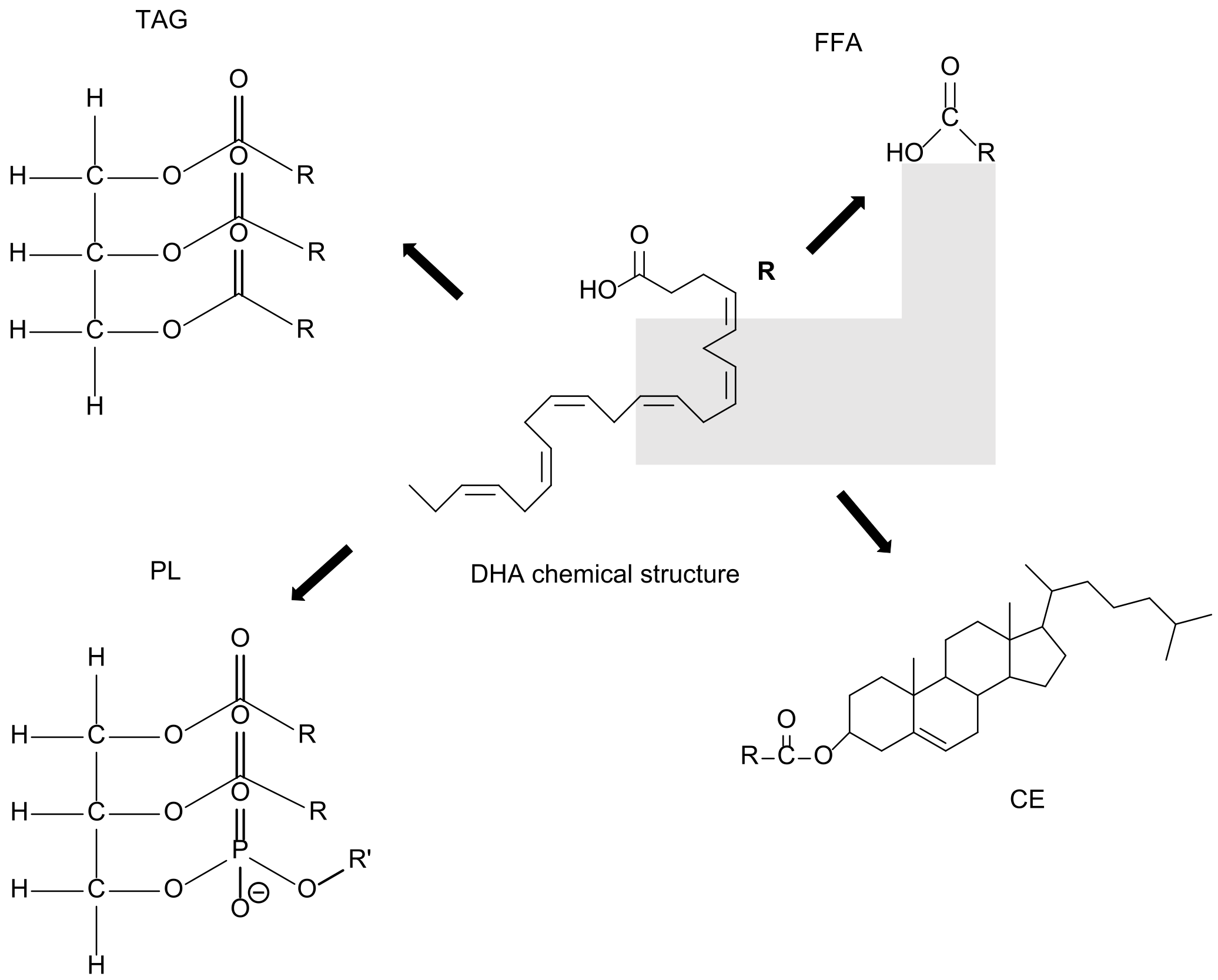
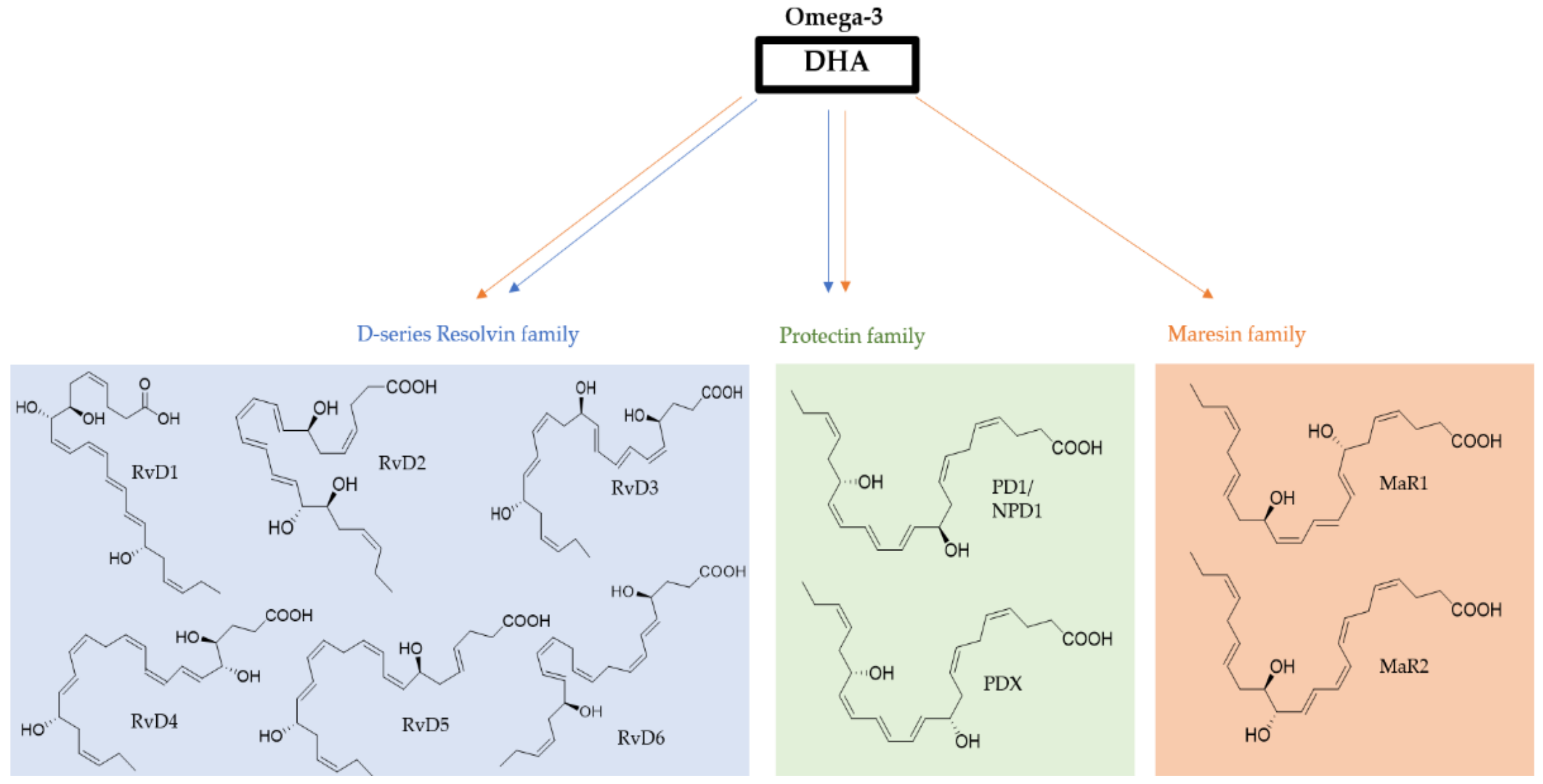







| Health Benefits of Dietary Supplementation | Reference |
|---|---|
| Reduce risks of macular degeneration and cancers | [7] |
| Protect against atherosclerotic heart disease and sudden coronary death | [8] |
| Have beneficial effect in patients with Meibomian gland dysfunction (MGD) | [9] |
| Reduce risks of developing cardiovascular disease (CVD), coronary heart disease (CHD), and myocardial infarction (MI) | [10] |
| Reduce risks of Parkinson’s and Alzheimer’s disease | [11] |
| Decrease systolic and diastolic blood pressure | [12] |
| Inhibit pro-inflammatory signalling cascades | [13] |
| SPM | Target | Bioaction | Reference |
|---|---|---|---|
| RvD1 | Polymorphonuclear leukocyte | Decreases infiltration in murine skin air pouch model; limits infiltration in renal ischaemic injury | [36,42,43] |
| Microglial cells | Inhibits IL-1β expression in vitro | [36] | |
| Vascular inflammation (arterial angioplasty) | Attenuates cell proliferation, leukocyte recruitment, and neointimal hyperplasia | [44] | |
| Alzheimer’s disease | Stimulates phagocytosis of Aβ by Alzheimer’s disease macrophages | [45] | |
| Pain | Controls inflammatory pain | [46] | |
| AT-RvD1 | Pain | Controls inflammatory pain | [47] |
| Temporomandibular joint inflammation | Limits PMN infiltration to CFA-inflamed TMJ | [48] | |
| Arthritis | Antihyperalgesic | [49] | |
| Fibromyalgia | Reduces mechanical allodynia and thermal sensitization and prevents depressive behaviour | [50] | |
| Postsurgical cognitive decline | Improves postoperative decline and attenuates memory | [51] | |
| PD1 | Polymorphonuclear leukocyte | Upregulates CCR5 expression; reduces tissue infiltration | [52,53] |
| Macrophages | Stimulates phagocytosis of apoptotic polymorphonuclear leukocyte | [54] | |
| T Cell | Promotes apoptosis in vitro | [55] | |
| Glial cells | Reduces cytokine production | [56] | |
| Epithelial cells | Protects from oxidative stress-induced apoptosis (retinal pigment epithelium) | [38] | |
| Eosinophils | Decreases recruitment in response to allergen | [57] | |
| RvD2 | Macroglia | Prohibits or reduces the activation of macroglia and microglia, respectively | [58] |
| - | Downregulates TNF-α, IL-1β, iNOS, NF-k, NO, and ROS production | [35] | |
| Burn wound | Prevents secondary thrombosis and necrosis | [48] | |
| RvD3 and AT-RvD3 | Neutrophils | Regulates neutrophils and mediators, reducing murine peritonitis and dermal inflammation | [59] |
| RvD4 | Protection and resolution of inflammation during bacterial infection | Stops leukocyte influx to the site of infection in the dorsal pouch cavity as well as the inflammation-initiating eicosanoids by reducing levels of PGD2 and LTB4 | [60] |
| RvD5 | Bacterial infection | Increases survival and lower antibiotic requirement | [48] |
| RvD6 | Corneal nerve | Decreases inflammation and increases wound healing and nerve regeneration by decreasing the expression of the ACE2 receptor, furin, and integrins. | [61] |
| MaR1 | Pain | Controls inflammatory pain | [37] |
| Tissue regeneration | Promotes tissue regeneration in planaria | [37] | |
| Neuroprotection | Treats amyotrophic lateral sclerosis and spinal muscular atrophy Accelerates clearance of neutrophils and a reduction in macrophage accumulation at the lesion site | [62,63] | |
| MaR2 | Macrophage phagocytosis | Anti-inflammatory activity | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, I.; Falcato, F.; Bandarra, N.; Rauter, A.P. Resolvins, Protectins, and Maresins: DHA-Derived Specialized Pro-Resolving Mediators, Biosynthetic Pathways, Synthetic Approaches, and Their Role in Inflammation. Molecules 2022, 27, 1677. https://doi.org/10.3390/molecules27051677
Ferreira I, Falcato F, Bandarra N, Rauter AP. Resolvins, Protectins, and Maresins: DHA-Derived Specialized Pro-Resolving Mediators, Biosynthetic Pathways, Synthetic Approaches, and Their Role in Inflammation. Molecules. 2022; 27(5):1677. https://doi.org/10.3390/molecules27051677
Chicago/Turabian StyleFerreira, Inês, Filipa Falcato, Narcisa Bandarra, and Amélia P. Rauter. 2022. "Resolvins, Protectins, and Maresins: DHA-Derived Specialized Pro-Resolving Mediators, Biosynthetic Pathways, Synthetic Approaches, and Their Role in Inflammation" Molecules 27, no. 5: 1677. https://doi.org/10.3390/molecules27051677
APA StyleFerreira, I., Falcato, F., Bandarra, N., & Rauter, A. P. (2022). Resolvins, Protectins, and Maresins: DHA-Derived Specialized Pro-Resolving Mediators, Biosynthetic Pathways, Synthetic Approaches, and Their Role in Inflammation. Molecules, 27(5), 1677. https://doi.org/10.3390/molecules27051677