Molecular Interactions Associated with Coagulation of Organic Pollutants by 2S Albumin of Plant Proteins: A Computational Approach
Abstract
:1. Introduction
2. Materials and Methods
3. Molecular Dynamics Simulation
4. Results and Discussion
4.1. Physicochemical Properties, Structural Composition, Quality and Stability of the Proteins under Study
4.2. Coagulation of the Organic Molecules to the Proteins under Study: Binding Affinity, Stability and Structural Quality of the Complexes
4.3. Moringa Oleifera
4.4. Arachis Hypogaea
4.5. Bertholletia Excelsa
4.6. Brassica napus
4.7. Helianthus annuus
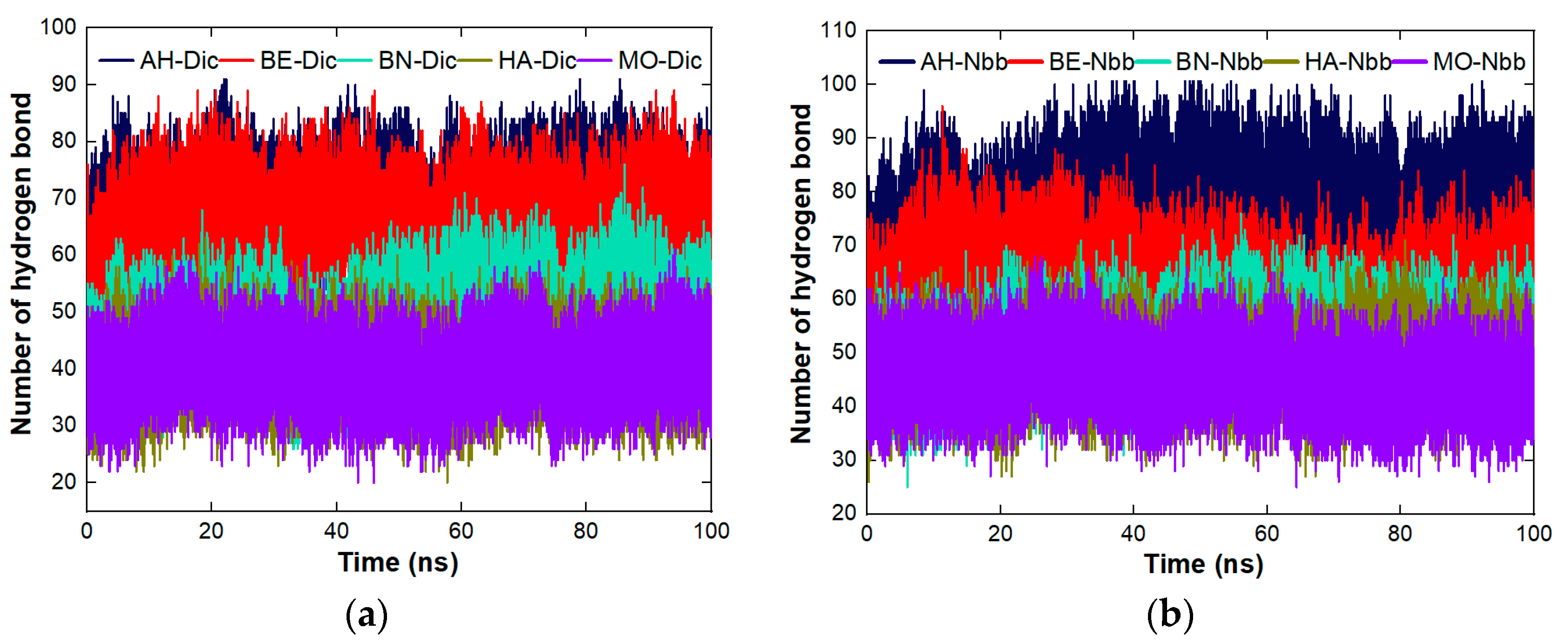
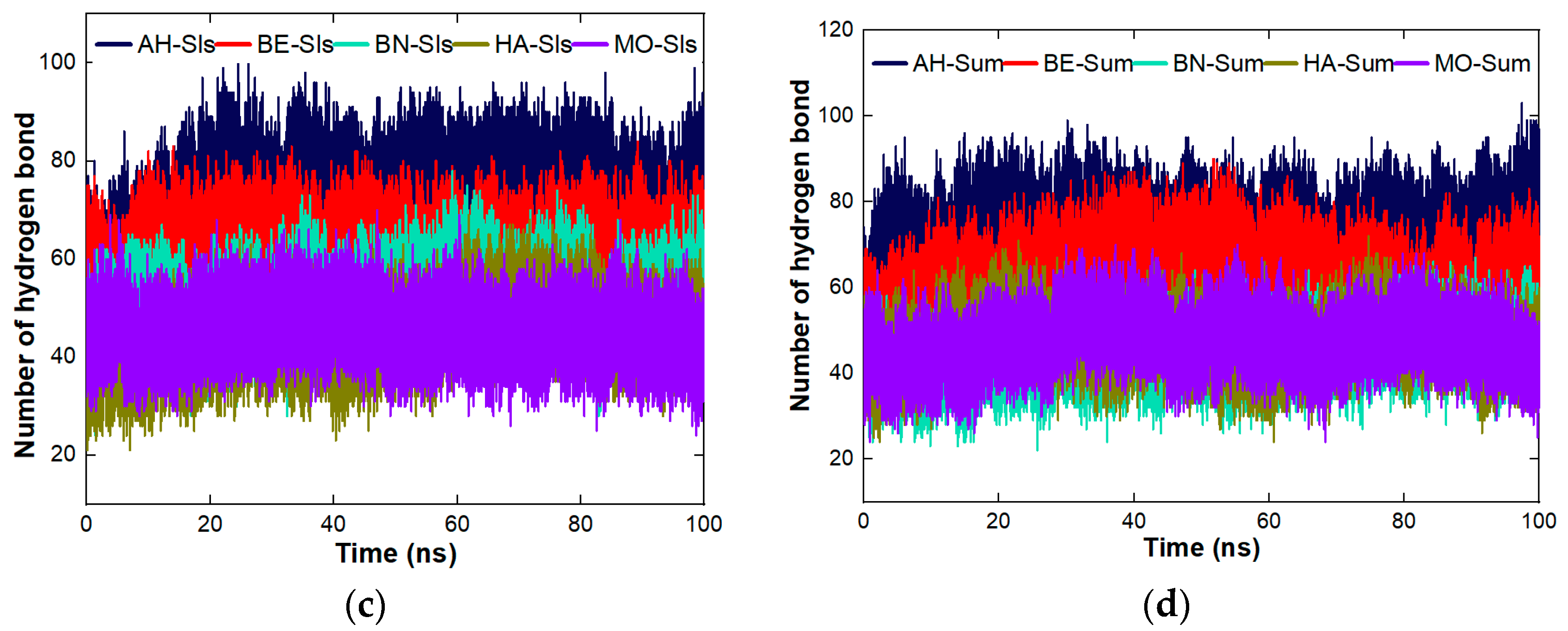
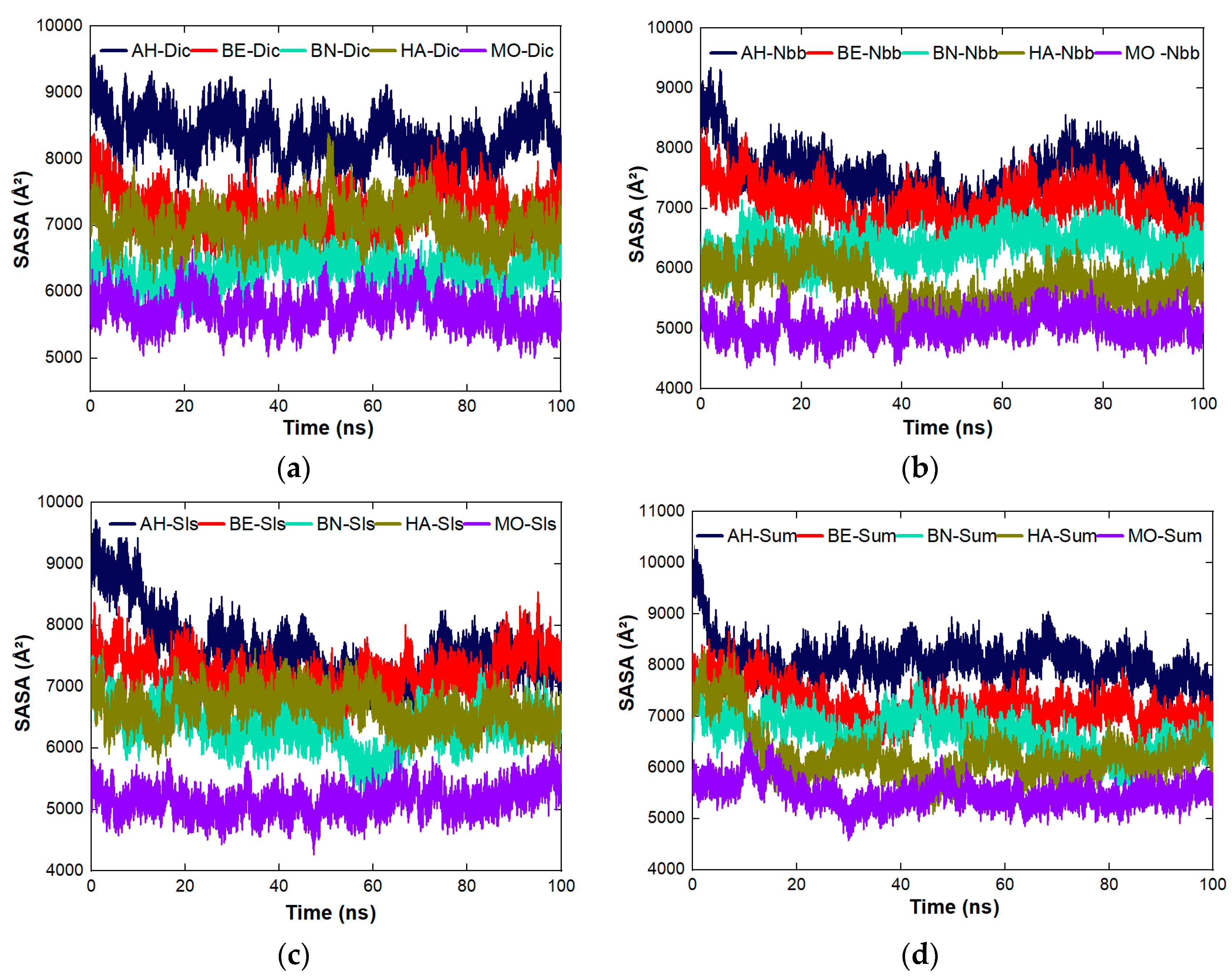
4.8. The Cross Performance of the Protein Molecules under Study for Dynamic Coagulation of Organic Molecules
4.8.1. Coagulation of Organic Pollutants by the Proteins
4.8.2. The Binding Affinity of the Proteins with the Organic Molecules
4.8.3. Stability Studies of the Complexes Formed between the Proteins and the Organic Molecules
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Robert, K.W.; Parris, T.M.; Leiserowitz, A.A. What is Sustainable Development? Goals, Indicators, Values, and Practice. Environ. Sci. Policy Sustain. Dev. 2005, 47, 8–21. [Google Scholar] [CrossRef]
- Alam, M.W.; Pandey, P.; Khan, F.; Souayeh, B.; Farhan, M. Study to Investigate the Potential of Combined Extract of Leaves and Seeds of Moringa oleifera in Groundwater Purification. Int. J. Environ. Res. Public Health 2020, 17, 7468. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Imron, M.F.; Chik, C.E.N.C.E.; Owodunni, A.A.; Ahmad, A.; Alnawajha, M.M.; Rahim, N.F.M.; Said, N.S.M.; Abdullah, S.R.S.; Kasan, N.A.; et al. What compound inside biocoagulants/bioflocculants is contributing the most to the coagulation and flocculation processes? Sci. Total Environ. 2022, 806, 150902. [Google Scholar] [CrossRef]
- Nouhi, S.; Kwaambwa, H.M.; Gutfreund, P.; Rennie, A.R. Comparative study of flocculation and adsorption behaviour of water treatment proteins from Moringa peregrina and Moringa oleifera seeds. Sci. Rep. 2019, 9, 17945. [Google Scholar] [CrossRef]
- Ullah, A.; Mariutti, R.; Masood, R.; Caruso, I.; Costa, G.H.G.; de Freita, C.M.; Santos, C.; Zanphorlin, L.M.; Mutton, M.J.R.; Murakami, M.T.; et al. Crystal structure of mature 2S albumin from Moringa oleifera seeds. Biochem. Biophys. Res. Commun. 2015, 468, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Huang, A.H.C. Occurrence of Low Molecular Weight and High Cysteine Containing Albumin Storage Proteins in Oilseeds of Diverse Species. Am. J. Bot. 1981, 68, 44–48. [Google Scholar] [CrossRef]
- Müntz, K.; Shutov, A.D. Legumains and their functions in plants. Trends Plant Sci. 2002, 7, 340–344. [Google Scholar] [CrossRef]
- Shewry, P.R.; Pandya, M.J. The 2S Albumin Storage Proteins BT—Seed Proteins; Shewry, P.R., Casey, R., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 563–586. [Google Scholar]
- Baptista, A.T.A.; Silva, M.O.; Gomes, R.G.; Bergamasco, R.; Vieira, M.F.; Vieira, A.M.S. Protein fractionation of seeds of Moringa oleifera lam and its application in superficial water treatment. Sep. Purif. Technol. 2017, 180, 114–124. [Google Scholar] [CrossRef]
- Gassenschmidt, U.; Jany, K.D.; Bernhard, T.; Niebergall, H. Isolation and characterization of a flocculating protein from Moringa oleifera Lam. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1995, 1243, 477–481. [Google Scholar] [CrossRef]
- Tan, M.; Nawaz, M.A.; Buckow, R. Functional and food application of plant proteins—A review. Food Rev. Int. 2021, 1–29. [Google Scholar] [CrossRef]
- Wan, H.; Chen, L.; Guo, J.; Li, Q.; Wen, J.; Yi, B.; Ma, C.; Tu, J.; Fu, T.; Shen, J. Genome-Wide Association Study Reveals the Genetic Architecture Underlying Salt Tolerance-Related Traits in Rapeseed (Brassica napus L.). Front. Plant Sci. 2017, 8, 593. [Google Scholar] [CrossRef]
- Choy, S.Y.; Prasad, K.M.N.; Wu, T.Y.; Ramanan, R.N. A review on common vegetables and legumes as promising plant-based natural coagulants in water clarification. Int. J. Environ. Sci. Technol. 2015, 12, 367–390. [Google Scholar] [CrossRef] [Green Version]
- Zamani, S.; Naderi, M.R.; Soleymani, A.; Nasiri, B.M. Sunflower (Helianthus annuus L.) biochemical properties and seed components affected by potassium fertilization under drought conditions. Ecotoxicol. Environ. Saf. 2020, 190, 110017. [Google Scholar] [CrossRef] [PubMed]
- Salgado, P.R.; Drago, S.R.; Ortiz, S.E.M.; Petruccelli, S.; Andrich, O.; González, R.J.; Mauri, A.N. Production and characterization of sunflower (Helianthus annuus L.) protein-enriched products obtained at pilot plant scale. LWT—Food Sci. Technol. 2012, 45, 65–72. [Google Scholar] [CrossRef]
- Lönnerdal, B.; Janson, J.-C. Studies on Brassica seed proteins: I. The low molecular weight proteins in rapeseed. Isolation and characterization. Biochim. Biophys. Acta—Protein Struct. 1972, 278, 175–183. [Google Scholar] [CrossRef]
- Nankya, R.; Mulumba, J.; Lwandasa, H.; Matovu, M.; Isabirye, B.; De Santis, P.; Jarvis, D. Diversity in Nutrient Content and Consumer Preferences of Sensory Attributes of Peanut (Arachis hypogaea L.) Varieties in Ugandan Agroecosystems. Sustainability 2021, 13, 2658. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Apolinário, D.; Bandeira, V.D.S.; Busse, A.L.; Magaldi, R.M.; Jacob-Filho, W.; Cozzolino, S.M.F. Effects of Brazil nut consumption on selenium status and cognitive performance in older adults with mild cognitive impairment: A randomized controlled pilot trial. Eur. J. Nutr. 2016, 55, 107–116. [Google Scholar] [CrossRef]
- Wang, K.; Sun, D.-W.; Pu, H.; Wei, Q. Principles and applications of spectroscopic techniques for evaluating food protein conformational changes: A review. Trends Food Sci. Technol. 2017, 67, 207–219. [Google Scholar] [CrossRef]
- Singh, A.; Vanga, S.K.; Orsat, V.; Raghavan, V. Application of molecular dynamic simulation to study food proteins: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2779–2789. [Google Scholar] [CrossRef]
- Wang, X.; Selvam, P.; Lv, C.; Kubo, M.; Miyamoto, A. A theoretical study on the cyclopropane adsorption onto the copper surfaces by density functional theory and quantum chemical molecular dynamics methods. J. Mol. Catal. A Chem. 2004, 220, 189–198. [Google Scholar] [CrossRef]
- Hossain, D.; Tschopp, M.A.; Ward, D.K.; Bouvard, J.L.; Wang, P.; Horstemeyer, M.F. Molecular dynamics simulations of deformation mechanisms of amorphous polyethylene. Polymer 2010, 51, 6071–6083. [Google Scholar] [CrossRef]
- Das, N.; Ojha, N.; Mandal, S.K. Wastewater treatment using plant-derived bioflocculants: Green chemistry approach for safe environment. Water Sci. Technol. 2021, 83, 1797–1812. [Google Scholar] [CrossRef]
- Kebede, E. Grain legumes production and productivity in Ethiopian smallholder agricultural system, contribution to livelihoods and the way forward. Cogent Food Agric. 2020, 6, 1722353. [Google Scholar] [CrossRef]
- Beltrán-Heredia, J.; Sánchez-Martín, J. Removal of sodium lauryl sulphate by coagulation/flocculation with Moringa oleifera seed extract. J. Hazard. Mater. 2009, 164, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Shirani, Z.; Santhosh, C.; Iqbal, J.; Bhatnagar, A. Waste Moringa oleifera seed pods as green sorbent for efficient removal of toxic aquatic pollutants. J. Environ. Manag. 2018, 227, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Sotheeswaran, S.; Nand, V.; Maata, M.; Koshy, K. Moringa oleifera and other local seeds in water purification in developing countries. Res. J. Chem. Environ. 2011, 15, 135–138. [Google Scholar]
- Flora, S.J.S. Arsenic and dichlorvos: Possible interaction between two environmental contaminants. J. Trace Elem. Med. Biol. 2016, 35, 43–60. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Patrolecco, L.; Pescatore, T.; Mariani, L.; Rolando, L.; Grenni, P.; Finizio, A.; Spataro, F.; Rauseo, J.; Ademollo, N.; Muzzini, V.G.; et al. Environmental Fate and Effects of Foaming Agents Containing Sodium Lauryl Ether Sulphate in Soil Debris from Mechanized Tunneling. Water 2020, 12, 2047. [Google Scholar] [CrossRef]
- Barra Caracciolo, A. Special Issue: Environmental Fate of Contaminants in the Aquatic Environment. Water 2021, 13, 1350. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Moulin, M.; Mossou, E.; Signor, L.; Kieffer-Jaquinod, S.; Kwaambwa, H.; Nermark, F.; Gutfreund, P.; Mitchell, E.; Haertlein, M.; Forsyth, V.; et al. Towards a molecular understanding of the water purification properties of Moringa seed proteins. J. Colloid Interface Sci. 2019, 554, 296–304. [Google Scholar] [CrossRef]
- Lehmann, K.; Schweimer, K.; Reese, G.; Randow, S.; Suhr, M.; Becker, W.-M.; Vieths, S.; Rösch, P. Structure and stability of 2S albumin-type peanut allergens: Implications for the severity of peanut allergic reactions. Biochem. J. 2006, 395, 463–472. [Google Scholar] [CrossRef]
- Rundqvist, L.; Tengel, T.; Zdunek, J.; Björn, E.; Schleucher, J.; Alcocer, M.; Larsson, G. Solution Structure, Copper Binding and Backbone Dynamics of Recombinant Ber e 1–The Major Allergen from Brazil Nut. PLoS ONE 2012, 7, e46435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantoja-Uceda, D.; Palomares, O.; Bruix, M.; Villalba, M.; Rodríguez, R.; Rico, M.; Santoro, J. Solution Structure and Stability against Digestion of rproBnIb, a Recombinant 2S Albumin from Rapeseed: Relationship to Its Allergenic Properties. Biochemistry 2004, 43, 16036–16045. [Google Scholar] [CrossRef]
- Pantoja-Uceda, D.; Shewry, P.R.; Bruix, M.; Tatham, A.S.; Santoro, J.; Rico, M. Solution Structure of a Methionine-Rich 2S Albumin from Sunflower Seeds: Relationship to Its Allergenic and Emulsifying Properties. Biochemistry 2004, 43, 6976–6986. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar] [CrossRef] [PubMed]
- Geourjon, C.; Deleage, G. SOPMA: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Bioinformatics 1995, 11, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35, W407–W410. [Google Scholar] [CrossRef] [Green Version]
- Colovos, C.; Yeates, T.O. Verification of protein structures: Patterns of nonbonded atomic interactions. Protein Sci. 1993, 2, 1511–1519. [Google Scholar] [CrossRef] [Green Version]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeleke, V.T.; Adeniyi, A.A.; Lokhat, D. Coagulation of organic pollutants by Moringa oleifera protein molecules: In silico approach. Environ. Sci. Water Res. Technol. 2021, 7, 1453–1464. [Google Scholar] [CrossRef]
- Case, D.A.; Ben-Shalom, I.Y.; Brozell, S.R.; Cerutti, D.S.; Cheatham III, T.E.; Cruzeiro, V.W.D.; Darden, T.A.; Duke, R.E.; Ghoreishi, D.; Gilson, M.K. AMBER 18; University of California: San Francisco, CA, USA, 2018. [Google Scholar]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Antechamber: An accessory software package for molecular mechanical calculations. J. Am. Chem. Soc. 2001, 222, U403. [Google Scholar]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Izaguirre, J.A.; Sweet, C.R.; Pande, V.S. multiscale dynamics of macromolecules using normal mode langevin. In Biocomputing 2010; World Scientific: Singapore, 2009; pp. 240–251. [Google Scholar]
- Literature, W.; Case, T.; Babin, V.; Berryman, J.; Betz, R.M.; Cai, Q.; Cerutti, D.S.; Cheatham, T.; Darden, T.; Duke, R.; et al. AMBER 14; University of California: San Francisco, CA, USA, 2014. [Google Scholar]
- Iqbal, A.; Khalil, I.A.; Ateeq, N.; Sayyar Khan, M. Nutritional quality of important food legumes. Food Chem. 2006, 97, 331–335. [Google Scholar] [CrossRef]
- Guckeisen, T.; Hosseinpour, S.; Peukert, W. Isoelectric Points of Proteins at the Air/Liquid Interface and in Solution. Langmuir 2019, 35, 5004–5012. [Google Scholar] [CrossRef]
- Hughes, C.S.; Moggridge, S.; Müller, T.; Sorensen, P.H.; Morin, G.B.; Krijgsveld, J. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 2019, 14, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Emami, F.; Vatanara, A.; Park, E.J.; Na, D.H. Drying Technologies for the Stability and Bioavailability of Biopharmaceuticals. Pharmaceutics 2018, 10, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akke, M.; Forsén, S. Protein stability and electrostatic interactions between solvent exposed charged side chains. Proteins Struct. Funct. Bioinform. 1990, 8, 23–29. [Google Scholar] [CrossRef]
- Pace, C.N.; Shirley, B.A.; McNutt, M.; Gajiwala, K. Forces contributing to the conformational stability of proteins. FASEB J. 1996, 10, 75–83. [Google Scholar] [CrossRef]
- Kebede, T.G.; Dube, S.; Nindi, M.M. Removal of multi-class antibiotic drugs from Wastewater using water-soluble protein of Moringa stenopetala seeds. Water 2019, 11, 595. [Google Scholar] [CrossRef] [Green Version]
- Wan, S.; Sinclair, R.C.; Coveney, P.V. Uncertainty quantification in classical molecular dynamics. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2021, 379, 20200082. [Google Scholar] [CrossRef]
- Knapp, B.; Ospina, L.; Deane, C.M. Avoiding False Positive Conclusions in Molecular Simulation: The Importance of Replicas. J. Chem. Theory Comput. 2018, 14, 6127–6138. [Google Scholar] [CrossRef]
- Huff, N.T.; Demiralp, E.; Çagin, T.; Goddard, W.A., III. Factors affecting molecular dynamics simulated vitreous silica structures. J. Non-Cryst. Solids 1999, 253, 133–142. [Google Scholar] [CrossRef]
- Hollingsworth, S.A.; Dror, R.O. Molecular Dynamics Simulation for All. Neuron 2018, 99, 1129–1143. [Google Scholar] [CrossRef] [Green Version]
- De Souza, T.N.V.; de Carvalho, S.M.L.; Vieira, M.G.A.; da Silva, M.G.C.; do Brasil, D.S.B. Adsorption of basic dyes onto activated carbon: Experimental and theoretical investigation of chemical reactivity of basic dyes using DFT-based descriptors. Appl. Surf. Sci. 2018, 448, 662–670. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, H.; Liu, G.; Qiao, J.; Wang, J.; Lu, H.; Yang, L.; Wu, Y. Methylene blue adsorption onto swede rape straw (Brassica napus L.) modified by tartaric acid: Equilibrium, kinetic and adsorption mechanisms. Bioresour. Technol. 2012, 125, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Fiala, H.; Bendjeffal, H.; Achraf, H.; Amina, L.; Yamina, B.; Zhour, H.; Abdelhak, G. Removal of Malachite Green Dye From Aqueous Media using Helianthus Annuus Seeds Shells as Eco-Friendly Adsorbent: Optimization, Equilibrium, and Kinetic Studies. Ann. Rom. Soc. Cell Biol. 2021, 25, 16488–16510. [Google Scholar]
- Bodlund, I.; Pavankumar, A.R.; Chelliah, R.; Kasi, S.; Sankaran, K.; Rajarao, G.K. Coagulant proteins identified in Mustard: A potential water treatment agent. Int. J. Environ. Sci. Technol. 2014, 11, 873–880. [Google Scholar] [CrossRef] [Green Version]
- Kpan, W.B.; Koné, M.W.; Bonfoh, B.; Kamanzi, K. Evaluation of eighteen west african plants for water purification, potential use for rural water treatment. J. Water Chem. Technol. 2017, 39, 310–316. [Google Scholar] [CrossRef]
- Hellsing, M.S.; Kwaambwa, H.M.; Nermark, F.M.; Nkoane, B.B.; Jackson, A.J.; Wasbrough, M.J.; Berts, I.; Porcar, L.; Rennie, A.R. Structure of flocs of latex particles formed by addition of protein from Moringa seeds. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 460–467. [Google Scholar] [CrossRef]
- Tambo, N.; Hozumi, H. Physical characteristics of flocs—II. Strength of floc. Water Res. 1979, 13, 421–427. [Google Scholar] [CrossRef]
- Yukselen, M.A.; Gregory, J.; Soyer, E. Formation and breakage of flocs using dual polymers. Water Sci. Technol. 2006, 53, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Coskun, O. Separation techniques: Chromatography. North. Clin. Istanb. 2016, 3, 156–160. [Google Scholar] [CrossRef] [Green Version]
- Ros, A.; Faupel, M.; Mees, H.; Van Oostrum, J.; Ferrigno, R.; Reymond, F.; Michel, P.; Rossier, J.S.; Girault, H.H. Protein purification by Off-Gel electrophoresis. Proteomics 2002, 2, 151–156. [Google Scholar] [CrossRef]
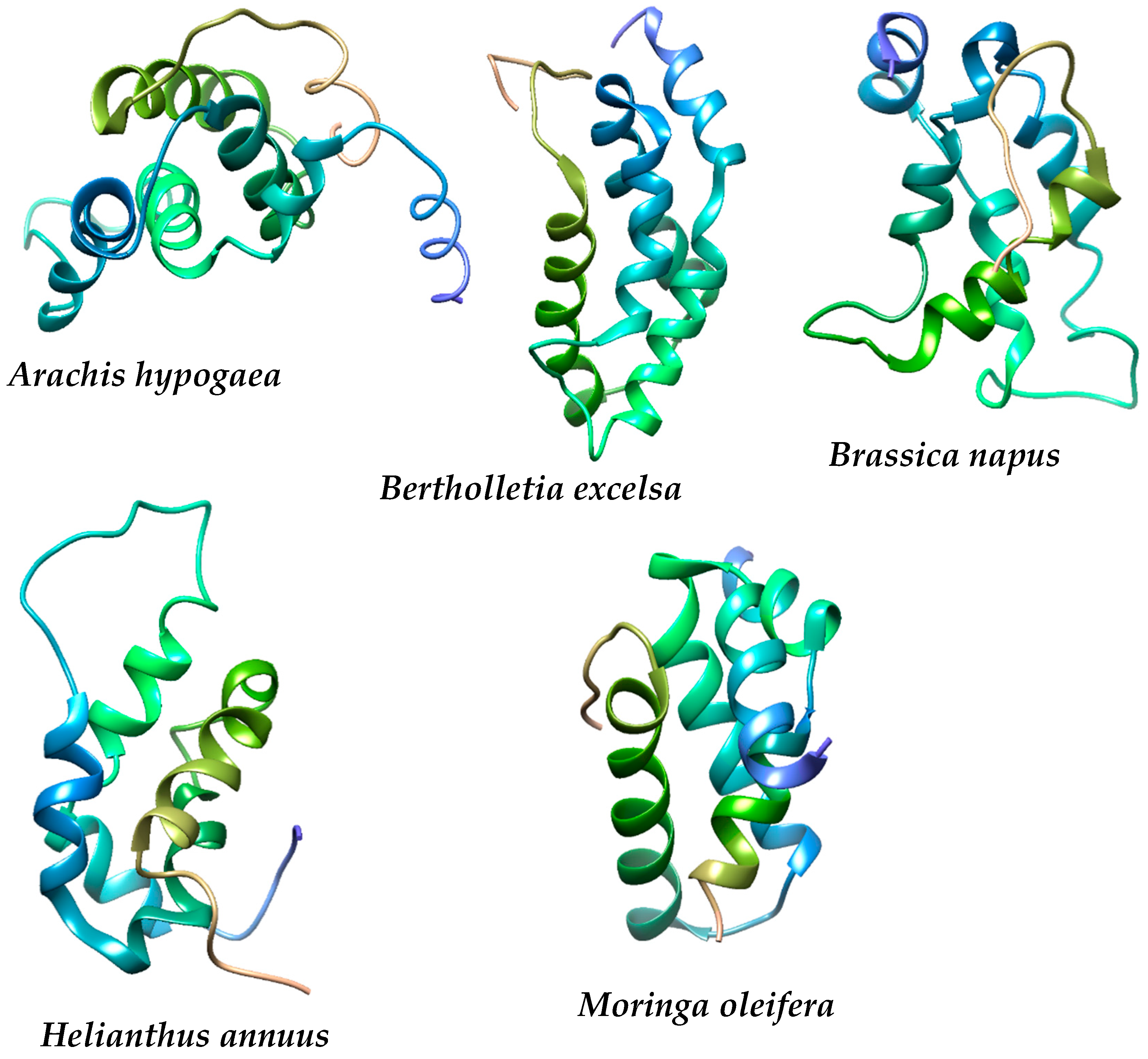
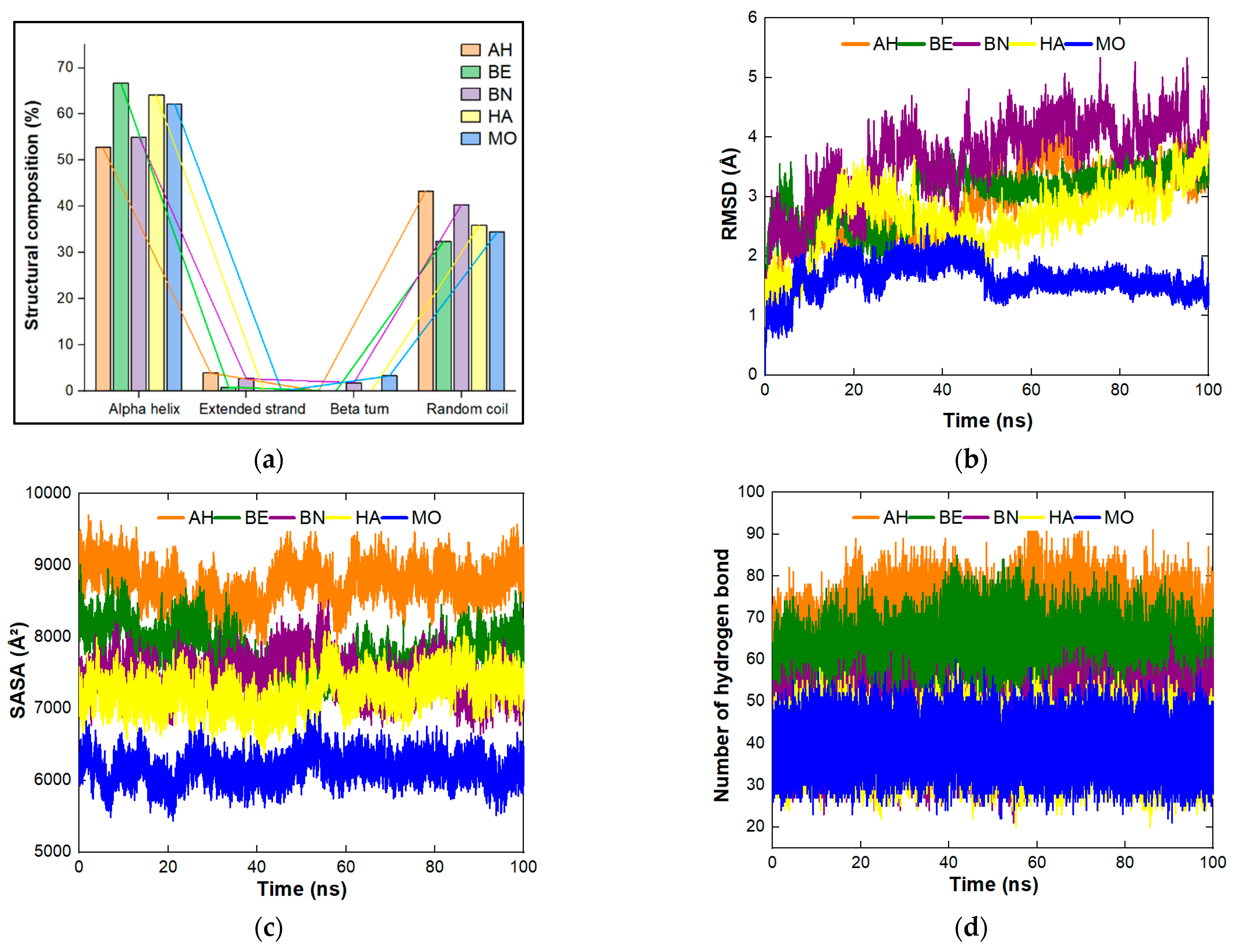
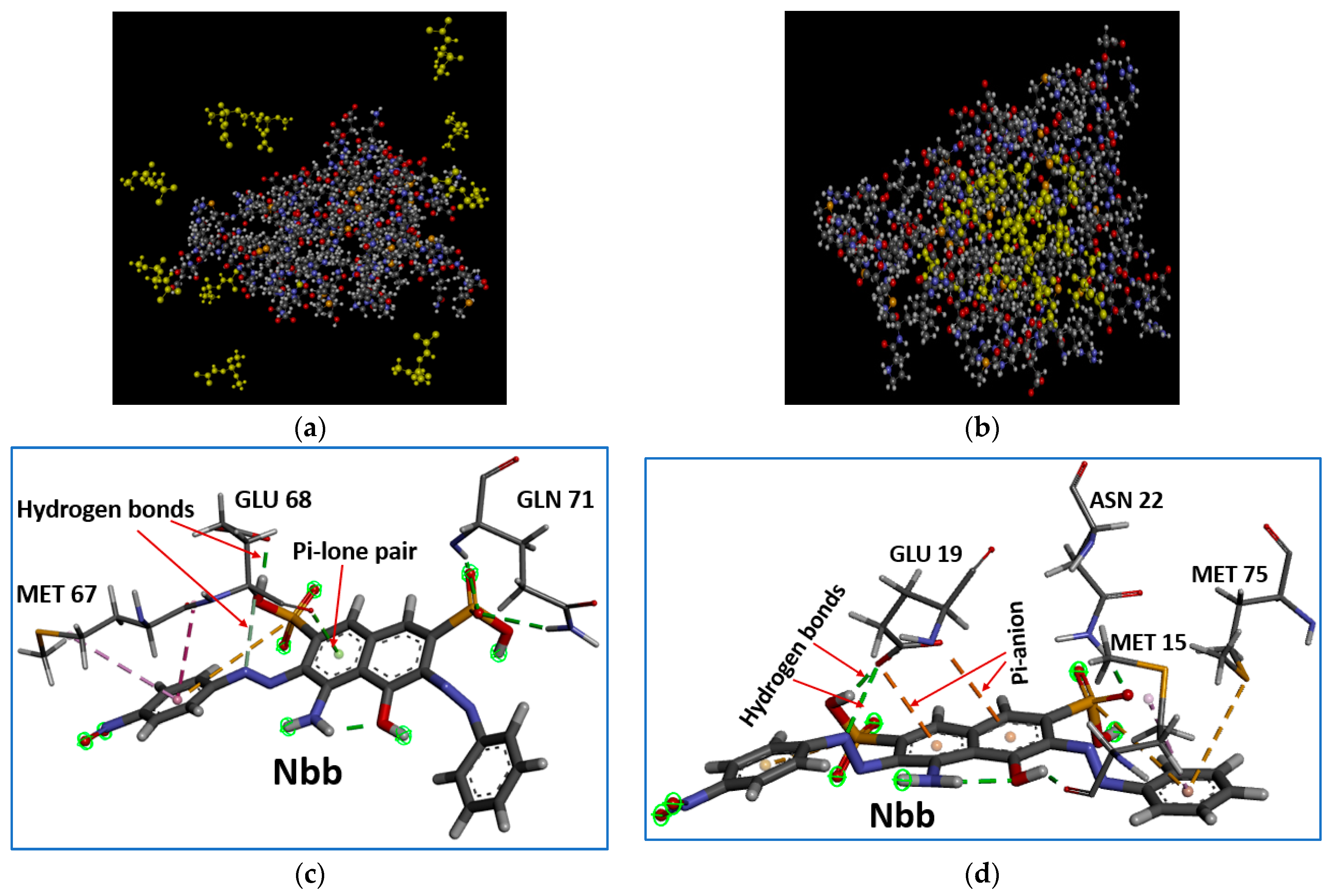

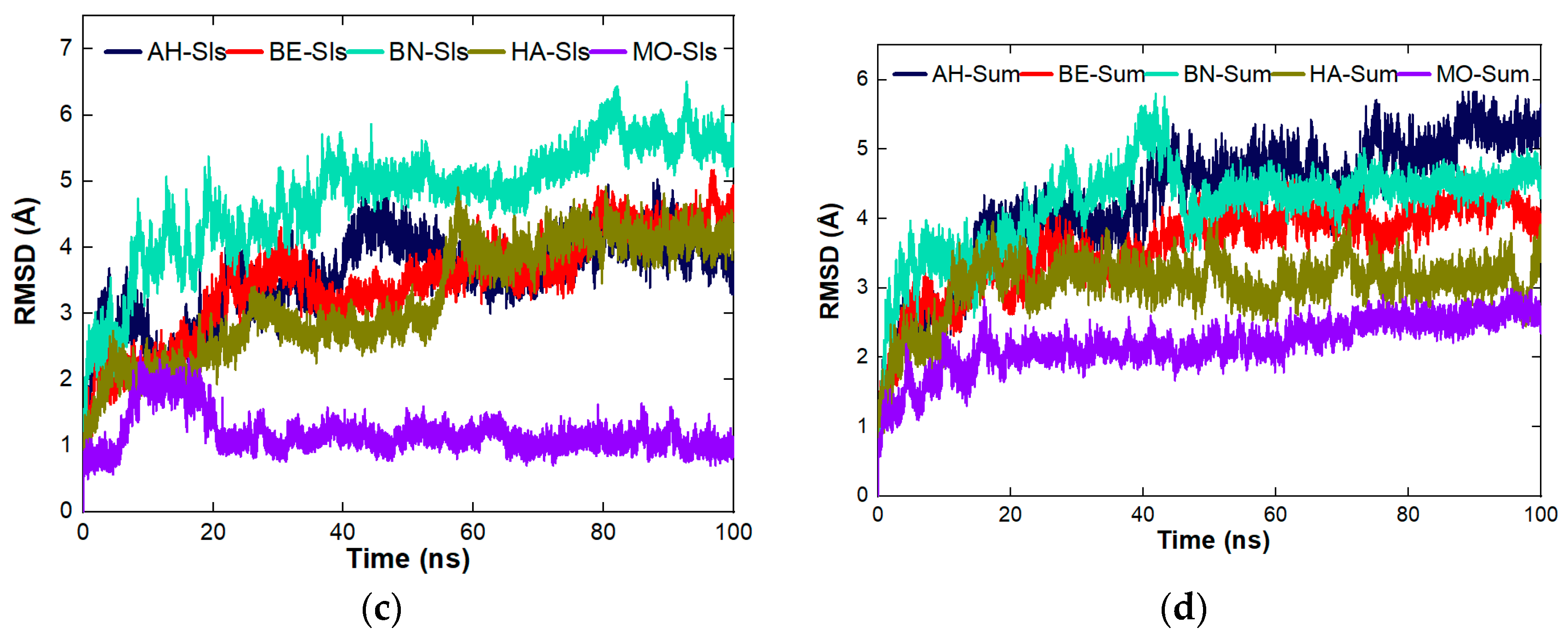
| Plant | Complex Formed | aa | MW (kDa) | Instability Index | Aliphatic Index | GRAVY | Z Score | Errat (%) | Ramachandran (Favoured Region) (%) |
|---|---|---|---|---|---|---|---|---|---|
| Arachishypogaea (AH) | 127 | 14.96 | 68.66 | 44.49 | −1.216 | −3.40 | 88.34 | 87.0 | |
| AH-Dic | −3.91 | 86.67 | 87.0 | ||||||
| AH-Nbb | −3.61 | 89.69 | 85.2 | ||||||
| AH-Sls | −4.54 | 95.23 | 88.7 | ||||||
| AH-Sum | −3.80 | 95.24 | 90.4 | ||||||
| Bertholletia excelsa (BE) | 114 | 13.63 | 77.74 | 27.46 | −1.016 | −3.7 | 91.25 | 88.9 | |
| BE-Dic | −4.33 | 90.24 | 90.90 | ||||||
| BE-Nbb | −4.69 | 92.21 | 92.9 | ||||||
| BE-Sls | −4.35 | 100.0 | 90.9 | ||||||
| BE-Sum | −4.49 | 87.34 | 87.9 | ||||||
| Brassica napus (BN) | 109 | 12.53 | 59.38 | 61.74 | −0.761 | −3.98 | 93.83 | 87.1 | |
| BN-Dic | −3.94 | 96.43 | 86.0 | ||||||
| BN-Nbb | −5.84 | 100.0 | 87.1 | ||||||
| BN-Sls | −4.72 | 94.87 | 87.1 | ||||||
| BN-Sum | −4.95 | 80.7 | 80.6 | ||||||
| Helianthus annuus (HA) | 103 | 12.16 | 67.95 | 53.98 | −0.586 | −4.78 | 92.19 | 84.6 | |
| HA-Dic | −4.52 | 100.00 | 83.5 | ||||||
| HA-Nbb | −5.56 | 87.10 | 90.1 | ||||||
| HA-Sls | −4.87 | 90.00 | 85.7 | ||||||
| HA-Sum | −5.66 | 94.74 | 91.2 | ||||||
| Moringa oleifera (MO) | 90 | 10.59 | 81.42 | 59.56 | −0.768 | −6.35 | 100.00 | 93.4 | |
| MO-Dic | −5.41 | 100.00 | 93.4 | ||||||
| MO-Nbb | −5.17 | 98.21 | 88.2 | ||||||
| MO-Sls | −5.32 | 100.00 | 88.2 | ||||||
| MO-Sum | −4.20 | 98.33 | 85.5 | ||||||
| Plant Proteins | Amino Acid Composition (%) | Isoelectric Point (PI) | Negatively Charged Residues (Asp + Glu) | Positively Charged Residues (Arg + Lys) | ||||
|---|---|---|---|---|---|---|---|---|
| Glutamic Acid (GLU) | Aspartic Acid (ASP) | Lysine (LYS) | Arginine (ARG) | Histidine (HIS) | ||||
| AH | 8.7 | 7.9 | 1.6 | 12.6 | 0.8 | 5.24 | 21.0 | 18.0 |
| BE | 14.0 | 0.9 | 0.9 | 13.2 | 1.8 | 6.20 | 17.0 | 16.0 |
| BN | 5.5 | 0.9 | 4.6 | 5.5 | 1.8 | 8.71 | 7.0 | 11.0 |
| HA | 9.7 | 2.9 | 3.9 | 6.8 | 2.9 | 5.91 | 13.0 | 11.0 |
| MO | 0.0 | 0.0 | 0.0 | 15.6 | 2.2 | 11.61 | 0.0 | 14.0 |
| Protein | Parameters | Organic Molecules | Average Coagulation (%) | |||
|---|---|---|---|---|---|---|
| Dic | Nbb | Sls | Sum | |||
| AH | % of the ligands coagulated | 70 | 90 | 60 | 60 | 70.00 |
| Types of bonds/interactions | Halogen, hydrogen, alkyl, π-alkyl | Hydrogen, π-alkyl, π-lone pair, Amide-π-stacked, π-π-stacked, van der Waals, π-cation, π-anion | Hydrogen, alkyl | Hydrogen, alkyl, π-alkyl, π-lone pair, Amide-π -stacked, π-anion | ||
| BE | % of the ligands coagulated | 90 | 70 | 70 | 50 | 70.00 |
| Types of interaction | Hydrogen, alkyl, π-alkyl, π-sigma | Hydrogen, π-alkyl, Amide-π-stacked, π-cation, π-anion, π-sulfur | Hydrogen, alkyl | Hydrogen, alkyl, π-alkyl, π-lone pair | ||
| BN | % of the ligands coagulated | 100 | 100 | 90 | 80 | 92.50 |
| Types of interaction | Hydrogen, alkyl, π -alkyl | Hydrogen, alkyl, π-alkyl, π-lone pair, Amide- π-stacked, π-π-stacked, π-sulfur, π-anion | Hydrogen, alkyl, π -alkyl | Hydrogen, alkyl, π-alkyl, π-cation, π-sulfur, π-π -stacked | ||
| HA | % of the ligands coagulated | 100 | 90 | 90 | 60 | 85.00 |
| Types of interaction | Hydrogen, alkyl, π-alkyl | Hydrogen, π-alkyl, π-lone pair, Amide- π-stacked, π-π -stacked, π-cation, π-sulfur, π-anion | Hydrogen, alkyl, π-alkyl | Hydrogen, alkyl, π-alkyl, π-cation, π-sulfur, π-anion | ||
| MO | % of the ligands coagulated | 100 | 90 | 50 | 70 | 77.50 |
| Types of interaction | Hydrogen, alkyl, π-alkyl | Hydrogen, π-alkyl, π-lone pair, Amide- π-stacked, π-cation, π-anion | Hydrogen, alkyl, π-alkyl, π-sigma | Hydrogen, alkyl, π-alkyl, π-π -stacked, π-cation, π-sulfur | ||
| Complex | Energy Component (kcal/mol) | ||||||
|---|---|---|---|---|---|---|---|
| ∆EVDW | ∆EELE | ∆EEGB | ∆ESURF | ∆Ggas | ∆Gsolv | ∆Ebind | |
| AH-Dic | −94.90 ± 0.67 | −10.70 ± 0.17 | 37.42 ± 0.25 | −13.47 ± 0.09 | −105.60 ± 0.75 | 23.95 ± 0.19 | −81.65 ± 0.64 |
| AH-Nbb | −154.12 ± 0.71 | −349.35 ± 1.66 | 401.03 ± 1.77 | −22.144 ± 0.09 | −503.47 ± 2.30 | 378.89 ± 1.69 | −124.58 ± 0.65 |
| AH-Sls | −101.87 ± 0.52 | −140.45 ± 1.05 | 167.04 ± 1.00 | −17.53 ± 0.09 | −242.32 ± 1.39 | 149.50 ± 0.94 | −92.81 ± 0.54 |
| AH-Sum | −94.80 ± 0.41 | −157.86 ± 1.01 | 194.04 ± 0.99 | −14.27 ± 0.06 | −252.66 ± 1.27 | 179.77 ± 0.94 | −72.99 ± 0.39 |
| BE-Dic | −142.58 ± 0.69 | −19.39 ± 0.19 | 54.88 ± 0.24 | −20.02 ± 0.09 | −161.97 ± 0.75 | 34.86 ± 0.20 | −127.10 ± 0.68 |
| BE-Nbb | −121.69 ± 0.65 | −236.03 ± 0.98 | 284.50 ± 0.09 | −17.72 ± 0.08 | −357.72 ± 1.44 | 266.78 ± 0.98 | −90.94 ± 0.50 |
| BE-Sls | −102.86 ± 0.60 | −117.03 ± 1.27 | 135.21 ± 1.09 | −16.95 ± 0.10 | −219.90 ± 1.75 | 118.26 ± 1.01 | −101.63 ± 0.79 |
| BE-Sum | −60.88 ± 0.39 | −121.11 ± 1.32 | 145.87 ± 1.25 | −9.56 ± 0.06 | −181.99 ± 1.55 | 136.31 ± 1.20 | −45.68 ± 0.39 |
| BN-Dic | −124.29 ± 0.47 | −21.99 ± 0.19 | 53.90 ± 0.22 | −17.07 ± 0.06 | −146.29 ± 0.56 | 36.83 ± 0.19 | −109.46 ± 0.44 |
| BN-Nbb | −167.18 ± 0.38 | −349.56 ± 1.48 | 409.71 ± 1.31 | −23.39 ± 0.05 | −516.74 ± 1.74 | 386.32 ± 1.26 | −130.42 ± 0.52 |
| BN-Sls | −170.54 ± 0.86 | −95.42 ± 0.87 | 145.10 ± 0.86 | −26.14 ± 0.13 | −265.97 ± 1.42 | 118.95 ± 0.78 | −147.02 ± 0.87 |
| BN-Sum | −109.00 ± 0.59 | −189.34 ± 1.35 | 237.59 ± 1.39 | −15.77 ± 0.9 | −298.34 ± 1.76 | 221.82 ± 1.32 | −76.52 ± 0.53 |
| HA-Dic | −138.76 ± 0.50 | −15.30 ± 0.19 | 48.69 ± 0.24 | −19.44 ± 0.07 | −154.06 ± 0.59 | 29.26 ± 0.20 | −124.80 ± 0.50 |
| HA-Nbb | −139.63 ± 0.34 | −251.35 ± 1.01 | 316.15 ± 0.99 | −20.90 ± 0.04 | −390.98 ± 1.17 | 295.26 ± 0.96 | −95.72 ± 0.28 |
| HA-Sls | −114.55 ± 0.57 | −155.87 ± 1.64 | 177.59 ± 1.49 | −19.00 ± 0.10 | −270.43 ± 2.08 | 158.59 ± 1.41 | −111.84 ± 0.72 |
| HA-Sum | −97.87 ± 0.52 | −153.42 ± 0.89 | 193.44 ± 0.91 | −14.40 ± 0.07 | −251.29 ± 1.17 | 179.04 ± 0.87 | −72.25 ± 0.41 |
| MO-Dic | −100.04 ± 0.40 | −28.87 ± 0.25 | 57.30 ± 0.29 | −13.99 ± 0.06 | −128.91 ± 0.55 | 43.30 ± 0.26 | −85.60 ± 0.36 |
| MO-Nbb | −169.10 ± 0.47 | −160.33 ± 0.85 | 248.02 ± 0.92 | −23.00 ± 0.06 | −329.43 ± 1.11 | 225.02 ± 0.87 | −104.41 ± 0.32 |
| MO-Sls | −123.97 ± 0.47 | −135.99 ± 1.29 | 167.30 ± 1.23 | −19.93 ± 0.08 | −259.97 ± 1.56 | 147.37 ± 1.17 | −112.60 ± 0.50 |
| MO-Sum | −97.12 ± 0.35 | −221.33 ± 1.54 | 257.84 ± 1.43 | −14.66 ± 0.05 | −318.44 ± 1.64 | 243.18 ± 1.41 | −75.27 ± 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeleke, V.T.; Madlala, N.E.; Adeniyi, A.A.; Lokhat, D. Molecular Interactions Associated with Coagulation of Organic Pollutants by 2S Albumin of Plant Proteins: A Computational Approach. Molecules 2022, 27, 1685. https://doi.org/10.3390/molecules27051685
Adeleke VT, Madlala NE, Adeniyi AA, Lokhat D. Molecular Interactions Associated with Coagulation of Organic Pollutants by 2S Albumin of Plant Proteins: A Computational Approach. Molecules. 2022; 27(5):1685. https://doi.org/10.3390/molecules27051685
Chicago/Turabian StyleAdeleke, Victoria T., Nkosinathi E. Madlala, Adebayo A. Adeniyi, and David Lokhat. 2022. "Molecular Interactions Associated with Coagulation of Organic Pollutants by 2S Albumin of Plant Proteins: A Computational Approach" Molecules 27, no. 5: 1685. https://doi.org/10.3390/molecules27051685
APA StyleAdeleke, V. T., Madlala, N. E., Adeniyi, A. A., & Lokhat, D. (2022). Molecular Interactions Associated with Coagulation of Organic Pollutants by 2S Albumin of Plant Proteins: A Computational Approach. Molecules, 27(5), 1685. https://doi.org/10.3390/molecules27051685







