Research on Preparation Methods of Carbon Nanomaterials Based on Self-Assembly of Carbon Quantum Dots
Abstract
:1. Introduction
2. Results and Discussion
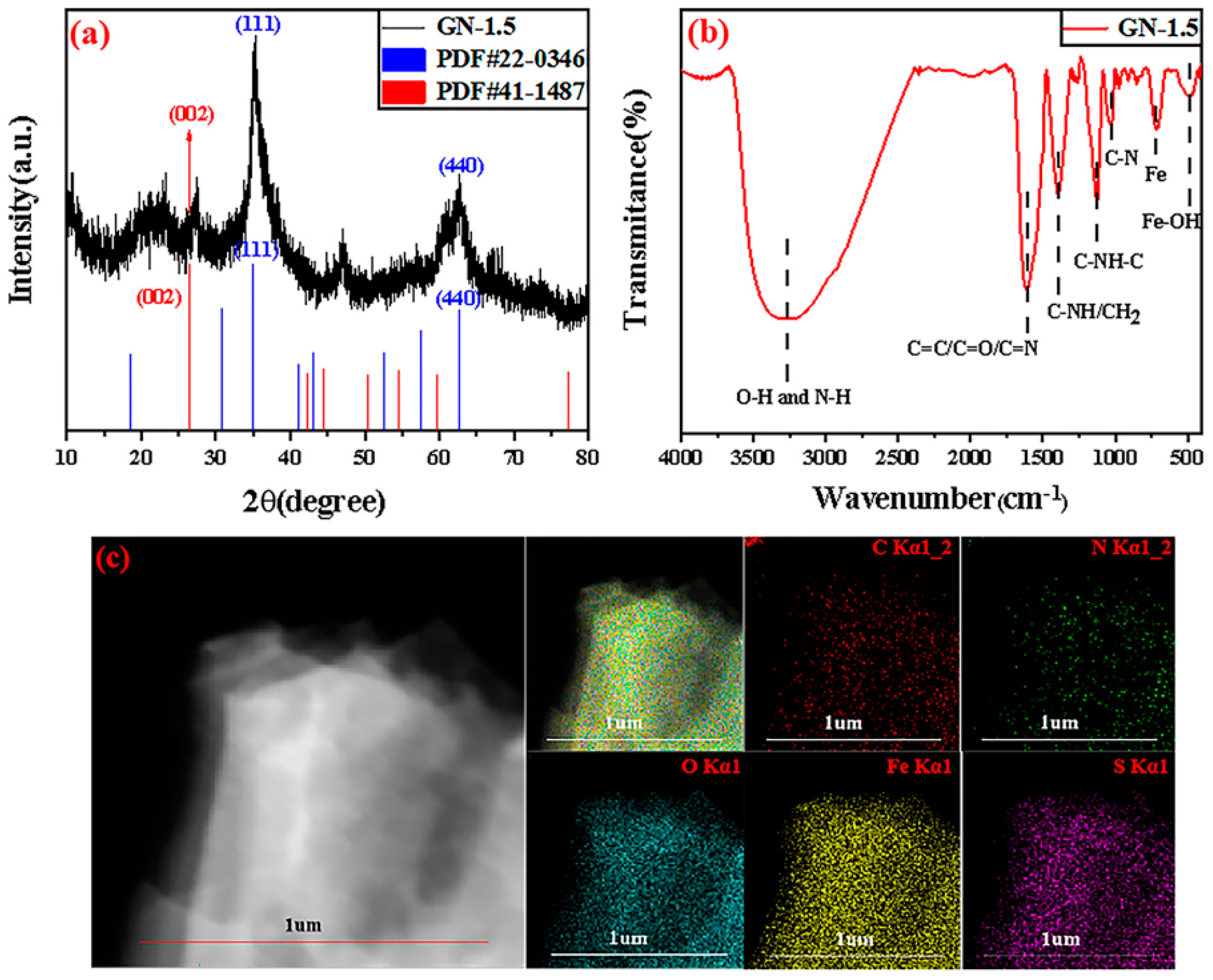
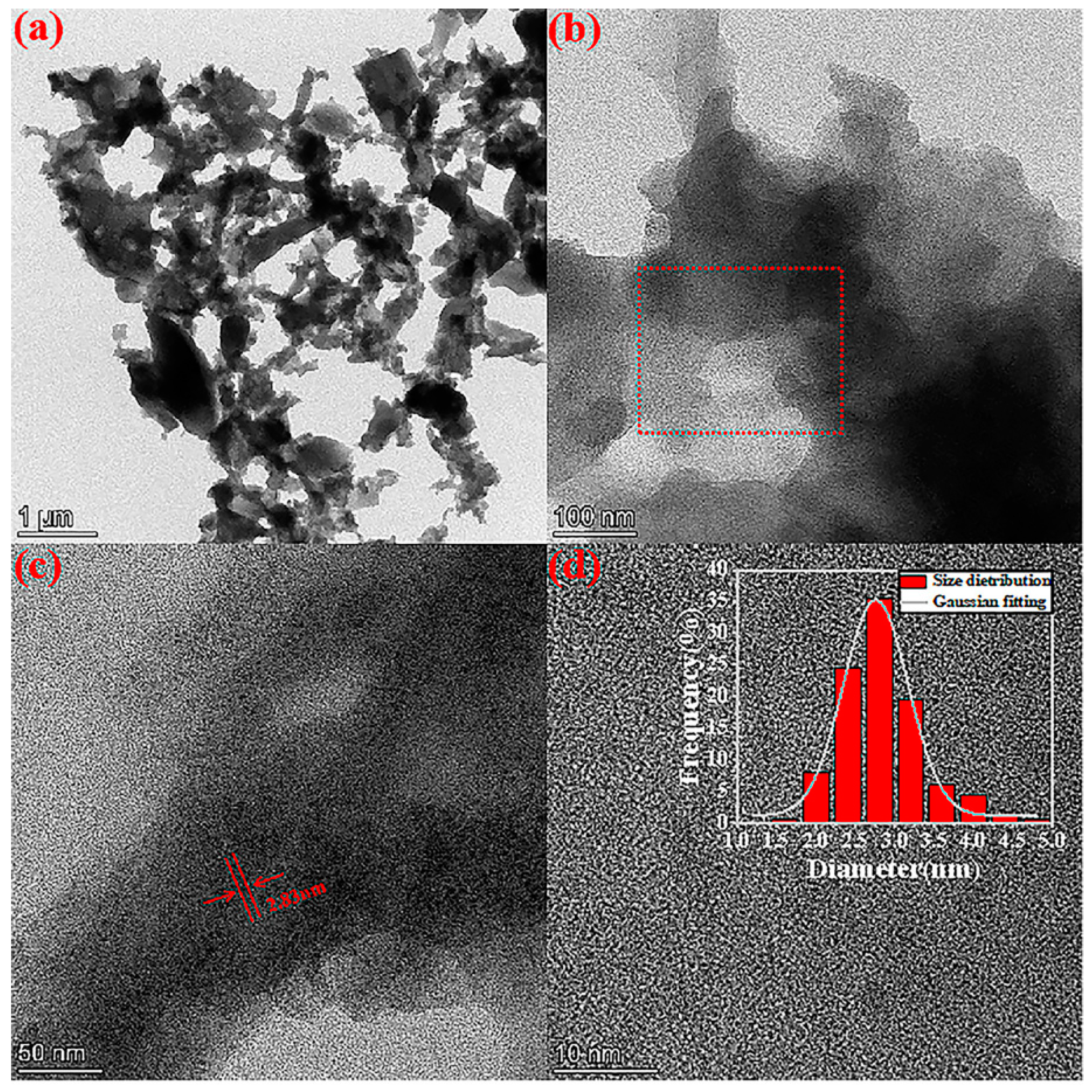
2.1. Structure of GN-1.5
2.2. Electrochemical Property Study for GN-1.5
2.3. Self-Assembly Mechanism of the Graphene-like Monolayer Film
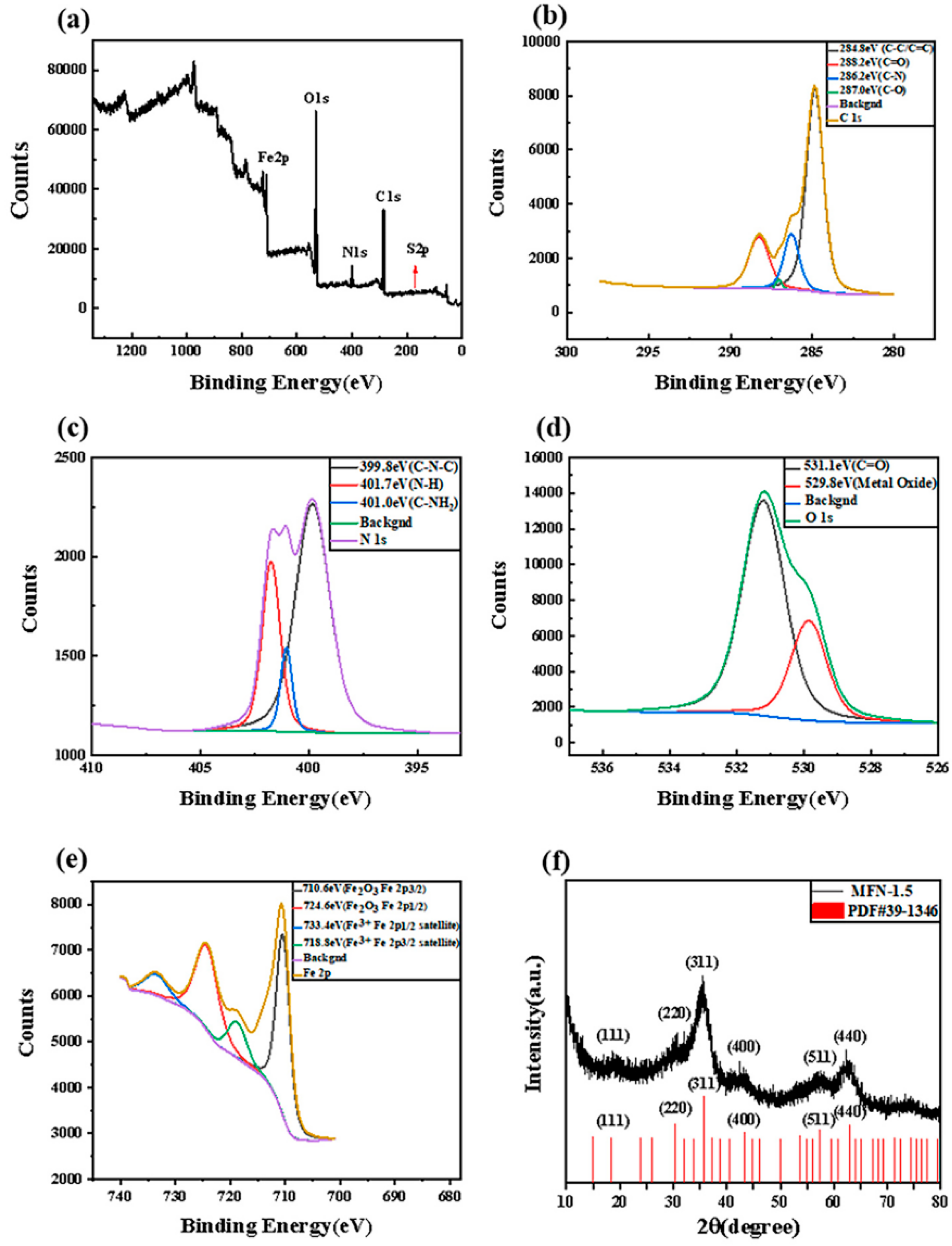
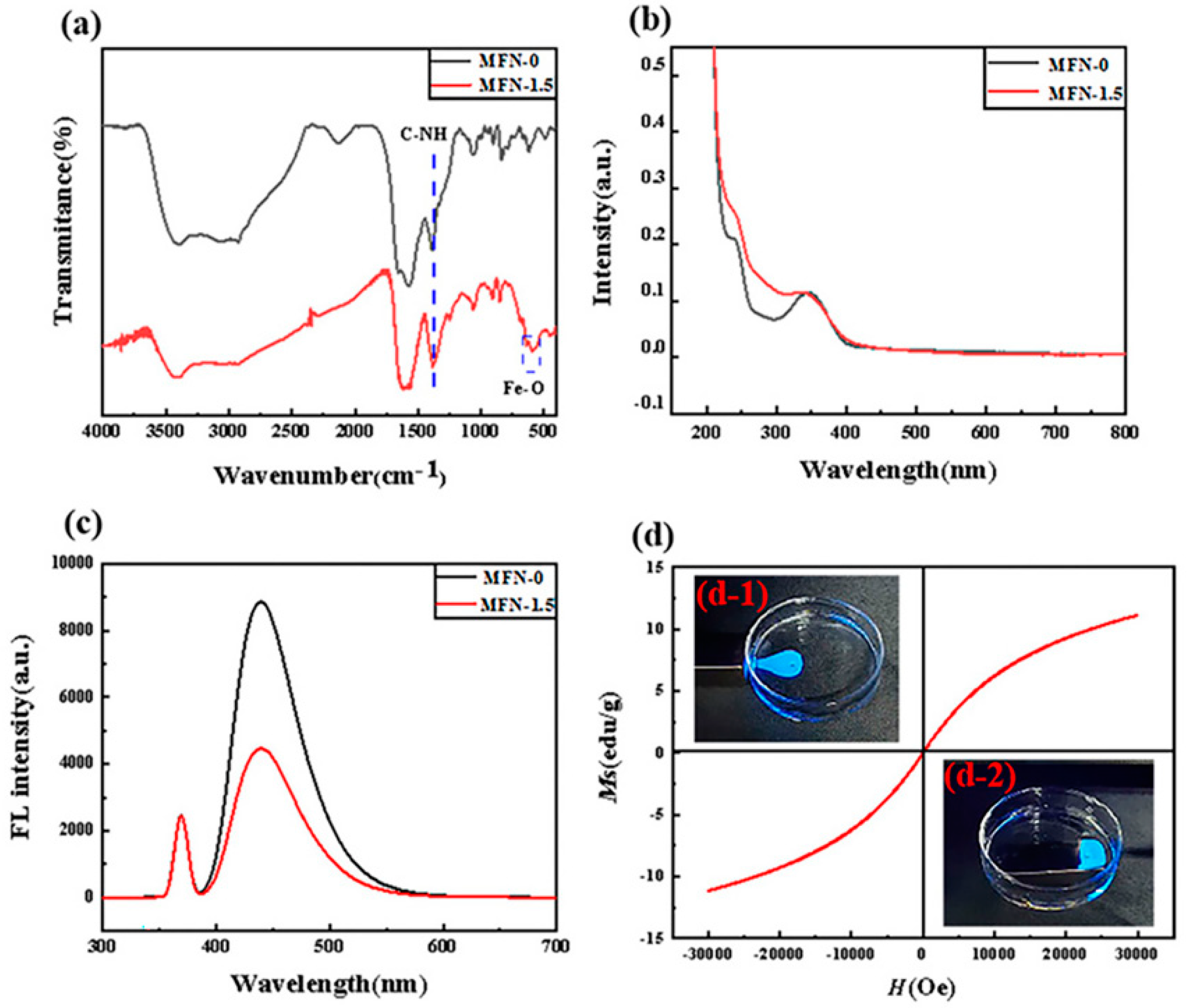
2.4. The Structure and Properties of MFN
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the Graphene-like Nano-Carbon Films
3.3. Synthesis of the Composite of γ-Fe2O3 and CDs
3.4. Characterization
3.5. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olabi, A.G.; Abdelkareem, M.A.; Wilberforce, T.; Sayed, E.T. Application of graphene in energy storage device—A review. Renew. Sustain. Energy Rev. 2021, 135, 110026. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Lee, Y.H.; Chang, K.H.; Li, J.M.; Simon, P.; Tang, J.; Torad, N.L.; Hu, C.C.; Yamauchi, Y. Nanoarchitectured Graphene-Based Supercapacitors for Next-Generation Energy-Storage Applications. Chem. Eur. J. 2014, 20, 13838–13852. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kim, J.H. Synthesis, toxicity, biocompatibility, and biomedical applications of graphene and graphene-related materials. Int. J. Nanomed. 2016, 11, 1927–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, B.M.; Shin, H.J.; Yoon, H.W.; Park, H.B. Graphene and Graphene Oxide and Their Uses in Barrier Polymers. J. Appl. Polym. Sci. 2014, 131, 39628. [Google Scholar] [CrossRef]
- Gui, Y.G.; Peng, X.; Liu, K.; Ding, Z.Y. Adsorption of C2H2, CH4 and CO on Mn-doped graphene: Atomic, electronic, and gas-sensing properties. Physica E 2020, 119, 113959. [Google Scholar] [CrossRef]
- Du, J.J.; Xu, N.; Fan, J.L.; Sun, W.; Peng, X.J. Carbon Dots for In Vivo Bioimaging and Theranostics. Small 2019, 15, 1805087. [Google Scholar] [CrossRef]
- Peng, Z.L.; Han, X.; Li, S.H.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; Leblanc, R.M. Carbon dots: Biomacromolecule interaction, bioimaging and nanomedicine. Coord. Chem. Rev. 2017, 343, 256–277. [Google Scholar] [CrossRef]
- Molaei, M.J. Principles, mechanisms, and application of carbon quantum dots in sensors: A review. Anal. Methods 2020, 12, 1266–1287. [Google Scholar] [CrossRef]
- Feng, T.; Ai, X.Z.; Ong, H.M.; Zhao, Y.L. Dual-Responsive Carbon Dots for Tumor Extracellular Microenvironment Triggered Targeting and Enhanced Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 18732–18740. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Guo, B.; Hao, L.Y.; Liu, N.; Lin, Y.F.; Guo, W.S.; Li, X.G.; Gu, B. Doxorubicin-loaded environmentally friendly carbon dots as a novel drug delivery system for nucleus targeted cancer therapy. Colloids Surf. B 2017, 159, 349–359. [Google Scholar] [CrossRef]
- Dong, S.Q.; Yuan, Z.Q.; Zhang, L.J.; Lin, Y.J.; Lu, C. Rapid Screening of Oxygen States in Carbon Quantum Dots by Chemiluminescence Probe. Anal. Chem. 2017, 89, 12520–12526. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.X.; Geng, F.L.; Di, F.; Guo, L.H.; Wan, B.; Yang, Y.; Zhang, H.; Sun, G.Z. Polyamine-functionalized carbon nanodots: A novel chemiluminescence probe for selective detection of iron(III) ions. RSC Adv. 2014, 4, 45768–45771. [Google Scholar] [CrossRef]
- Hu, C.; Li, M.Y.; Qiu, J.S.; Sun, Y.P. Design and fabrication of carbon dots for energy conversion and storage. Chem. Soc. Rev. 2019, 48, 2315–2337. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots. A review. Environ. Chem. Lett. 2020, 18, 703–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Pu, P.; Zhao, J.G.; Dong, C.B.; Gao, C.; Chen, Y.S.; Chen, J.R.; Liu, Y.; Zhou, H.J. Preparation of highly photoluminescent sulfur-doped carbon dots for Fe(III) detection. J. Mater. Chem. A 2015, 3, 542–546. [Google Scholar] [CrossRef]
- Qu, D.; Sun, Z.C. The formation mechanism and fluorophores of carbon dots synthesized via a bottom-up route. Mater. Chem. Front. 2020, 4, 400–420. [Google Scholar] [CrossRef]
- Feng, Z.B.; Li, Z.L.; Zhang, X.W.; Xu, G.Q.; Zhou, N. Fluorescent carbon dots with two absorption bands: Luminescence mechanism and ion detection. J. Mater. Sci. 2018, 53, 6459–6470. [Google Scholar] [CrossRef]
- Schneider, J.; Reckmeier, C.J.; Reckmeier, C.J.; Xiong, Y.; von Seckendorff, M.; Susha, A.S.; Kasak, P.; Rogach, A.L. Molecular fluorescence in citric acid-based carbon dots. J. Phys. Chem. C 2016, 121, 2014–2022. [Google Scholar] [CrossRef]
- Song, Y.B.; Zhu, S.J.; Zhang, S.T.; Fu, Y.; Wang, L.; Zhao, X.H.; Yang, B. Investigation from chemical structure to photoluminescent mechanism: A type of carbon dots from the pyrolysis of citric acid and an amine. J. Mater. Chem. C 2015, 3, 5976–5984. [Google Scholar] [CrossRef]
- Vallan, L.; Urriolabeitia, E.P.; Ruiperez, F.; Matxain, J.M.; Canton-Vitoria, R.; Tagmatarchis, N.; Benito, A.M.; Maser, W.K. Supramolecular-enhanced charge transfer within entangled polyamide chains as the origin of the universal blue fluorescence of polymer carbon dots. J. Am. Chem. Soc. 2018, 140, 12862–12869. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Zhang, Z.Q.; Zhang, W.; Zhou, L.Q.; Li, H.W.; Wang, H.M.; Andreazza-Vignolle, C.; Andreazza, P.; Zhao, D.X.; Wu, Y.H.; et al. Color-Switchable, Emission-Enhanced Fluorescence Realized by Engineering C-dot@C-dot Nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 20700–20708. [Google Scholar] [CrossRef]
- Wei, G.J.; Du, K.; Zhao, X.X.; Wang, Z.J.; Liu, M.; Li, C.; Wang, H.; An, C.H.; Xing, W. Carbon quantum dot-induced self-assembly of ultrathin Ni(OH)(2) nanosheets: A facile method for fabricating three-dimensional porous hierarchical composite micro-nanostructures with excellent supercapacitor performance. Nano Res. 2017, 10, 3005–3017. [Google Scholar] [CrossRef]
- Basavaprabhu, B.; Muniyappa, K.; Panguluri, N.R.; Veladi, P.; Sureshbabu, V.V. A simple and greener approach for the amide bond formation employing FeCl3 as a catalyst. New J. Chem. 2015, 39, 7746–7749. [Google Scholar] [CrossRef]
- Ishihara, K.; Ohara, S.; Yamamoto, H. 3,4,5-trifluorobenzeneboronic acid as an extremely active amidation catalyst. J. Org. Chem. 1996, 61, 4196–4197. [Google Scholar] [CrossRef]
- Ishihara, K.; Ohara, S.; Yamamoto, H. Direct polycondensation of carboxylic acids and amines catalyzed by 3,4,5-trifluorophenylboronic acid. Macromolecules 2000, 33, 3511–3513. [Google Scholar] [CrossRef]
- Ishihara, K.; Kondo, S.; Yamamoto, H. 3,5-Bis(perfluorodecyl)phenylboronic acid as an easily recyclable direct amide condensation catalyst. Synlett 2001, 9, 1371–1374. [Google Scholar] [CrossRef]
- Maki, T.; Ishihara, K.; Yamamoto, H. 4,5,6,7-Tetrachlorobenzo[d][1,3,2]dioxaborol-2-ol as an effective catalyst for the amide condensation of sterically demanding carboxylic acids. Org. Lett. 2006, 8, 1431–1434. [Google Scholar] [CrossRef] [PubMed]
- Arnold, K.; Batsanov, A.S.; Davies, B.; Whiting, A. Synthesis, evaluation and application of novel bifunctional N,N-di-isopropylbenzylamineboronic acid catalysts for direct amide formation between carboxylic acids and amines. Green Chem. 2008, 10, 124–134. [Google Scholar] [CrossRef]
- Lu, S.Y.; Sui, L.Z.; Liu, J.J.; Zhu, S.J.; Chen, A.M.; Jin, M.X.; Yang, B. Near-infrared photoluminescent polymer-carbon nanodots with two-photon fluorescence. Adv. Mater. 2017, 29, 1603443. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.L.; Xu, T.; Liao, H.B.; Yao, C.J.; Liu, Y.; Li, Z.; Chen, Z.W.; Pan, D.Y.; Sun, L.T.; et al. Gram-scale synthesis of single-crystalline graphene quantum dots with superior optical properties. Nat. Commun. 2014, 5, 5357. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; He, L.; Pan, S.; Liu, H.; Hu, X.L. Nitrogen-doped fluorescent carbon dots for highly sensitive and selective detection of tannic acid. Spectrochim. Acta A 2019, 210, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.F.; Yao, Y.X.; Zhou, H.M.; Zhang, J.; Pang, Z.Y.; Ao, K.L.; Cai, Y.B.; Wei, Q.F. Synthesis of novel nitrogen-doped carbon dots for highly selective detection of iron ion. Nanotechnology 2017, 28, 165502. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.B.; Jin, J.C.; Xu, Z.Q.; Jiang, Z.W.; Li, X.; Jiang, F.L.; Liu, Y. Single-step synthesis of highly photoluminescent carbon dots for rapid detection of Hg2+ with excellent sensitivity. J. Colloid Interface Sci. 2019, 551, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.K.; Sundaram, C.; Ngo, Y.-L.T.; Choi, W.M.; Chung, J.S.; Kim, E.J.; Hur, S.H. Pyromellitic acid-derived highly fluorescent N-doped carbon dots for the sensitive and selective determination of 4-nitrophenol. Dyes Pigments 2019, 165, 327–334. [Google Scholar] [CrossRef]
- Shi, J.X.; Han, H.J.; Xu, C.Y. A novel enhanced anaerobic biodegradation method using biochar and Fe(OH)(3)@biochar for the removal of nitrogen heterocyclic compounds from coal gasification wastewater. Sci. Total Environ. 2019, 697, 134052. [Google Scholar] [CrossRef] [PubMed]
- Kinge, S.; Crego-Calama, M.; Reinhoudt, D.N. Self-assembling nanoparticles at surfaces and interfaces. ChemPhysChem 2008, 9, 20–42. [Google Scholar] [CrossRef]
- Wu, P.; Xu, Y.X.; Zhan, J.Y.; Li, Y.; Xue, H.G.; Pang, H. The research development of quantum dots in electrochemical energy storage. Small 2018, 14, 1801479. [Google Scholar] [CrossRef]
- Jing, P.T.; Han, D.; Li, D.; Zhou, D.; Zhang, L.G.; Zhang, H.; Shen, D.Z.; Qu, S.N. Origin of Anisotropic Photoluminescence in Heteroatom-Doped Carbon Nanodots. Adv. Opt. Mater. 2017, 5, 1601049. [Google Scholar] [CrossRef]
- Li, Q.C.; Zhao, Z.F.; Yan, B.M.; Song, X.J.; Zhang, Z.P.; Li, J.; Wu, X.; Bian, Z.; Zou, X.; Zhang, Y. Nickelocene-precursor-facilitated fast growth of graphene/h-BN vertical heterostructures and its applications in OLEDs. Adv. Mater. 2017, 29, 1701325. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Y.H.; Cui, P.P.; Feng, X.T.; Chen, L.; Yang, Y.Z.; Liu, X.G. Water-soluble, nitrogen-doped fluorescent carbon dots for highly sensitive and selective detection of Hg2+ in aqueous solution. RSC Adv. 2015, 5, 40393–40401. [Google Scholar] [CrossRef]
- Lu, W.B.; Qin, X.Y.; Liu, S.; Chang, G.H.; Zhang, Y.W.; Luo, Y.L. Economical, green synthesis of fluorescent carbon nanoparticles and their use as probes for sensitive and selective detection of Mercury(II) Ions. Anal. Chem. 2012, 84, 5351–5357. [Google Scholar] [CrossRef]
- Huang, H.; Lv, J.J.; Zhou, D.L.; Bao, N.; Xu, Y.; Wang, A.J.; Feng, J.J. One-pot green synthesis of nitrogen-doped carbon nanoparticles as fluorescent probes for mercury ions. RSC Adv. 2013, 3, 21691–21696. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Yang, X.L.; Wang, X.Y.; Liu, X.Z.; Zhang, Y.J.; Song, W.G.; Shu, C.Y.; Jiang, L.; Wang, C.R. Preparation of graphene-like iron oxide nanofilm/silica composite with enhanced adsorption and efficient photocatalytic properties. J. Mater. Chem. A 2013, 1, 8332–8337. [Google Scholar] [CrossRef]
- Chen, J.Z.; Xu, J.L.; Zhou, S.; Zhao, N.; Wong, C.P. Template-grown graphene/porous Fe2O3 nanocomposite: A high-performance anode material for pseudocapacitors. Nano Energy 2015, 15, 719–728. [Google Scholar] [CrossRef]
- Liu, W.F.; Jia, H.S.; Zhang, J.; Tang, J.H.; Wang, J.; Fang, D.W. Preparation of nitrogen-doped carbon quantum dots (NCQDs) and application for non-enzymatic detection of glucose. Microchem. J. 2020, 158, 105187. [Google Scholar] [CrossRef]
- Dar, M.I.; Shivashankar, S.A. Single crystalline magnetite, maghemite, and hematite nanoparticles with rich coercivity. RSC Adv. 2014, 4, 4105–4113. [Google Scholar] [CrossRef]
- Fayazi, M.; Ghanei-Motlagh, M.; Taher, M.A. The adsorption of basic dye (Alizarin red S) from aqueous solution onto activated carbon/gamma-Fe2O3 nano-composite: Kinetic and equilibrium studies. Mater. Sci. Semicond. Process. 2015, 40, 35–43. [Google Scholar] [CrossRef]
- Hui, B.H.; Salimi, M.N. Production of Iron Oxide Nanoparticles by Co-Precipitation method with Optimization Studies of Processing Temperature, pH and Stirring Rate. IOP Conf. Ser. Mater. Sci. Eng. 2020, 743, 012036. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Sethuraman, M.G.; Shim, J.J.; Lee, Y.R. Microwave assisted green synthesis of fluorescent N-doped carbon dots: Cytotoxicity and bio-imaging applications. J. Photochem. Photobiol. B 2016, 161, 154–161. [Google Scholar] [CrossRef]
- Dong, Y.Q.; Pang, H.C.; Yang, H.B.; Guo, C.X.; Shao, J.W.; Chi, Y.W.; Li, C.M.; Yu, T. Carbon-based dots Co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew. Chem. Int. Ed. 2013, 52, 7800–7804. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Wang, L.; Sun, C.; Zhou, N. Research on Preparation Methods of Carbon Nanomaterials Based on Self-Assembly of Carbon Quantum Dots. Molecules 2022, 27, 1690. https://doi.org/10.3390/molecules27051690
Gao X, Wang L, Sun C, Zhou N. Research on Preparation Methods of Carbon Nanomaterials Based on Self-Assembly of Carbon Quantum Dots. Molecules. 2022; 27(5):1690. https://doi.org/10.3390/molecules27051690
Chicago/Turabian StyleGao, Xiaoqi, Lei Wang, Chao Sun, and Nan Zhou. 2022. "Research on Preparation Methods of Carbon Nanomaterials Based on Self-Assembly of Carbon Quantum Dots" Molecules 27, no. 5: 1690. https://doi.org/10.3390/molecules27051690






