Botanically-Derived Δ9-Tetrahydrocannabinol and Cannabidiol, and Their 1:1 Combination, Modulate Toll-like Receptor 3 and 4 Signalling in Immune Cells from People with Multiple Sclerosis
Abstract
:1. Introduction
2. Results
2.1. Demographic Data of Study Participants
2.2. Time-Dependent Effect of TLR3 and TLR4 Stimulation on CXCL10, IFN-β, and TNF-α Protein Expression in PBMCs
2.3. THC:CBD Inhibit TLR3-Induced CXCL10 Protein Expression in PBMCs from HCs and pwMS
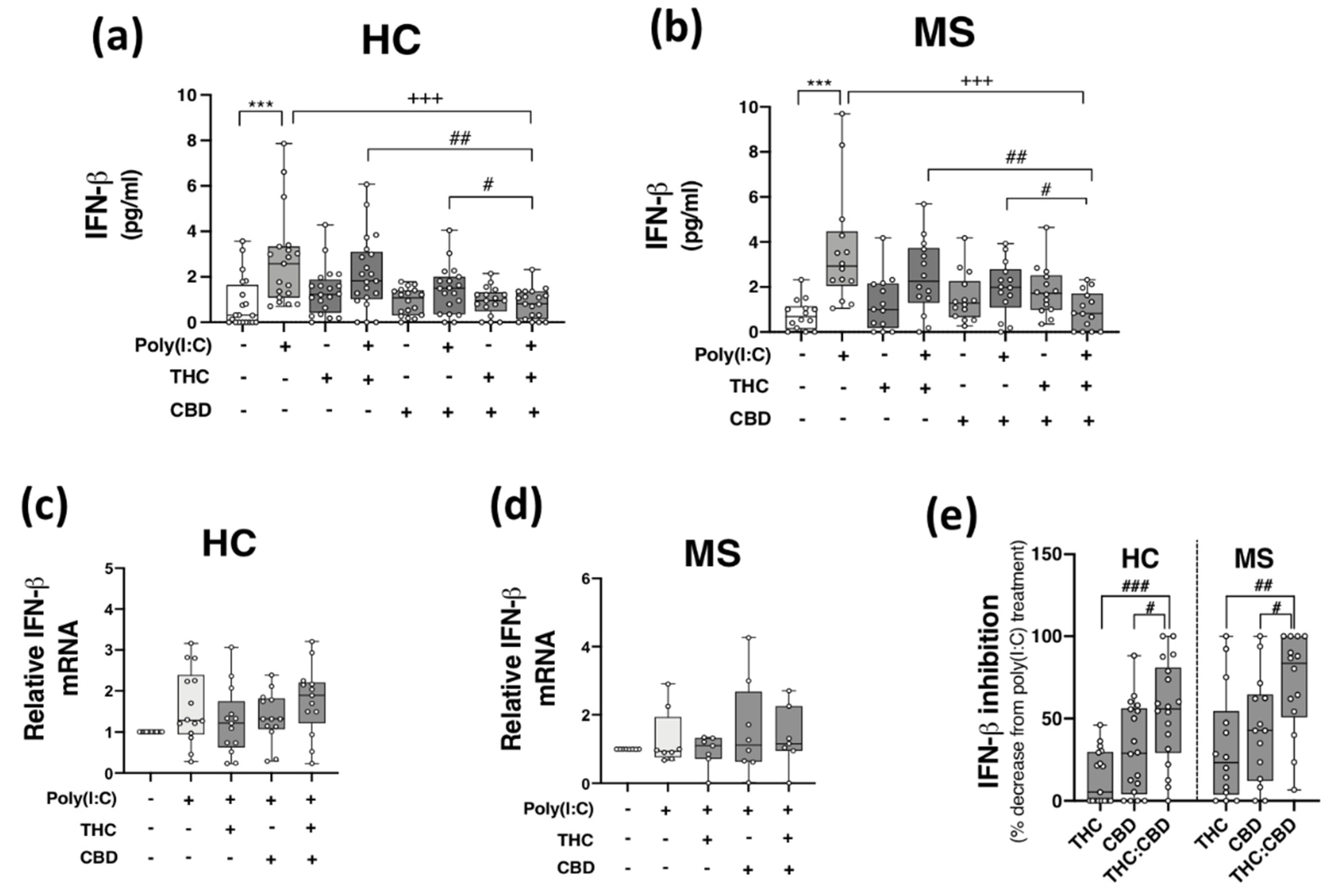
2.4. THC:CBD Inhibit TLR3-Induced IFN-β Protein Expression in PBMCs from HCs and pwMS
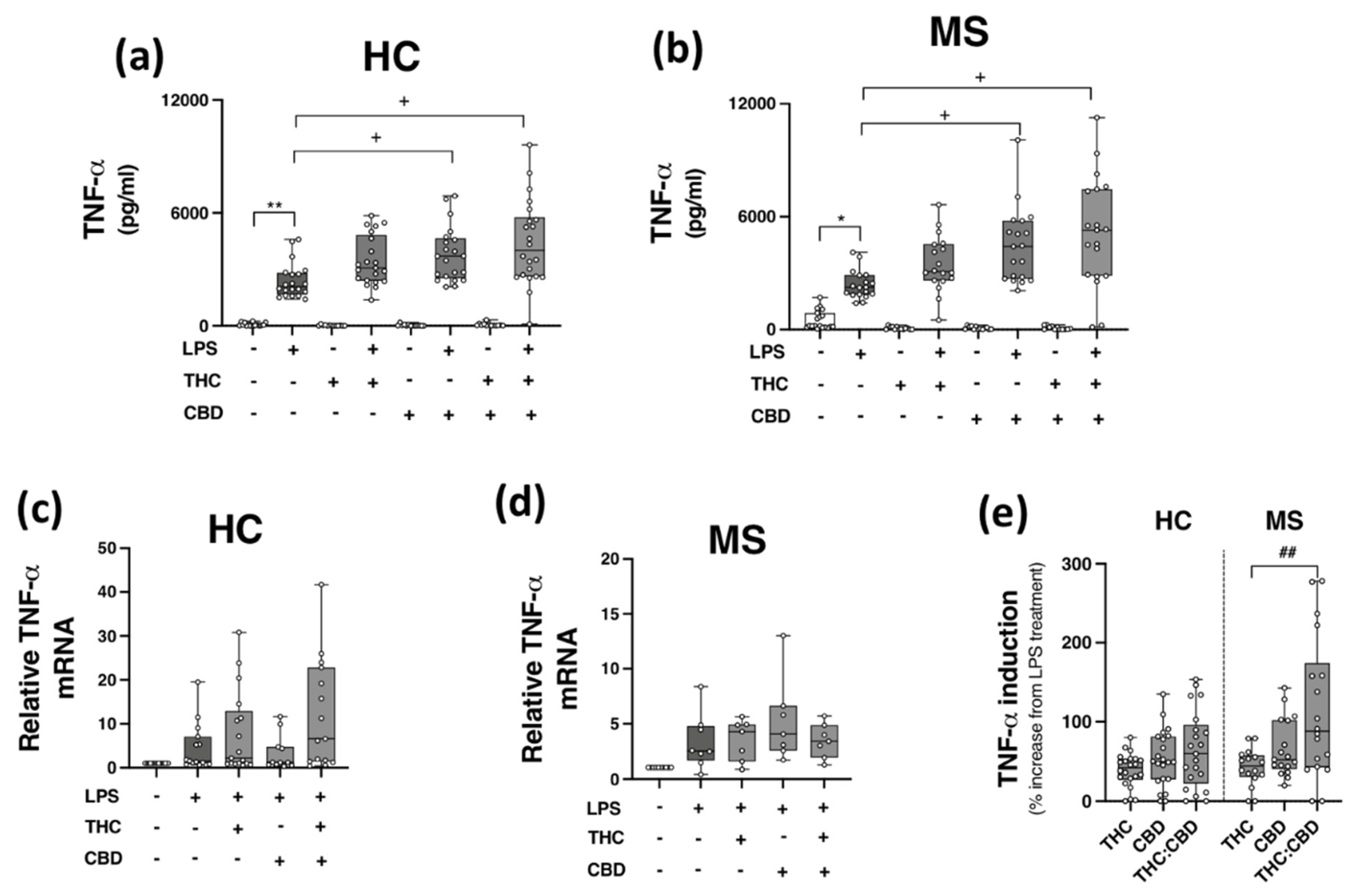
2.5. Phytocannabinoids Exacerbate TLR4-Induced TNF-α Expression in PBMCs from HCs and pwMS
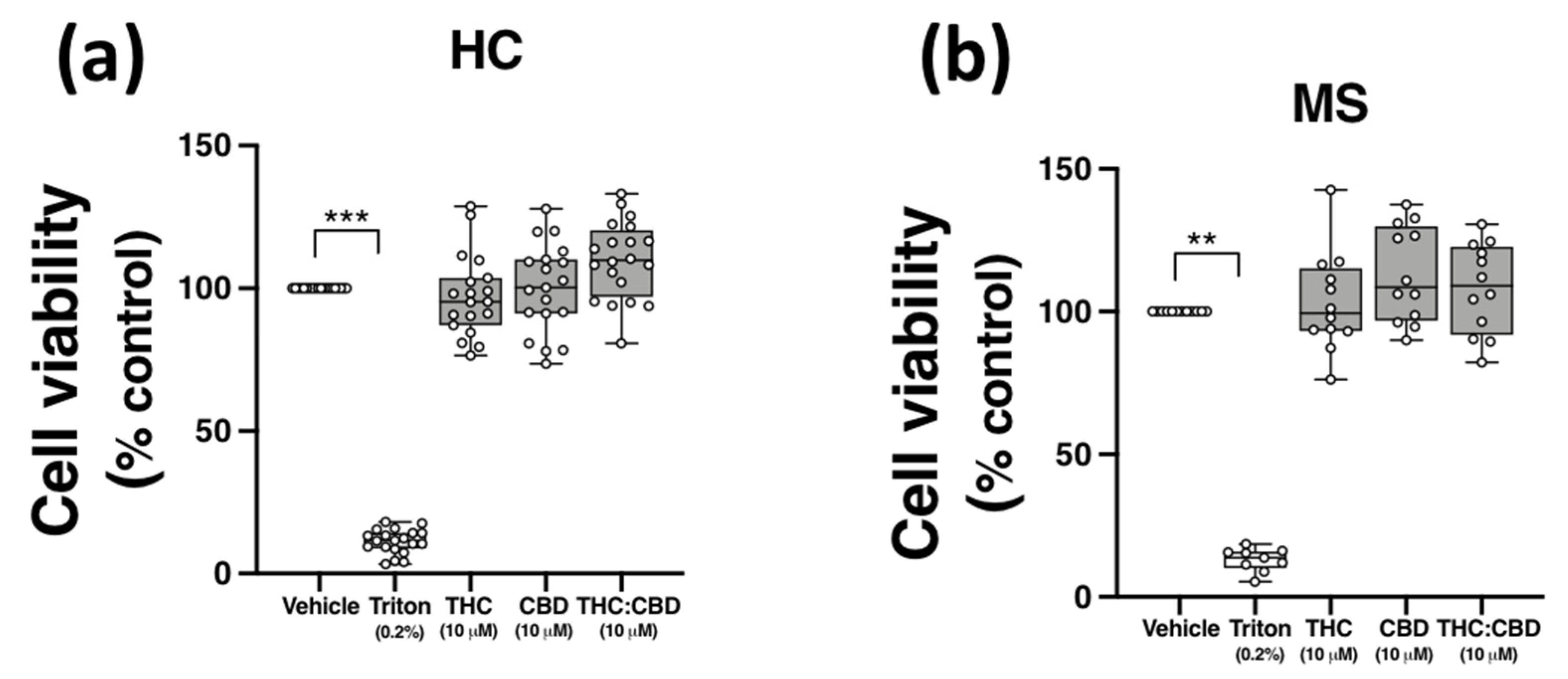
2.6. THC and CBD, When Delivered Alone and in 1:1 Combination, Are Not Cytotoxic to PBMCs from HCs and pwMS
2.7. Plasma C-Reactive Protein (CRP) and Haematological Parameters in HC and MS Cohorts
3. Discussion
4. Materials and Methods
4.1. Study Participants and Blood Samples
4.2. CRP Measurement in Plasma
4.3. Blood Counts
4.4. Materials
4.5. Cytokine Analysis in PBMC Culture Supernatants
4.6. Quantitative Real-Time PCR
4.7. Cell Viability Assay
4.8. QOL and Depressive Symptomatology
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, M.; Naito, S. Tissue-specific mRNA expression profiles of human toll-like receptors and related genes. Biol Pharm Bull 2005, 28, 886–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medzhitov, R.; Preston-Hurlburt, P.; Kopp, E.; Stadlen, A.; Chen, C.; Ghosh, S.; Janeway, C.A., Jr. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 1998, 2, 253–258. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Downer, E.J. Cannabinoids and innate immunity: Taking a toll on neuroinflammation. Sci. World J. 2011, 11, 855–865. [Google Scholar] [CrossRef] [Green Version]
- O′Brien, K.; Fitzgerald, D.C.; Naiken, K.; Alugupalli, K.R.; Rostami, A.M.; Gran, B. Role of the innate immune system in autoimmune inflammatory demyelination. Curr. Med. Chem. 2008, 15, 1105–1115. [Google Scholar] [CrossRef]
- Miranda-Hernandez, S.; Baxter, A.G. Role of toll-like receptors in multiple sclerosis. Am. J. Clin. Exp. Immunol. 2013, 2, 75–93. [Google Scholar]
- Fitzpatrick, J.K.; Downer, E.J. Toll-like receptor signalling as a cannabinoid target in Multiple Sclerosis. Neuropharmacology 2017, 113, 618–626. [Google Scholar] [CrossRef]
- Marta, M.; Andersson, A.; Isaksson, M.; Kampe, O.; Lobell, A. Unexpected regulatory roles of TLR4 and TLR9 in experimental autoimmune encephalomyelitis. Eur. J. Immunol. 2008, 38, 565–575. [Google Scholar] [CrossRef]
- Guo, B.; Chang, E.Y.; Cheng, G. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J. Clin. Investig. 2008, 118, 1680–1690. [Google Scholar] [CrossRef]
- Galligan, C.L.; Pennell, L.M.; Murooka, T.T.; Baig, E.; Majchrzak-Kita, B.; Rahbar, R.; Fish, E.N. Interferon-beta is a key regulator of proinflammatory events in experimental autoimmune encephalomyelitis. Mult. Scler. J. 2010, 16, 1458–1473. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Garbe, F.; Schmidt, H.; Mildner, A.; Gutcher, I.; Wolter, K.; Piesche, M.; Schroers, R.; Weiss, E.; Kirschning, C.J.; et al. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J. Clin. Investig. 2006, 116, 456–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lampropoulou, V.; Hoehlig, K.; Roch, T.; Neves, P.; Calderon Gomez, E.; Sweenie, C.H.; Hao, Y.; Freitas, A.A.; Steinhoff, U.; Anderton, S.M.; et al. TLR-activated B cells suppress T cell-mediated autoimmunity. J. Immunol. 2008, 180, 4763–4773. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, D.C.; O′Brien, K.; Young, A.; Fonseca-Kelly, Z.; Rostami, A.; Gran, B. Interferon regulatory factor (IRF) 3 is critical for the development of experimental autoimmune encephalomyelitis. J. Neuroinflamm. 2014, 11, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bsibsi, M.; Ravid, R.; Gveric, D.; van Noort, J.M. Broad expression of Toll-like receptors in the human central nervous system. J. Neuropathol. Exp. Neurol. 2002, 61, 1013–1021. [Google Scholar] [CrossRef] [Green Version]
- Andersson, A.; Covacu, R.; Sunnemark, D.; Danilov, A.I.; Dal Bianco, A.; Khademi, M.; Wallstrom, E.; Lobell, A.; Brundin, L.; Lassmann, H.; et al. Pivotal advance: HMGB1 expression in active lesions of human and experimental multiple sclerosis. J. Leukoc. Biol. 2008, 84, 1248–1255. [Google Scholar] [CrossRef] [Green Version]
- Crowley, T.; Fitzpatrick, J.M.; Kuijper, T.; Cryan, J.F.; O′Toole, O.; O′Leary, O.F.; Downer, E.J. Modulation of TLR3/TLR4 inflammatory signaling by the GABAB receptor agonist baclofen in glia and immune cells: Relevance to therapeutic effects in multiple sclerosis. Front. Cell Neurosci. 2015, 9, 284. [Google Scholar] [CrossRef] [Green Version]
- Downer, E.J.; Clifford, E.; Amu, S.; Fallon, P.G.; Moynagh, P.N. The synthetic cannabinoid R(+)WIN55,212-2 augments interferon-beta expression via peroxisome proliferator-activated receptor-alpha. J. Biol. Chem. 2012, 287, 25440–25453. [Google Scholar] [CrossRef] [Green Version]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. Prog. Chem. Org. Nat. Prod. 2017, 103, 1–36. [Google Scholar] [CrossRef]
- Shang, V.C.; Kendall, D.A.; Roberts, R.E. Delta(9)-Tetrahydrocannabinol reverses TNFalpha-induced increase in airway epithelial cell permeability through CB2 receptors. Biochem. Pharm. 2016, 120, 63–71. [Google Scholar] [CrossRef]
- Petrosino, S.; Verde, R.; Vaia, M.; Allara, M.; Iuvone, T.; Di Marzo, V. Anti-inflammatory Properties of Cannabidiol, a Nonpsychotropic Cannabinoid, in Experimental Allergic Contact Dermatitis. J. Pharm. Exp. Ther. 2018, 365, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Hu, F.; Wu, J.; Zhang, S. Cannabidiol attenuates OGD/R-induced damage by enhancing mitochondrial bioenergetics and modulating glucose metabolism via pentose-phosphate pathway in hippocampal neurons. Redox Biol. 2017, 11, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Vella, R.K.; Jackson, D.J.; Fenning, A.S. Delta(9)-Tetrahydrocannabinol Prevents Cardiovascular Dysfunction in STZ-Diabetic Wistar-Kyoto Rats. Biomed. Res. Int. 2017, 2017, 7974149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiurchiu, V.; Cencioni, M.T.; Bisicchia, E.; De Bardi, M.; Gasperini, C.; Borsellino, G.; Centonze, D.; Battistini, L.; Maccarrone, M. Distinct modulation of human myeloid and plasmacytoid dendritic cells by anandamide in multiple sclerosis. Ann. Neurol. 2013, 73, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.M.; Minogue, E.; Curham, L.; Tyrrell, H.; Gavigan, P.; Hind, W.; Downer, E.J. MyD88-dependent and -independent signalling via TLR3 and TLR4 are differentially modulated by Delta(9)-tetrahydrocannabinol and cannabidiol in human macrophages. J. Neuroimmunol. 2020, 343, 577217. [Google Scholar] [CrossRef] [PubMed]
- Centonze, D.; Bari, M.; Rossi, S.; Prosperetti, C.; Furlan, R.; Fezza, F.; De Chiara, V.; Battistini, L.; Bernardi, G.; Bernardini, S.; et al. The endocannabinoid system is dysregulated in multiple sclerosis and in experimental autoimmune encephalomyelitis. Brain 2007, 130, 2543–2553. [Google Scholar] [CrossRef] [Green Version]
- Jean-Gilles, L.; Feng, S.; Tench, C.R.; Chapman, V.; Kendall, D.A.; Barrett, D.A.; Constantinescu, C.S. Plasma endocannabinoid levels in multiple sclerosis. J. Neurol. Sci. 2009, 287, 212–215. [Google Scholar] [CrossRef]
- Pryce, G.; Ahmed, Z.; Hankey, D.J.; Jackson, S.J.; Croxford, J.L.; Pocock, J.M.; Ledent, C.; Petzold, A.; Thompson, A.J.; Giovannoni, G.; et al. Cannabinoids inhibit neurodegeneration in models of multiple sclerosis. Brain 2003, 126, 2191–2202. [Google Scholar] [CrossRef]
- Pryce, G.; Baker, D. Control of spasticity in a multiple sclerosis model is mediated by CB1, not CB2, cannabinoid receptors. Br. J. Pharmacol. 2007, 150, 519–525. [Google Scholar] [CrossRef] [Green Version]
- Rossi, S.; Furlan, R.; De Chiara, V.; Muzio, L.; Musella, A.; Motta, C.; Studer, V.; Cavasinni, F.; Bernardi, G.; Martino, G.; et al. Cannabinoid CB1 receptors regulate neuronal TNF-alpha effects in experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2011, 25, 1242–1248. [Google Scholar] [CrossRef]
- Palazuelos, J.; Davoust, N.; Julien, B.; Hatterer, E.; Aguado, T.; Mechoulam, R.; Benito, C.; Romero, J.; Silva, A.; Guzman, M.; et al. The CB(2) cannabinoid receptor controls myeloid progenitor trafficking: Involvement in the pathogenesis of an animal model of multiple sclerosis. J. Biol. Chem. 2008, 283, 13320–13329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, T. Toll-like receptors in neurodegeneration. Curr. Top Microbiol. Immunol. 2009, 336, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.; Gu, M.; Jiang, W.; Lin, W.; Xu, P.; Liu, Z.; Huang, H.; An, H.; Wang, X. Raf Kinase Inhibitor Protein Preferentially Promotes TLR3-Triggered Signaling and Inflammation. J. Immunol. 2017, 198, 4086–4095. [Google Scholar] [CrossRef] [Green Version]
- Zuniga, M.C.; Raghuraman, G.; Hitchner, E.; Weyand, C.; Robinson, W.; Zhou, W. PKC-epsilon and TLR4 synergistically regulate resistin-mediated inflammation in human macrophages. Atherosclerosis 2017, 259, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozela, E.; Pietr, M.; Juknat, A.; Rimmerman, N.; Levy, R.; Vogel, Z. Cannabinoids Delta(9)-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-kappaB and interferon-beta/STAT proinflammatory pathways in BV-2 microglial cells. J. Biol. Chem. 2010, 285, 1616–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajan, T.S.; Giacoppo, S.; Iori, R.; De Nicola, G.R.; Grassi, G.; Pollastro, F.; Bramanti, P.; Mazzon, E. Anti-inflammatory and antioxidant effects of a combination of cannabidiol and moringin in LPS-stimulated macrophages. Fitoterapia 2016, 112, 104–115. [Google Scholar] [CrossRef]
- Rao, R.; Nagarkatti, P.S.; Nagarkatti, M. Delta(9) Tetrahydrocannabinol attenuates Staphylococcal enterotoxin B-induced inflammatory lung injury and prevents mortality in mice by modulation of miR-17-92 cluster and induction of T-regulatory cells. Br. J. Pharmacol. 2015, 172, 1792–1806. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.M.; Scott, K.A.; Shamash, J.; Joel, S.; Powles, T.B. Enhancing the In Vitro cytotoxic activity of Delta9-tetrahydrocannabinol in leukemic cells through a combinatorial approach. Leuk. Lymphoma 2008, 49, 1800–1809. [Google Scholar] [CrossRef]
- Scott, K.A.; Dennis, J.L.; Dalgleish, A.G.; Liu, W.M. Inhibiting Heat Shock Proteins Can Potentiate the Cytotoxic Effect of Cannabidiol in Human Glioma Cells. Anticancer Res. 2015, 35, 5827–5837. [Google Scholar]
- Perry, V.H.; Cunningham, C.; Holmes, C. Systemic infections and inflammation affect chronic neurodegeneration. Nat. Rev. Immunol. 2007, 7, 161–167. [Google Scholar] [CrossRef]
- Hemmer, B.; Kerschensteiner, M.; Korn, T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol. 2015, 14, 406–419. [Google Scholar] [CrossRef]
- Gandhi, R.; Laroni, A.; Weiner, H.L. Role of the innate immune system in the pathogenesis of multiple sclerosis. J. Neuroimmunol. 2010, 221, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rostami, A.; Ciric, B. Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination. J. Neurol. Sci. 2013, 333, 76–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carstensen, M.; Christensen, T.; Stilund, M.; Moller, H.J.; Petersen, E.L.; Petersen, T. Activated monocytes and markers of inflammation in newly diagnosed multiple sclerosis. Immunol. Cell Biol. 2020, 98, 549–562. [Google Scholar] [CrossRef]
- Zozulya, A.L.; Clarkson, B.D.; Ortler, S.; Fabry, Z.; Wiendl, H. The role of dendritic cells in CNS autoimmunity. J. Mol. Med. 2010, 88, 535–544. [Google Scholar] [CrossRef] [Green Version]
- Kleiveland, C.R. Peripheral Blood Mononuclear Cells. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., Lopez-Exposito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 161–167. [Google Scholar]
- Zhu, Y.; Liang, L.; Qian, D.; Yu, H.; Yang, P.; Lei, B.; Peng, H. Increase in peripheral blood mononuclear cell Toll-like receptor 2/3 expression and reactivity to their ligands in a cohort of patients with wet age-related macular degeneration. Mol. Vis. 2013, 19, 1826–1833. [Google Scholar]
- Marta, M. Toll-like receptors in multiple sclerosis mouse experimental models. Ann. N. Y. Acad. Sci. 2009, 1173, 458–462. [Google Scholar] [CrossRef]
- Edvardsen, K.; Bjanesoy, T.; Hellesen, A.; Breivik, L.; Bakke, M.; Husebye, E.S.; Bratland, E. Peripheral Blood Cells from Patients with Autoimmune Addison′s Disease Poorly Respond to Interferons In Vitro, Despite Elevated Serum Levels of Interferon-Inducible Chemokines. J. Interferon Cytokine Res. 2015, 35, 759–770. [Google Scholar] [CrossRef] [Green Version]
- Brennan, K.; O′Leary, B.D.; Mc Laughlin, D.; Breen, E.P.; Connolly, E.; Ali, N.; O′Driscoll, D.N.; Ozaki, E.; Mahony, R.; Mulfaul, K.; et al. Type 1 IFN Induction by Cytosolic Nucleic Acid Is Intact in Neonatal Mononuclear Cells, Contrasting Starkly with Neonatal Hyporesponsiveness to TLR Ligation Due to Independence from Endosome-Mediated IRF3 Activation. J. Immunol. 2018, 201, 1131–1143. [Google Scholar] [CrossRef]
- Jansky, L.; Reymanova, P.; Kopecky, J. Dynamics of cytokine production in human peripheral blood mononuclear cells stimulated by LPS or infected by Borrelia. Physiol. Res. 2003, 52, 593–598. [Google Scholar]
- Ruhl, T.; Kim, B.S.; Beier, J.P. Cannabidiol restores differentiation capacity of LPS exposed adipose tissue mesenchymal stromal cells. Exp. Cell Res. 2018, 370, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Romano, B.; Pagano, E.; Orlando, P.; Capasso, R.; Cascio, M.G.; Pertwee, R.; Marzo, V.D.; Izzo, A.A.; Borrelli, F. Pure Delta(9)-tetrahydrocannabivarin and a Cannabis sativa extract with high content in Delta(9)-tetrahydrocannabivarin inhibit nitrite production in murine peritoneal macrophages. Pharmacol. Res. 2016, 113, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Peres, F.F.; Diana, M.C.; Suiama, M.A.; Justi, V.; Almeida, V.; Bressan, R.A.; Zuardi, A.W.; Hallak, J.E.; Crippa, J.A.; Abilio, V.C. Peripubertal treatment with cannabidiol prevents the emergence of psychosis in an animal model of schizophrenia. Schizophr. Res. 2016, 172, 220–221. [Google Scholar] [CrossRef] [PubMed]
- Osborne, A.L.; Solowij, N.; Babic, I.; Huang, X.F.; Weston-Green, K. Improved Social Interaction, Recognition and Working Memory with Cannabidiol Treatment in a Prenatal Infection (poly I:C) Rat Model. Neuropsychopharmacology 2017, 42, 1447–1457. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like receptor and RIG-I-like receptor signaling. Ann. N. Y. Acad. Sci. 2008, 1143, 1–20. [Google Scholar] [CrossRef]
- Tanaka, H.; Imaizumi, T. Inflammatory chemokine expression via Toll-like receptor 3 signaling in normal human mesangial cells. Clin. Dev. Immunol. 2013, 2013, 984708. [Google Scholar] [CrossRef]
- Liu, M.; Guo, S.; Hibbert, J.M.; Jain, V.; Singh, N.; Wilson, N.O.; Stiles, J.K. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 2011, 22, 121–130. [Google Scholar] [CrossRef]
- Blank, T.; Prinz, M. Type I interferon pathway in CNS homeostasis and neurological disorders. Glia 2017, 65, 1397–1406. [Google Scholar] [CrossRef]
- Hassanshahi, G.; Jafarzadeh, A.; Esmaeilzadeh, B.; Arababadi, M.K.; Yousefi, H.; Dickson, A.J. Assessment of NK cells response to hepatocyte derived chemotactic agents. Pak. J. Biol. Sci. 2008, 11, 1120–1125. [Google Scholar] [CrossRef] [Green Version]
- Vazirinejad, R.; Ahmadi, Z.; Kazemi Arababadi, M.; Hassanshahi, G.; Kennedy, D. The biological functions, structure and sources of CXCL10 and its outstanding part in the pathophysiology of multiple sclerosis. Neuroimmunomodulation 2014, 21, 322–330. [Google Scholar] [CrossRef]
- Sorensen, T.L.; Tani, M.; Jensen, J.; Pierce, V.; Lucchinetti, C.; Folcik, V.A.; Qin, S.; Rottman, J.; Sellebjerg, F.; Strieter, R.M.; et al. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J. Clin. Investig. 1999, 103, 807–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christophi, G.P.; Christophi, J.A.; Gruber, R.C.; Mihai, C.; Mejico, L.J.; Massa, P.T.; Jubelt, B. Quantitative differences in the immunomodulatory effects of Rebif and Avonex in IFN-beta 1a treated multiple sclerosis patients. J. Neurol. Sci. 2011, 307, 41–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comini-Frota, E.R.; Teixeira, A.L.; Angelo, J.P.; Andrade, M.V.; Brum, D.G.; Kaimen-Maciel, D.R.; Foss, N.T.; Donadi, E.A. Evaluation of serum levels of chemokines during interferon-beta treatment in multiple sclerosis patients: A 1-year, observational cohort study. CNS Drugs 2011, 25, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Zettl, U.K.; Hecker, M.; Aktas, O.; Wagner, T.; Rommer, P.S. Interferon beta-1a and beta-1b for patients with multiple sclerosis: Updates to current knowledge. Expert Rev. Clin. Immunol. 2018, 14, 137–153. [Google Scholar] [CrossRef]
- Ozenci, V.; Kouwenhoven, M.; Huang, Y.M.; Kivisakk, P.; Link, H. Multiple sclerosis is associated with an imbalance between tumour necrosis factor-alpha (TNF-alpha)- and IL-10-secreting blood cells that is corrected by interferon-beta (IFN-beta) treatment. Clin. Exp. Immunol. 2000, 120, 147–153. [Google Scholar] [CrossRef]
- Prat, A.; Biernacki, K.; Antel, J.P. Th1 and Th2 lymphocyte migration across the human BBB is specifically regulated by interferon beta and copolymer-1. J. Autoimmun. 2005, 24, 119–124. [Google Scholar] [CrossRef]
- Uematsu, S.; Akira, S. Toll-like receptors and Type I interferons. J. Biol. Chem. 2007, 282, 15319–15323. [Google Scholar] [CrossRef] [Green Version]
- Stark, G.R.; Darnell, J.E., Jr. The JAK-STAT pathway at twenty. Immunity 2012, 36, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Satoh, J.; Nanri, Y.; Tabunoki, H.; Yamamura, T. Microarray analysis identifies a set of CXCR3 and CCR2 ligand chemokines as early IFNbeta-responsive genes in peripheral blood lymphocytes In Vitro: An implication for IFNbeta-related adverse effects in multiple sclerosis. BMC Neurol. 2006, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- Sweet, M.J.; Hume, D.A. Endotoxin signal transduction in macrophages. J. Leukoc. Biol. 1996, 60, 8–26. [Google Scholar] [CrossRef]
- O′Neill, L.A.; Golenbock, D.; Bowie, A.G. The history of Toll-like receptors—Redefining innate immunity. Nat. Rev. Immunol. 2013, 13, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Do, Y.; McKallip, R.J.; Nagarkatti, M.; Nagarkatti, P.S. Activation through cannabinoid receptors 1 and 2 on dendritic cells triggers NF-kappaB-dependent apoptosis: Novel role for endogenous and exogenous cannabinoids in immunoregulation. J. Immunol. 2004, 173, 2373–2382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downer, E.J.; Fogarty, M.P.; Campbell, V.A. Tetrahydrocannabinol-induced neurotoxicity depends on CB1 receptor-mediated c-Jun N-terminal kinase activation in cultured cortical neurons. Br. J. Pharmacol. 2003, 140, 547–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porcella, A.; Gessa, G.L.; Pani, L. Delta9-tetrahydrocannabinol increases sequence-specific AP-1 DNA-binding activity and Fos-related antigens in the rat brain. Eur. J. Neurosci. 1998, 10, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Araiz, A.; Arevalo-Martin, A.; Gomez-Torres, O.; Navarro-Galve, B.; Garcia-Ovejero, D.; Suetterlin, P.; Sanchez-Heras, E.; Molina-Holgado, E.; Molina-Holgado, F. The endocannabinoid system modulates a transient TNF pathway that induces neural stem cell proliferation. Mol. Cell Neurosci. 2008, 38, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Hornung, V.; Rothenfusser, S.; Britsch, S.; Krug, A.; Jahrsdorfer, B.; Giese, T.; Endres, S.; Hartmann, G. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 2002, 168, 4531–4537. [Google Scholar] [CrossRef] [Green Version]
- Feliu, A.; Moreno-Martet, M.; Mecha, M.; Carrillo-Salinas, F.J.; de Lago, E.; Fernandez-Ruiz, J.; Guaza, C. A Sativex((R)) -like combination of phytocannabinoids as a disease-modifying therapy in a viral model of multiple sclerosis. Br. J. Pharmacol. 2015, 172, 3579–3595. [Google Scholar] [CrossRef] [Green Version]
- Rath, P.C.; Aggarwal, B.B. TNF-induced signaling in apoptosis. J. Clin. Immunol. 1999, 19, 350–364. [Google Scholar] [CrossRef]
- Downer, E.; Boland, B.; Fogarty, M.; Campbell, V. Delta 9-tetrahydrocannabinol induces the apoptotic pathway in cultured cortical neurones via activation of the CB1 receptor. Neuroreport 2001, 12, 3973–3978. [Google Scholar] [CrossRef]
- Mato, S.; Victoria Sanchez-Gomez, M.; Matute, C. Cannabidiol induces intracellular calcium elevation and cytotoxicity in oligodendrocytes. Glia 2010, 58, 1739–1747. [Google Scholar] [CrossRef]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibeas Bih, C.; Chen, T.; Nunn, A.V.; Bazelot, M.; Dallas, M.; Whalley, B.J. Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics 2015, 12, 699–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwamura, H.; Suzuki, H.; Ueda, Y.; Kaya, T.; Inaba, T. In Vitro and In Vivo pharmacological characterization of JTE-907, a novel selective ligand for cannabinoid CB2 receptor. J. Pharmacol. Exp. Ther. 2001, 296, 420–425. [Google Scholar]
- Rinaldi-Carmona, M.; Barth, F.; Heaulme, M.; Shire, D.; Calandra, B.; Congy, C.; Martinez, S.; Maruani, J.; Neliat, G.; Caput, D.; et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994, 350, 240–244. [Google Scholar] [CrossRef] [Green Version]
- Zygmunt, P.M.; Andersson, D.A.; Hogestatt, E.D. Delta 9-tetrahydrocannabinol and cannabinol activate capsaicin-sensitive sensory nerves via a CB1 and CB2 cannabinoid receptor-independent mechanism. J. Neurosci. 2002, 22, 4720–4727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, B.L.; Rockwell, C.E.; Kaminski, N.E. Evidence for cannabinoid receptor-dependent and -independent mechanisms of action in leukocytes. J. Pharmacol. Exp. Ther. 2003, 306, 1077–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kappos, L.; O′Connor, P.; Radue, E.W.; Polman, C.; Hohlfeld, R.; Selmaj, K.; Ritter, S.; Schlosshauer, R.; von Rosenstiel, P.; Zhang-Auberson, L.; et al. Long-term effects of fingolimod in multiple sclerosis: The randomized FREEDOMS extension trial. Neurology 2015, 84, 1582–1591. [Google Scholar] [CrossRef] [Green Version]
- O′Sullivan, S.A.; O′Sullivan, C.; Healy, L.M.; Dev, K.K.; Sheridan, G.K. Sphingosine 1-phosphate receptors regulate TLR4-induced CXCL5 release from astrocytes and microglia. J. Neurochem. 2018, 144, 736–747. [Google Scholar] [CrossRef] [Green Version]
- Paugh, S.W.; Cassidy, M.P.; He, H.; Milstien, S.; Sim-Selley, L.J.; Spiegel, S.; Selley, D.E. Sphingosine and its analog, the immunosuppressant 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol, interact with the CB1 cannabinoid receptor. Mol. Pharmacol. 2006, 70, 41–50. [Google Scholar] [CrossRef]
- Bettiga, A.; Aureli, M.; Colciago, G.; Murdica, V.; Moschini, M.; Luciano, R.; Canals, D.; Hannun, Y.; Hedlund, P.; Lavorgna, G.; et al. Bladder cancer cell growth and motility implicate cannabinoid 2 receptor-mediated modifications of sphingolipids metabolism. Sci. Rep. 2017, 7, 42157. [Google Scholar] [CrossRef] [Green Version]
- Janardhan, V.; Bakshi, R. Quality of life in patients with multiple sclerosis: The impact of fatigue and depression. J. Neurol. Sci. 2002, 205, 51–58. [Google Scholar] [CrossRef]
- Simpson, R.J.; McLean, G.; Guthrie, B.; Mair, F.; Mercer, S.W. Physical and mental health comorbidity is common in people with multiple sclerosis: Nationally representative cross-sectional population database analysis. BMC Neurol. 2014, 14, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, A.; Cronin, O.; Ryan, A.M.; Sweeney, B.; O′Toole, O.; Allen, A.P.; Clarke, G.; O′Halloran, K.D.; Downer, E.J. Impact of short-term cycle ergometer training on quality of life, cognition and depressive symptomatology in multiple sclerosis patients: A pilot study. Neurol. Sci. 2018, 39, 461–469. [Google Scholar] [CrossRef]
- Hung, Y.Y. Antidepressants Improve Negative Regulation of Toll-Like Receptor Signaling in Monocytes from Patients with Major Depression. Neuroimmunomodulation 2018, 25, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.M.; Chesney, S.A.; Lee, T.S.; Brasel, K.; Larson, C.L.; Hillard, C.J.; deRoon-Cassini, T.A. Circulating endocannabinoids and prospective risk for depression in trauma-injury survivors. Neurobiol. Stress 2021, 14, 100304. [Google Scholar] [CrossRef] [PubMed]
- Rapp, N.S.; Gilroy, J.; Lerner, A.M. Role of bacterial infection in exacerbation of multiple sclerosis. Am. J. Phys. Med. Rehabil. 1995, 74, 415–418. [Google Scholar] [CrossRef]
- Gao, L.; Liu, X.; Zhang, D.; Xu, F.; Chen, Q.; Hong, Y.; Feng, G.; Shi, Q.; Yang, B.; Xu, L. Early diagnosis of bacterial infection in patients with septicopyemia by laboratory analysis of PCT, CRP and IL-6. Exp. Ther. Med. 2017, 13, 3479–3483. [Google Scholar] [CrossRef] [Green Version]
- Ngaotepprutaram, T.; Kaplan, B.L.; Kaminski, N.E. Impaired NFAT and NFkappaB activation are involved in suppression of CD40 ligand expression by Delta(9)-tetrahydrocannabinol in human CD4(+) T cells. Toxicol. Appl. Pharmacol. 2013, 273, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [Green Version]
- Vickrey, B.G.; Hays, R.D.; Harooni, R.; Myers, L.W.; Ellison, G.W. A health-related quality of life measure for multiple sclerosis. Qual. Life Res. 1995, 4, 187–206. [Google Scholar] [CrossRef]
- Fischer, A.; Fischer, M.; Nicholls, R.A.; Lau, S.; Poettgen, J.; Patas, K.; Heesen, C.; Gold, S.M. Diagnostic accuracy for major depression in multiple sclerosis using self-report questionnaires. Brain Behav. 2015, 5, e00365. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Characteristics | HC (n = 26) | MS (n = 21) | p Value |
|---|---|---|---|
| Age, years, median (range) | 31.5 (25.0–40.8) | 37.0 (30.5–45.0) | 0.1543 |
| Gender | |||
| Female, n | 20 | 18 | |
| Male, n | 6 | 3 | |
| EDSS, median (range) | n/a | 1.8 (1.0–3.0) | |
| Disease duration, years, median (range) | n/a | 4.4 (2.4–5.8) | |
| MS-QOL54 composite score | |||
| Physical health, median (range) | 94.7 (89.9–95.8) | 71.8 (49.0–82.8) | <0.001 *** |
| Mental health, median (range) | 90.8 (88.0–94.9) | 73.4 (41.3–85.1) | <0.001 *** |
| QIDS-SR16 score | 2.0 (1.0–4.0) | 7.0 (3.0–11.5) | <0.001 *** |
| Symptom reported during study | |||
| Blood disorder, n | 1 | 1 | |
| Thyroid disease, n | 2 | - | |
| Non-MS autoimmune disease, n | 1 | 1 | |
| Allergies, n | 4 | 4 | |
| Asthma, n | 1 | 1 | |
| Infection, n | 1 | 2 | |
| Epilepsy, n | - | 2 | |
| Anxiety/depression, n | 2 | 2 | |
| Overactive bladder, n | - | 2 | |
| Kidney disease, n | - | 1 | |
| MS medication use in MS group | |||
| Peginterferon beta-1a (Plegridy®), n | - | 5 | |
| Natalizumab (Tysabri®), n | - | 3 | |
| Fingolimod (Gilenya®), n | - | 2 | |
| Rituximab (Rituxan®), n | - | 2 | |
| Dimethylfumarate (Tecfidera®), n | - | 1 | |
| Interferon beta-1a (Avonex®), n | - | 1 | |
| Glatiramer acetate (Copaxone®), n | - | 1 | |
| Other medication use | |||
| Anti-convulsant | - | 3 | |
| Muscle relaxant | - | 1 | |
| Analgesic | - | 1 | |
| Anti-depressant | 2 | 2 | |
| Antibiotic | 1 | - | |
| Thyroid medication | 2 | - | |
| Bladder medication | - | 2 | |
| Anti-asthmatic | 1 | - | |
| Contraceptive | 2 | 3 | |
| Vitamin D | - | 3 | |
| Folic acid | - | 2 | |
| Anti-allergy | - | 2 | |
| Smoker, n | - | 4 | |
| Cannabis use, n | - | 2 |
| Target Gene | HC | MS | p Value |
|---|---|---|---|
| TLR3 median delta Ct * | 20.9 (20.2–21.8) | 21.3 (19.4–21.5) | 0.9999 |
| TLR4 median delta Ct | 14.8 (14.2–17.9) | 14.8 (14.0–17.1) | 0.3095 |
| Cytokine/Chemokine | Basal | Treatment with Poly(I:C) | Mean Difference after Treatment (Fold Change) |
|---|---|---|---|
| CXCL10 (pg/mL) | |||
| HC | 74.6 ± 13.3 | 587.6 ± 94.1 *** | 513.0 (7.9) |
| MS | 28.0 ± 7.5 | 289.6 ± 71.6 # | 261.6 (10.3) |
| IFN-β (pg/mL) | |||
| HC | 0.68 ± 0.22 | 2.46 ± 0.39 * | 1.78 (3.6) |
| MS | 0.72 ± 0.18 | 3.39 ± 0.68 +++ | 2.67 (4.7) |
| Cytokine | Basal | Treatment with LPS | Mean Difference after Treatment (Fold Change) |
|---|---|---|---|
| TNF-α (pg/mL) | |||
| HC | 69.3 ± 19.8 | 2398 ± 208.2 *** | 2328.7 (34.6) |
| MS | 504.4 ± 113.3 | 2377 ± 177.6 +++ | 1872.6 (4.7) |
| Target Gene | HC | MS | p Value |
|---|---|---|---|
| CNR1 average delta Ct * | 20.8 ± 0.2 | 20.3 ± 0.9 | 0.4635 |
| CNR2 average delta Ct | 17.1 ± 0.2 | 16.6 ± 0.4 | 0.1867 |
| FAAH average delta Ct | 19.1 ± 0.2 | 18.7 ± 0.4 | 0.2802 |
| MGLL average delta Ct | 13.4 ± 0.3 | 13.1 ± 0.6 | 0.6103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitzpatrick, J.-M.; Hackett, B.; Costelloe, L.; Hind, W.; Downer, E.J. Botanically-Derived Δ9-Tetrahydrocannabinol and Cannabidiol, and Their 1:1 Combination, Modulate Toll-like Receptor 3 and 4 Signalling in Immune Cells from People with Multiple Sclerosis. Molecules 2022, 27, 1763. https://doi.org/10.3390/molecules27061763
Fitzpatrick J-M, Hackett B, Costelloe L, Hind W, Downer EJ. Botanically-Derived Δ9-Tetrahydrocannabinol and Cannabidiol, and Their 1:1 Combination, Modulate Toll-like Receptor 3 and 4 Signalling in Immune Cells from People with Multiple Sclerosis. Molecules. 2022; 27(6):1763. https://doi.org/10.3390/molecules27061763
Chicago/Turabian StyleFitzpatrick, John-Mark, Becky Hackett, Lisa Costelloe, William Hind, and Eric J. Downer. 2022. "Botanically-Derived Δ9-Tetrahydrocannabinol and Cannabidiol, and Their 1:1 Combination, Modulate Toll-like Receptor 3 and 4 Signalling in Immune Cells from People with Multiple Sclerosis" Molecules 27, no. 6: 1763. https://doi.org/10.3390/molecules27061763






