Experimental and Mathematical Modelling of Factors Influencing Carbon Dioxide Absorption into the Aqueous Solution of Monoethanolamine and 1-Butyl-3-methylimidazolium Dibutylphosphate Using Response Surface Methodology (RSM)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Density and Viscosity Measurement
2.3. Heat Capacity Measurement
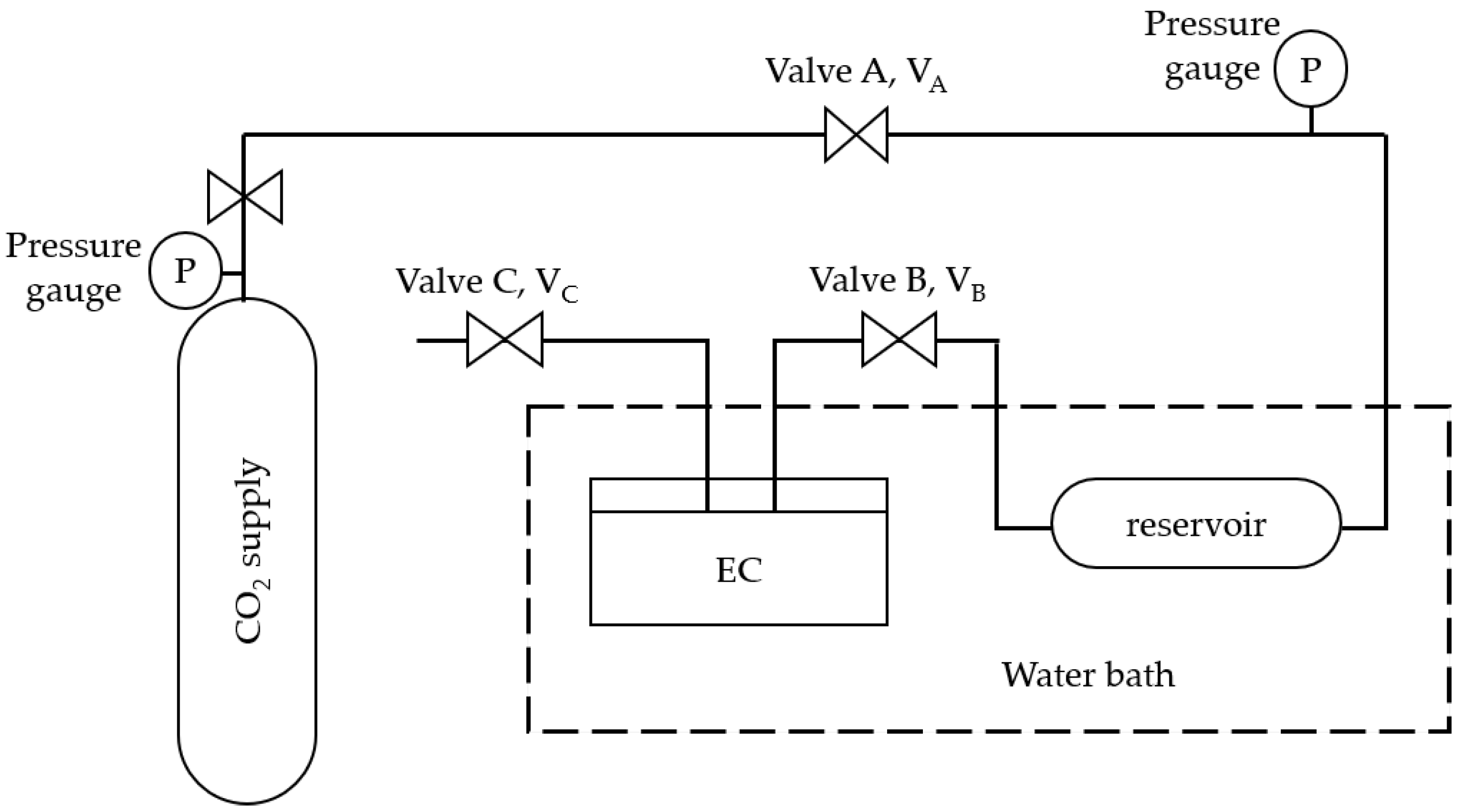
2.4. Solubility of CO2
2.5. CO2 Solubility in the Aqueous MEA-(BMIM)(DBP) Using RSM-CCD
3. Results and Discussion
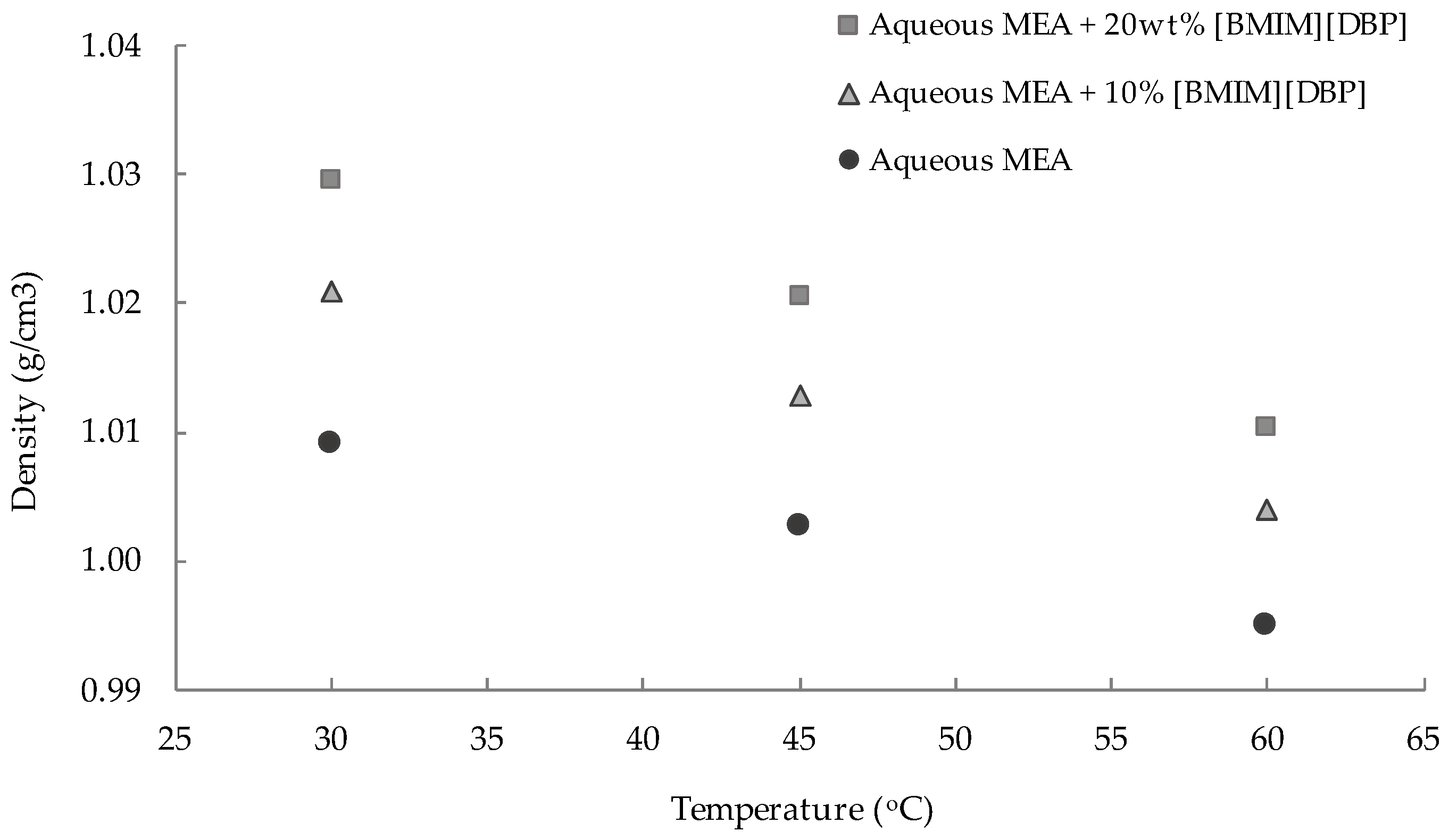
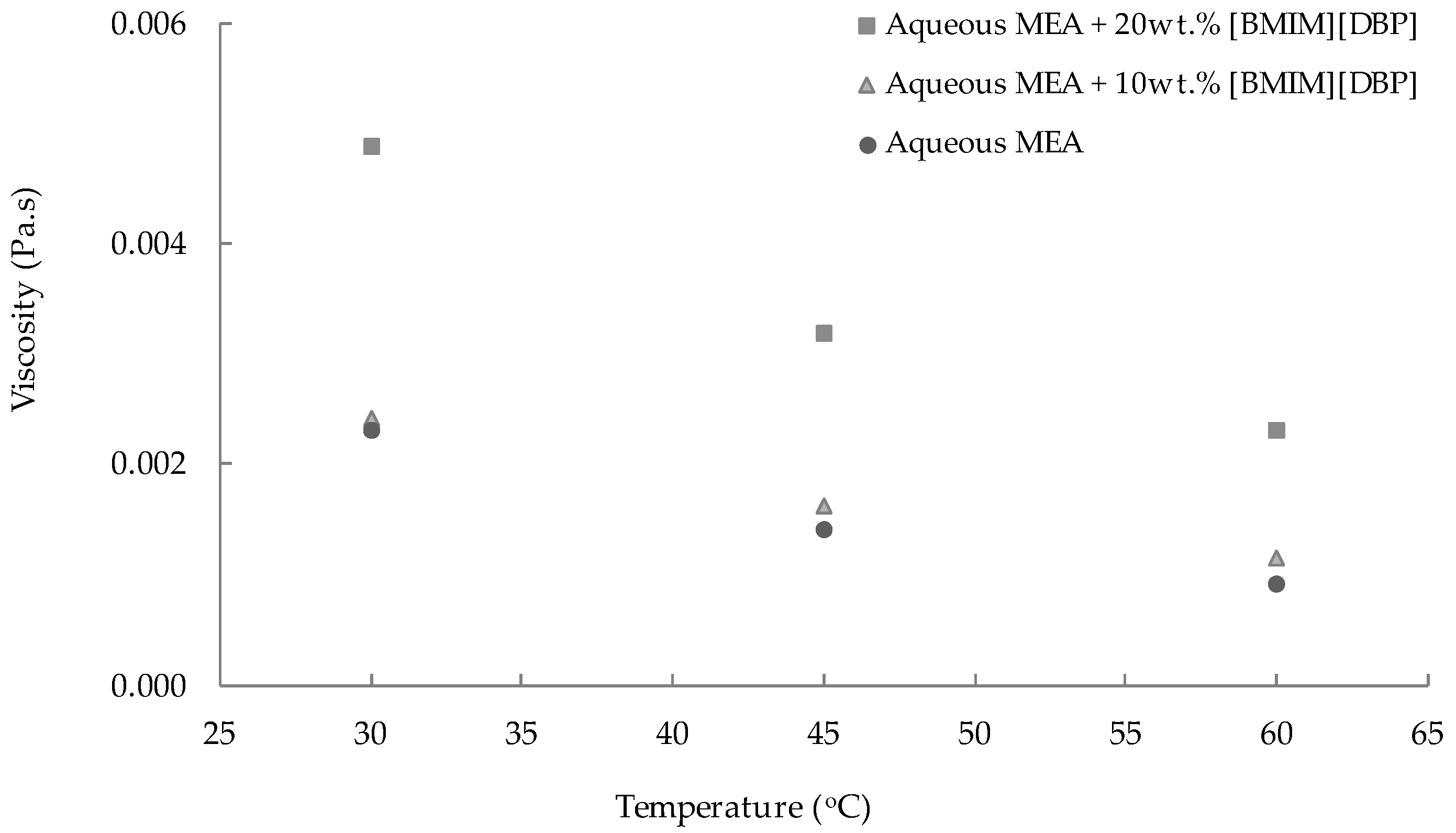
3.1. Density and Viscosity
3.2. Heat Capacity
3.3. CO2 Solubility in the Aqueous MEA-(BMIM)(DBP) Solvent Using RSM
3.3.1. Data Collection and Fit Summary Analysis
3.3.2. Validation of Empirical Model Adequacy
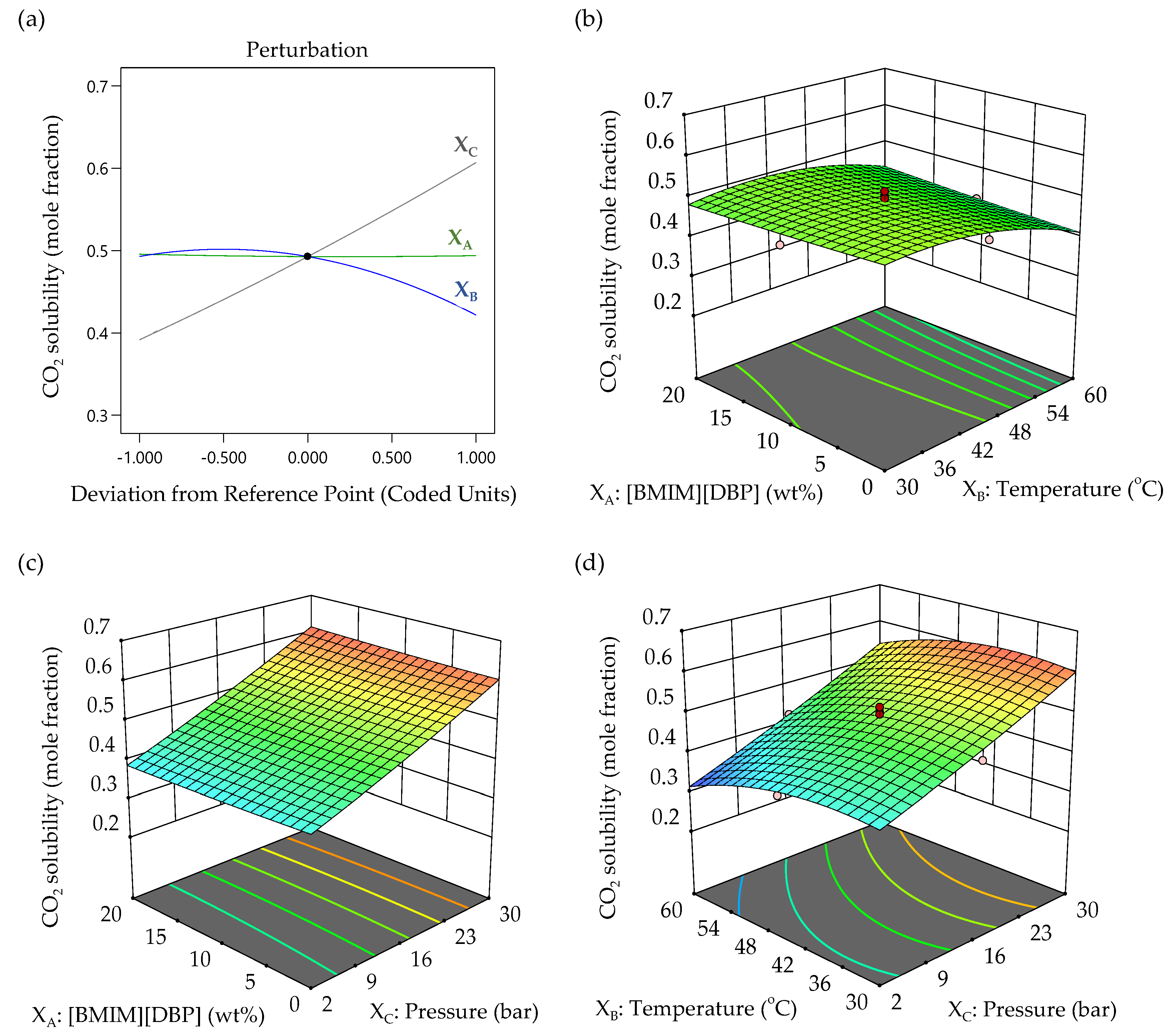
3.3.3. Effect of Variable Factors in CO2 Solubility
3.3.4. Optimized Simulation of the CO2 Absorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jacobson, T.A.; Kler, J.S.; Hernke, M.T.; Braun, R.K.; Meyer, K.C.; Funk, W.E. Direct human health risks of increased atmospheric carbon dioxide. Nat. Sustain. 2019, 2, 691–701. [Google Scholar] [CrossRef]
- Zeng, C.; Stringer, L.C.; Lv, T. The spatial spillover effect of fossil fuel energy trade on CO2 emissions. Energy 2021, 223, 120038. [Google Scholar] [CrossRef]
- Rufford, T.E.; Smart, S.; Watson, G.C.Y.; Graham, B.F.; Boxall, J.; Diniz da Costa, J.C.; May, E.F. The removal of CO2 and N2 from natural gas: A review of conventional and emerging process technologies. J. Pet. Sci. Eng. 2012, 94–95, 123–154. [Google Scholar] [CrossRef]
- de Ávila, S.G.; Logli, M.A.; Silva, L.C.C.; Fantini, M.C.A.; Matos, J.R. Incorporation of monoethanolamine (MEA), diethanolamine (DEA) and methyldiethanolamine (MDEA) in mesoporous silica: An alternative to CO2 capture. J. Environ. Chem. Eng. 2016, 4, 4514–4524. [Google Scholar] [CrossRef]
- Solangi, N.H.; Anjum, A.; Tanjung, F.A.; Mazari, S.A.; Mubarak, N.M. A review of recent trends and emerging perspectives of ionic liquid membranes for CO2 separation. J. Environ. Chem. Eng. 2021, 9, 105860. [Google Scholar] [CrossRef]
- Yang, J.; Yu, X.; Yan, J.; Tu, S.-T. CO2 Capture Using Amine Solution Mixed with Ionic Liquid. Ind. Eng. Chem. Res. 2014, 53, 2790–2799. [Google Scholar] [CrossRef]
- Gonzalez-Miquel, M.; Massel, M.; DeSilva, A.; Palomar, J.; Rodriguez, F.; Brennecke, J.F. Excess Enthalpy of Monoethanolamine + Ionic Liquid Mixtures: How Good are COSMO-RS Predictions? J. Phys. Chem. B 2014, 118, 11512–11522. [Google Scholar] [CrossRef]
- Feng, Z.; Cheng-Gang, F.; You-Ting, W.; Yuan-Tao, W.; Ai-Min, L.; Zhi-Bing, Z. Absorption of CO2 in the aqueous solutions of functionalized ionic liquids and MDEA. Chem. Eng. J. 2010, 160, 691–697. [Google Scholar] [CrossRef]
- Shojaeian, A.; Haghtalab, A. Solubility and density of carbon dioxide in different aqueous alkanolamine solutions blended with 1-butyl-3-methylimidazolium acetate ionic liquid at high pressure. J. Mol. Liq. 2013, 187, 218–225. [Google Scholar] [CrossRef]
- Notz, R.; Mangalapally, H.P.; Hasse, H. Post combustion CO2 capture by reactive absorption: Pilot plant description and results of systematic studies with MEA. Int. J. Greenh. Gas Control. 2012, 6, 84–112. [Google Scholar] [CrossRef]
- Palgunadi, J.; Kang, J.E.; Nguyen, D.Q.; Kim, J.H.; Min, B.K.; Lee, S.D.; Kim, H.; Kim, H.S. Solubility of CO2 in dialkylimidazolium dialkylphosphate ionic liquids. Thermochim. Acta 2009, 494, 94–98. [Google Scholar] [CrossRef]
- Palgunadi, J.; Kang, J.-E.; Cheong, M.-S.; Kim, H.-G.; Lee, H.-J.; Kim, H.-S. Fluorine-free imidazolium-based ionic liquids with a phosphorous-containing anion as potential CO2 absorbents. Bull. Korean Chem. Soc. 2009, 30, 1749–1754. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.-F.; Liu, Z.-C.; Xu, C.-M.; Zhang, X.-M. The molecular characteristics dominating the solubility of gases in ionic liquids. Chem. Soc. Rev. 2011, 40, 3802–3823. [Google Scholar] [CrossRef] [PubMed]
- Shannon, M.S.; Tedstone, J.M.; Danielsen, S.P.O.; Hindman, M.S.; Irvin, A.C.; Bara, J.E. Free Volume as the Basis of Gas Solubility and Selectivity in Imidazolium-Based Ionic Liquids. Ind. Eng. Chem. Res. 2012, 51, 5565–5576. [Google Scholar] [CrossRef]
- Mehdipoor, M.A.; Moosavirad, S.M. Effect of Holed Ferrum electrodes (HFE) on the efficiency of the electrocoagulation process for copper recovery and optimization of parameters, using RSM. Hydrometallurgy 2020, 194, 105313. [Google Scholar] [CrossRef]
- Morero, B.; Groppelli, E.S.; Campanella, E.A. Evaluation of biogas upgrading technologies using a response surface methodology for process simulation. J. Clean. Prod. 2017, 141, 978–988. [Google Scholar] [CrossRef]
- Pashaei, H.; Ghaemi, A.; Nasiri, M.; Karami, B. Experimental modeling and optimization of CO2 absorption into piperazine solutions using RSM-CCD methodology. ACS omega 2020, 5, 8432–8448. [Google Scholar] [CrossRef] [Green Version]
- Hasib-ur-Rahman, M.; Bouteldja, H.; Fongarland, P.; Siaj, M.; Larachi, F.Ç. Corrosion behavior of carbon steel in alkanolamine/Room-temperature ionic liquid based CO2 capture systems. Ind. Eng. Chem. Res. 2012, 51, 8711–8718. [Google Scholar] [CrossRef]
- Haider, M.B.; Hussain, Z.; Kumar, R. CO2 absorption and kinetic study in ionic liquid amine blends. J. Mol. Liq. 2016, 224, 1025–1031. [Google Scholar] [CrossRef]
- Jou, F.-Y.; Mather, A.E.; Otto, F.D. The solubility of CO2 in a 30 mass percent monoethanolamine solution. Can. J. Chem. Eng. 1995, 73, 140–147. [Google Scholar] [CrossRef]
- Kuhlmann, E.; Himmler, S.; Giebelhaus, H.; Wasserscheid, P. Imidazolium dialkylphosphates - a class of versatile, halogen-free and hydrolytically stable ionic liquids. Green Chem. 2007, 9, 233–242. [Google Scholar] [CrossRef]
- Xu, F.; Gao, H.; Dong, H.; Wang, Z.; Zhang, X.; Ren, B.; Zhang, S. Solubility of CO2 in aqueous mixtures of monoethanolamine and dicyanamide-based ionic liquids. Fluid Phase Equilibria 2014, 365, 80–87. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Li, C.; Wang, Z. Density Prediction of Ionic Liquids at Different Temperatures and Pressures Using a Group Contribution Equation of State Based on Electrolyte Perturbation Theory. Ind. Eng. Chem. Res. 2010, 49, 4420–4425. [Google Scholar] [CrossRef]
- Khan, S.N.; Hailegiorgis, S.M.; Man, Z.; Shariff, A.M.; Garg, S. Thermophysical properties of aqueous 1-butyl-3-methylimidazolium acetate [BMIM] [AC] + monoethanolamine (MEA) hybrid as a solvent for CO2 capture. Procedia Eng. 2016, 148, 1326–1331. [Google Scholar] [CrossRef] [Green Version]
- Zainul Anuar, M.U.; Taha, M.F.; Md Yunus, N.M.; Mat Ghani, S.M.; Idris, A. An Optimization Study of Carbon Dioxide Absorption into the Aqueous Solution of Monoethanolamine and Tetrabutylphosphonium Methanesulfonate Hybrid Solvent Using RSM-CCD Methodology. Processes 2021, 9, 1186. [Google Scholar] [CrossRef]
- Huang, K.; Chen, F.-F.; Tao, D.-J.; Dai, S. Ionic liquid–formulated hybrid solvents for CO2 capture. Curr. Opin. Green Sustain. Chem. 2017, 5, 67–73. [Google Scholar] [CrossRef]
- Khan, S.N.; Hailegiorgis, S.M.; Man, Z.; Garg, S.; Shariff, A.M.; Farrukh, S.; Ayoub, M.; Ghaedi, H. High-pressure absorption study of CO2 in aqueous N-methyldiethanolamine (MDEA) and MDEA-piperazine (PZ)-1-butyl-3-methylimidazolium trifluoromethanesulfonate [bmim][OTf] hybrid solvents. J. Mol. Liq. 2018, 249, 1236–1244. [Google Scholar] [CrossRef] [Green Version]
- Anuar, M.A.M.; Amran, N.A.; Ruslan, M.S.H. Optimization of Progressive Freezing for Residual Oil Recovery from a Palm Oil-Water Mixture (POME Model). ACS Omega 2021, 6, 2707–2716. [Google Scholar] [CrossRef]
- Durante, M.; Ferramosca, A.; Treppiccione, L.; Di Giacomo, M.; Zara, V.; Montefusco, A.; Piro, G.; Mita, G.; Bergamo, P.; Lenucci, M.S. Application of response surface methodology (RSM) for the optimization of supercritical CO2 extraction of oil from patè olive cake: Yield, content of bioactive molecules and biological effects in vivo. Food Chem. 2020, 332, 127405. [Google Scholar] [CrossRef]
- Abdulredha, M.M.; Hussain, S.A.; Abdullah, L.C. Optimization of the demulsification of water in oil emulsion via non-ionic surfactant by the response surface methods. J. Pet. Sci. Eng. 2020, 184, 106463. [Google Scholar] [CrossRef]
- Leuca, T.; Trip, N.; Silaghi, H.; Burd, A. Design of experiments approach for induction heating optimization. Rev. Roum. Des Sci. Tech. Série Électrotechnique Et 2016, 61, 169–172. [Google Scholar]
- Majaliwa, N.; Kibazohi, O.; Alminger, M. Optimization of process parameters for mechanical extraction of banana juice using response surface methodology. J Food Sci Technol 2019, 56, 4068–4075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, J.; Zhao, M.; Shi, J.; Wang, J.; Jiang, Y.; Cui, C.; Kakuda, Y.; Xue, S.J. Optimization of antioxidant peptide production from grass carp sarcoplasmic protein using response surface methodology. LWT-Food Sci. Technol. 2008, 41, 1624–1632. [Google Scholar] [CrossRef]
- Ahmady, A.; Hashim, M.A.; Aroua, M.K. Absorption of carbon dioxide in the aqueous mixtures of methyldiethanolamine with three types of imidazolium-based ionic liquids. Fluid Phase Equilibria 2011, 309, 76–82. [Google Scholar] [CrossRef]
- Torralba-Calleja, E.; Skinner, J.; Gutiérrez-Tauste, D. CO2 Capture in Ionic Liquids: A Review of Solubilities and Experimental Methods. J. Chem. 2013, 2013, 473584. [Google Scholar] [CrossRef] [Green Version]


| Factors | Unit | Levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| XA: (BMIM)(DBP) concentration | wt% | 0 | 11 | 20 |
| XB: Temperature | °C | 30 | 45 | 60 |
| XC: Pressure | bar | 2 | 16 | 30 |
| Solvent | A1 | A2 | 100AAD |
|---|---|---|---|
| Aqueous 30 wt%MEA-10 wt% (BMIM)(DBP) | 1.1940 | −0.0005 | 0.02 |
| Aqueous 30 wt%MEA-20 wt% (BMIM)(DBP) | 1.2228 | −0.0006 | 0.02 |
| Solvent | A3 | A4 | 100AAD |
|---|---|---|---|
| Aqueous 30 wt%MEA-10 wt% (BMIM)(DBP) | −7.3863 | 2.5059 | 0.52 |
| Aqueous 30 wt%MEA-20 wt% (BMIM)(DBP) | −6.7494 | 2.5235 | 1.29 |
| Standard | XA: IL Concentration (wt%) | XB: Temperature (°C) | XC: CO2 Pressure (bar) | Y: Mole Fraction Experimental | Y: Mole Fraction |
|---|---|---|---|---|---|
| Predicted | |||||
| 1 | 0 | 30 | 2 | 0.423 | 0.418 |
| 2 | 20 | 30 | 2 | 0.387 | 0.382 |
| 3 | 0 | 60 | 2 | 0.313 | 0.312 |
| 4 | 20 | 60 | 2 | 0.319 | 0.322 |
| 5 | 0 | 30 | 30 | 0.620 | 0.610 |
| 6 | 20 | 30 | 30 | 0.603 | 0.597 |
| 7 | 0 | 60 | 30 | 0.529 | 0.527 |
| 8 | 20 | 60 | 30 | 0.562 | 0.560 |
| 9 | 0 | 45 | 16 | 0.478 | 0.496 |
| 10 | 20 | 45 | 16 | 0.483 | 0.494 |
| 11 | 10 | 30 | 16 | 0.466 | 0.493 |
| 12 | 10 | 60 | 16 | 0.420 | 0.422 |
| 13 | 10 | 45 | 2 | 0.383 | 0.392 |
| 14 | 10 | 45 | 30 | 0.587 | 0.607 |
| 15 | 10 | 45 | 16 | 0.503 | 0.493 |
| 16 | 10 | 45 | 16 | 0.513 | 0.493 |
| 17 | 10 | 45 | 16 | 0.497 | 0.493 |
| 18 | 10 | 45 | 16 | 0.515 | 0.493 |
| Source | Standard Deviation | R2 | Adjusted R2 | Predicted R2 | Remarks |
|---|---|---|---|---|---|
| Linear | 0.0251 | 0.9358 | 0.922 | 0.9009 | |
| 2FI | 0.0256 | 0.9473 | 0.9186 | 0.8737 | |
| Quadratic | 0.0189 | 0.9791 | 0.9556 | 0.9049 | Suggested |
| Cubic | 0.0243 | 0.9828 | 0.927 | −20.9735 | Aliased |
| Source | Sum of Squares | dF | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 0.1344 | 9 | 0.0149 | 41.61 | <0.0001 | Significant |
| XA-IL Concentration | 8.10 × 10−6 | 1 | 8.10 × 10−6 | 0.0226 | 0.8843 | |
| XB-Temperature | 0.0127 | 1 | 0.0127 | 35.31 | 0.0003 | |
| XC-Pressure | 0.1158 | 1 | 0.1158 | 322.61 | <0.0001 | |
| XAXB | 0.0011 | 1 | 0.0011 | 2.95 | 0.1243 | |
| XAXC | 0.0003 | 1 | 0.0003 | 0.737 | 0.4156 | |
| XBXC | 0.0003 | 1 | 0.0003 | 0.737 | 0.4156 | |
| XA2 | 0 | 1 | 0 | 0.0324 | 0.8616 | |
| XB2 | 0.0034 | 1 | 0.0034 | 9.48 | 0.0152 | |
| XC2 | 0.0001 | 1 | 0.0001 | 0.3261 | 0.5837 | |
| Residual | 0.0029 | 8 | 0.0004 | |||
| Lack of Fit | 0.0027 | 5 | 0.0005 | 7.38 | 0.0654 | Not significant |
| Pure Error | 0.0002 | 3 | 0.0001 | |||
| Cor Total | 0.1373 | 17 | ||||
| Std. dev | 0.0189 | C.V.% | 3.96 | |||
| Mean | 0.4778 | Adeq precision | 21.098 |
| Run Factor | CO2 Solubility (Mole Fraction) | ||||
|---|---|---|---|---|---|
| XA: IL Concentration (wt%) | XB: Temperature (°C) | XC: Pressure (Bar) | Experimental Value | Lower Limit | Higher Limit |
| 30 | 30 | 30 | 0.639 | 0.544 | 0.689 |
| 20 | 30 | 16 | 0.489 | 0.425 | 0.489 |
| 10 | 30 | 02 | 0.402 | 0.340 | 0.402 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azhar, F.N.A.; Taha, M.F.; Mat Ghani, S.M.; Ruslan, M.S.H.; Md Yunus, N.M. Experimental and Mathematical Modelling of Factors Influencing Carbon Dioxide Absorption into the Aqueous Solution of Monoethanolamine and 1-Butyl-3-methylimidazolium Dibutylphosphate Using Response Surface Methodology (RSM). Molecules 2022, 27, 1779. https://doi.org/10.3390/molecules27061779
Azhar FNA, Taha MF, Mat Ghani SM, Ruslan MSH, Md Yunus NM. Experimental and Mathematical Modelling of Factors Influencing Carbon Dioxide Absorption into the Aqueous Solution of Monoethanolamine and 1-Butyl-3-methylimidazolium Dibutylphosphate Using Response Surface Methodology (RSM). Molecules. 2022; 27(6):1779. https://doi.org/10.3390/molecules27061779
Chicago/Turabian StyleAzhar, Fatin Nor Arissa, Mohd Faisal Taha, Siti Musliha Mat Ghani, Muhammad Syafiq Hazwan Ruslan, and Noor Mona Md Yunus. 2022. "Experimental and Mathematical Modelling of Factors Influencing Carbon Dioxide Absorption into the Aqueous Solution of Monoethanolamine and 1-Butyl-3-methylimidazolium Dibutylphosphate Using Response Surface Methodology (RSM)" Molecules 27, no. 6: 1779. https://doi.org/10.3390/molecules27061779






