Plumbagin Alleviates Intracerebroventricular-Quinolinic Acid Induced Depression-like Behavior and Memory Deficits in Wistar Rats
Abstract
:1. Introduction
2. Results
2.1. Effect of Plumbagin on Immobility Time during the Forced Swim Test
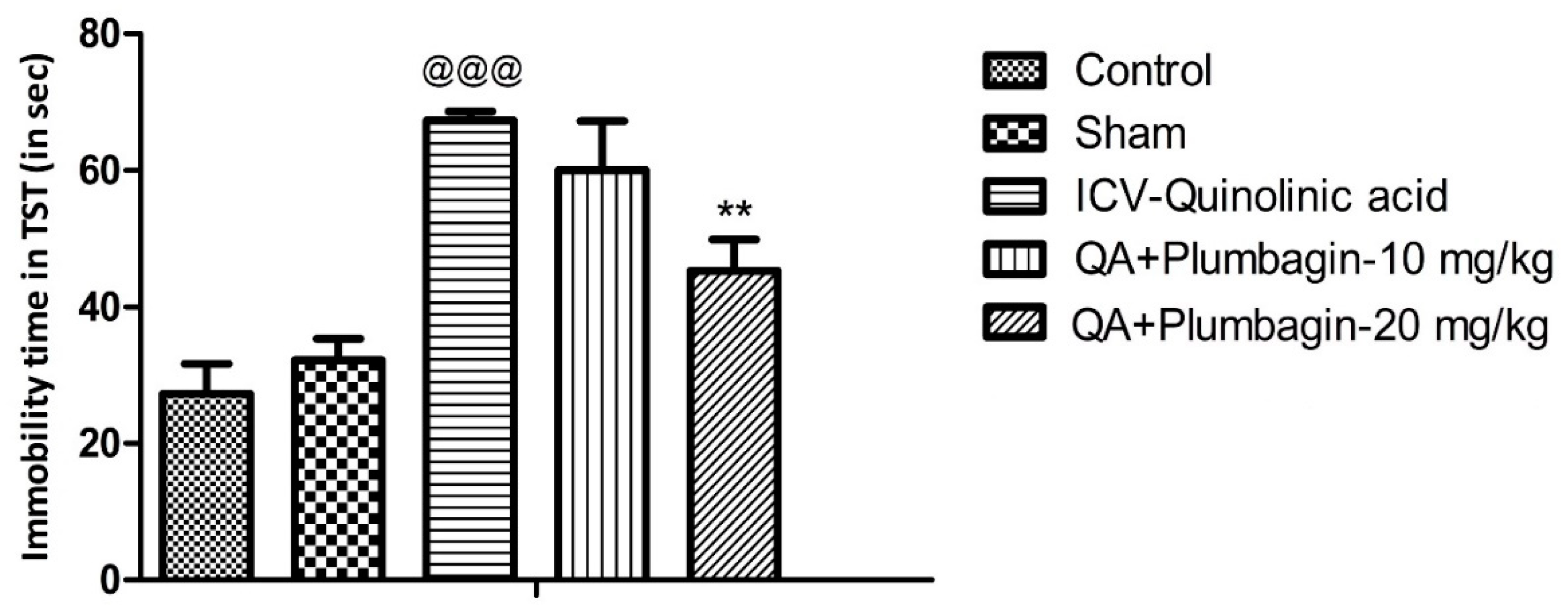
2.2. Effect of Plumbagin on Depression-like Behavior in the Tail Suspension Test
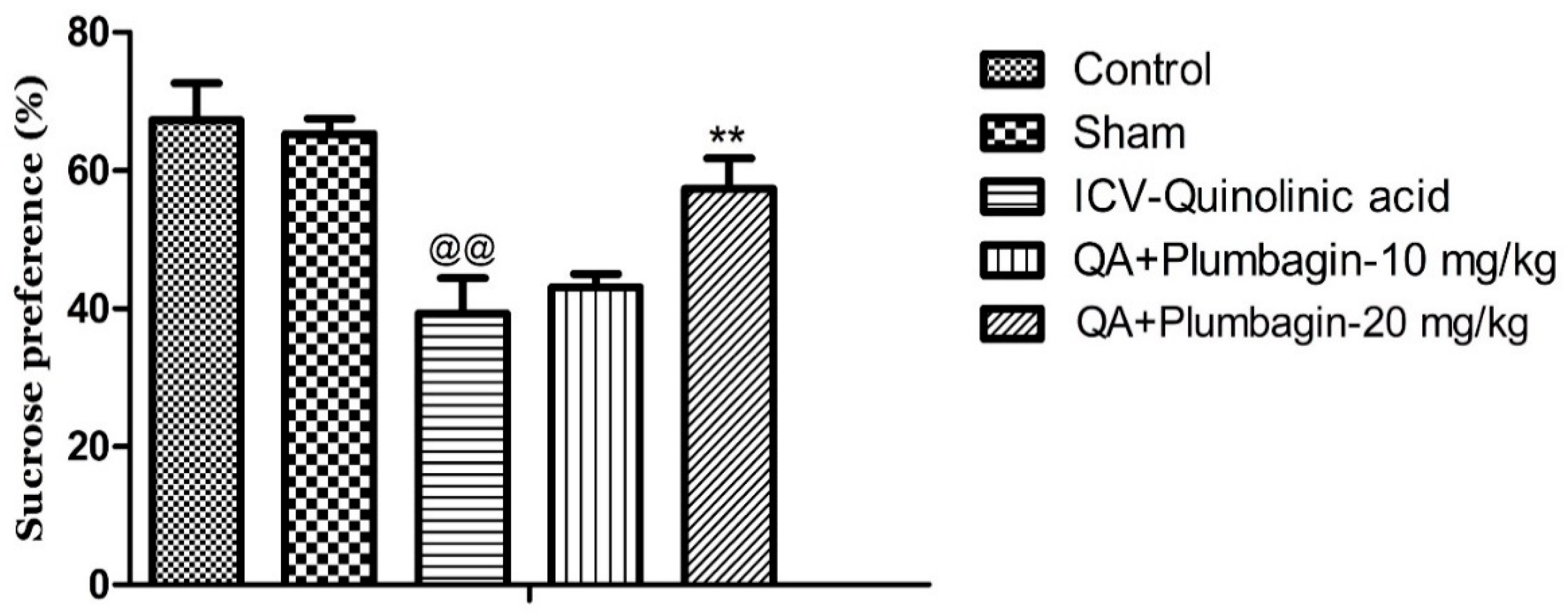
2.3. Effect of Plumbagin on Sucrose Consumption Test
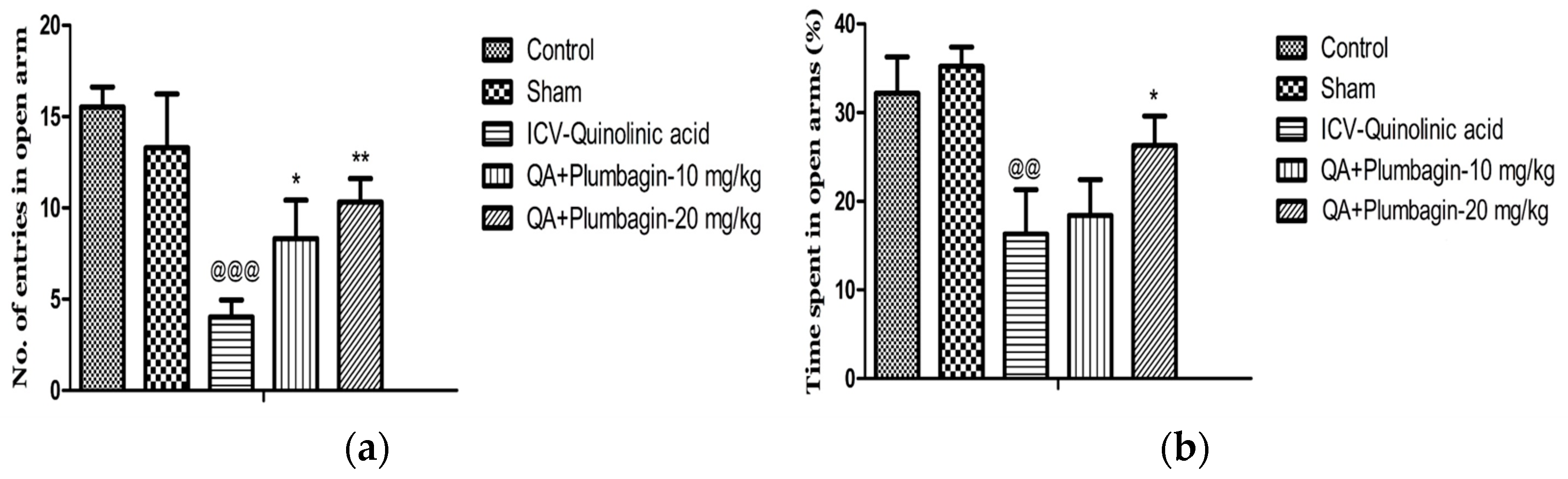
2.4. Effect of Plumbagin on Elevated Plus Maze
2.5. Effect of Plumbagin on Open Field Test
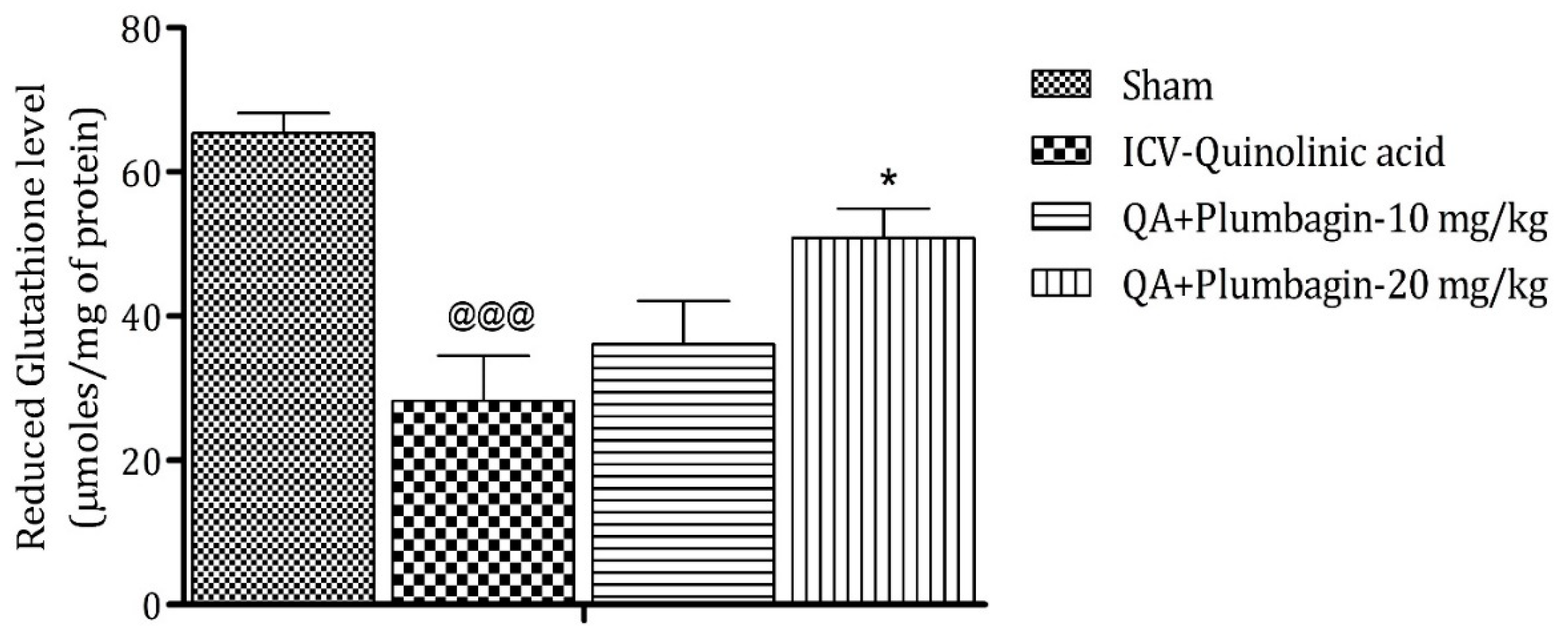
2.6. Effect of Plumbagin on ICV QA-Induced Differences in Reduced Glutathione (GSH) Level
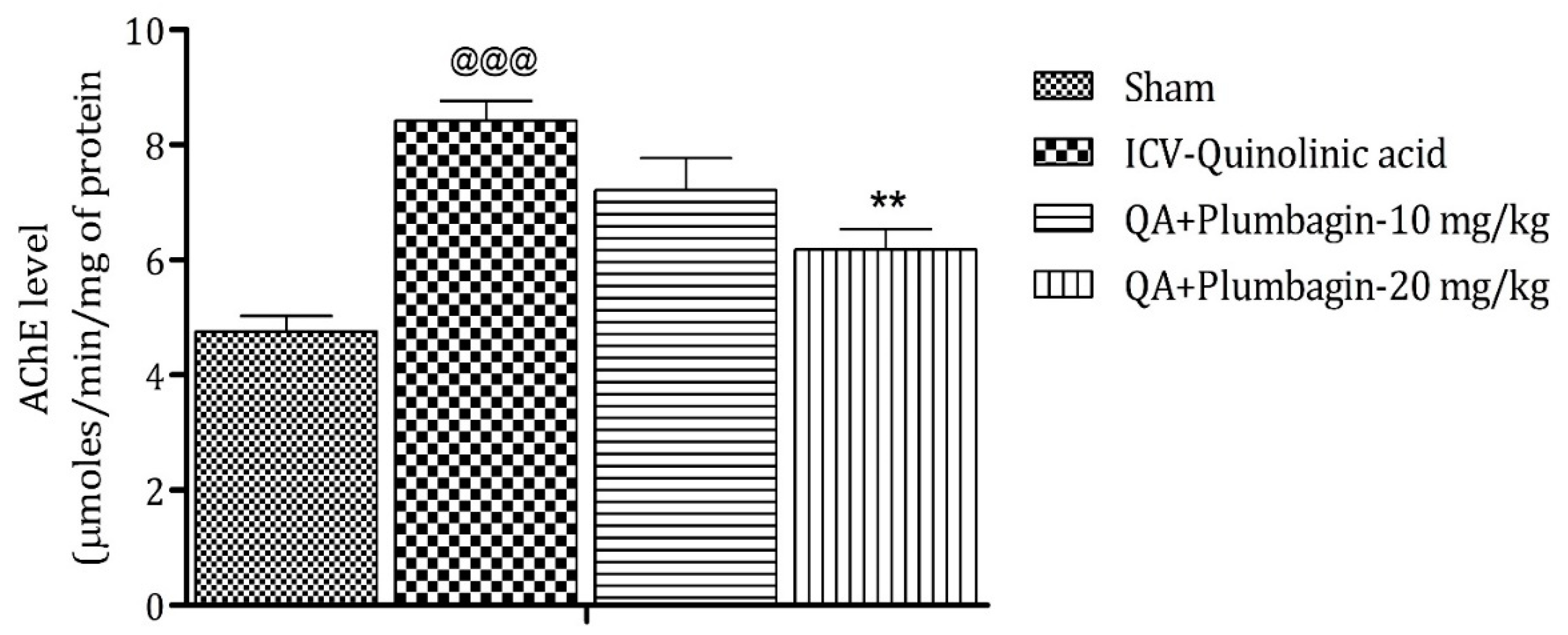
2.7. Effect of Plumbagin on ICV QA-Induced Differences in AChE Level
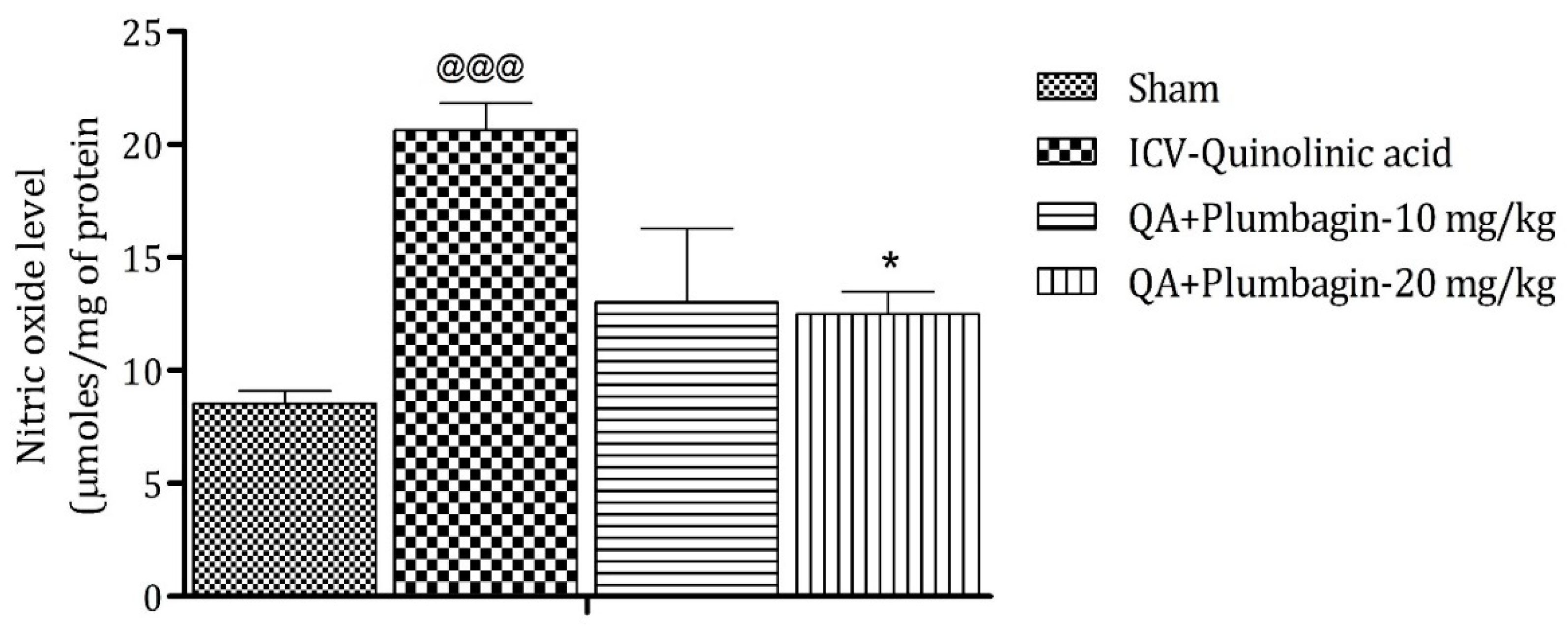
2.8. Effect of Plumbagin on ICV QA-Induced Differences in Nitric Oxide (NO) Level
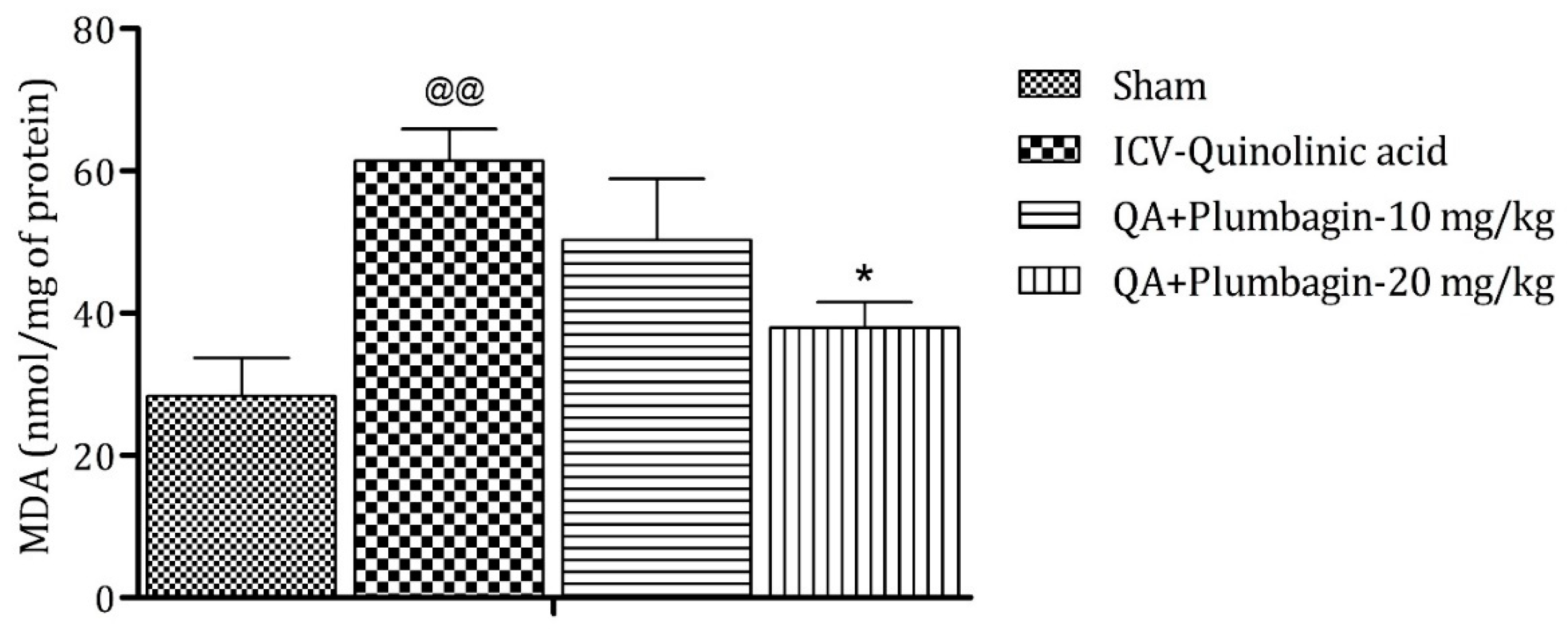
2.9. Effect of Plumbagin on ICV QA-Induced Differences in Malondialdehyde (MDA) Level
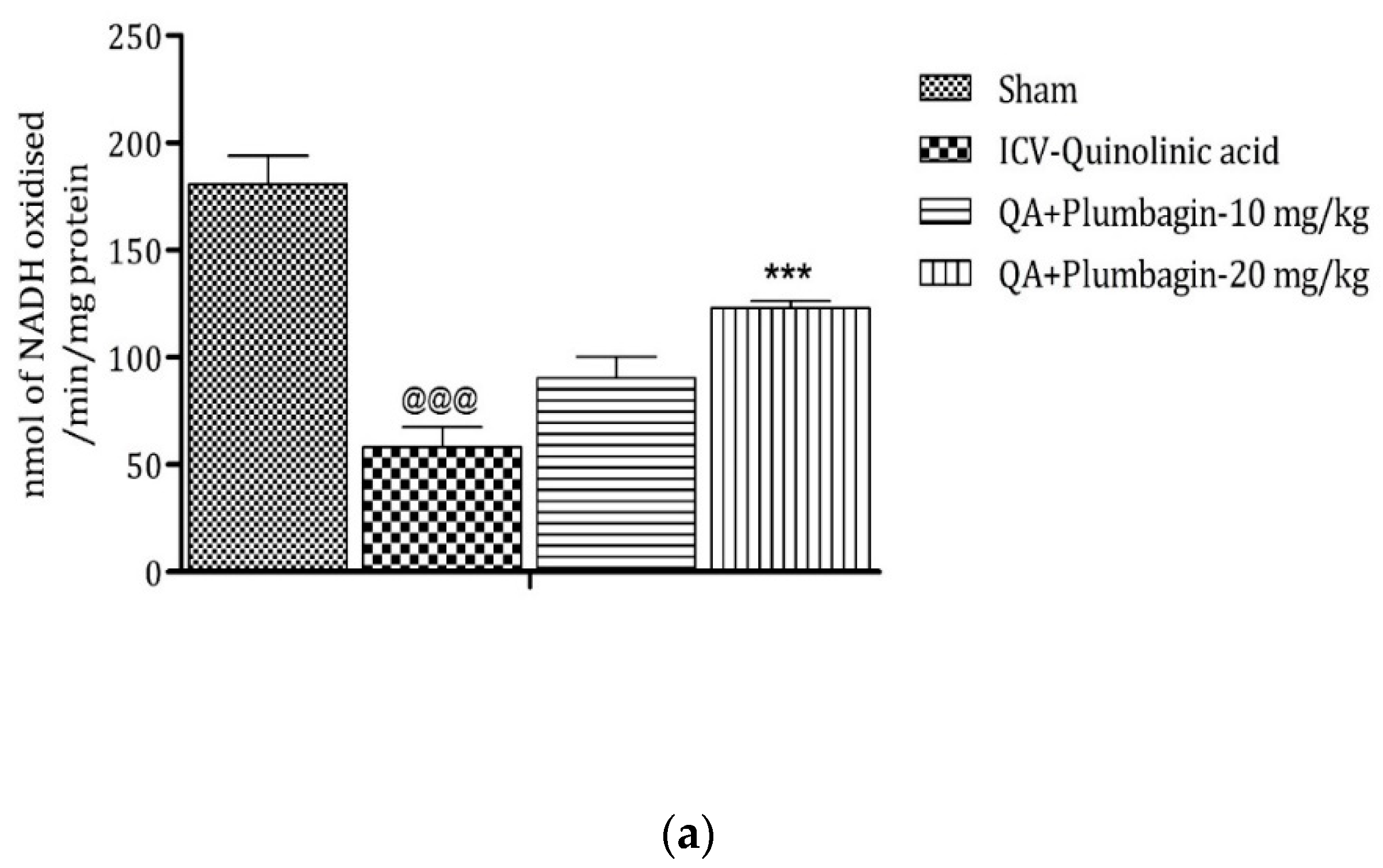
2.10. Effect of Plumbagin on ICV QA-Induced Differences in Mitochondrial Complexes I, II, and IV
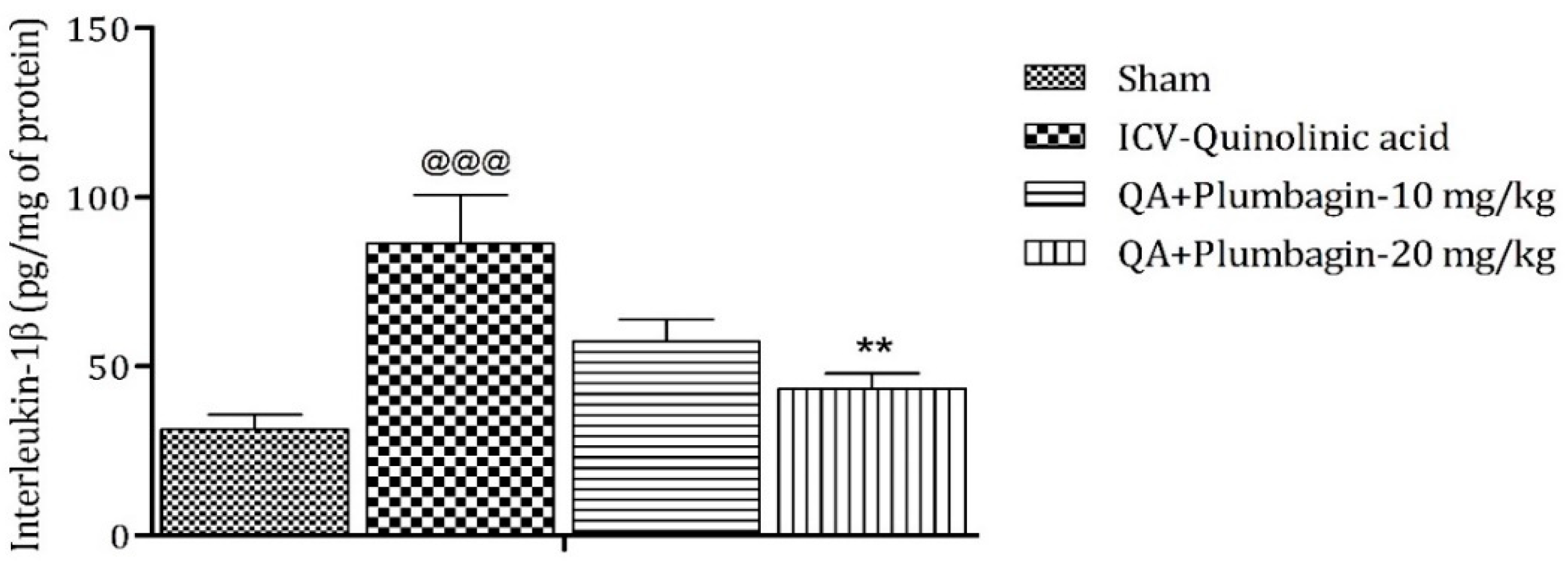
2.11. Effect of Plumbagin on ICV QA-Induced Differences in IL-1β Level
3. Discussion
4. Materials and Methods
4.1. Test Animals
4.2. Experimental Grouping and Dosing
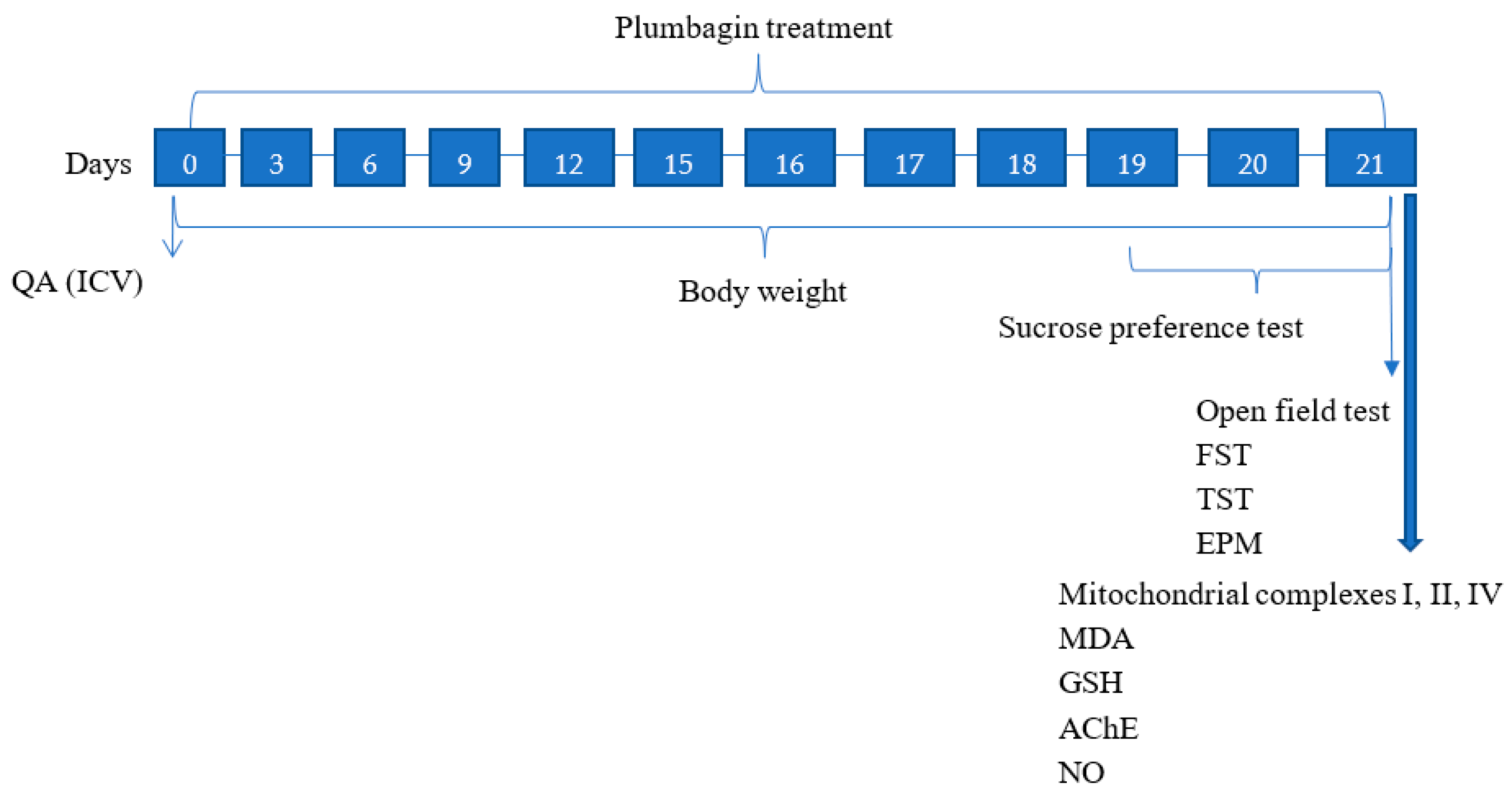
4.3. Study Design
4.4. Drugs and Chemicals
4.5. Surgical Procedure (Intracerebroventricular Injection)
4.6. Behavioral Parameters
4.6.1. Forced Swim Test (FST)
4.6.2. Tail Suspension Test (TST)
4.6.3. Sucrose Preference Test
4.6.4. Elevated Plus Maze (EPM)
4.6.5. Open Field Test (OFT)
4.7. Biochemical Parameters
4.7.1. Preparation of Tissue Homogenate
4.7.2. Lipid Peroxidation Estimation
4.7.3. Reduced Glutathione (GSH) Estimation
4.7.4. AChE Estimation
4.7.5. Nitric Oxide Estimation
4.7.6. Isolation and Preparation of Mitochondria
4.7.7. Complex I (NADH: Coenzyme Q Oxidoreductase)
4.7.8. Complex II (SDH Activity)
4.7.9. Complex IV (Assay of Cytochrome c Oxidase)
4.7.10. Interleukin-1β (IL-1β) Level Estimation
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jangra, A.; Sriram, C.S.; Lahkar, M. Lipopolysaccharide-induced behavioral alterations are alleviated by sodium phenylbutyrate via attenuation of oxidative stress and neuroinflammatory cascade. Inflammation 2016, 39, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Han, Z.; Bai, R.; Huang, S.; Ge, X.; Chen, F.; Lei, P. The accumulation of brain injury leads to severe neuropathological and neurobehavioral changes after repetitive mild traumatic brain injury. Brain Res. 2017, 1657, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Bromet, E.J. The epidemiology of depression across cultures. Annu. Rev. Public Health 2013, 34, 119–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grover, S.; Dutt, A.; Avasthi, A. An overview of Indian research in depression. Indian J. Psychiatry 2010, 52 (Suppl. 1), S178. [Google Scholar] [CrossRef]
- Lugo-Huitrón, R.; Ugalde Muñiz, P.; Pineda, B.; Pedraza-Chaverrí, J.; Ríos, C.; Pérez-de la Cruz, V. QA: An endogenous neurotoxin with multiple targets. Oxid. Med. Cell. Longev. 2013, 2013, 104024. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Gopar, P.E.; Pérez-Rodríguez, M.J.; Rodríguez-Manzo, G.; Garduño-Gutierrez, R.; Tristán-López, L.; Angeles-López, Q.D.; González-Espinosa, C.; Pérez-Severiano, F. Mast cells and histamine are involved in the neuronal damage observed in a quinolinic acid-induced model of Huntington’s disease. J. Neurochem. 2021, 160, 256–270. [Google Scholar] [CrossRef]
- Esmaeili, S.; Ghobadi, N.; Akbari, V.; Moradi, S.; Shahlaie, M.; Ghobadi, S.; Jalalvand, A.R.; Amani, M.; Khodarahmi, R. Pyridine-2,3-dicarboxylate, quinolinic acid, induces 1N4R Tau amyloid aggregation in vitro: Another evidence for the detrimental effect of the inescapable endogenous neurotoxin. Chem.-Biol. Interact. 2020, 315, 108884. [Google Scholar] [CrossRef]
- Pérez-De La Cruz, V.; Carrillo-Mora, P.; antamaría, A. Quinolinic Acid, an endogenous molecule combining excitotoxicity, oxidative stress and other toxic mechanisms. Int. J. Tryptophan Res. 2012, 5, 1–8. [Google Scholar] [CrossRef]
- Sumathi, T.; Vedagiri, A.; Ramachandran, S.; Purushothaman, B. QA-Induced Huntington Disease-Like Symptoms Mitigated by Potent Free Radical Scavenger Edaravone—A Pilot Study on Neurobehavioral, Biochemical, and Histological Approach in Male Wistar Rats. J. Mol. Neurosci. 2018, 66, 322–341. [Google Scholar] [CrossRef]
- Rahman, A.; Rao, M.S.; Khan, K.M. Intraventricular infusion of QA impairs spatial learning and memory in young rats: A novel mechanism of lead-induced neurotoxicity. J. Neuroinflamm. 2018, 15, 263. [Google Scholar] [CrossRef]
- Jhamandas, K.H.; Boegman, R.J.; Beninger, R.J.; Miranda, A.F.; Lipic, K.A. Excitotoxicity of QA: Modulation by endogenous antagonists. Neurotox. Res. 2000, 2, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Kubicova, L.; Hadacek, F.; Chobot, V. QA: Neurotoxin or oxidative stress modulator? Int. J. Mol. Sci. 2013, 14, 21328–21338. [Google Scholar] [CrossRef] [PubMed]
- Bansal, Y.; Singh, R.; Parhar, I.; Kuhad, A.; Soga, T. QA and Nuclear factor erythroid 2–related factor 2 in depression: Role in Neuroprogression. Front. Pharmacol. 2019, 10, 452. [Google Scholar] [CrossRef]
- Bordelon, Y.M.; Chesselet, M.F.; Nelson, D.; Welsh, F.; Erecińska, M. Energetic dysfunction in QA-lesioned rat striatum. J. Neurochem. 1997, 69, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Haug, L.S.; Ostvold, A.C.; Torgner, I.; Roberg, B.; Dvoráková, L.; St’astný, F.; Walaas, S.I. Intracerebroventricular administration of QA induces a selective decrease of inositol (1,4,5)-trisphosphate receptors in rat brain. Neurochem. Int. 1998, 33, 109–119. [Google Scholar] [CrossRef]
- Aziz, M.H.; Dreckschmidt, N.E.; Verma, A.K. Plumbagin, a medicinal plant–derived naphthoquinone, is a novel inhibitor of the growth and invasion of hormone-refractory prostate cancer. Cancer Res. 2008, 68, 9024–9032. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Dang, M.; Lin, Y.; Xue, F. Evaluation of wound healing activity of plumbagin in diabetic rats. Life Sci. 2019, 231, 116422. [Google Scholar] [CrossRef]
- Yuan, J.H.; Pan, F.; Chen, J.; Chen, C.E.; Xie, D.P.; Jiang, X.Z.; Guo, S.J.; Zhou, J. Neuroprotection by plumbagin involves BDNF-TrkB-PI 3K/Akt and ERK 1/2/JNK pathways in isoflurane-induced neonatal rats. J. Pharm. Pharmacol. 2017, 69, 896–906. [Google Scholar] [CrossRef]
- Periasamy, H.; Iswarya, S.; Pavithra, N.; Senthilnathan, S.; Gnanamani, A. In vitro antibacterial activity of plumbagin isolated from Plumbago zeylanica L. against methicillin-resistant Staphylococcus aureus. Lett. Appl. Microbiol. 2019, 69, 41–49. [Google Scholar] [CrossRef]
- Adusei, E.; Adosraku, R.K.; Oppong-Kyekyeku, J.; Amengor, C.D. Investigation of Acid-Base Indicator Property of Plumbagin from Plumbago zeylanica Linn. Int. J. Anal. Chem. 2019, 2019, 4061927. [Google Scholar] [CrossRef] [Green Version]
- Tilak, J.C.; Adhikari, S.; Devasagayam, T.P. Antioxidant properties of Plumbago zeylanica, an Indian medicinal plant and its active ingredient, plumbagin. Redox Rep. 2004, 9, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Messeha, S.S.; Zarmouh, N.O.; Mendonca, P.; Kolta, M.G.; Soliman, K.F. The attenuating effects of plumbagin on pro-inflammatory cytokine expression in LPS-activated BV-2 microglial cells. J. Neuroimmunol. 2017, 313, 129–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinho, B.R.; Sousa, C.; Valentão, P.; Andrade, P.B. Is nitric oxide decrease observed with naphthoquinones in LPS stimulated RAW 264.7 macrophages a beneficial property? PLoS ONE 2011, 6, e24098. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, D.K.; Kumar, D.; Kwatra, M.; Pandey, S.N.; Choubey, P.; Lahkar, M.; Jangra, A. Voluntary alcohol consumption exacerbated high fat diet-induced cognitive deficits by NF-κB-calpain dependent apoptotic cell death in rat hippocampus: Ameliorative effect of melatonin. Biomed. Pharm. 2018, 108, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Farooq, R.K.; Asghar, K.; Kanwal, S.; Zulqernain, A. Role of inflammatory cytokines in depression: Focus on interleukin-1β. Biomed. Rep. 2017, 6, 15–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Li, C.; Yu, H.; Cai, X.; Shen, X.; Sun, X.; Wang, J.; Zhang, Y.; Wang, C. Lentivirus-mediated interleukin-1β (IL-1β) knock-down in the hippocampus alleviates lipopolysaccharide (LPS)-induced memory deficits and anxiety- and depression-like behaviors in mice. J. Neuroinflamm. 2017, 14, 190. [Google Scholar] [CrossRef] [Green Version]
- Trojsi, F.; Christidi, F.; Migliaccio, R.; Santamaría-García, H.; Santangelo, G. Behavioural and cognitive changes in neurodegenerative diseases and brain injury. Behav. Neurol. 2018, 2018, 4935915. [Google Scholar] [CrossRef]
- Gulati, K.; Chakraborti, A.; Ray, A. Modulation of stress-induced neurobehavioral changes and brain oxidative injury by nitric oxide (NO) mimetics in rats. Behav. Brain Res. 2007, 183, 226–230. [Google Scholar] [CrossRef]
- Shear, D.A.; Dong, J.; Haik-Creguer, K.L.; Bazzett, T.J.; Albin, R.L.; Dunbar, G.L. Chronic administration of QA in the rat striatum causes spatial learning deficits in a radial arm water maze task. Exp. Neurol. 1998, 150, 305–311. [Google Scholar] [CrossRef]
- Sinz, E.H.; Kochanek, P.M.; Heyes, M.P.; Wisniewski, S.R.; Bell, M.J.; Clark, R.S.; DeKosky, S.T.; Blight, A.R.; Marion, D.W. QA is increased in CSF and associated with mortality after traumatic brain injury in humans. J. Cereb. Blood Flow Metab. 1998, 18, 610–615. [Google Scholar] [CrossRef]
- Heyes, M.P.; Ellis, R.J.; Ryan, L.; Childers, M.E.; Grant, I.; Wolfson, T.; Archibald, S.; Jernigan, T.L. Elevated cerebrospinal fluid QA levels are associated with region-specific cerebral volume loss in HIV infection. Brain 2001, 124, 1033–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massieu, L.; Rocamora, N.; Palacios, J.; Boddeke, H.W. Administration of quinolic acid in the rat hippocampus induces expression of c-fos and NGFI-A. Mol. Brain Res. 1992, 16, 88–96. [Google Scholar] [CrossRef]
- Kalonia, H.; Kumar, P.; Kumar, A. Pioglitazone ameliorates behavioral, biochemical and cellular alterations in QA induced neurotoxicity: Possible role of peroxisome proliferator activated receptor-γ (PPARγ) in Huntington’s disease. Pharmacol. Biochem. Behav. 2010, 96, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wang, G.; Kandhare, A.D.; Mukherjee-Kandhare, A.A.; Bodhankar, S.L. Neuroprotective effect of naringin, a flavone glycoside in QA-induced neurotoxicity: Possible role of PPAR-γ, Bax/Bcl-2, and caspase-3. Food Chem. Toxicol. 2018, 121, 95–108. [Google Scholar] [CrossRef]
- Latif-Hernandez, A.; Shah, D.; Ahmed, T.; Lo, A.C.; Callaerts-Vegh, Z.; Van der Linden, A.; Balschun, D.; D’Hooge, R. Quinolinic acid injection in mouse medial prefrontal cortex affects reversal learning abilities, cortical connectivity and hippocampal synaptic plasticity. Sci. Rep. 2016, 6, 36489. [Google Scholar] [CrossRef]
- Gill, J.S.; Jamwal, S.; Kumar, P.; Deshmukh, R. Sertraline and venlafaxine improves motor performance and neurobehavioral deficit in QA induced Huntington’s like symptoms in rats: Possible neurotransmitters modulation. Pharmacol. Rep. 2017, 69, 306–313. [Google Scholar] [CrossRef]
- Jangra, A.; Chadha, V.; Kumar, D.; Kumar, V.; Arora, M.K. Neuroprotective and acetylcholinesterase inhibitory activity of plumbagin in ICV-LPS induced behavioral deficits in rat. Curr. Res. Behav. Sci. 2021, 2, 100060. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhang, J.G.; Wu, L. Plumbagin inhibits neuronal apoptosis, intimal hyperplasia and also suppresses TNF-α/NF-κB pathway induced inflammation and matrix metalloproteinase-2/9 expression in rat cerebral ischemia. Saudi J. Biol. Sci. 2018, 25, 1033–1039. [Google Scholar] [CrossRef]
- Son, T.G.; Camandola, S.; Arumugam, T.V.; Cutler, R.G.; Telljohann, R.S.; Mughal, M.R.; Moore, T.A.; Luo, W.; Yu, Q.S.; Johnson, D.A.; et al. Plumbagin, a novel Nrf2/ARE activator, protects against cerebral ischemia. J. Neurochem. 2010, 112, 1316–1326. [Google Scholar] [CrossRef] [Green Version]
- Farkhondeh, T.; Mehrpour, O.; Forouzanfar, F.; Roshanravan, B.; Samarghandian, S. Oxidative stress and mitochondrial dysfunction in organophosphate pesticide-induced neurotoxicity and its amelioration: A review. Environ. Sci. Pollut. Res. Int. 2020, 27, 24799–24814. [Google Scholar] [CrossRef]
- Waldbaum, S.; Patel, M. Mitochondrial dysfunction and oxidative stress: A contributing link to acquired epilepsy? J. Bioenerget. Biomembr. 2010, 42, 449–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamwal, S.; Singh, S.; Kaur, N.; Kumar, P. Protective effect of spermidine against excitotoxic neuronal death induced by QA in rats: Possible neurotransmitters and neuroinflammatory mechanism. Neurotox. Res. 2015, 28, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Contreras, D.; Parra, C.; Freer, J.; Baeza, J.; Durán, N. Pulp mill effluent treatment by Fenton-type reactions catalyzed by iron complexes. Water Sci. Technol. 1999, 40, 351–355. [Google Scholar] [CrossRef]
- Sankar, R.; Devamanoharan, P.S.; Raghupathi, G.; Krishnasamy, M.; Devi, C.S. Lipid peroxidation in plumbagin administered rats. J. Biosci. 1987, 12, 267–271. [Google Scholar] [CrossRef]
- Gangabhagirathi, R.; Joshi, R. Antioxidant role of plumbagin in modification of radiation-induced oxidative damage. Oxid. Antioxid. Med. Sci. 2015, 4, 85–90. [Google Scholar] [CrossRef]
- Arora, M.K.; Kisku, A.; Jangra, A. Mangiferin ameliorates intracerebroventricular-quinolinic acid-induced cognitive deficits, oxidative stress, and neuroinflammation in Wistar rats. Indian J. Pharm. 2020, 52, 296–305. [Google Scholar]
- Dhingra, D.; Bansal, S. Antidepressant-like activity of plumbagin in unstressed and stressed mice. Pharm. Rep. 2015, 67, 1024–1032. [Google Scholar] [CrossRef]
- Arruri, V.; Komirishetty, P.; Areti, A.; Dungavath, S.K.N.; Kumar, A. Nrf2 and NF-κB modulation by plumbagin attenuates functional, behavioural and biochemical deficits in rat model of neuropathic pain. Pharm. Rep. 2017, 69, 625–632. [Google Scholar] [CrossRef]
- Glascock, J.J.; Osman, E.Y.; Coady, T.H.; Rose, F.F.; Shababi, M.; Lorson, C.L. Delivery of therapeutic agents through intracerebroventricular (ICV) and intravenous (IV) injection in mice. JoVE (J. Vis. Exp.) 2011, 3, e2968. [Google Scholar] [CrossRef]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The tail suspension test. JoVE (J. Vis. Exp.) 2012, 28, e3769. [Google Scholar] [CrossRef] [Green Version]
- Al-Yamani, M.J.; Asdaq, S.M.B.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M.; Alsalman, A.J.; Al Hawaj, M.A.; Alanazi, A.A.; Alanzi, K.D.; Imran, M. The role of serotonergic and catecholaminergic systems for possible antidepressant activity of apigenin. Saudi J. Biol. Sci. 2022, 29, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Jangra, A.; Sriram, C.S.; Dwivedi, S.; Gurjar, S.S.; Hussain, M.I.; Borah, P.; Lahkar, M. Sodium phenylbutyrate and edaravone abrogate chronic restraint stress-induced behavioral deficits: Implication of oxido-nitrosative, endoplasmic reticulum stress cascade, and neuroinflammation. Cell. Mol. Neurobiol. 2017, 37, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Jangra, A.; Lukhi, M.M.; Sulakhiya, K.; Baruah, C.C.; Lahkar, M. Protective effect of mangiferin against lipopolysaccharide-induced depressive and anxiety-like behaviour in mice. Eur. J. Pharmacol. 2014, 740, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Eyer, P.; Worek, F.; Kiderlen, D.; Sinko, G.; Stuglin, A.; Simeon-Rudolf, V.; Reiner, E. Molar absorption coefficients for the reduced Ellman reagent: Reassessment. Anal. Biochem. 2003, 312, 224–227. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Swamy, M.; Suhaili, D.; Sirajudeen, K.N.; Mustapha, Z.; Govindasamy, C. Propolis ameliorates tumor nerosis factor-α, nitric oxide levels, caspase-3 and nitric oxide synthase activities in kainic acid mediated excitotoxicity in rat brain. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Pinzón, M.A. Neuroprotective effects of ischemic preconditioning in brain mitochondria following cerebral ischemia. J. Bioenerget. Biomembr. 2004, 36, 323–327. [Google Scholar] [CrossRef]
- King, T.E.; Howard, R.L. Preparations and properties of soluble NADH dehydrogenases from cardiac muscle. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1967; Volume 10, pp. 275–294. [Google Scholar]
- Prakash, C.; Soni, M.; Kumar, V. Mitochondrial oxidative stress and dysfunction in arsenic neurotoxicity: A review. J. Appl. Toxicol. 2016, 36, 179–188. [Google Scholar] [CrossRef]
- Sottocasa, G.L.; Kuylenstierna, B.O.; Ernster, L.; Bergstrand, A. An electron-transport system associated with the outer membrane of liver mitochondria: A biochemical and morphological study. J. Cell Biol. 1967, 32, 415–438. [Google Scholar] [CrossRef]
- Hartree, E.F. Determination of protein: A modification of the Lowry method that gives a linear photometric response. Anal. Biochem. 1972, 48, 422–427. [Google Scholar] [CrossRef]



| Group I | Control (Normal Saline) (n = 10) |
| Group II | Sham control (n = 10) |
| Group III | QA (300 nM/4 μL QA (QA) in Normal saline) (n = 10) |
| Group IV | Plumbagin (10 mg/kg p.o. Low dose) + QA (300 nM/ 4 µL QA in Normal saline) (n = 10) |
| Group V | Plumbagin (20 mg/kg p.o. High dose) + QA (300 nM/ 4 µL QA in Normal saline) (n = 10) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar Arora, M.; Ratra, A.; Asdaq, S.M.B.; Alshamrani, A.A.; Alsalman, A.J.; Kamal, M.; Tomar, R.; Sahoo, J.; Ashok, J.; Imran, M. Plumbagin Alleviates Intracerebroventricular-Quinolinic Acid Induced Depression-like Behavior and Memory Deficits in Wistar Rats. Molecules 2022, 27, 1834. https://doi.org/10.3390/molecules27061834
Kumar Arora M, Ratra A, Asdaq SMB, Alshamrani AA, Alsalman AJ, Kamal M, Tomar R, Sahoo J, Ashok J, Imran M. Plumbagin Alleviates Intracerebroventricular-Quinolinic Acid Induced Depression-like Behavior and Memory Deficits in Wistar Rats. Molecules. 2022; 27(6):1834. https://doi.org/10.3390/molecules27061834
Chicago/Turabian StyleKumar Arora, Mandeep, Anish Ratra, Syed Mohammed Basheeruddin Asdaq, Ali A. Alshamrani, Abdulkhaliq J. Alsalman, Mehnaz Kamal, Ritu Tomar, Jagannath Sahoo, Jangra Ashok, and Mohd Imran. 2022. "Plumbagin Alleviates Intracerebroventricular-Quinolinic Acid Induced Depression-like Behavior and Memory Deficits in Wistar Rats" Molecules 27, no. 6: 1834. https://doi.org/10.3390/molecules27061834
APA StyleKumar Arora, M., Ratra, A., Asdaq, S. M. B., Alshamrani, A. A., Alsalman, A. J., Kamal, M., Tomar, R., Sahoo, J., Ashok, J., & Imran, M. (2022). Plumbagin Alleviates Intracerebroventricular-Quinolinic Acid Induced Depression-like Behavior and Memory Deficits in Wistar Rats. Molecules, 27(6), 1834. https://doi.org/10.3390/molecules27061834









