Synthesis and Structure Elucidation of Two Essential Metal Complexes: In-Vitro Studies of Their BSA/HSA-Binding Properties, Docking Simulations, and Anticancer Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Description of the Complexes
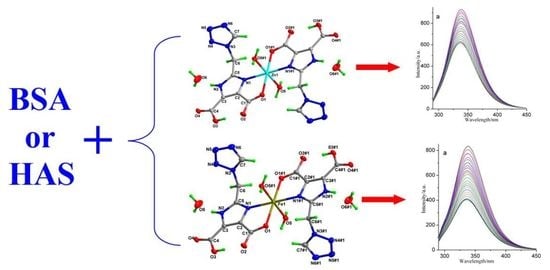
2.2. Interaction between Complexes and Albumins
2.2.1. Interaction between Complexes and BSA
2.2.2. Interaction between Complexes and HSA
2.3. Molecular Docking
2.4. In Vitro Anticancer Activities
3. Materials and Methods
3.1. Materials and Instrumentation
3.2. Syntheses of the Complexes
3.2.1. Syntheses of Complex [Zn(H2tmidc)2(H2O)2]·2H2O (1)
3.2.2. Syntheses of Complex [Fe(H2tmidc)2(H2O)2]·2H2O (2)
3.3. Preparation of the Related Solutions
3.3.1. Solutions Used in Protein Binding Studies
3.3.2. Solutions Used in Cytotoxic Activity Evaluation
3.4. Determination of Crystal Structure
3.5. Molecular Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esteghamat-Panah, R.; Hadadzadeh, H.; Farrokhpour, H.; Simpson, J.; Abdolmaleki, A.; Abyar, F. Synthesis, structure, DNA/protein binding, and cytotoxic activity of a rhodium(III) complex with 2,6-bis(2-benzimidazolyl)pyridine. Eur. J. Med. Chem. 2017, 127, 958–971. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shi, X.; Xu, M.; Li, Z.; Huang, L.; Bai, D.; Zeng, Z. Transition metal complexes of 2, 6-di ((phenazonyl-4-imino) me-thyl)-4-methylphenol: Structure and biological evaluation. Eur. J. Med. Chem. 2011, 46, 1638–1647. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-F.; Yang, Y.-Q.; Li, Y.-X.; Yang, H.-X.; Zhao, W.-F.; Meng, X.-R. Synthesis, crystal structure, and BSA binding studies of new Co(II) and Ni(II) complexes of 2-(hydroxymethyl)-1H-imidazole-4,5-dicarboxylate. Inorg. Chim. Acta 2020, 505, 119469. [Google Scholar] [CrossRef]
- Li, T.; Cheng, Z.; Cao, L.; Jiang, X.; Fan, L. Interactions of two food colourants with BSA: Analysis by Debye-Hückel theory. Food Chem. 2016, 211, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, L.; Pan, J. Probing the Binding of the Flavonoid Diosmetin to Human Serum Albumin by Multispectroscopic Techniques. J. Agric. Food Chem. 2012, 60, 2721–2729. [Google Scholar] [CrossRef]
- Li, X.-F.; Ma, L.-G.; Yang, Y.-Q.; Liu, Y.-J.; Meng, X.-R.; Yang, H.-X. Synthesis, crystal structure and bovine serum albumin–binding studies of a new Cd(II) complex incorporating 2,2′-(propane-1,3-diyl)bis(1H-imidazole-4,5-dicarboxylate). J. Chem. Res. 2020, 44, 198–205. [Google Scholar] [CrossRef]
- Raja, D.S.; Bhuvanesh, N.S.; Natarajan, K. Synthesis, crystal structure and pharmacological evaluation of two new Cu(II) complexes of 2-oxo-1,2-dihydroquinoline-3-carbaldehyde (benzoyl) hydrazone: A comparative investigation. Eur. J. Med. Chem. 2012, 47, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Annaraj, B.; Neelakantan, M. Synthesis, crystal structure, spectral characterization and biological exploration of water soluble Cu(II) complexes of vitamin B6 derivative. Eur. J. Med. Chem. 2015, 102, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Anjomshoa, M.; Torkzadeh-Mahani, M.; Sahihi, M.; Rizzoli, C.; Ansari, M.; Janczak, J.; Esfahani, S.S.; Ataei, F.; Dehkhodaei, M.; Amirheidari, B. Tris-chelated complexes of nickel(II) with bipyridine derivatives: DNA binding and cleavage, BSA binding, molecular docking, and cytotoxicity. J. Biomol. Struct. Dyn. 2019, 37, 3887–3904. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, L.; McArdle, P.; Erxleben, A.; Chiniforoshan, H. Nickel(II) and cobalt(II) complexes of lidocaine: Synthesis, structure and comparative in vitro evaluations of biological perspectives. Eur. J. Med. Chem. 2015, 103, 516–529. [Google Scholar] [CrossRef]
- Askerov, R.K.; Osmanov, V.K.; Kovaleva, O.N.; Baranov, E.V.; Fukin, G.K.; Fukina, D.G.; Boryakov, A.V.; Magerramov, A.M.; Borisov, A.V. Complexes of 1-(4-Methoxyphenyl)-1,4-Dihydro-5H-Tetrazole-5-Thione and 1-(2-Methoxyphenyl)-1,4-Dihydro-5H-Tetrazole-5-Thione with Cadmium Chloride: Synthesis and Molecular and Crystal Structures. Russ. J. Coord. Chem. 2021, 47, 741–750. [Google Scholar] [CrossRef]
- Eremina, J.; Lider, E.; Kuratieva, N.; Samsonenko, D.; Klyushova, L.; Sheven’, D.; Trifonov, R.; Ostrovskii, V. Synthesis and crystal structures of cytotoxic mixed-ligand copper(II) complexes with alkyl tetrazole and polypyridine derivatives. Inorg. Chim. Acta 2021, 516, 120169. [Google Scholar] [CrossRef]
- Eremina, J.A.; Smirnova, K.S.; Klyushova, L.S.; Berezin, A.S.; Lider, E.V. Synthesis and cytotoxicity evaluation of copper(II) complexes with polypyridines and 5-benzyltetrazole. J. Mol. Struct. 2021, 1245, 131024. [Google Scholar] [CrossRef]
- Gorbacheva, A.M.; Krutov, I.A.; Vorozhtsov, N.I.; Khrustalev, V.N.; Nenajdenko, V.G. New family of polydentate tetrazole-pyrazoline ligands prepared by the azido-Ugi reaction. Mendeleev Commun. 2021, 31, 48–50. [Google Scholar] [CrossRef]
- Voitekhovich, S.V.; Lyakhov, A.S.; Ivashkevich, L.S.; Lavrov, A.N.; Lavrenova, L.G.; Ivashkevich, O.A. Direct Synthesis and Characterization of Copper(II) 1-Phenyltetrazol-5-olates. Z. Anorg. Allg. Chem. 2021, 647, 1633–1638. [Google Scholar] [CrossRef]
- Serebryanskaya, T.V.; Lyakhov, A.S.; Ivashkevich, L.S.; Grigoriev, Y.V.; Kritchenkov, A.S.; Khrustalev, V.N.; Tskhovrebov, A.G.; Ivashkevich, O.A. Novel tetrazole PtII and PdII complexes with enhanced water solubility: Synthesis, structural characterization and evaluation of antiproliferative activity. Z. Für Krist. Cryst. Mater. 2021, 236, 23–32. [Google Scholar] [CrossRef]
- Eremina, J.A.; Ermakova, E.A.; Smirnova, K.S.; Klyushova, L.S.; Berezin, A.S.; Sukhikh, T.S.; Zubenko, A.A.; Fetisov, L.N.; Kononenko, K.N.; Lider, E.V. Cu(II), Co(II), Mn(II) complexes with 5-phenyltetrazole and polypyridyl ligands: Synthesis, characterization and evaluation of the cytotoxicity and antimicrobial activity. Polyhedron 2021, 206, 115352. [Google Scholar] [CrossRef]
- Protas, A.V.; Popova, E.A.; Suslonov, V.V.; Trifonov, R.E. Novel water soluble palladium(II) complexes featuring tetrazolylacetic acids and their esters. Polyhedron 2017, 124, 131–138. [Google Scholar] [CrossRef]
- Ermakova, E.A.; Eremina, J.A.; Smirnova, K.S.; Klyushova, L.S.; Kal’Nyi, D.B.; Sukhikh, T.S.; Zubenko, A.; Fetisov, L.; Kononenko, K.; Lider, E.V. Mixed-ligand manganese(II) complexes with 5-phenyltetrazole and polypyridine derivatives: Synthesis, crystal structures and biological activity. Results Chem. 2021, 3, 100239. [Google Scholar] [CrossRef]
- Annaraj, B.; Balakrishnan, C.; Neelakantan, M. Synthesis, structure information, DNA/BSA binding affinity and in vitro cytotoxic studies of mixed ligand copper(II) complexes containing a phenylalanine derivative and diimine co-ligands. J. Photochem. Photobiol. B Biol. 2016, 160, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, L.; Chiniforoshan, H.; Tavakol, H. New mixed ligand palladium(II) complexes based on the antiepileptic drug sodium valproate and bioactive nitrogen-donor ligands: Synthesis, structural characterization, binding interactions with DNA and BSA, in vitro cytotoxicity studies and DFT calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 141, 16–26. [Google Scholar] [CrossRef]
- Guo, X.-Y.; Zhang, J.-D.; Li, Y.-Y.; Li, X.-J.; Meng, X.-R. Synthesis, structure, and BSA binding studies of a new Co(II) complex based on 2-(1H-tetrazol-1-methyl)-1H-imidazole-4,5-dicarboxylic acid. Inorg. Chem. Commun. 2020, 119, 108055. [Google Scholar] [CrossRef]
- Yan, H.-Y.; Li, Y.-X.; Yang, H.-X.; Li, X.-J. Synthesis, molecular structure and BSA-binding properties of a new binuclear Cd(II) complex based on 2-(1H-tetrazol-1-methyl)-1H-imidazole-4,5-dicarboxylic acid. Z. Für Nat. B 2020, 75, 537–544. [Google Scholar] [CrossRef]
- Xie, W.; Li, R.; Wang, C.-Q. Crystal structure of catena-poly[triqua-bis(µ2-4-carboxy-2-(1H-tetrazol-1- yl)-1H-imidazole-5-carboxylato-k3N,O:O’)barium(II)] tetra¬hydrate, C14H14BaN12O15. Z. Kristallogr. NCS 2021, 236, 227–229. [Google Scholar]
- Huang, M.Z.; Huang, X. Trace elements and human health. Stud. Trace Elem. Health 2010, 27, 58–62. [Google Scholar]
- Thamilarasan, V.; Jayamani, A.; Sengottuvelan, N. Synthesis, molecular structure, biological properties and molecular docking studies on MnII, CoII and ZnII complexes containing bipyridine–azide ligands. Eur. J. Med. Chem. 2015, 89, 266–278. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Wu, F.-J.; Gong, Y.; Hu, C.-W.; Zhang, Y.-H.; Gan, M.-Y. Synthesis, Characterization and Activity againstStaphylococcus of Metal(II)-Gatifloxacin Complexes. Chin. J. Chem. 2007, 25, 1809–1814. [Google Scholar] [CrossRef]
- Zhou, Q.; Hambley, T.W.; Kennedy, B.J.; Lay, P.A.; Turner, P.; Warwick, B.; Biffin, J.R.; Regtop, H.L. Syntheses and Characterization of Anti-inflammatory Dinuclear and Mononuclear Zinc Indomethacin Complexes. Crystal Structures of [Zn2(Indomethacin)4(L)2] (L = N,N-Dimethylacetamide, Pyridine, 1-Methyl-2-pyrrolidinone) and [Zn(Indomethacin)2(L1)2] (L1 = Ethanol, Methanol). Inorg. Chem. 2000, 39, 3742–3748. [Google Scholar] [CrossRef]
- Zhu, L.; Peng, B.; Ling, Y.; Lin, Y.B. Interaction of complex [Co2(EGTB)Cl2]∙(BF4)2∙5H2O with DNA. Acta Chim. Sin. 2008, 66, 2705–2711. [Google Scholar]
- Kolenko, V.; Teper, E.; Kutikov, A.; Uzzo, R. Zinc and zinc transporters in prostate carcinogenesis. Nat. Rev. Urol. 2013, 10, 219–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Yi, C.; Liu, H.; Li, H.; Li, Q.; Yuan, Z.; Wei, G. Syntheses, crystal structures and in vitro anticancer activities of oxovanadium(IV) complexes of amino acid Schiff base and 1,10-phenanthroline ligands. Transit. Met. Chem. 2016, 41, 531–538. [Google Scholar] [CrossRef]
- Mu, B.; Li, Q.; Lv, L.; Yang, D.-D.; Wang, Q.; Huang, R.-D. Assembly and property research on seven 0D–3D complexes derived from imidazole dicarboxylate and 1,2-bi(pyridin-4-yl)ethene. J. Solid State Chem. 2015, 226, 1–10. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, X.; Li, T.; Meng, X. Construction of zinc–organic frameworks by flexible aliphatic dicarboxylates plus 2-(1H-imidazolyl-1-methyl)-1H-benzimidazole ligand. J. Coord. Chem. 2015, 68, 88–105. [Google Scholar] [CrossRef]
- Huang, Q.-Y.; Liu, W.; Yang, Y.; Meng, X.-R. A new one-dimensional ZnII coordination polymer based on 2-[(1H-imidazol-1-yl)methyl]-1H-benzimidazole and benzene-1,2-dicarboxylate. Acta Cryst. 2015, C71, 1017–1021. [Google Scholar]
- Yang, Y.-Q.; Su, C.-F.; Zhang, J.-D.; Yang, H.-X.; Zhang, G.-Y.; Meng, X.-R. Construction of Cd(II) complexes based on 2-(1H-imidazol-1-methyl)-1H-benzimidazole and 1,4-benzenedicarboxylate. J. Coord. Chem. 2016, 69, 3762–3775. [Google Scholar] [CrossRef]
- Yang, H.-X.; Liang, Z.; Hao, B.-L.; Meng, X.-R. Syntheses, crystal structures, and characterization of three 1D, 2D and 3D complexes based on mixed multidentate N- and O-donor ligands. J. Solid State Chem. 2014, 218, 23–31. [Google Scholar] [CrossRef]
- Shao, Z.-C.; Meng, X.-R.; Hou, H.-W. Effect of pH on the construction of CdII coordination polymers involving the 1,1′-[1,4-phenylenebis(methylene)]bis(3,5-dicarboxylatopyridinium) ligand. Acta Crystallogr. Sect. C Struct. Chem. 2019, 75, 1142–1149. [Google Scholar] [CrossRef]
- Machicote, R.G.; Pacheco, M.E.; Bruzzone, L. Binding of several benzodiazepines to bovine serum albumin: Fluorescence study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 77, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Khodarahmi, R.; Karimi, S.A.; Kooshk, M.R.A.; Ghadamia, S.A.; Ghobadic, S.; Amani, M. Comparative spectroscopic studies on drug binding characteristics and protein surface hydrophobicity of native and modified forms of bovine serum albumin: Possible relevance to change in protein structure/function upon non-enzymatic glycation. Spectrochim. Acta 2012, A89, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Huang, J.; Lin, J.; Li, Z.; Liu, F.; Jiang, Z.; Sun, Y. A study of the binding of C.I. Mordant Red 3 with bovine serum albumin using fluorescence spectroscopy. Dye. Pigment. 2009, 82, 65–70. [Google Scholar] [CrossRef]
- Samari, F.; Shamsipur, M.; Hemmateenejad, B.; Khayamian, T.; Gharaghani, S. Investigation of the interaction between amodiaquine and human serum albumin by fluorescence spectroscopy and molecular modeling. Eur. J. Med. Chem. 2012, 54, 255–263. [Google Scholar] [CrossRef]
- Naseri, A.; Hosseini, S.; Rasoulzadeh, F.; Rashidi, M.-R.; Zakery, M.; Khayamian, T. Interaction of norfloxacin with bovine serum albumin studied by different spectrometric methods; displacemen tstudies, molecular modeling and chemometrics approaches. J. Lumin. 2015, 157, 104–112. [Google Scholar] [CrossRef]
- Eswaran, R.; Bertani, R.; Sgarbossa, P.; Karuppannan, N.; Bhuvanesh, N.S.P. Synthesis, crystal structure, DNA and protein binding studies of novel binuclear Pd(II) complex of 6-methoxy-2-oxo-1,2-dihydroquinoline-3-carbaldehyde-4(N,N)-dimethylthiosemicarbazone. J. Inorg. Biochem. 2016, 155, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Icsel, C.; Yilmaz, V.T.; Aydinlik, Ş.; Aygun, M. New manganese(II), iron(II), cobalt(II), nickel(II) and copper(II) saccharinate complexes of 2,6-bis(2-benzimidazolyl)pyridine as potential anticancer agents. Eur. J. Med. Chem. 2020, 202, 112535. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Su, C.; Schulz, C.E.; Fan, Y.; Bian, Y.; Li, J. Perspectives on Ligand Properties of N-Heterocyclic Carbenes in Iron Porphyrin Complexes. Inorg. Chem. 2022, 61, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Takjoo, R.; Ramasami, P.; Rhyman, L.; Ahmadi, M.; Rudbari, H.A.; Bruno, G. Structural and theoretical studies of iron(III) and copper(II) complexes of dianion N1,N4-bis(salicylidene)-S-alkyl-thiosemicarbazide. J. Mol. Struct. 2022, 1255, 132388. [Google Scholar] [CrossRef]
- Sushila; Patra, H.A.; Dhamija, S.; Patra, M.; Pécaut, J.; Kataria, R.; Goswami, S.; Bhowmik, S.; Patra, R. Probing the structural features and magnetic behaviors in dinuclear cobalt (II) and trinuclear iron(III) complexes. Inorg. Chim. Acta 2022, 535, 120852. [Google Scholar] [CrossRef]
- El-Sayed, D.S.; HElbadawy, A.; Khalil, T.E. Rational modulation of N and O binding in Fe(III) complex formation derived from hydroxychloroquine: Synthesis, spectroscopic, computational, and docking simulation with human thrombin plasma. J. Mol. Struct. 2022, 1254, 132268. [Google Scholar] [CrossRef]
- Danilescu, O.; Bulhac, I.; Bourosh, P.N.; Croitor, L. Anion-assisted Fe(III)-coordination supramolecular systems based on 2,6-diacetylpyridine dihydrazone. Polyhedron 2022, 215, 115679. [Google Scholar] [CrossRef]
- El-Sonbati, A.; Diab, M.; Morgan, S.; Abbas, S.; Mohamed, G.G. Synthesis, theoretical study, molecular docking and biological activity of nano tridentate (E)-2-((3-hydoxyphenyl)methyl)phenol metal complexes. Inorg. Chem. Commun. 2022, 137, 109193. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed]










| 1 | 2 | |
|---|---|---|
| Empirical formula | C14H18N12O12Zn | C14H18FeN12O12 |
| Formula weight | 611.77 | 602.25 |
| Temperature, K | 293(2) | 298(2) |
| Crystal size, mm3 | 0.20 × 0.19 × 0.17 | 0.22 × 0.20 × 0.17 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/C | P21/C |
| a, Å | 9.1846(5) | 9.2443(3) |
| b, Å | 18.9797(12) | 18.8504(7) |
| c, Å | 6.9919(5) | 6.9777(3) |
| α, deg | 90 | 90 |
| β, deg | 111.887(2) | 111.5800(10) |
| γ, deg | 90 | 90 |
| Volume, Å3 | 891.8(3) | 1130.69(7) |
| Z | 2 | 2 |
| Calculated density, g cm−3 | 1.796 | 1.769 |
| Absorption coefficient, mm−1 | 1.177 | 0.758 |
| F(000), e | 624 | 616 |
| θ range for data collection, deg | 3.213–27.561 | 3.207–27.573 |
| Index ranges | −11 ≤ h ≤ 11 | −9 ≤ h ≤ 12 |
| −24 ≤ k ≤ 24 | −24 ≤ k ≤ 24 | |
| −8 ≤ l ≤ 9 | −9 ≤ l ≤ 9 | |
| Reflections collected/unique | 15196/2599 | 16235/2596 |
| Rint | 0.0225 | 0.0234 |
| Data/restraints/parameters | 2599/0/179 | 2596/0/178 |
| Final indices R1/wR2 [I > 2σ(I)] | 0.0267/0.0672 | 0.0288/0.0712 |
| Final indices R1/wR2 (all data) | 0.0307/0.0692 | 0.0339/0.0735 |
| Goodness-of-fit on (F2) | 1.056 | 1.073 |
| Δρfin (max/min), e.Å−3 | 0.299/−0.377 | 0.383/−0.277 |
| CCDC number | 2024298 | 2024304 |
| Complex 1 | Complex 2 | ||
|---|---|---|---|
| Zn(1)-O(5) | 2.1361(11) | Fe(1)-O(5) | 2.1031(11) |
| Zn(1)-O(5)#1 | 2.1361(11) | Fe(1)-O(5)#1 | 2.1032(11) |
| Zn(1)-N(1) | 2.0760(11) | Fe(1)-N(1) | 2.1407(11) |
| Zn(1)-N(1)#1 | 2.0760(11) | Fe(1)-N(1)#1 | 2.1408(11) |
| Zn(1)-O(1) | 2.1465(11) | Fe(1)-O(1) | 2.1840(10) |
| Zn(1)-O(1)#1 | 2.1465(11) | Fe(1)-O(1)#1 | 2.1840(10) |
| O(5)-Zn(1)-O(5)#1 | 180 | O(5)-Fe(1)-O(5)#1 | 180 |
| N(1)-Zn(1)-O(5) | 91.61(4) | O(5)-Fe(1)-N(1) | 90.85(4) |
| N(1)-Zn(1)-O(5)#1 | 88.39(4) | O(5)#1-Fe(1)-N(1) | 89.15(4) |
| N(1)#1-Zn(1)-O(5) | 88.39(4) | O(5)-Fe(1)-N(1)#1 | 89.15(4) |
| N(1)#1-Zn(1)-O(5)#1 | 91.61(4) | O(5)#1-Fe(1)-N(1)#1 | 90.85(4) |
| N(1)-Zn(1)-N(1)#1 | 180 | N(1)-Fe(1)-N(1)#1 | 180 |
| O(5)-Zn(1)-O(1)#1 | 88.99(4) | O(5)-Fe(1)-O(1)#1 | 88.44(4) |
| O(5)#1-Zn(1)-O(1)#1 | 91.00(4) | O(5)#1-Fe(1)-O(1)#1 | 91.56(4) |
| N(1)-Zn(1)-O(1)#1 | 100.43(4) | N(1)-Fe(1)-O(1)#1 | 102.61(4) |
| N(1)#1-Zn(1)-O(1)#1 | 79.58(4) | N(1)#1-Fe(1)-O(1)#1 | 77.39(4) |
| O(5)-Zn(1)-O(1) | 91.00(4) | O(5)-Fe(1)-O(1) | 91.56(4) |
| O(5)#1-Zn(1)-O(1) | 89.00(4) | O(5)#1-Fe(1)-O(1) | 88.44(4) |
| N(1)-Zn(1)-O(1) | 79.58(4) | N(1)-Fe(1)-O(1) | 77.39(4) |
| N(1)#1-Zn(1)-O(1) | 100.42(4) | N(1)#1-Fe(1)-O(1) | 102.61(4) |
| O(1)-Zn(1)-O(1)#1 | 180 | O(1)#1-Fe(1)-O(1) | 180 |
| D-H⋯A | d(D-H) (Å) | d(H⋯A) (Å) | d(D⋯A) (Å) | (D-H⋯A)(°) |
|---|---|---|---|---|
| Complex 1 | ||||
| O(3)-H(3)…O(2) | 0.82 | 1.67 | 2.4937(16) | 178.8 |
| N(2)-H(2A)…O(6) | 0.86 | 1.89 | 2.7094(19) | 159.7 |
| O(5)-H(5A)…O(4)#2 | 0.85 | 1.99 | 2.8061(16) | 159.8 |
| O(5)-H(5B)…O(2)#3 | 0.85 | 2.01 | 2.8319(16) | 161.6 |
| O(6)-H(6A)…N(6)#4 | 0.85 | 2.09 | 2.904(2) | 160.8 |
| O(6)-H(6B)…O(4)#5 | 0.85 | 2.14 | 2.977(2) | 169.3 |
| Complex 2 | ||||
| O(3)-H(3)…O(2) | 0.82 | 1.67 | 2.4887(16) | 179.3 |
| N(2)-H(2A)…O(6) | 0.86 | 1.89 | 2.7159(18) | 159.3 |
| O(5)-H(5A)…O(4)#2 | 0.85 | 1.95 | 2.7757(16) | 162.0 |
| O(5)-H(5B)…O(2)#3 | 0.85 | 1.97 | 2.8060(15) | 168.2 |
| O(6)-H(6A)…N(6)#4 | 0.85 | 2.09 | 2.910(2) | 161.3 |
| O(6)-H(6B)…O(4)#5 | 0.85 | 2.12 | 2.9592(19) | 168.3 |
| Complex | T/K | Ksv (L∙mol−1) | Kq (L∙mol−1∙s−1) | Kb/(L·mol−1) | n | ΔH (kJ·mol−1) | ΔS (J·mol−1·K−1) | ΔG (kJ·mol−1) |
|---|---|---|---|---|---|---|---|---|
| 1 | 298 | 1.06 × 104 | 1.06 × 1012 | 2.20 × 104 | 0.99 | −24.59 | ||
| 308 | 6.69 × 103 | 6.69 × 1011 | 4.50 × 103 | 0.97 | −98.31 | −247.38 | −22.12 | |
| 313 | 6.22 × 103 | 6.22 × 1011 | 3.56 × 103 | 0.95 | −20.88 | |||
| 2 | 298 | 2.22 × 104 | 2.22 × 1012 | 2.62 × 105 | 1.24 | −30.87 | ||
| 308 | 2.06 × 104 | 2.06 × 1012 | 5.94 × 104 | 1.11 | −108.59 | −260.80 | −28.26 | |
| 313 | 1.98 × 104 | 1.98 × 1012 | 3.26 × 104 | 1.05 | −26.96 |
| Complex | T/K | Ksv (L∙mol−1) | Kq (L∙mol−1∙s−1) | Kb/(L·mol−1) | n | ΔH (kJ·mol−1) | ΔS (J·mol−1·K−1) | ΔG (kJ·mol−1) |
|---|---|---|---|---|---|---|---|---|
| 1 | 298 | 1.04 × 104 | 1.04 × 1012 | 3.33 × 103 | 0.90 | −20.84 | ||
| 308 | 1.02 × 104 | 1.02 × 1012 | 1.43 × 103 | 0.81 | −164.05 | −480.56 | −16.04 | |
| 313 | 5.57 × 103 | 5.57 × 1011 | 9.53 × 101 | 0.59 | −11.26 | |||
| 2 | 298 | 2.78 × 104 | 2.78 × 1012 | 4.80 × 104 | 1.06 | −27.77 | ||
| 308 | 2.56 × 104 | 2.56 × 1012 | 2.51 × 104 | 0.99 | −189.87 | −543.95 | −22.33 | |
| 313 | 1.74 × 104 | 1.74 × 1012 | 7.18 × 102 | 0.64 | −19.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.-L.; Liu, G.-Y.; Wang, R.-Y.; Sun, S.-X. Synthesis and Structure Elucidation of Two Essential Metal Complexes: In-Vitro Studies of Their BSA/HSA-Binding Properties, Docking Simulations, and Anticancer Activities. Molecules 2022, 27, 1886. https://doi.org/10.3390/molecules27061886
Guo J-L, Liu G-Y, Wang R-Y, Sun S-X. Synthesis and Structure Elucidation of Two Essential Metal Complexes: In-Vitro Studies of Their BSA/HSA-Binding Properties, Docking Simulations, and Anticancer Activities. Molecules. 2022; 27(6):1886. https://doi.org/10.3390/molecules27061886
Chicago/Turabian StyleGuo, Jun-Li, Guang-Yu Liu, Rui-Ying Wang, and Shu-Xiang Sun. 2022. "Synthesis and Structure Elucidation of Two Essential Metal Complexes: In-Vitro Studies of Their BSA/HSA-Binding Properties, Docking Simulations, and Anticancer Activities" Molecules 27, no. 6: 1886. https://doi.org/10.3390/molecules27061886
APA StyleGuo, J.-L., Liu, G.-Y., Wang, R.-Y., & Sun, S.-X. (2022). Synthesis and Structure Elucidation of Two Essential Metal Complexes: In-Vitro Studies of Their BSA/HSA-Binding Properties, Docking Simulations, and Anticancer Activities. Molecules, 27(6), 1886. https://doi.org/10.3390/molecules27061886