Stable Zinc-Based Metal-Organic Framework Photocatalyst for Effective Visible-Light-Driven Hydrogen Production
Abstract
:1. Introduction
2. Results
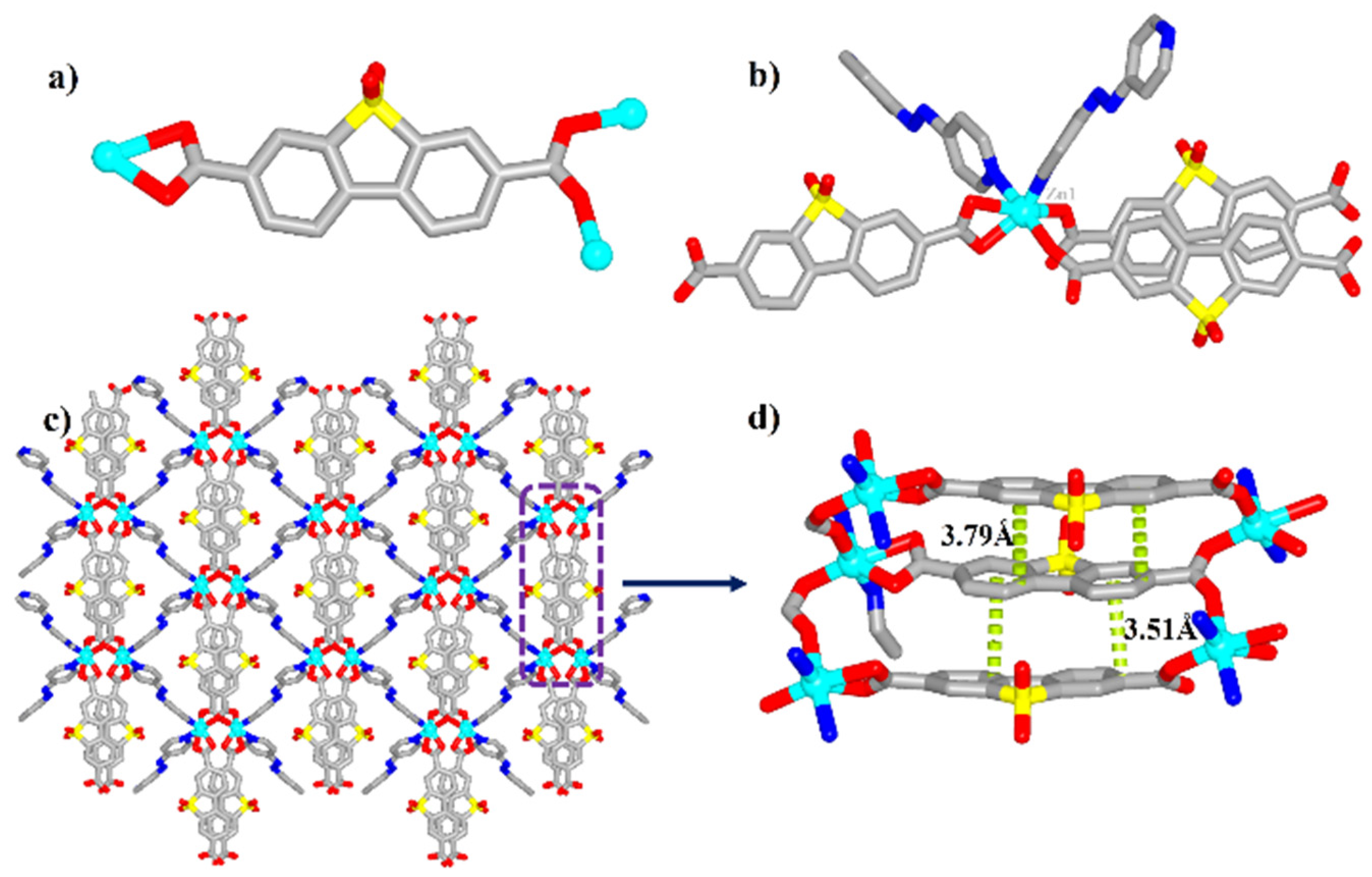
2.1. The Structure of Complex 1
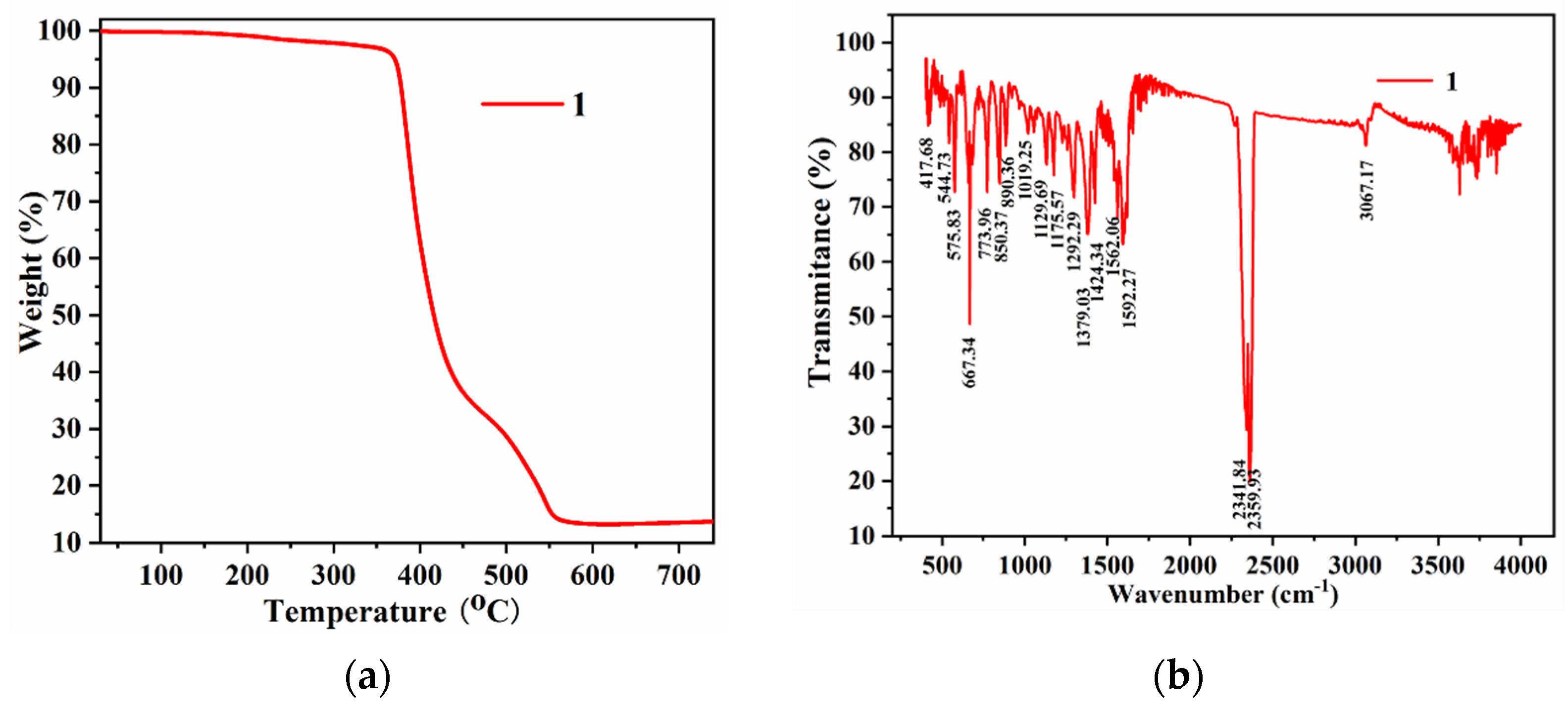
2.2. The TGA and Stability Exploration of 1
2.3. The UV-Vis Absorption and Fluorescence Emission Spectra
2.4. The Photocurrent Response Performance of Complex 1
2.5. The Visible-Light-Driven H2 Production
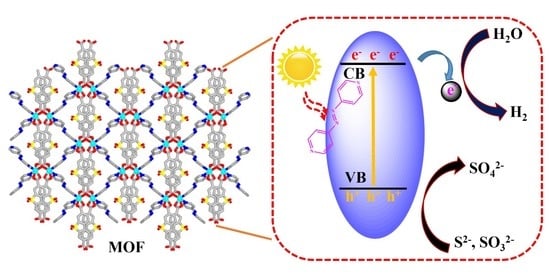
2.6. The Photocatalytic Mechanism Study of Hydrogen Production
3. Experiment
3.1. Materials and Methods
3.2. Synthesis of Complex 1
3.3. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hisatomi, T.; Kubota, J.; Domen, K. Recent Advances in Semiconductors for Photocatalytic and Photoelectrochemical Water Splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Shi, J.; Wang, Z.; Li, C. Photoelectrocatalytic Water Splitting: Significance of Cocatalysts, Electrolyte, and Interfaces. ACS Catal. 2017, 7, 675–688. [Google Scholar] [CrossRef]
- Lianos, P. Review of Recent Trends in Photoelectrocatalytic Conversion of Solar Energy to Electricity and Hydrogen. Appl. Catal. B Environ. 2017, 210, 235–254. [Google Scholar] [CrossRef]
- Gao, C.; Wang, J.; Xu, H.; Xiong, Y. Coordination Chemistry in the Design of Heterogeneous Photocatalysts. Chem. Soc. Rev. 2017, 46, 2799–2823. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Y.; Li, J.; Zhang, G. Boosting Interfacial Charge Separation of Ba5Nb4O15/g-C3N4 Photocatalysts by 2D/2D Nano-junction towards Efficient Visible-Light Driven H2 Generation. Appl. Catal. B Environ. 2020, 263, 117730. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, B.; Liu, M.; Zhang, L.; Yu, J.; Zhou, M. Direct Z-Scheme ZnO/CdS Hierarchical Photocatalyst for Enhanced Photocatalytic H2 Production Activity. Appl. Catal. B Environ. 2019, 243, 19–26. [Google Scholar] [CrossRef]
- Li, T.; Dang, L.; Zhao, C.; Lv, Z.; Yang, X.; Zhao, Y.; Zhang, S. A self-sensitized Co (II)-MOF for efficient visible-light-driven hydrogen evolution without additional cocatalysts. J. Solid State Chem. 2021, 304, 122609–122614. [Google Scholar] [CrossRef]
- Qin, J.; Xu, P.; Huang, Y.; Xiao, L.; Lu, W.; Yang, X.; Ma, L.; Zang, S. High loading of Mn(II)-metalated porphyrin in MOF for photocatalytic CO2 reduction in gas–solid condition. Chem. Commun. 2021, 57, 8468–8471. [Google Scholar] [CrossRef]
- Wang, W.; Xu, X.; Zhou, W.; Shao, Z. Recent Progress in Metal-Organic Frameworks for Applications in Electrocatalytic and Photocatalytic Water Splitting. Adv. Sci. 2017, 4, 1600371. [Google Scholar] [CrossRef]
- Zeng, L.; Guo, X.; He, C.; Duan, C. Metal-Organic Frameworks: Versatile Materials for Heterogeneous Photocatalysis. ACS Catal. 2016, 6, 7935–7947. [Google Scholar] [CrossRef]
- Qin, J.-H.; Qin, W.-J.; Xiao, Z.; Yang, J.-K.; Wang, H.-R.; Yang, X.-G.; Li, D.-S.; Ma, L.-F. Efficient energy-transfer-induced high photoelectric conversion in a dye-encapsulated ionic pyrene-based metal–organic framework. Inorg. Chem. 2021, 60, 18593–18597. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, A.; Cao, C.; Zhao, B. Applications of MOFs: Recent Advances in Photocatalytic Hydrogen Production from Water. Coord. Chem. Rev. 2019, 390, 50–75. [Google Scholar] [CrossRef]
- Zhang, T.; Jin, Y.; Shi, Y.; Li, M.; Li, J.; Duan, C. Modulating Photoelectronic Performance of Metal-Organic Frameworks for Premium Photocatalysis. Coord. Chem. Rev. 2019, 380, 201–229. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, J.; Wang, X.; Cao, R. Multifunctional Metal-Organic Framework Catalysts: Synergistic Catalysis and Tandem Reactions. Chem. Soc. Rev. 2017, 46, 126–157. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xiong, X.; Xiong, J.; Krishna, R.; Li, L.; Fan, Y.; Luo, F.; Chen, B. A robust Th-azole framework for highly efficient purification of C2H4 from a C2H4/C2H2/C2H6 mixture. Nat. Commun. 2020, 11, 3163. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Yang, C.; Dang, L.; Krishna, R.; Zhou, W.; Wu, H.; Dong, X.; Han, Y. UTSA-74: A MOF-74 isomer with two accessible binding sites per metal center for highly selective gas separation. J. Am. Chem. Soc. 2016, 138, 5678–5684. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kuppler, R.; Zhou, H. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Yang, G.; Hou, L.; Ma, L.; Wang, Y. Investigation on the prime factors influencing the formation of entangled metal–organic frameworks. CrystEngComm 2013, 15, 2561–2578. [Google Scholar] [CrossRef]
- Wu, X.; Fu, H.; Han, M.; Zhou, L.; Ma, L. Tetraphenylethylene Immobilized Metal–Organic Frameworks: Highly Sensitive Fluorescent Sensor for the Detection of Cr2O72− and Nitroaromatic Explosives. Cryst. Growth Des. 2017, 17, 6041–6048. [Google Scholar] [CrossRef]
- Han, M.; Duan, Y.; Li, D.; Xu, G.; Wu, Y.; Zhao, J. A series of divalent metal coordination polymers based on isomeric tetracarboxylic acids: Synthesis, structures and magnetic properties. Dalton Trans. 2014, 43, 17519–17527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Han, M.; Qin, J.; Wang, L.; Wang, L.; Du, M. Mn-II coordination polymers based on Bi-, Tri- and tetranuclear and polymeric chain building units: Crystal structures and magnetic properties. Inorg. Chem. 2012, 51, 9431–9442. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luo, Z.; Pan, Y.; Singh, A.; Trivedi, M.; Kumar, A. Recent developments in luminescent coordination polymers: Designing strategies, sensing application and theoretical evidences. Coord. Chem. Rev. 2020, 406, 213145. [Google Scholar] [CrossRef]
- Chang, X.H.; Qin, J.H.; Han, M.L.; Ma, L.F.; Wang, L.Y. Exploring the structural diversities and magnetic properties of copper (II) and manganese (II) complexes based on 5-methoxyisophthalate and flexible bis (imidazole) ligands. CrystEngComm 2014, 16, 870–882. [Google Scholar] [CrossRef]
- Meng, X.; Wang, H.; Song, S.; Zhang, H. Proton-Conducting Crystalline Porous Materials. Chem. Soc. Rev. 2017, 46, 464–480. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Aung, T.; Guo, N.; Weichselbaum, R.; Lin, W. Nanoscale Metal-Organic Frameworks for Therapeutic, Imaging, and Sensing Applications. Adv. Mater. 2018, 30, 1707634. [Google Scholar] [CrossRef]
- Chang, X.H.; Zhao, Y.; Han, M.L.; Ma, L.F.; Wang, L.Y. Five Cd (II) coordination polymers based on 2,3′,5,5′-biphenyltetracarboxylic acid and N-donor coligands: Syntheses, structures and fluorescent properties. CrystEngComm 2014, 16, 6417–6424. [Google Scholar] [CrossRef]
- Chang, X.H.; Qin, W.J.; Zhang, X.Y.; Jin, X.; Yang, X.G.; Dou, C.X.; Ma, L.F. Angle-Dependent Polarized Emission and Photoelectron Performance of Dye-Encapsulated Metal-Organic Framework. Inorg. Chem. 2021, 60, 10109–10113. [Google Scholar] [CrossRef]
- Sutherland, B.; Hoogland, S.; Adachi, M.; Kanjanaboos, P.; Wong, C.; McDowell, J.; Xu, J.; Voznyy, O.; Ning, Z.; Houtepen, A.; et al. Perovskite Thin Films via Atomic Layer Deposition. Adv. Mater. 2015, 27, 53–58. [Google Scholar] [CrossRef]
- Yang, X.; Zhai, Z.; Lu, X.; Qin, J.; Li, F.; Ma, L. Hexanuclear Zn (II)-Induced Dense π-Stacking in a Metal–Organic Framework Featuring Long-Lasting Room Temperature Phosphorescence. Inorg. Chem. 2020, 59, 10395–10399. [Google Scholar] [CrossRef]
- Yang, X.; Lu, X.; Zhai, Z.; Qin, J.; Chang, X.; Han, M.; Li, F.; Ma, L. π-Type halogen bonding enhanced the long-lasting room temperature phosphorescence of Zn (II) coordination polymers for photoelectron response applications. Inorg. Chem. Front. 2020, 7, 2224–2230. [Google Scholar] [CrossRef]
- Chang, X.H.; Ling, X.L.; Lu, X.M.; Yang, X.G.; Li, F.F.; Guo, Y.M. Near-infrared phosphorescence emission of three-fold interpenetrated MOF based on 1,4-bis (imidazole-1-ylmethyl) benzene: Syntheses, structure and photoelectron performance. J. Solid State Chem. 2020, 292, 121694. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, H.; Sun, P.; Huang, Y.; Shen, Q.; Yang, X.; Ma, L. Ionic liquid induced highly dense assembly of porphyrin in MOF nanosheets for photodynamic therapy. Dalton Trans. 2020, 49, 17772–17778. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Lu, X.; Yang, C.; Fan, N.; Yang, Z.; Wang, L.; Ma, L. {Zn6} cluster based metal-organic framework with enhanced room-temperature phosphorescence and optoelectronic performances. Inorg Chem. 2019, 58, 6215–6221. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.; Li, T.; Cui, Z.; Sui, D.; Ma, L.; Jin, G. Selective construction and stability studies of molecular trefoil knot and Solomon link. Dalton Trans. 2021, 50, 16984–16989. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, L.; Fan, N.; Han, M.; Yang, G.; Ma, L. Porous Zn(II)-based metal–organic frameworks decorated with carboxylate groups exhibiting high gas adsorption and separation of organic dyes. Cryst. Growth & Des. 2018, 18, 7114–7121. [Google Scholar]
- Dang, L.; Li, T.; Zhao, C.; Zhang, T.; Ye, X.; Sun, X.; Wang, H.; Ma, L. Supramolecular Rh6 catalytic system promoting directed [4 + 4] cycloaddition reaction of anthracene under UV irradiation. J. Solid State Chem. 2022, 306, 122785–122792. [Google Scholar] [CrossRef]
- Meyer, K.; Ranocchiari, M.; van Bokhoven, J.A. Metal organic frameworks for photo-catalytic water splitting. Energy Environ. Sci. 2015, 8, 1923–1937. [Google Scholar] [CrossRef]
- Lan, M.; Guo, R.M.; Dou, Y.B.; Zhou, J.; Zhou, A.; Li, J.R. Fabrication of porous Pt-doping heterojunctions by using bimetallic MOF template for photocatalytic hydrogen generation. Nano Energy. 2017, 33, 238–246. [Google Scholar] [CrossRef]
- Liao, W.-M.; Zhang, J.-H.; Wang, Z.; Yin, S.-Y.; Pan, M.; Wang, H.-P.; Su, C.-Y. Post-synthetic exchange (PSE) of UiO-67 frameworks by Ru/Rh half-sandwich units for visible-light-driven H2 evolution and CO2 reduction. J. Mater. Chem. A 2018, 6, 11337–11345. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.H.; Garcia, H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef] [PubMed]
- Zhen, W.L.; Ma, J.T.; Lu, G.X. Small-sized Ni (1 1 1) particles in metal-organic frameworks with low over-potential for visible photocatalytic hydrogen generation. Appl. Catal. B Environ. 2016, 190, 12–25. [Google Scholar] [CrossRef]
- Nasalevich, M.; Becker, R.; Ramos-Fernandez, E.; Castellanos, S.; Veber, S.; Fedin, M.; Kapteijn, F.; Reek, J.; van der Vlugt, J.; Gascon, J. Co@NH2-MIL-125 (Ti): Cobaloxime-derived metal-organic framework-based composite for light-driven H2 production. Energy Environ. Sci. 2015, 8, 364–375. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Guo, X.; Cheng, L.; He, C.; Wang, J.; Duan, C. Photoactive Metal-Organic Framework and Its Film for Light-Driven Hydrogen Production and Carbon Dioxide Reduction. Inorg. Chem. 2016, 55, 8153–8159. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chen, C.; Liu, Z.; Fei, B.; Lin, P.; Li, Q.; Sun, S.; Du, S. Application of a Ni mercaptopyrimidine MOF as highly efficient catalyst for sunlight-driven hydrogen generation. J. Mater. Chem. A 2015, 3, 7163–7169. [Google Scholar] [CrossRef]
- Liao, W.; Zhang, J.; Hou, Y.; Wang, H.; Pan, M. Visible-light-driven CO2 photo-catalytic reduction of Ru (II) and Ir (III) coordination complexes. Inorg. Chem. Commun. 2016, 73, 80–89. [Google Scholar] [CrossRef]
- Liao, W.; Zhang, J.; Wang, Z.; Lu, Y.; Yin, S.; Wang, H.; Fan, Y.; Pan, M.; Su, C. Semiconductive Amine-Functionalized Co (II)-MOF for Visible-Light-Driven Hydrogen Evolution and CO2 Reduction. Inorg. Chem. 2018, 57, 11436–11442. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Zheng, R.; Sun, M.; Cao, X.; Sun, C.; Cui, C.; Liu, C.; Zhao, J.; Du, M. Semiconductive Copper (I)-Organic Frameworks for Efficient Light-Driven Hydrogen Generation Without Additional Photosensitizers and Cocatalysts. Angew. Chem. Int. Ed. 2017, 56, 14637–14641. [Google Scholar] [CrossRef] [PubMed]
- Luo. M., J.; Wang. Y., L.; Huang, T.B.; Su, T.; Fu, D.H.; Yue, S.T.; Zeng, H.P. Application of an Mn-MOF as a highly efficient catalyst for sunlight-driven hydrogen generation. Phase Transitions. 2018, 91, 1179–1187. [Google Scholar] [CrossRef]
- Wen, M.; Mori, K.; Kamegawa, T.; Yamashita, H. Amine-functionalized MIL-101 (Cr) with imbedded platinum nanoparticles as a durable photocatalyst for hydrogen production from water. Chem. Commun. 2014, 50, 11645–11648. [Google Scholar] [CrossRef]
- Zhang, S.; Li, L.; Zhao, S.; Sun, Z.; Luo, J. Construction of interpenetrated ruthenium metal-organic frameworks as stable photocatalysts for CO2 reduction. Inorg. Chem. 2015, 54, 8375–8379. [Google Scholar] [CrossRef]
- Yang, G.; Che, X.; Hou, S.; Cao, C.; Zhao, B. Photocatalytic Hydrogen Evolution Based on Cobalt–Organic Framework with High Water Vapor Adsorption. Inorg. Chem. 2021, 60, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Jia, Y.; Li, H.; Zhao, B.; Wu, D.; Zang, S.; Hou, H.; Fan, Y. Conversion from a Heterochiral [2 + 2] Coaxially Nested Double-Helical Column to a Cationic Spiral Staircase Stimulated by an Ionic Liquid Anion. Inorg. Chem. 2014, 53, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Ma, L.; Ke, F.; Peng, F.; Xu, G.; Shen, Y.; Zhu, J.; Qiu, L.; Yuan, Y. Metal–organic frameworks MIL-88A hexagonal microrods as a new photocatalyst for efficient decolorization of methylene blue dye. Dalton Trans. 2014, 43, 3792–3798. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, C.; Sun, Y.; Chen, Q.; He, L.; Zhang, K.; Zhang, J.; Liu, B.; Chen, L. Design of metal-organic framework-based photocatalysts for hydrogen generation. Coord. Chem. Rev. 2020, 413, 213266–213283. [Google Scholar] [CrossRef]
- Toyao, T.; Saito, M.; Horiuchi, Y.; Mochizuki, K.; Iwata, M.; Higashimura, H.; Matsuoka, M. Efficient hydrogen production and photocatalytic reduction of nitrobenzene over a visible-light-responsive metal–organic framework photocatalyst. Catal. Sci. Technol. 2013, 3, 2092–2097. [Google Scholar] [CrossRef]
- Zhou, T.; Du, Y.; Borgna, A.; Hong, J.; Wang, Y.; Han, J.; Zhang, W.; Xu, R. Post-synthesis modification of a metal–organic framework to construct a bifunctional photocatalyst for hydrogen production. Energy Environ. Sci. 2013, 6, 3229–3234. [Google Scholar] [CrossRef]
- Lin, R.; Shen, L.; Ren, Z.; Wu, W.; Tan, Y.; Fu, H.; Zhang, J.; Wu, L. Enhanced photocatalytic hydrogen production activity via dual modification of MOF and reduced graphene oxide on CdS. Chem. Commun. 2014, 50, 8533–8535. [Google Scholar] [CrossRef]
- Yuan, Y.; Yin, L.; Cao, S.; Xu, G.; Li, C.; Xue, C. Improving photocatalytic hydrogen production of metal–organic framework UiO-66 octahedrons by dye-sensitization. Appl. Catal. B Environ. 2015, 572, 168–169. [Google Scholar] [CrossRef]
- Toyao, T.; Saito, M.; Dohshi, S.; Mochizuki, K.; Iwata, M.; Higashimura, H.; Horiuchi, Y.; Matsuoka, Y. Development of a Ru complex-incorporated MOF photocatalyst for hydrogen production under visible-light irradiation. Chem. Commun. 2014, 50, 6779–6781. [Google Scholar] [CrossRef]
- He, J.; Wang, J.; Chen, Y.; Zhang, J.; Duan, D.; Wang, Y.; Yan, Z. A dye-sensitized Pt@UiO-66(Zr) metal–organic framework for visible-light photocatalytic hydrogen production. Chem. Commun. 2014, 50, 7063–7066. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, J.; Liu, J.; Shi, W.; Yang, G.; Wang, G.; Cheng, P. An Efficient, Visible-Light-Driven, Hydrogen Evolution Catalyst NiS/ZnxCd1-xS Nanocrystal Derived from a Metal-Organic Framework. Angew. Chem. Int. Ed. 2018, 26, 9790–9794. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yu, S.; Jiang, H. From UV to Near-Infrared Light-Responsive Metal–Organic Framework Composites: Plasmon and Upconversion Enhanced Photocatalysis. Adv. Mater. 2018, 30, 1707377. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Shang, Q.; Wang, Y.; Jiao, L.; Yao, T.; Li, Y.; Zhang, Q.; Luo, Y.; Jiang, H. Single Pt Atoms Confined into a Metal–Organic Framework for Efficient Photocatalysis. Adv. Mater. 2018, 30, 1705112. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, J.; Jiang, H. Encapsulating a Co(II) Molecular Photocatalyst in Metal−Organic Framework for Visible-Light-Driven H2 Production: Boosting Catalytic Efficiency via Spatial Charge Separation. ACS Catal. 2016, 6, 5359–5365. [Google Scholar] [CrossRef]
- Lan, G.; Zhu, Y.; Veroneau, S.; Xu, Z.; Micheroni, D.; Lin, W. Electron Injection from Photoexcited Metal−Organic Framework Ligands to Ru2 Secondary Building Units for Visible-Light-Driven Hydrogen Evolution. J. Am. Chem. Soc. 2018, 140, 5326–5329. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Qin, J.; Xu, H.; Su, J.; Rossi, D.; Chen, Y.; Zhang, L.; Lollar, C.; Wang, Q.; Jiang, H.; et al. [Ti8Zr2O12(COO)16] Cluster: An Ideal Inorganic Building Unit for Photoactive Metal−Organic Frameworks. ACS Cent. Sci. 2018, 4, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.D.; Shang, Q.; Xiong, Y.; Zhang, Q.; Luo, Y.; Yu, S.H.; Jiang, H.L. Boosting Photocatalytic Hydrogen Production of a Metal−Organic Framework Decorated with Platinum Nanoparticles: The Platinum Location Matters. Angew. Chem. Int. Ed. 2016, 55, 9389–9393. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Phase annealing in SHELX-90: Direct methods for larger structures. Acta Crystallogr. Sect. A Found. Crystallogr. 1990, 46, 467–473. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, L.-L.; Zhang, T.-T.; Li, T.-T.; Chen, T.; Zhao, Y.; Zhao, C.-C.; Ma, L.-F. Stable Zinc-Based Metal-Organic Framework Photocatalyst for Effective Visible-Light-Driven Hydrogen Production. Molecules 2022, 27, 1917. https://doi.org/10.3390/molecules27061917
Dang L-L, Zhang T-T, Li T-T, Chen T, Zhao Y, Zhao C-C, Ma L-F. Stable Zinc-Based Metal-Organic Framework Photocatalyst for Effective Visible-Light-Driven Hydrogen Production. Molecules. 2022; 27(6):1917. https://doi.org/10.3390/molecules27061917
Chicago/Turabian StyleDang, Li-Long, Ting-Ting Zhang, Ting-Ting Li, Tian Chen, Ying Zhao, Chen-Chen Zhao, and Lu-Fang Ma. 2022. "Stable Zinc-Based Metal-Organic Framework Photocatalyst for Effective Visible-Light-Driven Hydrogen Production" Molecules 27, no. 6: 1917. https://doi.org/10.3390/molecules27061917
APA StyleDang, L.-L., Zhang, T.-T., Li, T.-T., Chen, T., Zhao, Y., Zhao, C.-C., & Ma, L.-F. (2022). Stable Zinc-Based Metal-Organic Framework Photocatalyst for Effective Visible-Light-Driven Hydrogen Production. Molecules, 27(6), 1917. https://doi.org/10.3390/molecules27061917