Experimental Investigation of the Biofunctional Properties of Nickel–Titanium Alloys Depending on the Type of Production
Abstract
:1. Introduction
2. Material and Methods
2.1. Sample Preparation
- NiTi-1—3 mL HF, 6 mL HNO3, 100 mL—etching time 30 s;
- NiTi-2—3 mL HF, 6 mL HNO3, 100 mL—etching time 30 s.
2.2. The Determination of Chemical Composition and Phase Distribution by Scanning Electron Microscopy (SEM)
- (i)
- For NiTi-1: In the central part of the sample, two segments were chosen. In the first segment, the measurement was performed at five measuring points, and in the second segment at four points. In the marginal part of the sample, measurement was performed at four points of the measuring segment.
- (ii)
- For NiTi-2: In the central part of the sample three measuring segments with three measuring points were chosen. In the marginal part there were two measuring segments, one with four and one with two measuring points.
2.3. Hardness
2.4. Immersion Testing and ICP-MS Analysis
2.5. FIB Cross-Section of the Immersed Samples
2.6. In Vitro Determination of Biocompatibility
2.7. Statistical Analysis
3. Results
3.1. Chemical Composition (SEM/EDX and SEM/XRF Analyses)
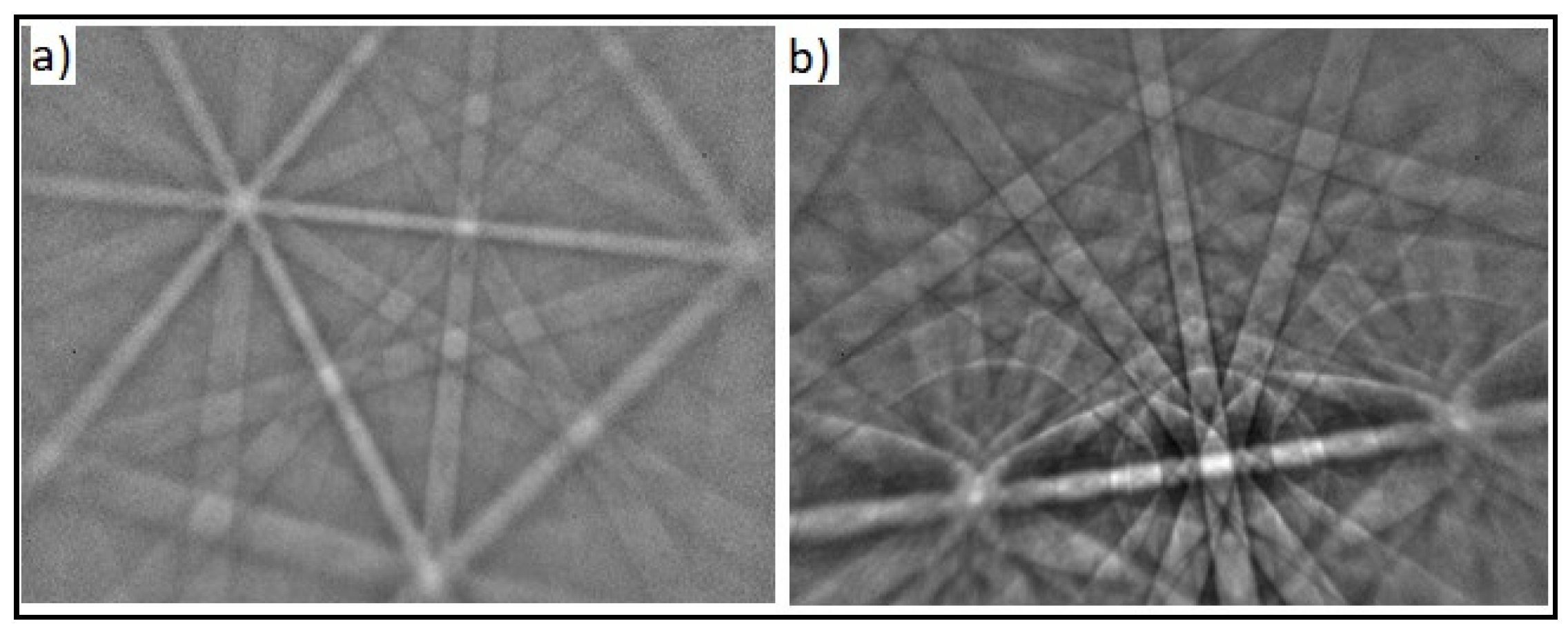
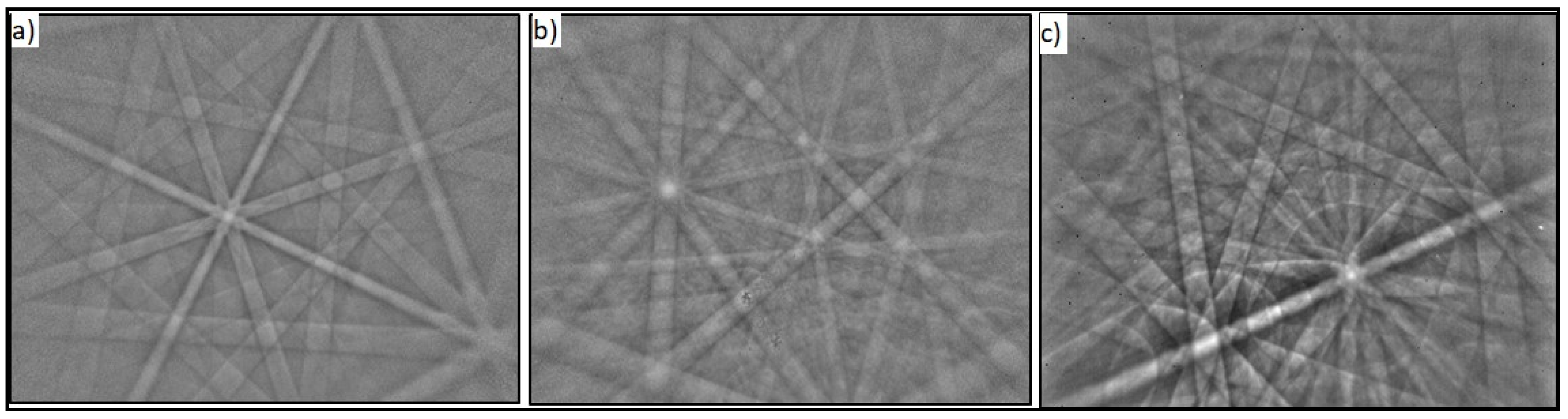
3.1.1. Phase Identification (EBSD Analysis)
3.1.2. Grain Size Measurement
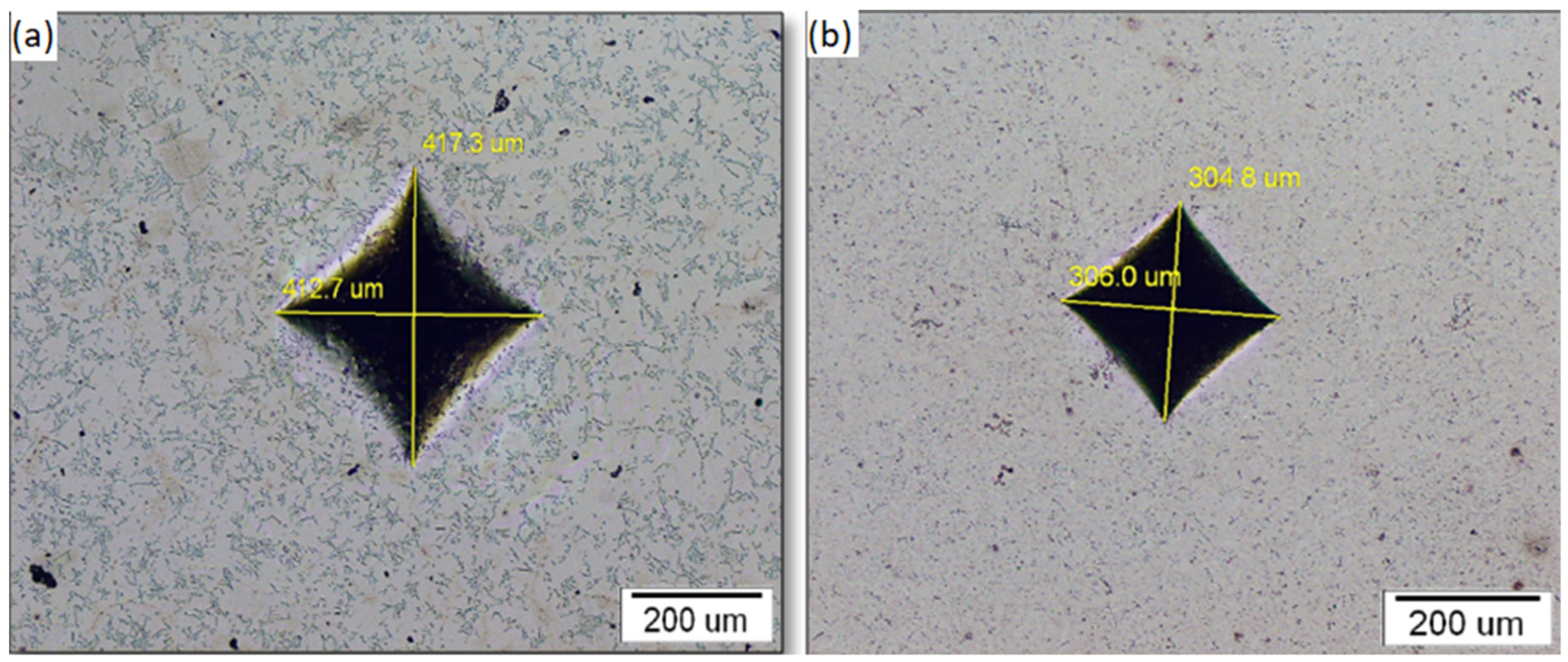
3.2. Sample Hardness
3.3. ICP Analysis of Solutions after Immersion Testing
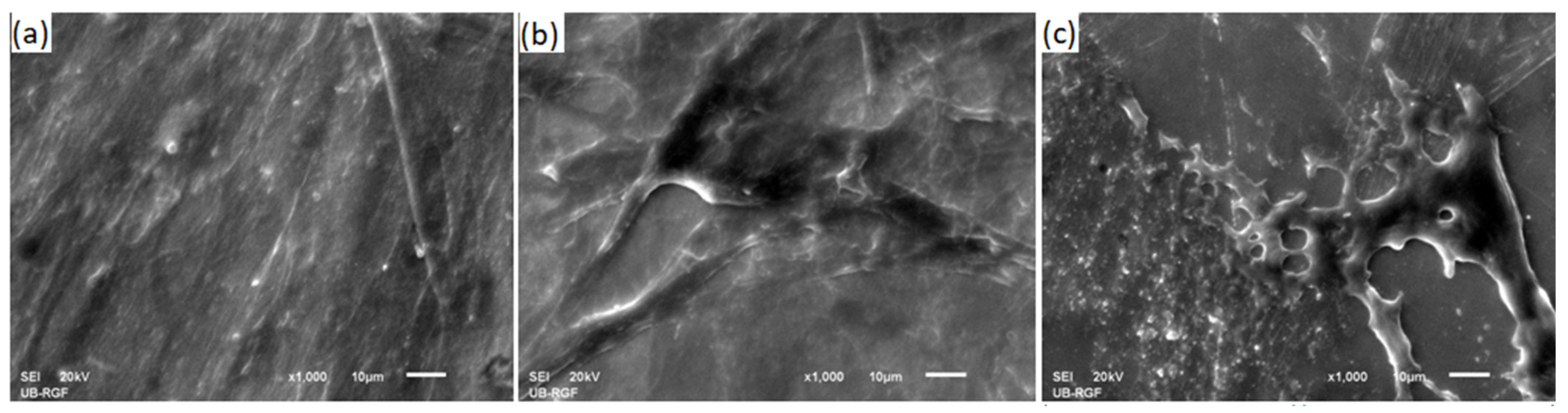
3.4. FIB Cross-Section Analysis
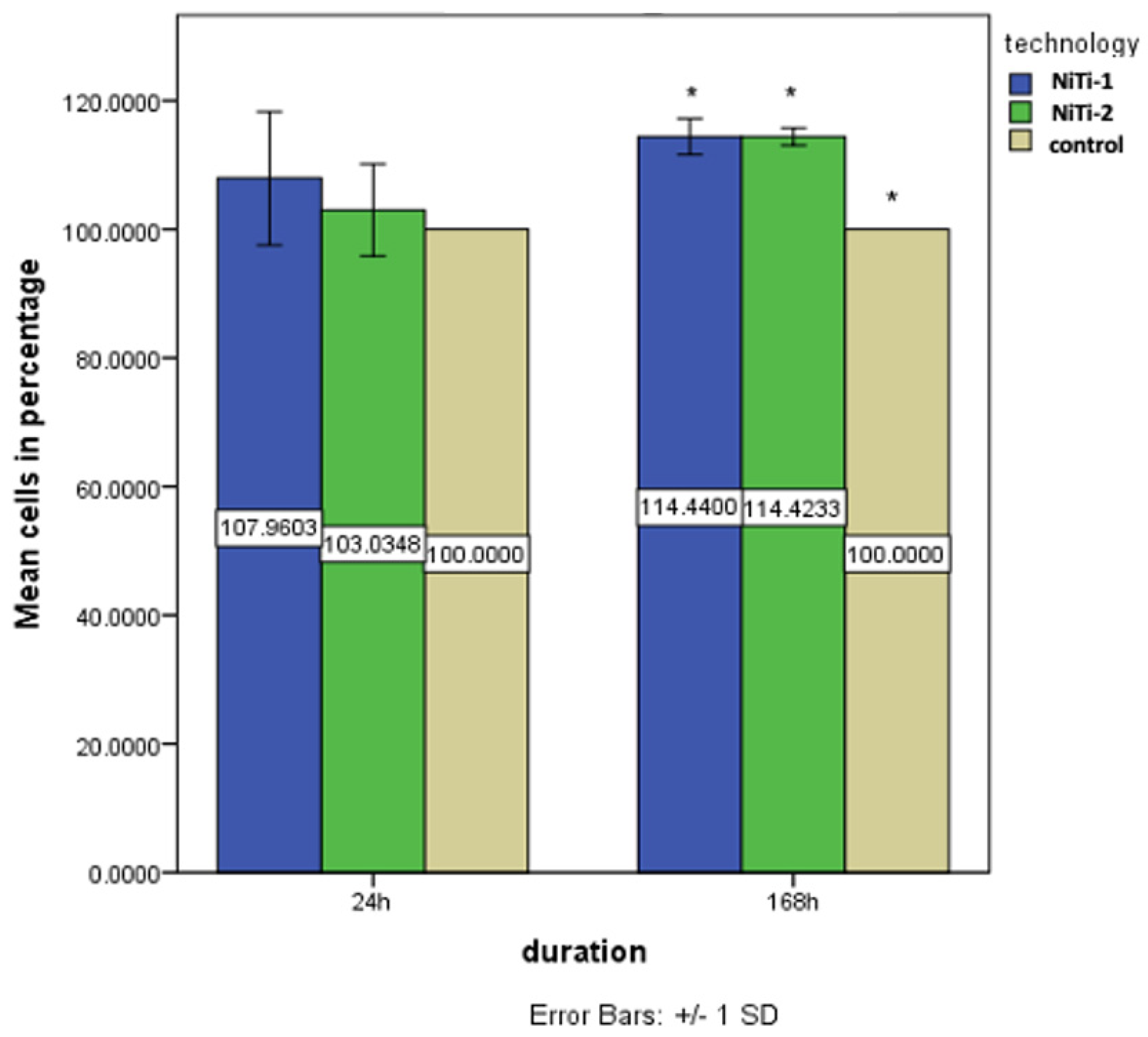
3.5. Biocompatibility Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABAM | Antibiotic-antimycotic |
| ANOVA | Analysis of variance |
| DMEM | Dulbecco′s Modified Eagle′s Medium |
| DTM | Difficult-to-machine |
| EBSD | Electron backscatter diffraction |
| EDM | Electrical discharge machining |
| EDX | Energy dispersive X-ray analysis |
| FBS | Fetal bovine serum |
| FIB | Focused ion beam |
| HGCs | Human gingival cells |
| HV | Hardness according to Vickers |
| ICP-MS | Inductively coupled plasma-mass spectrometry |
| MR | Magnetic resonance |
| MTT | Colorimetric assay for measuring cell metabolic activity |
| NiTi-1 | Commercial nitinol |
| NiTi-2 | Nickel–titanium alloy produced by continuous casting |
| SEM | Scanning electron microscope |
| SMAs | Shape memory alloys |
| TT | Transformation temperature |
References
- Kumar, P.; Lagoudas, D. Shape Memory Alloys: Modeling and Engeenering Applications, 1st ed.; Springer Nature: Basingstoke, UK, 2008; pp. 23–28. [Google Scholar]
- Arun, D.I.; Chakravarthy, P.; Arockia Kumar, R.; Santhosh, B. Shape Memory Materials, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 17–18. [Google Scholar]
- Jensen, O.T.; Jansen, C.E.; Seo, Y.; Yellich, G. Guided Nitinol-Retained (Smileloc) Single-Tooth Dental Restorations. Oral. Maxillofac. Surg. Clin. N. Am. 2019, 31, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, G.F.; Hilleman, T.B. An evaluation of 55 cobalt substituted Nitinol wire for use in orthodontics. J. Am. Dent. Assoc. 1971, 82, 1373–1375. [Google Scholar] [CrossRef] [PubMed]
- Walia, H.M.; Brantley, W.A.; Gerstein, H. An initial investigation of the bending and torsional properties of Nitinol root canal files. J. Endod. 1988, 14, 346–351. [Google Scholar] [CrossRef]
- Shah, K.C.; Chao, D.; Wu, B.M.; Jensen, O.T. Shape-Memory Retained Complete Arch Guided Implant Treatment Using Nitinol (Smileloc) Abutments. Oral. Maxillofac. Surg. Clin. N. Am. 2019, 31, 427–435. [Google Scholar] [CrossRef]
- Wadood, A. Brief Overview on Nitinol as Biomaterial. Adv. Mater. Sci. Eng. 2016, 2016, 4173138. [Google Scholar] [CrossRef] [Green Version]
- Morgan, N. Medical shape memory alloy applications—the market and its products. Mater. Sci. Eng. A 2004, 378, 16–23. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of metallic biomaterials: A review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [Green Version]
- Trepanier, C.; Venugopalan, R.; Pelton, A.R. Corrosion Resistance and Biocompatibility of Passivated NiTi. In Shape Memory Implants, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 35–45. [Google Scholar] [CrossRef]
- Shabalovskaya, S.; Anderegg, J.; Van Humbeeck, J. Critical overview of Nitinol surfaces and their modifications for medical applications. Acta Biomater. 2008, 4, 447–467. [Google Scholar] [CrossRef]
- Chavatte, L.; Juan, M.; Mounicou, S.; Leblanc Noblesse, E.; Pays, K.; Nizard, C.; Bulteau, A.L. Elemental and molecular imaging of human full thickness skin after exposure to heavy metals. Metallomics 2020, 12, 1555–1562. [Google Scholar] [CrossRef]
- Wisgrill, L.; Werner, P.; Jalonen, E.; Berger, A.; Lauerma, A.; Alenius, H.; Fyhrquist, N. Integrative transcriptome analysis deciphers mechanisms of nickel contact dermatitis. Allergy 2021, 76, 804–815. [Google Scholar] [CrossRef]
- Akçan, R.; Aydogan, H.C.; Yildirim, M.Ş.; Taştekin, B.; Sağlam, N. Nanotoxicity: A challenge for future medicine. Turk. J. Med. Sci. 2020, 50, 1180–1196. [Google Scholar] [CrossRef] [PubMed]
- Hudecki, A.; Kiryczyński, G.; Łos, M.J. Biomaterials, Definition, Overview. Stem Cells and Biomaterials for Regenerative Medicine, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 85–98. [Google Scholar] [CrossRef]
- Katić, V.; Ćurković, H.O.; Semenski, D.; Baršić, G.; Marušić, K.; Špalj, S. Influence of surface layer on mechanical and corrosion properties of nickel–titanium orthodontic wires. Angle Orthod. 2014, 84, 1041–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lojen, G.; Stambolić, A.; ŠetinaBatič, B.; Rudolf, R. Experimental Continuous Casting of Nitinol. Metals 2020, 10, 505. [Google Scholar] [CrossRef] [Green Version]
- Mehta, K.; Gupta, K. Fabrication and Processing of Shape Memory Alloys, 1st ed.; Springer: Cham, Switzerland, 2018; pp. 9–15. [Google Scholar] [CrossRef]
- Sevcikova, J.; Pavkova Goldbergova, M. Biocompatibility of NiTi alloys in the cell behaviour. Biometals 2017, 30, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Jangra, K.K.; Raj, T. Fabrication of NiTi alloy: A review. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2015, 232, 250–269. [Google Scholar] [CrossRef]
- Sharma, N.; Gupta, K.; Davim, J.P. On wire spark erosion machining induced surface integrity of Ni55.8Ti shape memory alloys. Archiv. Civ. Mech. Eng. 2019, 19, 680–693. [Google Scholar] [CrossRef]
- Manjaiah, M.; Narendranath, S.; Basavarajappa, S. A review on machining of titanium based alloys using EDM and WEDM. Rev. Adv. Mater. Sci. 2014, 36, 89–111. [Google Scholar]
- Ghafari-Nazari, A.; Mozatarzadeh, F.; Jalali, N.; Moztarzadeh, S.; Mozafari, M. Electrical discharge machining characteristics of nickel–titanium shape memory alloybased on full factorial design. J. Intell. Mater. Syst. Struct. 2013, 24, 1546–1556. [Google Scholar]
- Theisen, W.; Schuermann, A. Electro discharge machining of nickel–titanium shape memory alloys. Mater. Sci. Eng. A 2004, 378, 200–204. [Google Scholar] [CrossRef]
- Ivošević, Š.; Majerić, P.; Vukićević, M.; Rudolf, R. A study of the possible use of materials with shape memory effect in shipbuilding. J. Marit. Transp. Sci. 2020, 3, 265–278. [Google Scholar] [CrossRef]
- Kovač, N.; Ivošević, Š.; Vastag, G.; Vukelić, G.; Rudolf, R. Statistical Approach to the Analysis of the Corrosive Behaviour of NiTi Alloys under the Influence of Different Seawater Environments. Appl. Sci. 2021, 11, 8825. [Google Scholar] [CrossRef]
- Markhoff, J.; Krogull, M.; Schulze, C.; Rotsch, C.; Hunger, S.; Bader, R. Biocompatibility and Inflammatory Potential of Titanium Alloys Cultivated with Human Osteoblasts, Fibroblasts and Macrophages. Materials 2017, 10, 52. [Google Scholar] [CrossRef] [Green Version]
- Laplanche, G.; Kazuch, A.; Eggeler, G. Processing of NiTi shape memory sheets e Microstructural heterogeneity and evolution of texture. J. Alloys Compd. 2015, 651, 333–339. [Google Scholar] [CrossRef]
- Alavi, S.; Kachuie, M. Assessment of the hardness of different orthodontic wires and brackets produced by metal injection molding and conventional methods. Dent. Res. J. 2017, 14, 282–287. [Google Scholar] [CrossRef]
- Fadlallah, S.A.; El-Bagoury, N.; Gad El-Rab, S.M.F.; Ahmed, R.A.; El-Ousamii, G. An overview of NiTi shape memory alloy: Corrosion resistance and antibacterial inhibition for dental application. J. Alloys Compd. 2014, 583, 455–464. [Google Scholar] [CrossRef]
- Cioffi, M.; Gilliland, D.; Ceccone, G.; Chiesa, R.; Cigada, A. Electrochemical release testing of nickel–titanium orthodontic wires in artificial saliva using thin layer activation. Acta Biomater. 2005, 1, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Močnik, P.; Kosec, T.; Kovač, J.; Bizjak, M. The effect of pH, fluoride and tribocorrosion on the surface properties of dental archwires. Mater. Sci. Eng. C 2017, 78, 682–689. [Google Scholar] [CrossRef]
- Huang, H. Ion release from NiTi orthodontic wires in artificial saliva with various acidities. Biomaterials 2003, 24, 3585–3592. [Google Scholar] [CrossRef]
- Taqa, A.; Fathi, W.; Mohammad, R. Evaluation of Nickel Ion Release from Orthodontic Wires in Different Types of Artificial Saliva. Al–Rafidain Dent. J. 2014, 14, 182–188. [Google Scholar] [CrossRef]
- Lee, T.H.; Huang, T.K.; Lin, S.Y.; Chen, L.K.; Chou, M.Y.; Huang, H.H. Corrosion resistance of different nickel–titanium archwires in acidic fluoride-containing artificial saliva. Angle Orthod. 2010, 80, 547–553. [Google Scholar] [CrossRef] [Green Version]
- Katic, V.; Curkovic, L.; Ujevic Bosnjak, M.; Spalj, S. Determination of corrosion rate of orthodontic wires based on nickel–titanium alloy in artificial saliva. Materialwiss. Werkst. 2014, 45, 99–105. [Google Scholar] [CrossRef] [Green Version]
- RincicMlinaric, M.; Durgo, K.; Katic, V.; Spalj, S. Cytotoxicity and oxidative stress induced by nickel and titanium ions from dental alloys on cells of gastrointestinal tract. Toxicol. Appl. Pharmacol. 2019, 383, 114784. [Google Scholar] [CrossRef] [PubMed]
- Ryhänen, J. Biocompatibility Evaluation of Nickel-Titanium Shape Memory Metal Alloy. Ph.D. Thesis, University of Oulu, Oulu, Finland, 1999. Available online: http://herkules.oulu.fi/isbn9514252217/html/index.html (accessed on 16 August 2018).
- Heim, K. Nickel Allergy and EU Nickel Restriction. In Proceedings of the Nickel Institute Workshop, Brussels, Belgium, 27 June 2017; Available online: https://nickelinstitute.org/media/3861/201709-nacd-ws-report-brussels-final.pdf (accessed on 21 September 2021).

| Element (wt.%) | NiTi-1 | NiTi-2 |
|---|---|---|
| XRF: | ||
| Nickel | 55.20 | 62.50–63.60 |
| Titanium | 44.80 | 35.90 |
| Iron | Not detected | 1.40 |
| EDX: | ||
| Nickel | 47.62 | 63.2 |
| Titanium | 52.38 | 34.4 |
| Iron | Not detected | 1.53 |
| Sample | Grain Number G | Number of Grains per mm2 | Mean Number of Intersections (mm) |
|---|---|---|---|
| NiTi-1 | 5 | 256 | 0.0527 |
| NiTi-2 | 7 | 1024 | 0.0234 |
| NiTi-1 | NiTi-2 | |
|---|---|---|
| Mean | 317 | 624 |
| St. Dev. | 23 | 20 |
| Min | 289 | 586 |
| Max | 357 | 644 |
| N | 8 | 8 |
| Sample | Al | Cu | Ni | Ti | |
|---|---|---|---|---|---|
| Blank solution | 0.06 | 0.08 | <0.01 | <0.01 | |
| Artificial | Niti-1 | Not detected | Not detected | 0.05 | <0.01 |
| Saliva pH 6.5 | NiTi-2 | Not detected | Not detected | 0.04 | <0.01 |
| Blank solution | 0.05 | 0.37 | 0.02 | 0.01 | |
| Lactic | Niti-1 | 0.01 | 0.37 | 2.33 | 2.17 |
| acid pH 2.3 | NiTi-2 | 0.06 | 0.45 | 1.2 | 0.63 |
| NiTi-1 | NiTi-2 | |
|---|---|---|
| Mean | 34 | 35 |
| St. Dev. | 8 | 5 |
| Min | 27 | 25 |
| Max | 55 | 50 |
| N | 20 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miličić Lazić, M.; Majerič, P.; Lazić, V.; Milašin, J.; Jakšić, M.; Trišić, D.; Radović, K. Experimental Investigation of the Biofunctional Properties of Nickel–Titanium Alloys Depending on the Type of Production. Molecules 2022, 27, 1960. https://doi.org/10.3390/molecules27061960
Miličić Lazić M, Majerič P, Lazić V, Milašin J, Jakšić M, Trišić D, Radović K. Experimental Investigation of the Biofunctional Properties of Nickel–Titanium Alloys Depending on the Type of Production. Molecules. 2022; 27(6):1960. https://doi.org/10.3390/molecules27061960
Chicago/Turabian StyleMiličić Lazić, Minja, Peter Majerič, Vojkan Lazić, Jelena Milašin, Milica Jakšić, Dijana Trišić, and Katarina Radović. 2022. "Experimental Investigation of the Biofunctional Properties of Nickel–Titanium Alloys Depending on the Type of Production" Molecules 27, no. 6: 1960. https://doi.org/10.3390/molecules27061960






