Abstract
DNA methylation, as one of the major means of epigenesis change, makes a large difference in the spatial structure of chromatin, transposable element activity and, fundamentally, gene transcription. It has been confirmed that DNA methylation is closely related to innate immune responses. Decitabine, the most efficient available DNA methyltransferase inhibitor, has demonstrated exhilarating immune activation and antiviral effects on multiple viruses, including HIV, HBV, HCV, HPV and EHV1. This review considers the role of decitabine in regulating innate immune responses and antiviral ability. Understanding the complex transcriptional and immune regulation of decitabine could help to identify and validate therapeutic methods to reduce pathogen infection-associated morbidity, especially virus infection-induced morbidity and mortality.
1. Background
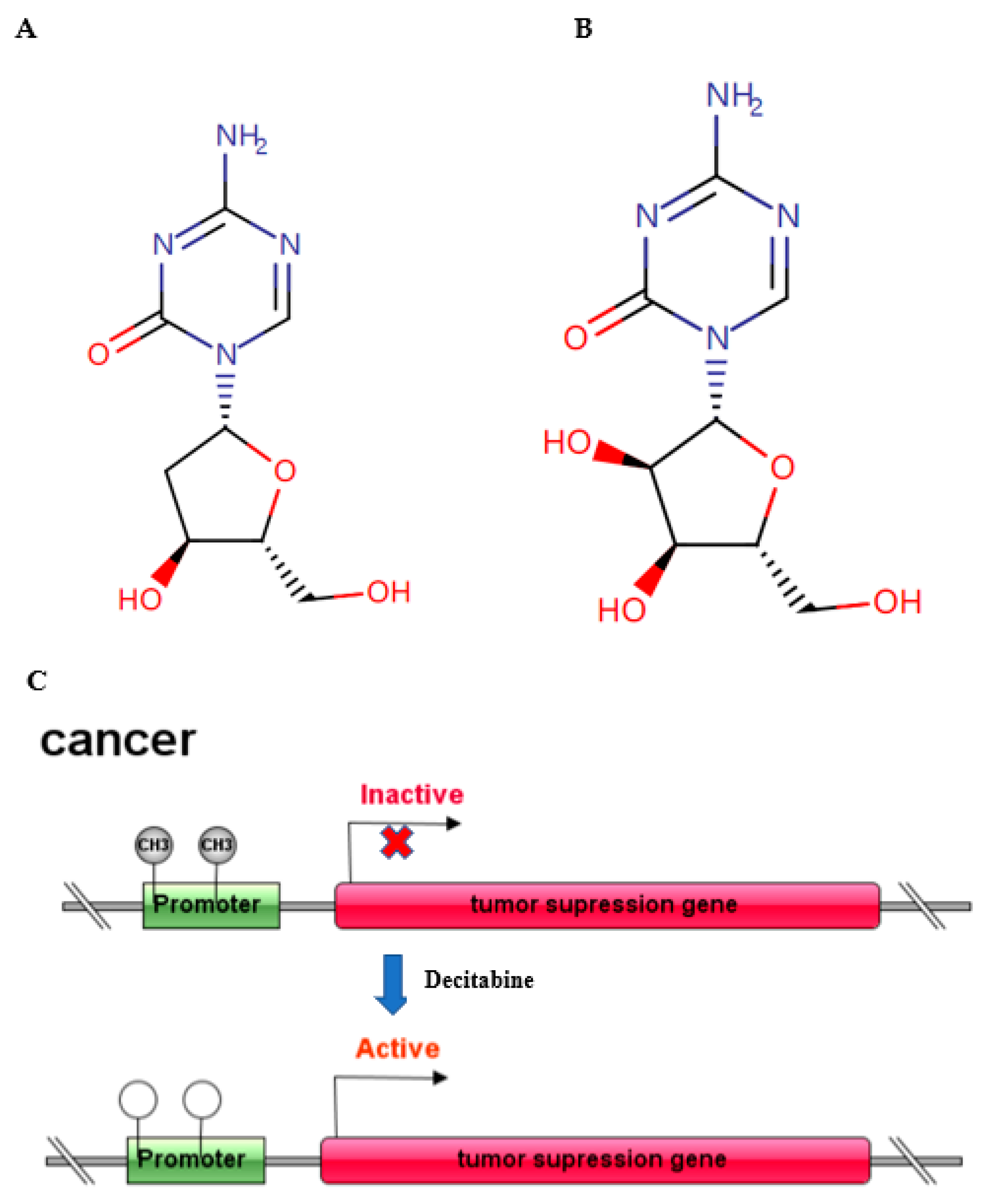
Epigenetic regulation occurs at multiple levels, including through DNA methylation, histone modification, RNA interference, nucleosome remodelling and modulation of the 3D chromatin structure, and contains almost all molecular mechanisms affecting gene expression in a reversible, transmissible, and adaptive way without altering the genomic DNA sequence [1]. These dynamic epigenetic regulations play a significant role in transcriptional regulation, genomic integrity, cell fate and physiological control of tissue and organ development. DNA methylation is established through the addition of a methyl group from S-adenosyl-methionine to the 5′ position on a cytosine within a cytosine–guanine (CG) dinucleotide. In general, CpG islands are rare in mammalian DNA with a typical expected: observed ratio of 30% or lower. In promoters with CpG islands, this ratio is 60% or higher [2]. Promoter hypermethylation is an epigenetic mechanism of gene regulation known to silence gene expression. Various factors, such as ageing, differentiation, and environmental stress, can alter DNA methylation patterns in mammalian cells [3], including immune-related cells [4]. DNA methylation is deposited and maintained through the concerted activity of three essential DNA methyltransferases, mainly DNMT1, DNMT3A and DNMT3B [5]. Mounting evidence suggests that 5-aza-2′-deoxycytidine (5-AZA-dC, decitabine, DAC) (Figure 1A), the most widely used inhibitor of DNA methyltransferases (DNMTs), induces demethylation of DNA, leading to consecutive reactivation of epigenetically silenced tumour suppressor genes mainly in practice for haematological tumours, and it is being developed for solid tumours. While exploring the antitumour effect of DAC, an array of studies has revealed that in addition to inhibiting cell proliferation, inducing cell apoptosis and regulating tumour immunity, DAC shows a crucial function in the innate immune response [6,7,8,9]. Moreover, as a nucleic acid analogue, DAC has demonstrated potential antiviral activity by upregulating innate antiviral immune responses. In 2006, decitabine (DAC) was approved by the FDA for the treatment of patients with myelodysplastic syndrome (MDS) [10]. Therefore, it is highly valuable to explore its new applications in addition to antitumour functions, especially in antiviral activity.
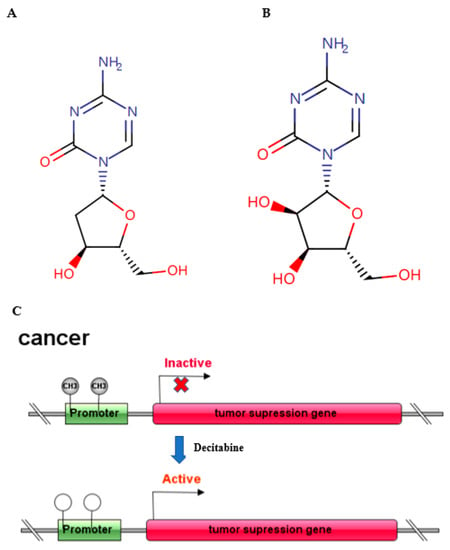
Figure 1.
Molecular structure of decitabine (A) and 5-azacytidine; (B) schematic diagram of antitumor mechanism (C).
3. Decitabine and Its Role in the Immune Modulation of Viral Diseases
In 1964, the azanucleosides 5-azacytidine (azacytidine, AZA) and 2′-deoxy-5-azacytidine (5-aza-dC, decitabine, DAC) were first synthesized as classical cytostatic agents [23] (Figure 1A,B). As nucleic acid analogues, detection results of the in vitro stability of decitabine in a neutral aqueous solution indicated considerable chemical stability (half-life time of 7 days at 4 °C, 96 h at 20 °C and 21 h at 37 °C), and even storing the solution at room temperature effectively inhibited DNA methylation. At 37 °C, the half-life times were 7 h for azacytidine and 21 h for decitabine [24]. DNMT inhibitors (DNMTis), such as 5-azacytidine (azacytidine) and decitabine, are the most frequently used epigenetic modulators employed in routine clinical practice for the treatment of malignant diseases. DAC can have direct or indirect effects on gene expression. The effect is direct when DAC incorporation into a gene significantly alters its methylation and expression status; promoter and gene body demethylation are two such examples. The effects are considered indirect when gene expression is altered without the gene itself undergoing any marked change in methylation.
As a cytosine analogue, DAC can be incorporated into DNA and trap DNA methyltransferases (DNMTs), resulting in their proteasomal degradation and global DNA demethylation [25]. As mentioned in the preceding part of the text, DNA methylation is a dynamic epigenetic modification with a prominent role in the immune system [13], indicating that decitabine, one of the most effective inhibitors of DNMTs, plays an important role in the regulation of the interferon signalling pathway. Both DAC and DNMT1 siRNA caused overall hypomethylation, and hypomethylation at the promoters of many histones and hypermethylation at multiple sites genome wide were unique to DAC treatment [8]. DAC has been shown to be a powerful inducer of human endogenous retrovirus, HERV-Fc1 in cells previously not expressing HERV-Fc1, or with a low expression level, and at the same time, it strongly inhibits methylation of DNA [26]. Transient treatment of HCT116 colorectal cancer cells with a low dose of DAC induces an increase in dsRNAs and durable DNA demethylation-independent activation of the det gene enriched for interferon-responsive genes and the MDA5/MAVS/IRF7 pathway [7]. Additionally, DAC-induced transcripts of human endogenous retroviruses (ERVs), which constitute more than 8% of the human genome, can activate interferon signalling-mediated viral defence responses in epithelial ovarian cancer (EOC) [6]. Hung-Yu Lin reported that DAC effectively induced a RIG-I-related innate immune response and apoptotic signalling primarily in SK-N-AS NB (human neuroblastoma cells) cells by hypomethylating the DDX58/RIG-I promoter, elevated mtROS and increased dsRNA [15]. These reports suggest that decitabine is a promising compound for innate immune response regulation. In addition, inflammation affects immunoregulation. Bioinformatics analysis showed upregulated DNMT1 expression and suggested upregulated NF-κB signalling pathway-related genes in patients with sepsis. Degrading intracellular DNMT protein levels by decitabine improved the inflammatory response and survival in mice with severe sepsis induced by caecal ligation and puncture (CLP) [18]. GO (gene ontology) analysis of the genes demonstrated that IKK/NF-κB cascade-related genes such as Bst2, Rnf31, Zc3hav1 and Ubd were dramatically upregulated upon inhibition of DNA methylation with 1 μM DAC on colon tumour organoids [9]. Low-dose decitabine treatment enhanced IκBα degradation and induced NF-κB activation in CD4 T cells from patients with a response to decitabine-primed chemotherapy rather than those without a response [27]. DAC also regulated the inflammatory response by the significant upregulation of p-IKKα/β, p-IκBα, p-p65, p-p38 and p-ERK in lipoteichoic acid (LTA)-stimulated human odontoblast-like cells (hOBs) [28]. A recent study showed that, in B cell lymphomas, decitabine repressed B cell-specific gene transcription and activated NF-κB signalling; during osteoclastogenesis, decitabine conversely inhibited the activity of NF-κB, AP-1 and extracellular signal-regulated kinase (ERK) but not the PI3K/Akt pathway [29]. Taken together, decitabine showed a shifting function on the NF-κB pathway, mainly regulating the inflammatory response, but showed a concentrated character on the interferon response pathway, which makes decitabine an ideal drug candidate for interferon-related diseases such as pathogen infection.
5. Conclusions and Future Prospects
As one of the DNMTs, DAC interferes with the epigenetic control of gene expression in cells by impeding DNMTs. DAC can reactivate epigenetically silenced genes and has a role in cancer chemotherapy. However, DAC is also a nucleic acid analogue that shares analogous functions with other nucleic acid antiviral drugs, such as acyclovir (ACV) [49] and ganciclovir (GCV) [50], and much more than this, DAC is able to regulate the antiviral innate immune response in various tumour cells. These results have endowed DAC with particular and promising functions in antiviral therapy regimens. In addition, DAC was approved for the treatment of myelodysplastic syndrome subtypes by the FDA in 2006 and Europe in 2009 and progressively spread to different countries worldwide [10], indicating its obvious advantages in medicinal properties. To date, DAC has been reported to have antiviral effects on HIV, hepatitis virus, EHV1, B19V, HPV16 and FeLV. These studies suggest that decitabine may share a similar function in other types of viruses, and because it is a listed drug, decitabine may be a potential drug for antiviral therapy.
In summary, DAC possesses a spectrum of antiviral activity. However, it is difficult to achieve stable pharmacokinetics with decitabine because of their rapid deamination by cytidine deaminase in vivo and spontaneous hydrolytic cleavage. With the improved understanding of the DAC mechanism of action, researchers have discovered that even nanomolar doses could achieve effective inhibition of DNA methylation while also improving tolerability [51]. Decitabine has demonstrated rapid deamination by cytidine deaminase in vivo and spontaneous hydrolytic cleavage. Developing more stable derivatives of decitabine is a demanding prompt solution. 5′-O-trialkylsilylated DACs-OR-2003 and OR-2100 were confirmed to completely deplete DNA methyltransferase 1 and induce both gene-specific and genome-wide demethylation and were comparable to that of DAC, with fewer adverse effects in vivo [52]. Guadecitabine (SGI-110), an investigational drug for the treatment of myelodysplastic syndrome and acute myeloid leukaemia, is a second-generation DNA methylation inhibitor that was designed to overcome the instability of DAC, with the potential to improve pharmacodynamics, clinical efficacy, and safety [53]. At the same time, as an antitumour drug, DAC’s main role is to inhibit cell growth and induce cell apoptosis. Therefore, high-dose and high-frequency administration has a certain toxicity and side effects. Therefore, reducing the side effects of DAC is an important development direction, and the role of guadecitabine in the field of antiviral therapy is worthy of further exploration. The effectiveness of decitabine therapy is also influenced by the relative transport capacities of the target tissue, and four different classes of proteins participate in the transportation process of nucleosides across membranes in human cells [54]. There was also a statistically significant correlation between the expression level of the equilibrative transporter ENT-1 and the sensitivity of mononuclear cells cultured in vitro from acute myelocytic leukaemia (AML) patients [55]. Therefore, it is a new direction to explore the relationship between host cell nucleotide transporter proteins such as ENT-1 and viral infection. Correspondingly, viruses have also evolved various mechanisms to evade host immunity to ensure efficient viral replication and persistence. Several viruses, such as Ebola virus (EBV), HBV, HPV and Kaposi’s sarcoma-associated herpesvirus (KSHV), can modulate host DNA methyltransferases for epigenetic dysregulation of immune-related gene expression in host cells [56]. Hypomethylation of CpG islands in the interferon regulatory factor 5 (IRF-5) promoter was observed in EBV type III latent infected Burkitt’s lymphoma and gastric carcinoma cell lines to restrain IFR5 expression [57]. Further detailed explorations are required for a more thorough understanding of the molecular mechanism of decitabine immunoregulation and feasible treatments for virus infection.
Author Contributions
J.X. participated in data analysis and drafting and editing of the manuscript. P.L. contributed to the data collection and edited the manuscript. Y.W. (Yifei Wang) and Z.R. contributed to the critical review and revision of the manuscript. All authors J.X., P.L., Y.W. (Yiliang Wang), Y.Z., Q.Z., X.H., Z.R. and Y.W. (Yifei Wang) have reviewed and supported the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by grants from the National Natural Science Foundation of China (grant no. 82072274) and Guangdong Natural Science Foundation (grant no. 2019A1515010046).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| DAC | 5-aza-2′-deoxycytidine, decitabine |
| DNMTs | DNA methyltransferases |
| MDS | myelodysplastic syndrome |
| HSV-1 | Herpes simplex virus type 1 |
| RIG-I | retinoic acid-inducible gene I |
| IRF-7 | interferon regulatory factor-7 |
| IRF-5 | interferon regulatory factor 5 |
| IFN | interferon |
| TLRs | Toll-like receptors |
| sPTL | spontaneous preterm labour |
| TNL | term not in labour |
| TL | term in labour |
| AZA | 5-azacytidine, azacytidine |
| DNMTis | DNMT inhibitors |
| ERVs | endogenous retroviruses |
| EOC | epithelial ovarian cancer |
| CLP | caecal ligation and puncture |
| GO | gene ontology |
| LTA | lipoteichoic acid |
| hOBs | stimulated human odontoblast-like cells |
| ERK | extracellular signal-regulated kinase |
| HIV | human immunodeficiency virus |
| AIDS | acquired immunodeficiency syndrome |
| MuLV | murine leukaemia virus |
| dNTP | deoxyribonucleoside triphosphate |
| HDACIs | histone deacetylase inhibitors |
| HBV | hepatitis B virus |
| ApoA1 | apolipoprotein A1 |
| CHB | chronic hepatitis B |
| HCVcc | HCV cell culture |
| E6AP | E6-associated protein |
| AcMNPV | Autographa californica nuclear polyhedrosis virus |
| B19V | human parvovirus B19 |
| EHV-1 | Equid herpesvirus-1 |
| FeLV | feline leukaemia virus |
| ACV | acyclovir |
| GCV | ganciclovir |
| AML | acute myelocytic leukaemia |
| EBV | Ebola virus |
| KSHV | herpesvirus |
References
- Mehdi, A.; Rabbani, S. Role of Methylation in Pro- and Anti-Cancer Immunity. Cancers 2021, 13, 545. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Au, W.-C.; Yeow, W.-S.; Hageman, N.; Pitha, P.M. Regulation of the Promoter Activity of Interferon Regulatory Factor-7 Gene. Activation by interferon snd silencing by hypermethylation. J. Biol. Chem. 2000, 275, 31805–31812. [Google Scholar] [CrossRef] [PubMed]
- Raddatz, G.; Hagemann, S.; Aran, D.; Söhle, J.; Kulkarni, P.P.; Kaderali, L.; Hellman, A.; Winnefeld, M.; Lyko, F. Aging is associated with highly defined epigenetic changes in the human epidermis. Epigenet. Chromatin 2013, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.D.; Conneely, K.N. The role of DNA methylation and hydroxymethylation in immunosenescence. Ageing Res. Rev. 2019, 51, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Lyko, F. The DNA methyltransferase family: A versatile toolkit for epigenetic regulation. Nat. Rev. Genet. 2018, 19, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Chiappinelli, K.B.; Strissel, P.L.; Desrichard, A.; Li, H.; Henke, C.; Akman, B.; Hein, A.; Rote, N.S.; Cope, L.M.; Snyder, A.; et al. Inhibiting DNA Methylation Causes an Interferon Response in Cancer via dsRNA Including Endogenous Retroviruses. Cell 2015, 162, 974–986. [Google Scholar] [CrossRef]
- Roulois, D.; Loo Yau, H.; Singhania, R.; Wang, Y.; Danesh, A.; Shen, S.Y.; Han, H.; Liang, G.; Jones, P.A.; Pugh, T.J.; et al. DNA-Demethylating Agents Target Colorectal Cancer Cells by Inducing Viral Mimicry by Endogenous Transcripts. Cell 2015, 162, 961–973. [Google Scholar] [CrossRef]
- Mackin, S.-J.; O’Neill, K.M.; Walsh, C.P. Comparison of DNMT1 inhibitors by methylome profiling identifies unique signature of 5-aza-2′deoxycytidine. Epigenomics 2018, 10, 1085–1101. [Google Scholar] [CrossRef]
- Saito, Y.; Nakaoka, T.; Sakai, K.; Muramatsu, T.; Toshimitsu, K.; Kimura, M.; Kanai, T.; Sato, T.; Saito, H. Inhibition of DNA Methylation Suppresses Intestinal Tumor Organoids by Inducing an Anti-Viral Response. Sci. Rep. 2016, 6, 25311. [Google Scholar] [CrossRef]
- Kaminskas, E.; Farrell, A.; Abraham, S.; Baird, A.; Hsieh, L.; Lee, S.; Leighton, J.K.; Patel, H.; Rahman, A.; Sridhara, R.; et al. Approval summary: Azacitidine for treatment of myelodysplastic syndrome subtypes. Clin. Cancer Res. 2005, 11, 3604–3608. [Google Scholar] [CrossRef]
- Saxonov, S.; Berg, P.; Brutlag, D.L. A genome-wide analysis of CpG dinucleotides in the human genome distinguishes two distinct classes of promoters. Proc. Natl. Acad. Sci. USA 2006, 103, 1412–1417. [Google Scholar] [CrossRef]
- Kimbrell, D.A.; Beutler, B. The evolution and genetics of innate immunity. Nat. Rev. Genet. 2001, 2, 256–267. [Google Scholar] [CrossRef]
- Morales-Nebreda, L.; McLafferty, F.; Singer, B. DNA methylation as a transcriptional regulator of the immune system. Transl. Res. 2019, 204, 1–18. [Google Scholar] [CrossRef]
- Gao, Z.-J.; Li, W.-P.; Mao, X.-T.; Huang, T.; Wang, H.-L.; Li, Y.-N.; Liu, B.-Q.; Zhong, J.-Y.; Renjie, C.; Jin, J. Single-nucleotide methylation specifically represses type I interferon in antiviral innate immunity. J. Exp. Med. 2021, 218, e20201798. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Chuang, J.-H.; Wang, P.-W.; Lin, T.-K.; Wu, M.-T.; Hsu, W.-M.; Chuang, H.-C. 5-aza-2′-Deoxycytidine Induces a RIG-I-Related Innate Immune Response by Modulating Mitochondria Stress in Neuroblastoma. Cells 2020, 9, 1920. [Google Scholar] [CrossRef]
- Varanasi, S.K.; Reddy, P.B.J.; Bhela, S.; Jaggi, U.; Gimenez, F.; Rouse, B.T. Azacytidine Treatment Inhibits the Progression of Herpes Stromal Keratitis by Enhancing Regulatory T Cell Function. J. Virol. 2017, 91, e02367-16. [Google Scholar] [CrossRef]
- de Cubas, A.; Dunker, W.; Zaninovich, A.; Hongo, R.; Bhatia, A.; Panda, A.; Beckermann, K.E.; Bhanot, G.; Ganesan, S.; Karijolich, J.; et al. DNA hypomethylation promotes transposable element expression and activation of immune signaling in renal cell cancer. JCI Insight 2020, 5, e137569. [Google Scholar] [CrossRef]
- Cao, L.; Zhu, T.; Lang, X.; Jia, S.; Yang, Y.; Zhu, C.; Wang, Y.; Feng, S.; Wang, C.; Zhang, P.; et al. Inhibiting DNA Methylation Improves Survival in Severe Sepsis by Regulating NF-κB Pathway. Front. Immunol. 2020, 11, 1360. [Google Scholar] [CrossRef]
- Rowles, D.L.; Tsai, Y.-C.; Greco, T.M.; Lin, A.; Li, M.; Yeh, J.; Cristea, I.M. DNA methyltransferase DNMT3A associates with viral proteins and impacts HSV-1 infection. Proteomics 2015, 15, 1968–1982. [Google Scholar] [CrossRef]
- Roulois, D.; Yau, H.L.; De Carvalho, D.D. Pharmacological DNA demethylation: Implications for cancer immunotherapy. OncoImmunology 2016, 5, e1090077. [Google Scholar] [CrossRef]
- Benakanakere, M.; Abdolhosseini, M.; Hosur, K.; Finoti, L.; Kinane, D. TLR2 Promoter Hypermethylation Creates Innate Immune Dysbiosis. J. Dent. Res. 2015, 94, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.W.; Chumble, A.A.; Washington, S.L.; Archer, K.J.; Sahingur, S.E.; Strauss, J.F. Increased expression of toll-like receptors 2 and 9 is associated with reduced DNA methylation in spontaneous preterm labor. J. Reprod. Immunol. 2017, 121, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Šorm, F.; Pískala, A.; Čihák, A.; Veselý, J. 5-Azacytidine, a new, highly effective cancerostatic. Experientia 1964, 20, 202–203. [Google Scholar] [CrossRef] [PubMed]
- Stresemann, C.; Lyko, F. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. Int. J. Cancer 2008, 123, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Hackanson, B.; Daskalakis, M. Decitabine. Recent Results Cancer Res. 2014, 201, 269–297. [Google Scholar] [PubMed]
- Laska, M.J.; Nissen, K.K.; Nexø, B.A. (Some) Cellular Mechanisms Influencing the Transcription of Human Endogenous Retrovirus, HERV-Fc1. PLoS ONE 2013, 8, e53895. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dong, L.; Liu, J.; Wang, C.; Zhang, Y.; Mei, Q.; Han, W.; Xie, P.; Nie, J. Low-Dose Decitabine Augments the Activation and Anti-Tumor Immune Response of IFN-γ CD4 T Cells Through Enhancing IκBα Degradation and NF-κB Activation. Front. Cell Dev. Biol. 2021, 9, 647713. [Google Scholar] [CrossRef]
- Feng, Z.; Meng, R.; Li, Q.; Li, D.; Xu, Q. 5-aza-2’-deoxycytidine may regulate the inflammatory response of human odontoblast-like cells through the NF-κB pathway. Int. Endod. J. 2021, 54, 1105–1117. [Google Scholar] [CrossRef]
- Guan, H.; Mi, B.; Li, Y.; Wu, W.; Tan, P.; Fang, Z.; Li, J.; Zhang, Y.; Li, F. Decitabine represses osteoclastogenesis through inhibition of RANK and NF-κB. Cell. Signal. 2015, 27, 969–977. [Google Scholar] [CrossRef]
- Kauder, S.E.; Bosque, A.; Lindqvist, A.; Planelles, V.; Verdin, E. Epigenetic Regulation of HIV-1 Latency by Cytosine Methylation. PLOS Pathog. 2009, 5, e1000495. [Google Scholar] [CrossRef]
- Bouchard, J.; Walker, M.C.; Leclerc, J.M.; Lapointe, N.; Beaulieu, R.; Thibodeau, L. 5-azacytidine and 5-azadeoxycytidine inhibit human immunodeficiency virus type 1 replication in vitro. Antimicrob. Agents Chemother. 1990, 34, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Rawson, J.M.O.; Daly, M.B.; Xie, J.; Clouser, C.L.; Landman, S.R.; Reilly, C.S.; Bonnac, L.; Kim, B.; Patterson, S.E.; Mansky, L.M. 5-Azacytidine Enhances the Mutagenesis of HIV-1 by Reduction to 5-Aza-2′-Deoxycytidine. Antimicrob. Agents Chemother. 2016, 60, 2318–2325. [Google Scholar] [CrossRef] [PubMed]
- Rawson, J.M.O.; Landman, S.R.; Reilly, C.S.; Bonnac, L.; Patterson, S.E.; Mansky, L.M. Lack of Mutational Hot Spots during Decitabine-Mediated HIV-1 Mutagenesis. Antimicrob. Agents Chemother. 2015, 59, 6834–6843. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clouser, C.L.; Chauhan, J.; Bess, M.A.; van Oploo, J.L.; Zhou, D.; Dimick-Gray, S.; Mansky, L.M.; Patterson, S.E. Anti-HIV-1 activity of resveratrol derivatives and synergistic inhibition of HIV-1 by the combination of resveratrol and decitabine. Bioorganic Med. Chem. Lett. 2012, 22, 6642–6646. [Google Scholar] [CrossRef] [PubMed]
- Clouser, C.L.; Patterson, S.E.; Mansky, L.M. Exploiting Drug Repositioning for Discovery of a Novel HIV Combination Therapy. J. Virol. 2010, 84, 9301–9309. [Google Scholar] [CrossRef] [PubMed]
- Clouser, C.L.; Holtz, C.M.; Mullett, M.; Crankshaw, D.L.; Briggs, J.E.; O’Sullivan, M.G.; Patterson, S.E.; Mansky, L.M. Activity of a Novel Combined Antiretroviral Therapy of Gemcitabine and Decitabine in a Mouse Model for HIV-1. Antimicrob. Agents Chemother. 2012, 56, 1942–1948. [Google Scholar] [CrossRef] [PubMed]
- Clouser, C.L.; Bonnac, L.; Mansky, L.M.; Patterson, S.E. Characterization of Permeability, Stability and Anti-HIV-1 Activity of Decitabine and Gemcitabine Divalerate Prodrugs. Antivir. Chem. Chemother. 2014, 23, 223–230. [Google Scholar] [CrossRef][Green Version]
- Fernandez, G.; Zeichner, S.L. Cell line-dependent variability in HIV activation employing DNMT inhibitors. Virol. J. 2010, 7, 266. [Google Scholar] [CrossRef] [PubMed]
- Bouchat, S.; Delacourt, N.; Kula, A.; Darcis, G.; Van Driessche, B.; Corazza, F.; Gatot, J.S.; Melard, A.; Vanhulle, C.; Kabeya, K.; et al. Sequential treatment with 5-aza-2’-deoxycytidine and deacetylase inhibitors reactivates HIV-1. EMBO Mol. Med. 2016, 8, 117–138. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, J.; Liu, X.; Wang, H.; Zeng, X.; Yang, J.; Li, L.; Kuang, X.; Zhang, T. The mechanism of apoliprotein A1 down-regulated by Hepatitis B virus. Lipids Health Dis. 2016, 15, 64. [Google Scholar] [CrossRef]
- Chen, C.; Pan, D.; Deng, A.-M.; Huang, F.; Sun, B.-L.; Yang, R.-G. DNA methyltransferases 1 and 3B are required for hepatitis C virus infection in cell culture. Virology 2013, 441, 57–65. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kwak, J.; Shim, J.H.; Tiwari, I.; Jang, K.L. Hepatitis C virus core protein inhibits E6AP expression via DNA methylation to escape from ubiquitin-dependent proteasomal degradation. Cancer Lett. 2016, 380, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Bach, C.; Cramer, A.; Scholtissek, C. Effect of methyltransferase inhibitors on the regulation of baculovirus protein synthesis. J. Gen. Virol. 1995, 76, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yin, J.; Huang, X.; Zhong, J. DNA methyltransferase inhibitors increase baculovirus-mediated gene expression in mammalian cells when applied before infection. Anal. Biochem. 2010, 396, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hao, S.; Zhang, J.; Chen, Z.; Wang, H.; Guan, W. The formation and modification of chromatin-like structure of human parvovirus B19 regulate viral genome replication and RNA processing. Virus Res. 2017, 232, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Thieulent, C.; Hue, E.S.; Sutton, G.; Fortier, C.; Dallemagne, P.; Zientara, S.; Munier-Lehmann, H.; Hans, A.; Paillot, R.; Vidalain, P.-O.; et al. Identification of antiviral compounds against equid herpesvirus-1 using real-time cell assay screening: Efficacy of decitabine and valganciclovir alone or in combination. Antivir. Res. 2020, 183, 104931. [Google Scholar] [CrossRef]
- Morel, A.; Baguet, A.; Perrard, J.; Demeret, C.; Jacquin, E.; Guenat, D.; Mougin, C.; Prétet, J.-L. 5azadC treatment upregulates miR-375 level and represses HPV16 E6 expression. Oncotarget 2017, 8, 46163–46176. [Google Scholar] [CrossRef]
- Greggs, W.M.; Clouser, C.L.; Patterson, S.E.; Mansky, L.M. Discovery of drugs that possess activity against feline leukemia virus. J. Gen. Virol. 2012, 93, 900–905. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Resistance of Herpes Simplex Viruses to Nucleoside Analogues: Mechanisms, Prevalence, and Management. Antimicrob. Agents Chemother. 2011, 55, 459–472. [Google Scholar] [CrossRef]
- Sadowski, L.; Upadhyay, R.; Greeley, Z.; Margulies, B. Current Drugs to Treat Infections with Herpes Simplex Viruses-1 and -2. Viruses 2021, 13, 1228. [Google Scholar] [CrossRef]
- Christman, J.K.; Mendelsohn, N.; Herzog, D.; Schneiderman, N. Effect of 5-azacytidine on differentiation and DNA methylation in human promyelocytic leukemia cells (HL-60). Cancer Res. 1983, 43, 763–769. [Google Scholar] [PubMed]
- Hattori, N.; Sako, M.; Kimura, K.; Iida, N.; Takeshima, H.; Nakata, Y.; Kono, Y.; Ushijima, T. Novel prodrugs of decitabine with greater metabolic stability and less toxicity. Clin. Epigenetics 2019, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Daher-Reyes, G.S.; Merchan, B.M.; Yee, K.W.L. Guadecitabine (SGI-110): An investigational drug for the treatment of myelodysplastic syndrome and acute myeloid leukemia. Expert Opin. Investig. Drugs 2019, 28, 835–849. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Anglada, M.; Cano-Soldado, P.; Arcas, M.M.; Lostao, M.P.; Larráyoz, I.; Martínez-Picado, J.; Casado, F.J. Cell entry and export of nucleoside analogues. Virus Res. 2005, 107, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Hubeek, I.; Stam, R.W.; Peters, G.J.; Broekhuizen, R.; Meijerink, J.P.P.; Van Wering, E.R.; Gibson, B.E.S.; Creutzig, U.; Zwaan, C.M.; Cloos, J.; et al. The human equilibrative nucleoside transporter 1 mediates in vitro cytarabine sensitivity in childhood acute myeloid leukaemia. Br. J. Cancer 2005, 93, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Kuss-Duerkop, S.K.; Westrich, J.A.; Pyeon, D. DNA Tumor Virus Regulation of Host DNA Methylation and Its Implications for Immune Evasion and Oncogenesis. Viruses 2018, 10, 82. [Google Scholar] [CrossRef]
- Dong, S.M.; Lee, H.G.; Cho, S.-G.; Kwon, S.-H.; Yoon, H.; Kwon, H.-J.; Lee, J.H.; Kim, H.; Park, P.-G.; Kim, H.; et al. Hypermethylation of the interferon regulatory factor 5 promoter in Epstein-Barr virus-associated gastric carcinoma. J. Microbiol. 2015, 53, 70–76. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).