Positive Effect of α-Asaronol on the Incidence of Post-Stroke Epilepsy for Rat with Cerebral Ischemia-Reperfusion Injury
Abstract
:1. Introduction
2. Results and Discussion
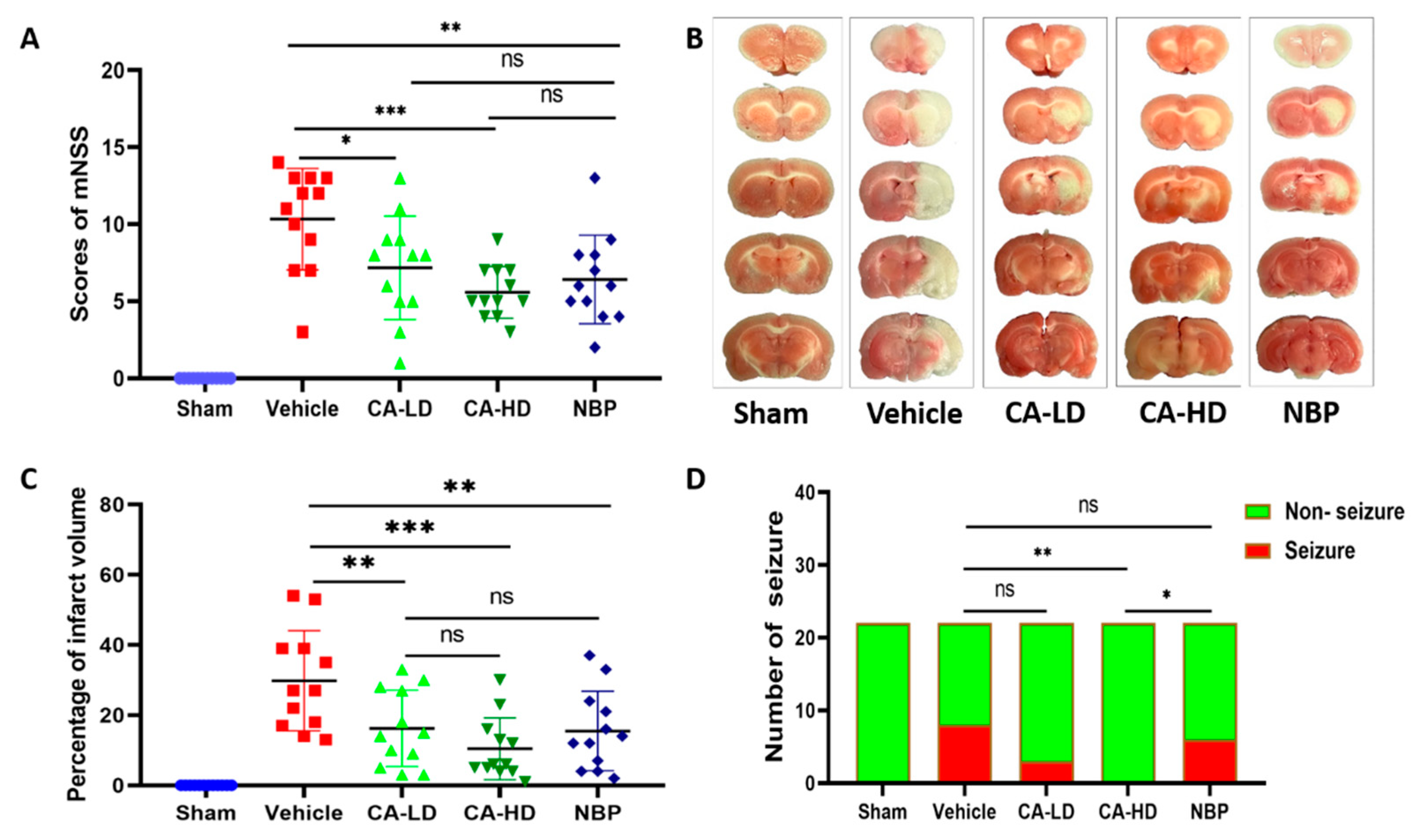
2.1. The Effect of ASOL on mNSS
2.2. The Effect of ASOL on Infarct Volume
2.3. The Effect of ASOL on the Incidence of Post-Stroke Epilepsy
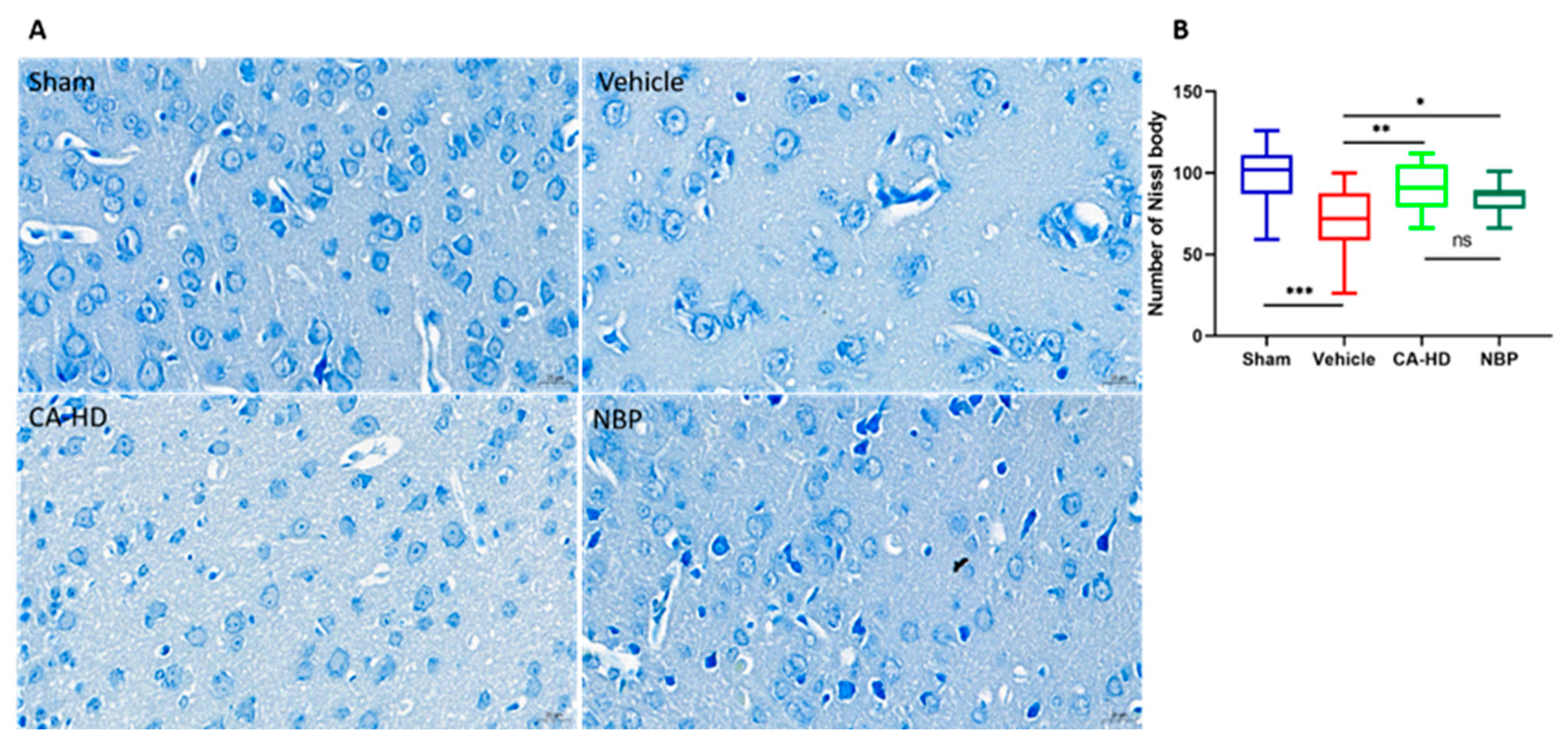
2.4. The Effect of ASOL on the Results of Nissl Staining
2.5. The Effect of ASOL on the Results of TUNEL Staining
2.6. Immunohistochemical Analysis of Caspase-3 Expression
3. Conclusions
4. Materials and Methods
4.1. Drug
4.2. Animals
4.3. Middle Cerebral Artery Occlusion (MCAO) and Treatments
4.4. Evaluation of the Neurological Deficits
4.5. Determination of Infarct Volume
4.6. Evaluation of Post-Stroke Epilepsy
4.7. Brain Tissue Preparation
4.8. Nissl Staining
4.9. TUNEL Staining
4.10. Caspase-3 Activity Determination
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Bian, D.; Yu, J.; Li, M.; Zhao, D. Using machine learning models to improve stroke risk level classification methods of China national stroke screening. BMC Med. Inform. Decis. Mak. 2019, 19, 261. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Li, T.; Kury, L.T.A.; Zeb, A.; Khatoon, S.; Liu, G.; Yang, X.; Liu, F.; Yao, H.; Khan, A.U.; et al. Pathological Comparisons of the Hippocampal Changes in the Transient and Permanent Middle Cerebral Artery Occlusion Rat Models. Front. Neurol. 2019, 10, 1178. [Google Scholar] [CrossRef] [PubMed]
- Guekht, A.; Bornstein, N.M. Seizures after stroke. Handb. Clin. Neurol. 2012, 108, 569–583. [Google Scholar] [CrossRef]
- Xie, W.J.; Dong, M.; Liu, Q.; Meng, H.M. Early predictors and prevention for post-stroke epilepsy: Changes in neurotransmitter levels. Transl. Neurosci. 2016, 7, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.; Zhang, K.; Tong, T.; Cui, R. The Progress of Epilepsy after Stroke. Curr. Neuropharmacol. 2018, 16, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Bladin, C.F.; Alexandrov, A.V.; Bellavance, A.; Bornstein, N.; Chambers, B.; Coté, R.; Lebrun, L.; Pirisi, A.; Norris, J.W. Seizures after stroke: A prospective multicenter study. Arch. Neurol. 2000, 57, 1617–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velioğlu, S.K.; Ozmenoğlu, M.; Boz, C.; Alioğlu, Z. Status epilepticus after stroke. Stroke 2001, 32, 1169–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waterhouse, E.J.; Vaughan, J.K.; Barnes, T.Y.; Boggs, J.G.; Towne, A.R.; Kopec-Garnett, L.; DeLorenzo, R.J. Synergistic effect of status epilepticus and ischemic brain injury on mortality. Epilepsy Res. 1998, 29, 175–183. [Google Scholar] [CrossRef]
- Holtkamp, M.; Beghi, E.; Benninger, F.; Kälviäinen, R.; Rocamora, R.; Christensen, H. European Stroke Organisation guidelines for the management of post-stroke seizures and epilepsy. Eur. Stroke J. 2017, 2, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Morgenstern, L.B.; Hemphill, J.C.; Anderson, C.; Becker, K.; Broderick, J.P.; Connolly, E.S.; Greenberg, S.M.; Huang, J.N.; MacDonald, R.L.; Messé, S.R.; et al. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2010, 41, 2108–2129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naidech, A.M.; Garg, R.K.; Liebling, S.; Levasseur, K.; Macken, M.P.; Schuele, S.U.; Batjer, H.H. Anticonvulsant use and outcomes after intracerebral hemorrhage. Stroke 2009, 40, 3810–3815. [Google Scholar] [CrossRef] [PubMed]
- Temkin, N.R.; Dikmen, S.S.; Anderson, G.D.; Wilensky, A.J.; Holmes, M.D.; Cohen, W.; Newell, D.W.; Nelson, P.; Awan, A.; Winn, H.R. Valproate therapy for prevention of posttraumatic seizures: A randomized trial. J. Neurosurg. 1999, 91, 593–600. [Google Scholar] [CrossRef] [PubMed]
- He, L.Y.; Hu, M.B.; Li, R.L.; Zhao, R.; Fan, L.H.; Wang, L.; Peng, W.; Liu, Y.J.; Wu, C.J. The Effect of Protein-Rich Extract from Bombyx Batryticatus against Glutamate-Damaged PC12 Cells Via Regulating γ-Aminobutyric Acid Signaling Pathway. Molecules 2020, 25, 553. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.T.; Shi, X.J.; Yuan, Y.X.; Qiu, Y.Y.; Zou, Y.; Liu, C.; Yu, H.; He, X.; Xu, K.; Yin, P.H. Bufalin reverses ABCB1-mediated drug resistance in colorectal cancer. Oncotarget 2017, 8, 48012–48026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Wu, Z.M.; Yang, Y.P.; Shaukat, A.; Yang, J.; Guo, Y.F.; Zhang, T.; Zhu, X.Y.; Qiu, J.X.; Deng, G.Z.; et al. Catalpol ameliorates LPS-induced endometritis by inhibiting inflammation and TLR4/NF-κB signaling. J. Zhejiang Univ. Sci. B 2019, 20, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Li, W.G.; Zhang, X.B.; Wang, L.; Xu, T.L.; Wu, D.; Li, Y. α-asarone from Acorus gramineus alleviates epilepsy by modulating A-type GABA receptors. Neuropharmacology 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Levinson, S.R.; Sun, L.; Heinbockel, T. Identification of both GABAA receptors and voltage-activated Na (+) channels as molecular targets of anticonvulsant α-asarone. Front. Pharmacol. 2014, 5, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cartus, A.T.; Schrenk, D. Metabolism of the carcinogen alpha-asarone in liver microsomes. Food Chem. Toxicol. 2016, 87, 103–112. [Google Scholar] [CrossRef]
- He, X.; Bai, Y.; Zeng, M.; Zhao, Z.; Zhang, Q.; Xu, N.; Qin, F.; Wei, X.; Zhao, M.; Wu, N.; et al. Anticonvulsant activities of α-asaronol ((E)-3’-hydroxyasarone), an active constituent derived from α-asarone. Pharmacol. Rep. 2018, 70, 69–74. [Google Scholar] [CrossRef]
- Han, Q.Y.; Zhang, H.; Zhang, X.; He, D.S.; Wang, S.W.; Cao, X.; Dai, Y.T.; Xu, Y.; Han, L.-J. dl-3-n-butylphthalide preserves white matter integrity and alleviates cognitive impairment in mice with chronic cerebral hypoperfusion. CNS Neurosci. Ther. 2019, 25, 1042–1053. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Lu, W.; Zhu, L.; Zeng, L.; Shi, C.; Jing, Z.; Xiang, Y.; Li, W.; Tsang, C.K.; Ruan, Y.; et al. Dl-3-n-Butylphthalide Treatment Enhances Hemodynamics and Ameliorates Memory Deficits in Rats with Chronic Cerebral Hypoperfusion. Front. Aging Neurosci. 2017, 9, 238. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Li, G.; Zhao, D.; Yang, P.; Shabier, T.; Tuerxun, T. Association between IL-1β and recurrence after the first epileptic seizure in ischemic stroke patients. Sci. Rep. 2020, 10, 13505. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Rajah, G.; Guo, A.; Wang, Y.; Wang, Q. Pathogenesis of epileptic seizures and epilepsy after stroke. Neurol. Res. 2018, 40, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, B.; Lv, X.; Liu, S.; Liu, Y.; Tang, R.; Lang, Y.; Huang, Q.; He, J. Low-Intensity Focused Ultrasound-Mediated Attenuation of Acute Seizure Activity Based on EEG Brain Functional Connectivity. Brain Sci. 2021, 11, 711. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, G.t. The novel squamosamide derivative FLZ enhances BDNF/TrkB/CREB signaling and inhibits neuronal apoptosis in APP/PS1 mice. Acta Pharmacol. Sin. 2010, 31, 265–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, W.S.; Lin, I.H.; Feng, K.M.; Chang, Z.Y.; Huang, Y.C.; Lu, D.W. Neuroprotective effects of exogenous erythropoietin in Wistar rats by downregulating apoptotic factors to attenuate N-methyl-D-aspartate-mediated retinal ganglion cells death. PLoS ONE 2020, 15, e0223208. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, D.W.; Ali, A.; Thornberry, N.A.; Vaillancourt, J.P.; Ding, C.K.; Gallant, M.; Gareau, Y.; Griffin, P.R.; Labelle, M.; Lazebnik, Y.A. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 1995, 376, 37–43. [Google Scholar] [CrossRef]
- Krajewska, M.; Wang, H.G.; Krajewski, S.; Zapata, J.M.; Shabaik, A.; Gascoyne, R.; Reed, J.C. Immunohistochemical analysis of in vivo patterns of expression of CPP32 (Caspase-3), a cell death protease. Cancer Res. 1997, 57, 1605–1613. [Google Scholar] [PubMed]
- Sakurai, M.; Nagata, T.; Abe, K.; Horinouchi, T.; Itoyama, Y.; Tabayashi, K. Survival and death-promoting events after transient spinal cord ischemia in rabbits: Induction of Akt and caspase3 in motor neurons. J. Thorac. Cardiovasc. Surg. 2003, 125, 370–377. [Google Scholar] [CrossRef] [Green Version]
- Esposito, E.; Li, W.; Mandeville, E.T.; Park, J.H.; Şencan, I.; Guo, S.; Shi, J.; Lan, J.; Lee, J.; Hayakawa, K.; et al. Potential circadian effects on translational failure for neuroprotection. Nature 2020, 582, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Li, Y.; Wang, L.; Zhang, Z.; Lu, D.; Lu, M.; Chopp, M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 2001, 32, 1005–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khamse, S.; Sadr, S.S.; Roghani, M.; Hasanzadeh, G.; Mohammadian, M. Rosmarinic acid exerts a neuroprotective effect in the kainate rat model of temporal lobe epilepsy: Underlying mechanisms. Pharm. Biol. 2015, 53, 1818–1825. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Hu, X. Positive Effect of α-Asaronol on the Incidence of Post-Stroke Epilepsy for Rat with Cerebral Ischemia-Reperfusion Injury. Molecules 2022, 27, 1984. https://doi.org/10.3390/molecules27061984
Jiang L, Hu X. Positive Effect of α-Asaronol on the Incidence of Post-Stroke Epilepsy for Rat with Cerebral Ischemia-Reperfusion Injury. Molecules. 2022; 27(6):1984. https://doi.org/10.3390/molecules27061984
Chicago/Turabian StyleJiang, Lan, and Xiangnan Hu. 2022. "Positive Effect of α-Asaronol on the Incidence of Post-Stroke Epilepsy for Rat with Cerebral Ischemia-Reperfusion Injury" Molecules 27, no. 6: 1984. https://doi.org/10.3390/molecules27061984
APA StyleJiang, L., & Hu, X. (2022). Positive Effect of α-Asaronol on the Incidence of Post-Stroke Epilepsy for Rat with Cerebral Ischemia-Reperfusion Injury. Molecules, 27(6), 1984. https://doi.org/10.3390/molecules27061984





