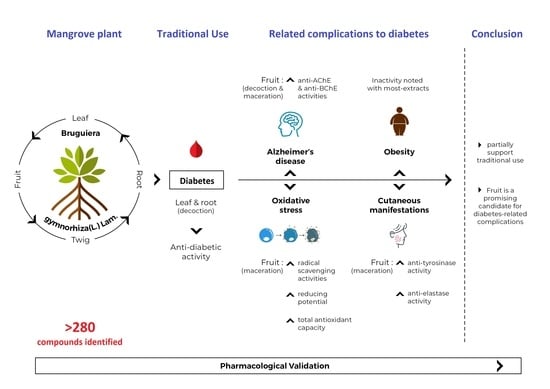
Towards the Pharmacological Validation and Phytochemical Profiling of the Decoction and Maceration of Bruguiera gymnorhiza (L.) Lam.—A Traditionally Used Medicinal Halophyte
Abstract
1. Introduction
2. Results
2.1. Bioactive Compounds
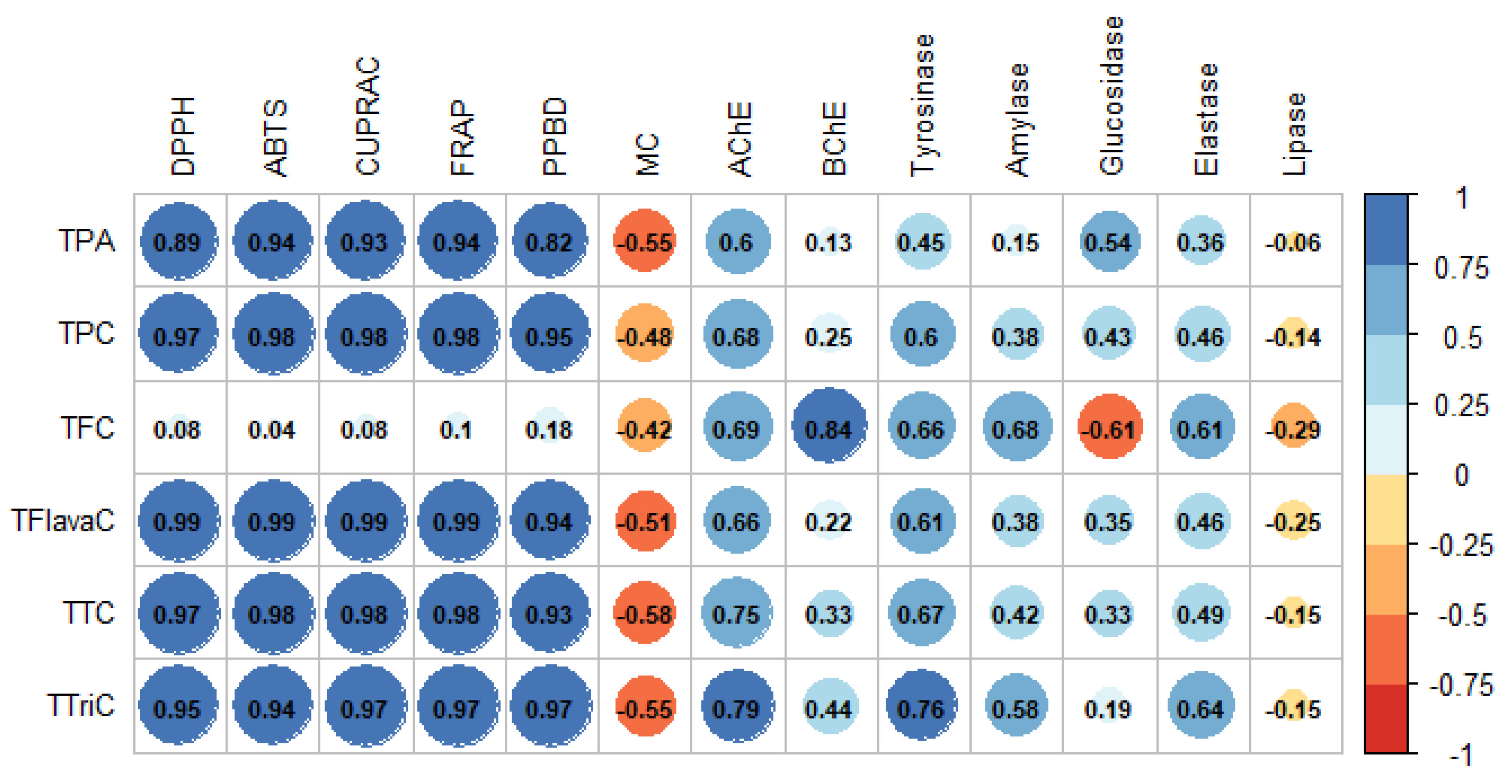
2.2. Antioxidant Assays
2.3. Enzymatic Inhibitory Effects
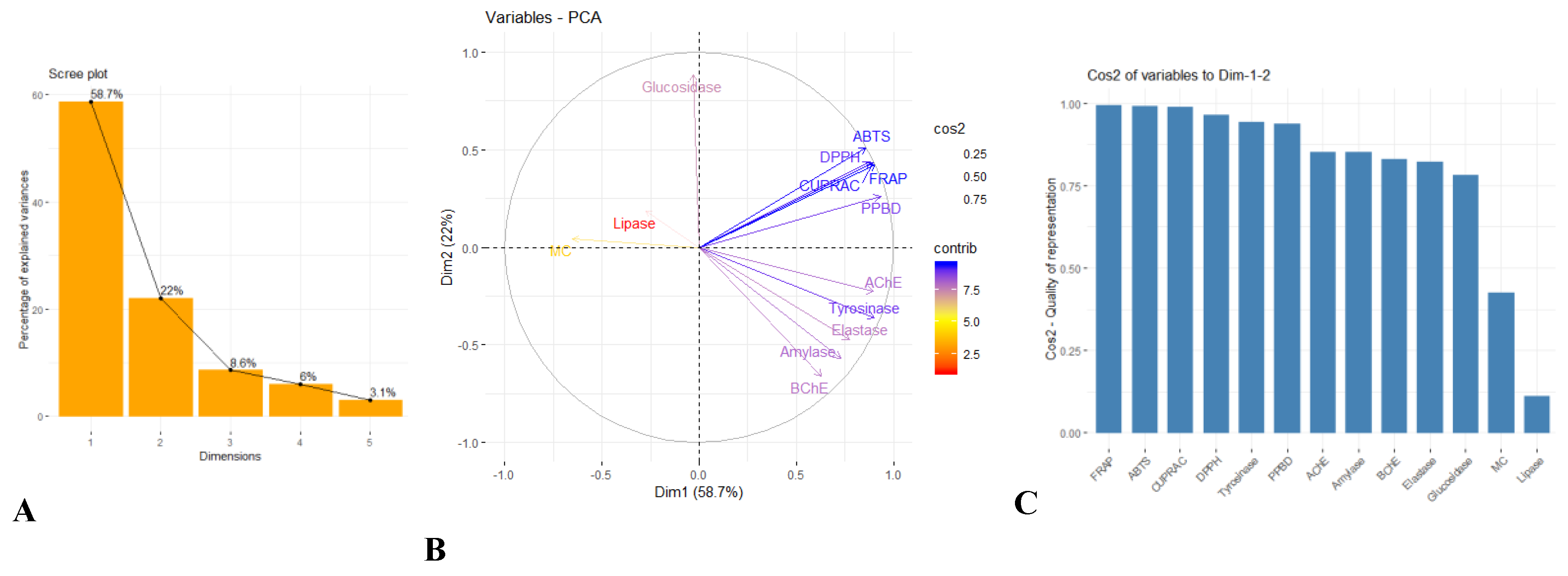
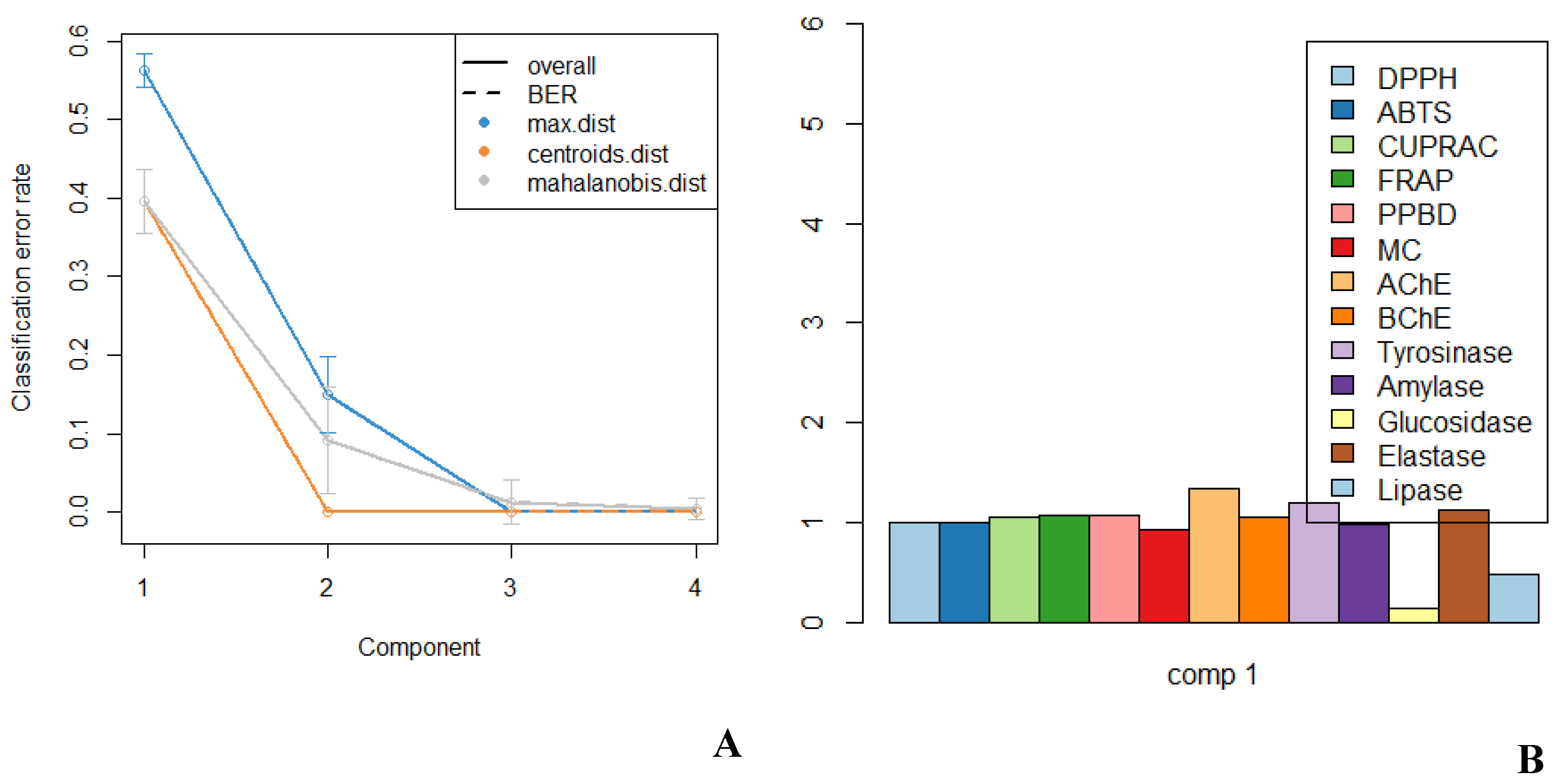
2.4. Multivariate Analysis
2.5. In Silico Docking
3. Materials and Methods
3.1. Collection of Plant Materials
3.2. Extraction
3.3. Profile of Bioactive Compounds
3.4. Determination of Antioxidant and Enzyme Inhibitory Effects
3.5. Statistical Analyses
3.6. In Silico Docking Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mahomoodally, M.F.; Mootoosamy, A.; Wambugu, S. Traditional therapies used to manage diabetes and related complications in Mauritius: A comparative ethnoreligious study. Evid.-Based Complement. Altern. Med. 2016, 2016, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Suroowan, S.; Pynee, K.B.; Mahomoodally, M.F. A comprehensive review of ethnopharmacologically important medicinal plant species from Mauritius. South. Afr. J. Bot. 2019, 122, 189–213. [Google Scholar] [CrossRef]
- Sadeer, N.B.; Zengin, G.; Mahomoodally, F.M. Biotechnological applications of mangrove plants and their isolated compounds in medicine-a mechanistic overview. Crit. Rev. Biotechnol. 2022. [Google Scholar] [CrossRef]
- Sadeer, N.B.; Sinan, K.I.; Cziáky, Z.; Jekő, J.; Zengin, G.; Jeewon, R.; Abdallah, H.H.; Rengasamy, K.R.; Mahomoodally, M.F. Assessment of the Pharmacological Properties and Phytochemical Profile of Bruguiera gymnorhiza (L.) Lam Using In Vitro Studies, In Silico Docking, and Multivariate Analysis. Biomolecules 2020, 10, 731. [Google Scholar] [CrossRef]
- Cefalu, W.T. “TODAY” Reflects on the Changing “Faces” of Type 2 Diabetes. Diabetes Care 2013, 36, 1732. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Amir, B.; Mohammed, A.D. Anti-diabetic medications: How to make a choice? Sudan J. Paediatr. 2017, 17, 11–20. [Google Scholar]
- Toniolo, A.; Cassani, G.; Puggioni, A.; Rossi, A.; Colombo, A.; Onodera, T.; Ferrannini, E. The diabetes pandemic and associated infections: Suggestions for clinical microbiology. Rev. Med. Microbiol. 2019, 30, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Shahid, I.Z.; Gain, N.C.; Reza, S.; Sadhu, S. Antinociceptive and antidiarrhoeal activities of Bruguiera gymnorrhiza. Orient. Pharm. Exp. Med. 2007, 7, 280–285. [Google Scholar] [CrossRef]
- Haq, M.; Sani, W.; Hossain, A.B.M.S.; Taha, R.; Khandaker, M. Total phenolic contents, antioxidant and antimicrobial activities of Bruguiera gymnorrhiza. J. Med. Plants 2011, 5, 4112–4118. [Google Scholar]
- Ravindran, K.C.; Venkatesan, K.; Balakrishnan, V.; Chellapan, K.P.; Balasubramanian, T. Ethnomedicinal studies of Pichavaram mangroves of East coast, Tamil Nadu. Indian J. Tradit. Knowl. 2005, 4, 409–411. [Google Scholar]
- Seepana, R.; Perumal, K.; Kada, N.M.; Chatragadda, R.; Raju, R.; Annamalai, V. Evaluation of antimicrobial properties from the mangrove Rhizophora apiculata and Bruguiera gymnorrhiza of Burmanallah coast, South Andaman, India. J. Coast. Life Med. 2016, 4, 475–478. [Google Scholar] [CrossRef]
- Nabeelah Bibi, S.; Fawzi, M.M.; Gokhan, Z.; Rajesh, J.; Nadeem, N.; Rengasamy, R.R.K.; Albuquerque, R.D.D.G.; Pandian, S.K. Ethnopharmacology, Phytochemistry, and Global Distribution of Mangroves―A Comprehensive Review. Mar. Drugs 2019, 17, 231. [Google Scholar] [CrossRef] [PubMed]
- Ado, M.A.; Abas, F.; Leong, S.W.; Shaari, K.; Ismail, I.S.; Ghazali, H.M.; Lajis, N.H. Chemical constituents and biological activities of Callicarpa maingayi leaves. South. Afr. J. Bot. 2016, 104, 98–104. [Google Scholar] [CrossRef]
- Banerjee, D.; Chakrabarti, S.; Hazra, A.; Banerjee, S.; Ray, J.; Mukherjee, B. Antioxidant activity and total phenolics of some mangroves in Sundarbans. Afr. J. Biotechnol. 2008, 7, 4–7. [Google Scholar]
- Karimulla, S.; Balagani, P. Anti diabetic and Anti hyperlipidemic activity of bark of Bruguiera gymnorrhiza on streptozotocin induced diabetic rats. Asian J. Pharm. Sci. Technol. 2011, 1, 4. [Google Scholar]
- Uddin, S.J.; Grice, I.D.; Tiralongo, E. Cytotoxic effects of bangladeshi medicinal plant extracts. Evid.-Based Complement. Altern. Med. 2011, 2011, 578092. [Google Scholar] [CrossRef]
- Ciudin, A.; Espinosa, A.; Simó-Servat, O.; Ruiz, A.; Alegret, M.; Hernández, C.; Boada, M.; Simó, R. Type 2 diabetes is an independent risk factor for dementia conversion in patients with mild cognitive impairment. J. Diabetes Its Complicat. 2017, 31, 1272–1274. [Google Scholar] [CrossRef]
- Azizian, Z.; Behrangi, E.; Hasheminasabzavareh, R.; Kazemlo, H.; Esmaeeli, R.; Hassani, P. Prevalence Study of Dermatologic Manifestations among Diabetic Patients. Adv. Prev. Med. 2019, 2019, 5. [Google Scholar] [CrossRef]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef]
- Afsharpour, F.; Javadi, M.; Hashemipour, S.; Koushan, Y.; Haghighian, H.K. Propolis supplementation improves glycemic and antioxidant status in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled study. Complementary Ther. Med. 2019, 43, 283–288. [Google Scholar] [CrossRef]
- Balbi, M.E.; Tonin, F.S.; Mendes, A.M.; Borba, H.H.; Wiens, A.; Fernandez-Llimos, F.; Pontarolo, R. Antioxidant effects of vitamins in type 2 diabetes: A meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 2018, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Halim, M.; Halim, A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Morgantini, C.; Natali, A.; Boldrini, B.; Imaizumi, S.; Navab, M.; Fogelman, A.M.; Ferrannini, E.; Reddy, S.T. Anti-inflammatory and Antioxidant Properties of HDLs Are Impaired in Type 2 Diabetes. Diabetes 2011, 60, 2617. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Khan, A. Antioxidants and diabetes. Indian J. Endocrinol. Metab. 2012, 16, S267. [Google Scholar] [CrossRef] [PubMed]
- Kashino, I.; Serafini, M.; Kurotani, K.; Akter, S.; Mizoue, T.; Ishihara, J.; Kotemori, A.; Sawada, N.; Inoue, M.; Iwasaki, M.; et al. Relationship between dietary non-enzymatic antioxidant capacity and type 2 diabetes risk in the Japan Public Health Center-based Prospective Study. Nutrition 2019, 66, 62–69. [Google Scholar] [CrossRef]
- Rajendiran, D.; Packirisamy, S.; Gunasekaran, K. A review on role of antioxidants in diabetes. Asian J. Pharm. Clin. Res. 2018, 11, 48–53. [Google Scholar] [CrossRef]
- Leung, L.K.; Su, Y.; Chen, R.; Zhang, Z.; Huang, Y.; Chen, Z.Y. Theaflavins in black tea and catechins in green tea are equally effective antioxidants. J. Nutr. 2001, 131, 2248–2251. [Google Scholar] [CrossRef]
- Saedisomeolia, A.; Ashoori, M. Riboflavin in human health: A review of current evidences. Adv. Food Nutr. Res. 2018, 83, 57–81. [Google Scholar]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A dietary molecule with diverse biological activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef]
- Watson, R.R. Polyphenols in Plants: Isolation, purification and Extract Preparation, 2nd ed.; Academic Press: London, UK, 2019. [Google Scholar]
- Banjarnahor, S.D.; Artanti, N. Antioxidant properties of flavonoids. Med. J. Indones. 2015, 23, 239–244. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.-C.; Yong, A.-L.; Ting, E.P.-S.; Khoo, S.-C.; Ong, H.-C.; Chai, T.-T. Antioxidant, metal chelating, anti-glucosidase activities and phytochemical analysis of selected tropical medicinal plants. Iran. J. Pharm Res. 2014, 13, 1409–1415. [Google Scholar]
- Khan, A.; Awan, F. Metals in the pathogenesis of type 2 diabetes. J. Diabetes Metab. Disord. 2014, 13, 16. [Google Scholar] [CrossRef]
- Jiang, R.; Manson, J.E.; Meigs, J.B.; Ma, J.; Rifai, N.; Hu, F.B. Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. Jama 2004, 291, 711–717. [Google Scholar] [CrossRef]
- Thengyai, S.; Thiantongin, P.; Sontimuang, C.; Ovatlarnporn, C.; Puttarak, P. α-Glucosidase and α-amylase inhibitory activities of medicinal plants in Thai antidiabetic recipes and bioactive compounds from Vitex glabrata R. Br. stem bark. J. Herb. Med. 2019, 19, 100302. [Google Scholar] [CrossRef]
- Akata, I.; Zengin, G.; Picot, C.M.N.; Mahomoodally, M.F. Enzyme inhibitory and antioxidant properties of six mushroom species from the Agaricaceae family. South. Afr. J. Bot. 2019, 120, 95–99. [Google Scholar] [CrossRef]
- Chipiti, T.; Ibrahim, M.A.; Singh, M.; Islam, M.S. In vitro α-amylase and α-glucosidase inhibitory effects and cytotoxic activity of Albizia antunesiana extracts. Pharmacogn. Mag. 2015, 11, S231–S236. [Google Scholar]
- Brzezinski, P.; Chiriac, A.E.; Pinteala, T.; Foia, L.; Chiriac, A. Diabetic dermopathy (“shin spots”) and diabetic bullae (“bullosis diabeticorum”) at the same patient. Pak. J. Med. Sci 2015, 31, 1275–1276. [Google Scholar] [CrossRef]
- Duff, M.; Demidova, O.; Blackburn, S.; Shubrook, J. Cutaneous manifestations of diabetes mellitus. Clin. Diabetes 2015, 33, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Engelke, M.; Jensen, J.M.; Ekanayake-Mudiyanselage, S.; Proksch, E. Effects of xerosis and ageing on epidermal proliferation and differentiation. Br. J. Dermatol. 1997, 137, 219–225. [Google Scholar] [CrossRef]
- Baselga Torres, E.; Torres-Pradilla, M. Cutaneous Manifestations in Children with Diabetes Mellitus and Obesity. Actas Dermo-Sifiliográficas (Engl. Ed.) 2014, 105, 546–557. [Google Scholar] [CrossRef]
- Ando, H.; Kondoh, H.; Ichihashi, M.; Hearing, V.J. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J. Investig. Dermatol. 2007, 127, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Shin, H. Anti-melanogenesis effect of quercetin. Cosmetics 2016, 3, 18. [Google Scholar] [CrossRef]
- Sun, L.; Guo, Y.; Zhang, Y.; Zhuang, Y. Antioxidant and anti-tyrosinase activities of phenolic extracts from rape bee pollen and inhibitory melanogenesis by cAMP/MITF/TYR pathway in B16 mouse melanoma cells. Front. Pharmacol. 2017, 8, 104. [Google Scholar] [CrossRef]
- Pientaweeratch, S.; Panapisal, V.; Tansirikongkol, A. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: An in vitro comparative study for anti-aging applications. Pharm. Biol. 2016, 54, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. 2014, 7, 587–591. [Google Scholar] [CrossRef]
- Karpe, F.; Dickmann, J.R.; Frayn, K.N. Fatty acids, obesity, and insulin resistance: Time for a reevaluation. Diabetes 2011, 60, 2441–2449. [Google Scholar] [CrossRef]
- Verma, S.; Hussain, M.E. Obesity and diabetes: An update. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, 73–79. [Google Scholar] [CrossRef]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s disease. Nat. Rev. Dis. Primers 2015, 1, 15056. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.; Strachan, M.W.J.; Visseren, F.L.J.; Kappelle, L.J.; Whitmer, R.A. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: Towards targeted interventions. Lancet Diabetes Endocrinol. 2014, 2, 246–255. [Google Scholar] [CrossRef]
- Kandimalla, R.; Thirumala, V.; Reddy, P.H. Is Alzheimer’s disease a Type 3 Diabetes? A critical appraisal. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2017, 1863, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.; Sato, N. Bidirectional interactions between diabetes and Alzheimer’s disease. Neurochem. Int. 2017, 108, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Verdile, G.; Fuller, S.J.; Martins, R.N. The role of type 2 diabetes in neurodegeneration. Neurobiol. Dis. 2015, 84, 22–38. [Google Scholar] [CrossRef]
- Heneka, M.T.; Fink, A.; Doblhammer, G. Effect of pioglitazone medication on the incidence of dementia. Ann. Neurol. 2015, 78, 284–294. [Google Scholar] [CrossRef]
- Ng, T.; Feng, L.; Yap, K.; Lee, T.; Tan, C.H.; Winblad, B. Long-term metformin usage and cognitive function among older adults with diabetes. J. Alzheimer’s Dis. 2014, 41, 61–68. [Google Scholar] [CrossRef]
- Budson, A.E.; Solomon, P.R. Appendix A: Cognitive Test and Questionnaire Forms, Instructions, and Normative Data for Evaluating Memory Loss, Alzheimer’s Disease, and Dementia. In Memory Loss, Alzheimer’s Disease, and Dementia, 2nd ed.; Budson, A.E., Solomon, P.R., Eds.; Elsevier: London, UK, 2016; pp. e1–e4. [Google Scholar]
- Colović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Bais, S.; Kumari, R.; Prashar, Y. Ameliorative Effect of Trans-Sinapic Acid and its Protective Role in Cerebral Hypoxia in Aluminium Chloride Induced Dementia of Alzheimer’s Type. CNS Neurol. Disord. Drug Targets 2018, 17, 144–154. [Google Scholar] [CrossRef]
- Shahmohamady, P.; Eidi, A.; Mortazavi, P.; Panahi, N.; Minai-Tehrani, D. Effect of sinapic acid on memory deficits and neuronal degeneration induced by intracerebroventricular administration of streptozotocin in rats. Pol. J. Pathol. 2018, 69, 266–277. [Google Scholar] [CrossRef]
- Farrés, M.; Platikanov, S.; Tsakovski, S.; Tauler, R. Comparison of the variable importance in projection (VIP) and of the selectivity ratio (SR) methods for variable selection and interpretation. J. Chemom. 2015, 29, 528–536. [Google Scholar] [CrossRef]
- Zengin, G.; Sarikurkcu, C.; Uyar, P.; Aktumsek, A.; Uysal, S.; Kocak, M.S.; Ceylan, R. Crepis foetida L. subsp. rhoeadifolia (Bieb.) Celak. as a source of multifunctional agents: Cytotoxic and phytochemical evaluation. J. Funct. Foods 2015, 17, 698–708. [Google Scholar] [CrossRef]
- Vladimir-Knežević, S.; Blažeković, B.; Bival Štefan, M.; Alegro, A.; Kőszegi, T.; Petrik, J. Antioxidant activities and polyphenolic contents of three selected Micromeria species from Croatia. Molecules 2011, 16, 1454–1470. [Google Scholar] [CrossRef]
- Zengin, G.; Aktumsek, A. Investigation of antioxidant potentials of solvent extracts from different anatomical parts of Asphodeline anatolica E. Tuzlaci: An endemic plant to Turkey. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 481–488. [Google Scholar] [CrossRef]
- Chlapanidas, T.; Faragò, S.; Lucconi, G.; Perteghella, S.; Galuzzi, M.; Mantelli, M.; Avanzini, M.A.; Tosca, M.C.; Marazzi, M.; Vigo, D.; et al. Sericins exhibit ROS-scavenging, anti-tyrosinase, anti-elastase, and in vitro immunomodulatory activities. Int. J. Biol. Macromol. 2013, 58, 47–56. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Zengin, G. A study on in vitro enzyme inhibitory properties of Asphodeline anatolica: New sources of natural inhibitors for public health problems. Ind. Crop. Prod. 2016, 83, 39–43. [Google Scholar] [CrossRef]



| Yield (%) | Total Phenolic Acid (mg CAE/g) | Total Phenolic Content (mg GAE/g) | Total Flavonoid Content (mg RE/g) | Total Flavanol (mg CE/g) | Total Tannin (mg CE/g) | Total Triterpenoids (mg OAE/g) | |
|---|---|---|---|---|---|---|---|
| BLD | 18.70 | 0.71 ± 0.04 g | 23.57 ± 0.03 f | 1.73 ± 0.03 d | 1.23 ± 0.02 e | 11.04 ± 0.07 d | 2.41 ± 0.51 f g |
| BRD | 7.38 | 14.69 ± 0.39 a | 177.09 ± 0.91 b | 0.47 ± 0.06 e | 13.38 ± 0.14 a | 90.49 ± 5.32 a | 29.24 ± 0.54 b |
| BTD | 7.78 | 5.09 ± 0.20 d | 84.28 ± 1.44 c | 0.64 ± 0.09 e | 2.93 ± 0.02 d | 35.92 ± 3.25 c | 9.77 ± 0.80 e |
| BFD | 8.24 | 3.46 ± 0.13 e | 84.40 ± 0.36 c | 5.26 ± 0.15 a | 3.99 ± 0.01 c | 45.00 ± 1.31 b | 12.87 ± 0.49 d |
| BLA | 11.04 | 0.92 ± 0.02 g | 47.64 ± 0.07 e | 1.41 ± 0.27 d | 1.28 ± 0.03 e | 14.00 ± 0.22 d | 1.41 ± 0.06 g |
| BRA | 4.28 | 10.29 ± 0.18 b | 184.18 ± 0.49 a | 2.19 ± 0.13 c | 13.32 ± 0.12 b | 98.01 ± 1.94 a | 23.27 ± 0.18 c |
| BTA | 4.26 | 2.15 ± 0.13 f | 77.78 ± 1.02 d | 0.39 ± 0.08 e | 3.09 ± 0.02 d | 17.66 ± 0.40 d | 4.08 ± 0.20 f |
| BFA | 11.12 | 9.23 ± 0.58 c | 185.33 ± 1.03 a | 3.12 ± 0.07 b | 13.84 ± 0.16 a | 97.85 ± 4.41 a | 36.84 ± 2.30 a |
| Compound Name | Retention Time | Decoction | Aqueous |
|---|---|---|---|
| Quinic acid | 1.21 | + | + |
| Citric acid | 1.56 | + | + |
| Brugierol | 1.64 | + | + |
| Gallocatechin (Casuarin, Gallocatechol) | 4.58 | + | + |
| Protocatechuic acid (3,4-Dihydroxybenzoic acid) | 4.65 | + | + |
| Neochlorogenic acid (5-O-Caffeoylquinic acid) | 8.64 | + | + |
| Procyanidin B isomer 1 | 12.05 | + | + |
| Procyanidin B isomer 2 | 12.05 | − | − |
| 3-O-(4-Coumaroyl) quinic acid | 12.45 | + | + |
| Catechin | 13.32 | + | + |
| Epigallocatechin (Epigallocatechol) | 13.62 | + | + |
| Chlorogenic acid (3-O-Caffeoylquinic acid) | 14.23 | + | − |
| 3-O-Feruloylquinic acid | 14.50 | + | + |
| Ampelopsin (Ampeloptin, Dihydromyricetin) | 14.71 | + | + |
| Procyanidin B isomer 3 | 15.51 | − | − |
| Vanillin | 15.57 | + | − |
| Chryptochlorogenic acid (4-O-Caffeoylquinic acid) | 17.08 | + | + |
| Epicatechin | 17.55 | + | + |
| 4-O-(4-Coumaroyl) quinic acid | 17.75 | + | − |
| 3-(Benzoyloxy)-2-hydroxypropylglucuronic acid | 18.24 | − | + |
| 4-Coumaric acid | 18.52 | + | + |
| Antiarol (3,4,5-Trimethoxyphenol) | 12.45 | − | + |
| Loliolide or Isololiolide | 13.32 | + | + |
| 4-O-Feruloylquinic acid | 13.62 | + | + |
| Riboflavin | 18.63 | − | + |
| Indole-3-lactic acid | 18.78 | − | + |
| Ferulic acid | 19.28 | + | + |
| Loliolide or Isololiolide | 19.31 | + | + |
| 4-Hydroxy-3-methoxycinnamaldehyde (Coniferyl aldehyde) | 19.50 | − | − |
| Sinapic acid (Sinapinic acid) | 19.86 | + | + |
| Myricetin-3-O-rutinoside | 21.04 | + | + |
| Cinchonain I isomer 1 | 22.28 | − | − |
| Theaflavin | 21.65 | − | + |
| Dihydrokaempferol (Aromadendrin, Katuranin) | 21.88 | − | + |
| Cinchonain I isomer 2 | 22.29 | − | + |
| Methoxy-tetrahydroxy(iso)flavone isomer 1 | 22.68 | − | − |
| Isoquercitrin (Hirsutrin, Quercetin-3-O-glucoside) | 22.93 | − | − |
| Rutin (Quercetin-3-O-rutinoside) | 23.01 | + | + |
| Myricetin (Cannabisetin, Myricetol, 3,3′,4′,5,5′,7-hexahydroflavone) | 24.19 | − | + |
| Azelaic acid | 24.47 | + | + |
| Methoxy-trihydroxy(iso)flavone | 24.84 | − | − |
| Kaempferol-3-O-rutinoside (Nicotiflorin) | 25.01 | + | + |
| Gramrione (5,5′-Dimethoxy-3′,4′,7-trihydroxyflavone) | 26.89 | + | + |
| Dihydroxy-trimethoxy(iso)flavone | 26.98 | − | + |
| Quercetin (3,3′,4′,5,7-Penthahyroxyflavone) | 27.19 | − | + |
| Naringenin (4′,5,7-Trihydroxyflavanone) | 27.19 | + | + |
| Sebacic acid | 27.96 | − | + |
| Methoxy-tetrahydroxy(iso)flavone isomer 2 | 30.85 | − | − |
| Bruguierol A | 36.05 | + | + |
| Lupeol caffeate | 52.73 | − | − |
| Lupeol coumarate | 54.53 | − | − |
| Compound Name | Retention Time | Decoction | Aqueous |
|---|---|---|---|
| Quinic acid | 1.24 | + | − |
| Brugierol | 1.67 | + | + |
| Gallocatechin | 4.54 | + | − |
| Protocatechuic acid (3,4-Dihydroxybenzoic acid) | 4.71 | + | + |
| Catechol | 5.12 | − | + |
| Genistic acid (2,5-Dihydroxybenzoic acid) | 8.97 | − | + |
| Neochlorogenic acid (5-O-Caffeoylquinic acid) | 8.66 | + | − |
| Procyanidin B isomer 1 | 12.07 | + | − |
| 3-O-(4-Coumaroyl) quinic acid | 12.46 | + | − |
| Catechin | 13.31 | + | − |
| Epigallocatechin | 13.62 | + | − |
| Chlorogenic acid (3-O-Caffeoylquinic acid) | 14.24 | + | − |
| Dihydroxybenzoic acid isomer | 14.34 | − | + |
| Caffeic acid | 14.48 | + | + |
| 3-O-Feruloylquinic acid | 15.22 | + | + |
| Procyanidin B | 15.49 | + | − |
| Vanillin | 15.57 | + | − |
| Chryptochlorogenic acid (4-O-Caffeoylquinic acid) | 17.08 | + | + |
| Epicatechin | 17.52 | + | − |
| 4-O-(4-Coumaroyl) quinic acid | 17.60 | + | − |
| 3-(Benzoyloxy)-2-hydroxypropylglucuronic acid | 14.34 | + | − |
| 4-Coumaric acid | 17.73 | + | − |
| Antiarol (3,4,5-Trimethoxyphenol) | 17.92 | + | + |
| Loliolide or Isololiolide | 18.23 | + | + |
| 4-O-Feruloylquinic acid | 18.49 | + | + |
| Riboflavin | 18.64 | + | − |
| Cinchonain I isomer 1 | 18.90 | + | − |
| Ferulic acid | 19.26 | + | − |
| Taxifolin (Didydroquercetin) | 19.29 | − | − |
| Loliolide or Isololiolide | 19.50 | + | + |
| Dimethoxy-trihydroxy(iso)flavone-O-hexoside isomer 1 | 20.14 | + | − |
| Dimethoxy-trihydroxy(iso)flavone-O-hexoside isomer | 20.72 | + | − |
| Isoferulic acid | 20.30 | − | − |
| Dihydroxy-methoxy(iso)flavone-O-hexoside | 20.72 | − | + |
| Quercetin-O-dirhamnosylhexoside | 20.81 | + | − |
| Myricetin-3-O-rutinoside | 21.03 | + | − |
| Cinchonain I isomer 2 | 21.30 | + | − |
| Kaempferol-O-dirhamnosylhexoside | 22.11 | + | − |
| Cinchonain I isomer 3 | 22.30 | + | − |
| Methoxy-tetrahydroxy(iso)flavone isomer 1 | 22.67 | + | + |
| Isoquercitrin (Hirsutrin, Quercetin-3-O-glucoside) | 22.93 | + | − |
| Dimethoxy-trihydroxy(iso)flavone-O-hexoside isomer 2 | 23.01 | + | − |
| Rutin (Quercetin-3-O-rutinoside) | 23.05 | + | − |
| Apigenin-O-rhamnosylhexoside | 24.48 | + | − |
| Azelaic acid | 24.48 | + | + |
| Methoxy-trihydroxy(iso)flavone | 24.70 | + | + |
| Kaempferol-3-O-rutinoside (Nicotiflorin) | 24.85 | + | − |
| Cinchonain I isomer 4 | 24.95 | + | − |
| Gramrione (5,5′-Dimethoxy-3′,4′,7-trihydroxyflavone) | 25.03 | + | + |
| Dimethoxy-trihydroxy(iso)flavone-O-hexoside isomer 3 | 26.00 | + | − |
| Dihydroxy-methoxy(iso)flavone | 26.51 | + | + |
| Dihydroxy-dimethoxy(iso)flavone | 26.85 | + | + |
| Dihydroxy-trimethoxy(iso)flavone | 26.89 | + | + |
| Quercetin (3,3′, 4′, 5, 7-Pentahydroxylflavone) | 28.08 | − | − |
| Naringenin (4′,5,7-Trihydroxyflavanone) | 29.16 | − | − |
| Luteolin (3′,4′,5,7-Tetrahydroxyflavone) | 20.72 | − | + |
| Methoxy-tetrahydroxy(iso)flavone isomer 2 | 20.81 | + | + |
| Dimethoxy-trihydroxy(iso)flavone-O-rhamnoside | 21.03 | + | − |
| Kaempferol (3,4′,5,7-Tetrahydroxyflavone) | 29.30 | − | − |
| Apigenin (4′,5,7-Trihydroxyflavone) | 29.66 | − | + |
| Tricin (3′,5′-Dimethoxy-4′,5,7-trihydroxyflavone) | 29.84 | − | − |
| Bruguierol A | 36.06 | + | + |
| Compound Name | Retention Time | Decoction | Aqueous |
|---|---|---|---|
| Quinic acid | 1.27 | + | + |
| Citric acid | 1.58 | + | + |
| Brugierol | 1.64 | + | + |
| Gallocatechin | 4.55 | + | + |
| Unidentified compound | 4.64 | + | + |
| Protocatechuic acid (3,4-Dihydroxybenzoic acid) | 4.67 | + | + |
| Neochlorogenic acid (5-O-Caffeoylquinic acid) | 8.59 | + | + |
| Syringic acid-O-hexoside isomer 1 | 10.62 | + | + |
| Prodelphinidin C | 10.83 | + | + |
| Unidentified compound | 13.12 | − | + |
| Catechin | 13.30 | + | + |
| Epigallocatechin | 13.61 | + | + |
| Chlorogenic acid (3-O-Caffeoylquinic acid) | 14.22 | + | + |
| Caffeic acid | 14.33 | − | + |
| Unidentified compound | 15.00 | − | + |
| Procyanidin B isomer 1 | 16.96 | + | + |
| Vanillin | 15.54 | + | − |
| Chryptochlorogenic acid (4-O-Caffeoylquinic acid) | 15.56 | + | + |
| Syringic acid | 15.81 | − | − |
| Procyanidin C | 16.96 | + | + |
| Epicatechin | 17.07 | + | + |
| 4-Coumaric acid | 17.71 | − | − |
| 3-(Benzoyloxy)-2-hydroxypropylglucuronic acid | 17.59 | + | + |
| Antiarol (3,4,5-Trimethoxyphenol) | 17.91 | + | + |
| 3,4-Dihydro-3-hydroxy-7-methoxy-2H-1,5-benzodithiepine-6,9-dione | 18.23 | − | + |
| Cinchonain I isomer 1 | 18.90 | + | + |
| Epiafzelechin | 19.04 | + | + |
| Ferulic acid | 19.27 | + | + |
| Taxifolin (Dihydroquercetin) | 17.91 | − | + |
| Isoferulic acid | 20.32 | − | − |
| 4-Hydroxy-3-methoxycinnamaldehyde (Coniferyl aldehyde) | 20.02 | + | − |
| Procyanidin B isomer 2 | 20.56 | − | − |
| Trihydroxystilbene | 20.86 | − | − |
| Myricetin-3-O-rutinoside | 21.03 | + | + |
| Cinchonain I isomer 2 | 21.07 | + | + |
| Cinchonain I isomer 3 | 22.30 | + | + |
| Rutin (Quercetin-3-O-rutinoside) | 23.01 | + | + |
| Azelaic acid | 24.47 | + | + |
| Methoxy-trihydroxy(iso)flavone | 24.73 | + | − |
| Kaempferol-3-O-rutinoside (Nicotiflorin) | 24.85 | − | − |
| Cinchonain I isomer 4 | 24.95 | + | + |
| Gramrione (5,5′-Dimethoxy-3′,4′,7-trihydroxyflavone) | 25.04 | + | − |
| Quercetin (3,3′,4′,5,7-Pentahydroxyflavone) | 26.97 | − | − |
| Naringenin (4′,5,7-Trihydroxyflavanone) | 27.19 | − | − |
| 3-O-Methylellagic acid-4′-O-rhamnoside | 25.33 | − | − |
| Sebacic acid | 28.00 | + | + |
| Phloretin | 28.14 | − | + |
| Tricin (3′,5′-Dimethoxy-4′,5,7-trihydroxyflavone) | 29.84 | − | − |
| Norstictic acid | 32.46 | − | + |
| Methyl-trihydroxyxanthone | 32.88 | − | − |
| 16,17-Dihydroxy-9(11)-kauren-19-al or Steviol | 33.77 | + | + |
| 1-Hydroxy-8(14)-isopimaren-1,15,16-triol or isomer | 33.86 | + | + |
| 13-Hydroxy-16-kauren-19-al or isomer | 35.50 | + | + |
| Bruguierol A | 36.07 | + | + |
| 1-Hydroxy-8(14)-isopimaren-1,15,16-triol or isomer | 36.39 | + | + |
| Dihydroxy-methoxy-methylxanthone | 37.84 | − | + |
| 1-Hydroxy-8(14)-isopimaren-1,15,16-triol or isomer | 38.07 | + | + |
| 13-Hydroxy-16-kauren-19-al or isomer | 39.38 | + | + |
| Unidentified xanthone | 43.29 | + | + |
| Isopimar-7-en-15,16-diol or isomer | 43.71 | + | + |
| Compound Name | Retention Time | Decoction | Aqueous |
|---|---|---|---|
| Quinic acid | 1.19 | + | − |
| Citric acid | 1.55 | + | − |
| Brugierol | 1.66 | + | + |
| Gallocatechin | 4.58 | + | − |
| Protocatechuic acid (3,4-Dihydroxybenzoic acid) | 4.70 | + | + |
| Neochlorogenic acid (5-O-Caffeoylquinic acid) | 8.69 | + | − |
| Syringic acid-O-hexoside isomer 1 | 9.93 | + | − |
| Syringic acid-O-hexoside isomer 2 | 10.66 | − | − |
| Catechin | 13.33 | + | − |
| Epigallocatechin | 13.64 | + | − |
| Chlorogenic acid (3-O-Caffeoylquinic acid) | 14.21 | + | − |
| Caffeic acid | 14.32 | + | + |
| Vanillin | 15.19 | − | − |
| Procyanidin B | 15.21 | + | − |
| Chryptochlorogenic acid (4-O-Caffeoylquinic acid) | 15.56 | + | − |
| Syringic acid | 15.81 | + | − |
| Ehyl syringate | 16.95 | − | − |
| Procyanidin C | 17.07 | + | − |
| Epicatechin | 17.59 | + | − |
| 3-(Benzoyloxy)-2-hydroxypropylglucuronic acid | 17.75 | + | − |
| 4-Coumaric acid | 17.93 | + | − |
| Antiarol (3,4,5-Trimethoxyphenol) | 18.25 | + | + |
| 3,4-Dihydro-3-hydroxy-7-methoxy-2H-1,5-benzodithiepine-6,9-dione | 18.64 | + | − |
| Riboflavin | 18.90 | − | + |
| Cinchonain I isomer 1 | 19.29 | + | − |
| Ferulic acid | 19.50 | + | − |
| Loliolide or Isololiolide | 15.56 | + | − |
| Coumarin | 19.73 | − | − |
| 4-Hydroxy-3-methoxycinnamaldehyde (Coniferyl aldehyde) | 19.99 | − | − |
| 3,5-Dimethoxy-4-hydroxycinnamaldehyde (Sinapyl aldehyde) | 20.44 | − | − |
| Isoquercitrin (Hirsutin, Quercetin-3-O-glucoside) | 22.94 | − | − |
| Cinchonain I isomer 2 | 24.95 | + | − |
| Cinchonain I isomer 3 | 22.29 | − | − |
| Rutin (Quercetin-3-O-rutinoside) | 23.04 | + | − |
| Azelaic acid | 24.48 | + | + |
| Methoxy-trihydroxy(iso)flavone | 24.72 | + | − |
| Kaempferol-3-O-rutinoside (Nicotiflorin) | 24.86 | + | − |
| Cinchonain I isomer 4 | 24.95 | − | − |
| Gramrione (5,5′-Dimethoxy-3′,4′,7-trihydroxyflavone) | 25.04 | + | − |
| Dihydroxy-methoxy(iso)flavone | 26.51 | + | − |
| Dihydroxy-dimethoxy(iso)flavone | 26.85 | − | − |
| Dihydroxy-trimethoxy(iso)flavone | 26.91 | − | − |
| Naringenin (4′,5,7-Trihydroxyflavanone) | 27.20 | − | − |
| 16,17-Dihydroxy-9(11)-kauran-19-al | 32.40 | + | + |
| 16,17-Dihydroxy-9(11)-kauren-19-al or Steviol | 33.78 | + | + |
| 1-Hydroxy-8(14)-isopimaren-1,15,16-triol or isomer | 34.79 | − | − |
| Methyl 16α,17-dihydroxy-9(11)-kauren-19-oate or isomer | 35.51 | + | − |
| 13-Hydroxy-16-kauren-19-al or isomer | 35.67 | + | − |
| 16,17-Dihydroxy-9(11)-kauran-19-al isomer | 36.08 | + | − |
| Bruguierol A | 36.14 | + | + |
| Methyl 16α,17-dihydroxy-9(11)-kauren-19-oate or isomer | 34.79 | + | − |
| 1-Hydroxy-8(14)-isopimaren-1,15,16-triol or isomer | 36.38 | − | − |
| 13-Hydroxy-16-kauren-19-al or isomer | 38.06 | − | − |
| Isopimar-7-en-15,16-diol or isomer | 38.31 | − | − |
| Lupeol caffeate | 52.71 | − | − |
| Lupeol coumarate | 54.51 | − | − |
| DPPH (mg TE/g) | ABTS (mg TE/g) | CUPRAC (mg TE/g) | FRAP (mg TE/g) | Phosphomolybdenum (mmol TE/g) | Chelating Activity (mg EDTAE/g) | |
|---|---|---|---|---|---|---|
| BLD | 28.30 ± 0.30 f | 28.82 ± 1.28 e | 67.14 ± 0.17 g | 46.08 ± 1.06 h | 0.40 ± 0.01 e | 23.69 ± 0.40 b |
| BRD | 451.72 ± 3.71 c | 448.09 ± 18.47 a | 877.30 ± 13.82 b | 547.22 ± 7.59 b | 3.70 ± 0.08 b | 7.35 ± 1.06 d |
| BTD | 90.34 ± 1.05 d | 123.16 ± 3.90 b | 246.52 ± 6.65 d e | 167.10 ± 1.03 e | 1.67 ± 0.03 c | 21.90 ± 1.08 b |
| BFD | 84.01 ± 3.35 d | 113.79 ± 5.58 b c | 271.14 ± 7.96 d | 185.60 ± 5.35 d | 1.89 ± 0.06 c | 10.41 ± 1.86 c d |
| BLA | 47.93 ± 2.07 e f | 58.95 ± 1.01 d e | 116.57 ± 1.67 f | 79.43 ± 0.33 g | 1.22 ± 0.05 d | 12.57 ± 2.60 c |
| BRA | 472.62 ± 14.58 b | 437.34 ± 19.60 a | 814.82 ± 33.09 c | 497.53 ± 8.49 c | 3.57 ± 0.04 b | 14.47 ± 0.18 c |
| BTA | 56.12 ± 0.76 e | 87.40 ± 2.40 c d | 211.26 ± 6.44 e | 105.33 ± 0.72 f | 1.66 ± 0.05 c | 31.45 ± 1.95 a |
| BFA | 547.75 ± 10.99 a | 439.59 ± 19.13 a | 956.04 ± 11.90 a | 577.26 ± 4.55 a | 5.34 ± 0.24 a | 14.02 ± 2.27 c |
| AChE Inhibition (mg GALAE/g) | BChE Inhibition (mg GALAE/g) | Tyrosinase Inhibition (mg KAE/g) | Amylase Inhibition (mmol ACAE/g) | Glucosidase Inhibition (mmol ACAE/g) | Elastase Inhibition (mg CAE/g) | Lipase Inhibition (mg OE/g) | |
|---|---|---|---|---|---|---|---|
| BLD | na | 0.30 ± 0.04 b | 22.39 ± 3.17 f | 0.09 ± 0.01 f | na | 0.56 ± 0.08 c d | na |
| BRD | 2.56 ± 0.46 b | 0.57 ± 0.05 b | 74.16 ± 3.70 c | 0.10 ± 0.01 e f | 30.87 ± 0.27 a b | 1.85 ± 0.26 b | na |
| BTD | 1.17 ± 0.18 c | 0.72 ± 0.11 b | 31.06 ± 1.95 e | 0.09 ± 0.01 e f | 28.40 ± 2.55 b | 0.35 ± 0.05 d | 13.81 ± 5.13 a |
| BFD | 3.90 ± 0.14 a | 2.85 ± 0.74 a | 101.93 ± 2.45 b | 0.70 ± 0.01 b | na | 2.76 ± 0.52 a | na |
| BLA | na | na | 58.72 ± 3.21 d | 0.10 ± 0.01 e | 1.41 ± 0.14 d | na | na |
| BRA | 2.13 ± 0.34 b | 0.32 ± 0.05 b | 60.74 ± 1.73 d | 0.13 ± 0.01 d | 31.18 ± 0.06 a | 0.39 ± 0.02 d | na |
| BTA | na | na | 11.77 ± 1.36 g | 0.21 ± 0.01 c | 24.25 ± 0.56 c | 1.04 ± 0.10 c | na |
| BFA | 3.75 ± 0.03 a | 2.19 ± 0.13 a | 147.01 ± 0.78 a | 1.22 ± 0.01 a | na | 3.14 ± 0.08 a | na |
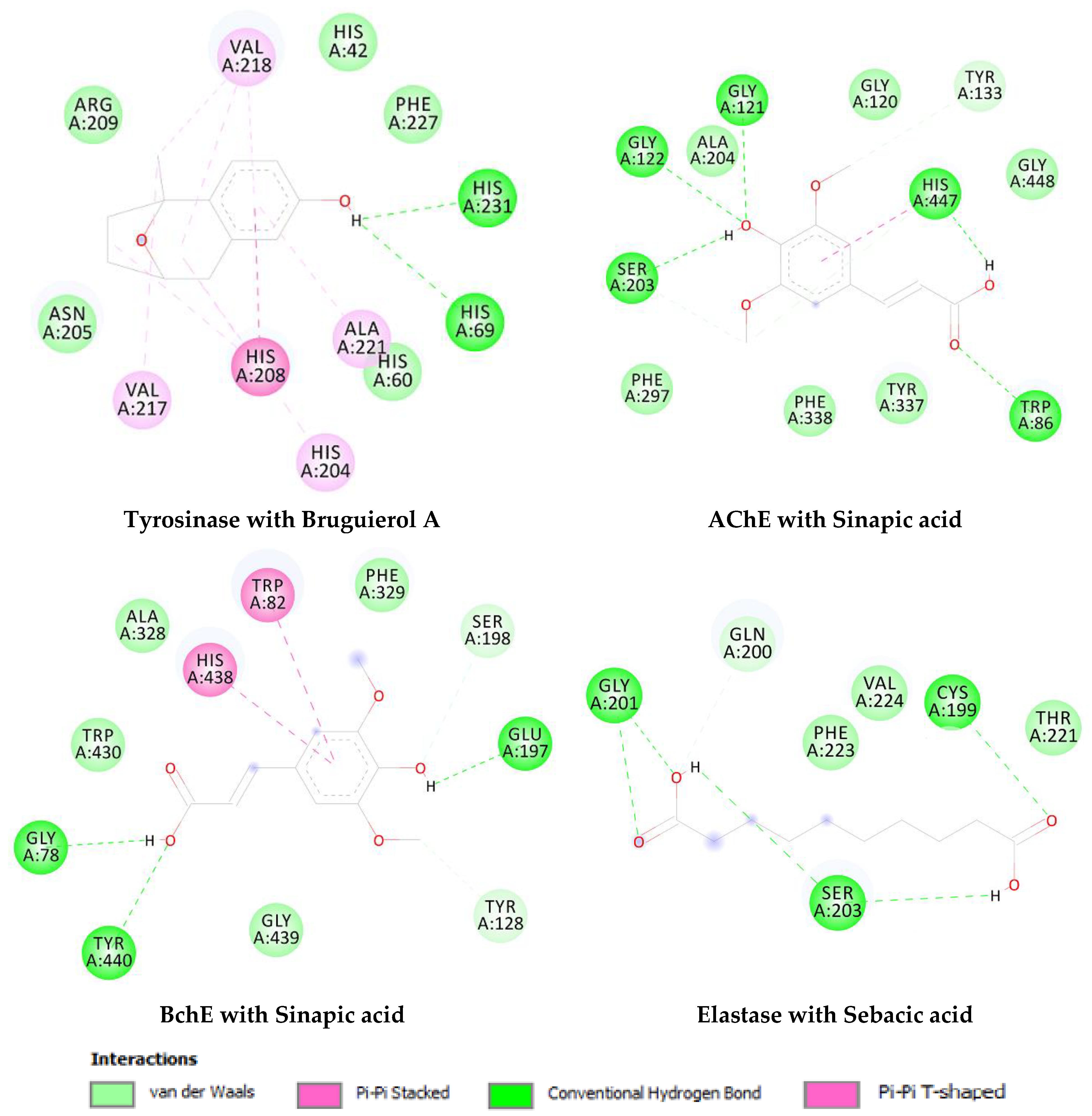
| Enzyme | Compound | Chemical Structure |
|---|---|---|
| Tyrosinase | Brugierol | 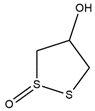 |
| Theaflavin | 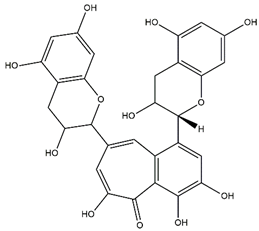 | |
| Bruguierol A | 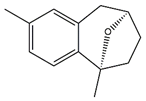 | |
| Sebacic acid | 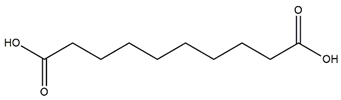 | |
| AchE | Sinapic acid |  |
| BchE | Sinapic acid |  |
| Elastase | Sebacic acid | 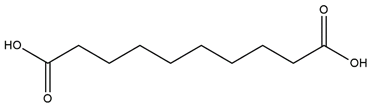 |
| Enzyme | Compound | Binding Free Energy (kcal/mol) | Inhibition Constant (Ki) |
|---|---|---|---|
| Tyrosinase | Brugierol | −2.34 | 19.23 mM |
| Theaflavin | −6.59 | 14.86 µM | |
| Bruguierol A | −6.70 | 12.26 µM | |
| Sebacic acid | −5.26 | 139.04 µM | |
| AchE | Sinapic acid | −6.74 | 11.56 µM |
| BchE | Sinapic acid | −6.36 | 21.80 µM |
| Elastase | Sebacic acid | −4.41 | 581.30 µM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bibi Sadeer, N.; Sinan, K.I.; Cziáky, Z.; Jekő, J.; Zengin, G.; Jeewon, R.; Abdallah, H.H.; AlDhaheri, Y.; Eid, A.H.; Mahomoodally, M.F. Towards the Pharmacological Validation and Phytochemical Profiling of the Decoction and Maceration of Bruguiera gymnorhiza (L.) Lam.—A Traditionally Used Medicinal Halophyte. Molecules 2022, 27, 2000. https://doi.org/10.3390/molecules27062000
Bibi Sadeer N, Sinan KI, Cziáky Z, Jekő J, Zengin G, Jeewon R, Abdallah HH, AlDhaheri Y, Eid AH, Mahomoodally MF. Towards the Pharmacological Validation and Phytochemical Profiling of the Decoction and Maceration of Bruguiera gymnorhiza (L.) Lam.—A Traditionally Used Medicinal Halophyte. Molecules. 2022; 27(6):2000. https://doi.org/10.3390/molecules27062000
Chicago/Turabian StyleBibi Sadeer, Nabeelah, Kouadio Ibrahime Sinan, Zoltán Cziáky, József Jekő, Gokhan Zengin, Rajesh Jeewon, Hassan H. Abdallah, Yusra AlDhaheri, Ali H. Eid, and Mohamad Fawzi Mahomoodally. 2022. "Towards the Pharmacological Validation and Phytochemical Profiling of the Decoction and Maceration of Bruguiera gymnorhiza (L.) Lam.—A Traditionally Used Medicinal Halophyte" Molecules 27, no. 6: 2000. https://doi.org/10.3390/molecules27062000
APA StyleBibi Sadeer, N., Sinan, K. I., Cziáky, Z., Jekő, J., Zengin, G., Jeewon, R., Abdallah, H. H., AlDhaheri, Y., Eid, A. H., & Mahomoodally, M. F. (2022). Towards the Pharmacological Validation and Phytochemical Profiling of the Decoction and Maceration of Bruguiera gymnorhiza (L.) Lam.—A Traditionally Used Medicinal Halophyte. Molecules, 27(6), 2000. https://doi.org/10.3390/molecules27062000