Olive Oil Traceability Studies Using Inorganic and Isotopic Signatures: A Review
Abstract
:1. Introduction
2. Olive Oil Geographical Authentication by Means of Trace Elements
2.1. Reported Content of Trace Elements in Olive Oil
2.2. Analytical Procedures of Quantification of Trace Elements in Olive Oil
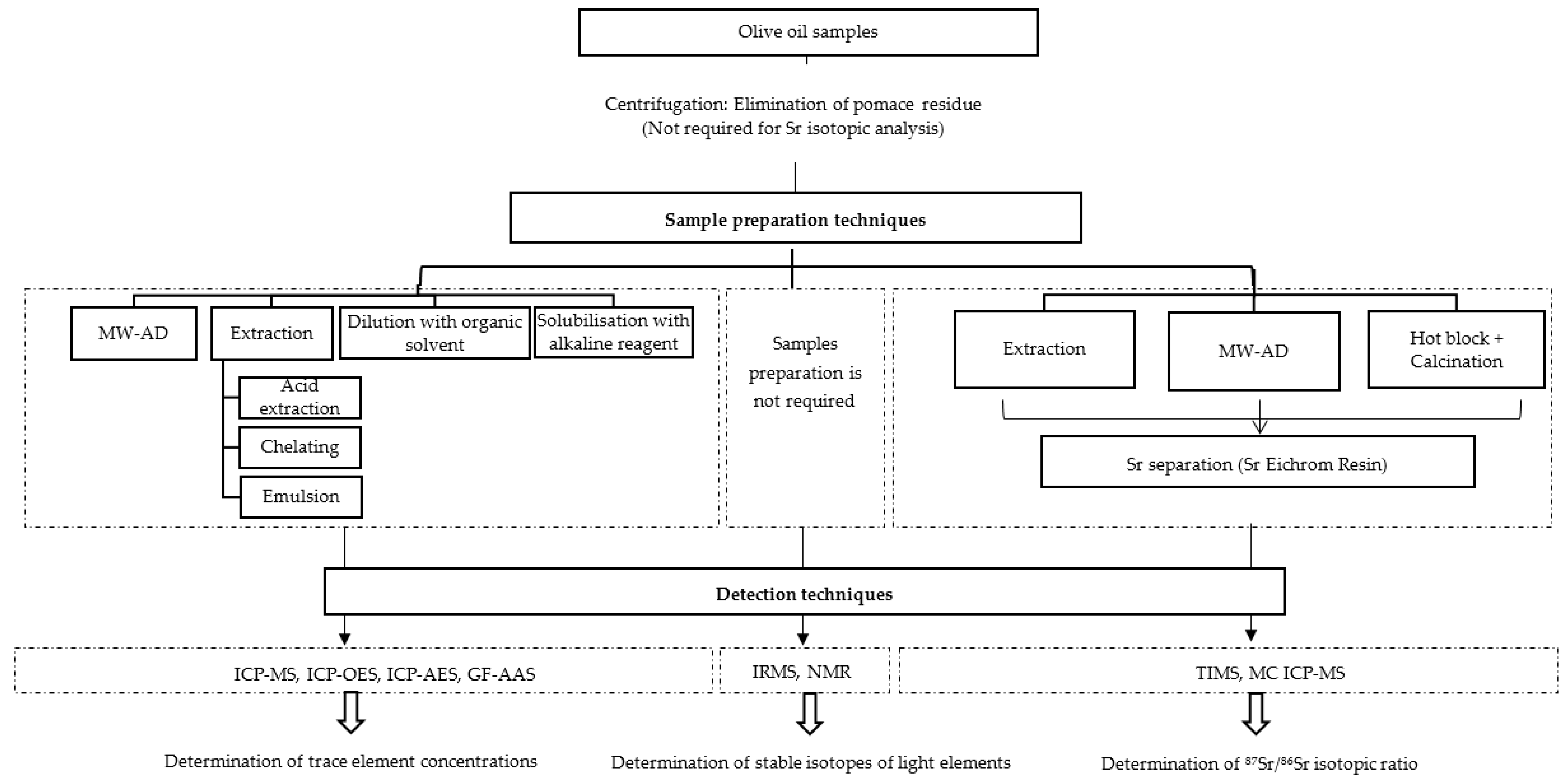
2.2.1. Sample Preparation Techniques
- (i)
- Microwave-assisted digestion (MW-AD)
| Sample Preparation Techniques | Oil Type/Quality | Number of Samples | Origin | Reagents | Detection Technique | Analytes | Limit of Detection | Material/Method Used for Validation | Accuracy (ACC)/Recovery (R) (%) | Chemometric Method | Purpose | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Microwave-assisted digestion | Extra-Virgin Olive Oil (EVOO) | 110 | Italy | HNO3 and H2O2 | ICP-MS | Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Rb, Sr, Cd, Sb, Ba, W, Tl, Pb, Th, U and REE | - | BCR-668 (musel tissue) | - | PCA, LDA | Authentication, traceability | [60] |
| Virgin Olive Oil (VOO) | 82 | Spain | HNO3, H2O2 and HCl | ICP-MS | Al, As, Ba, Ca, Co, Cr, Cs, Cu, Fe, Ga, Hf, K, Li, Mo, Mg, Mn, Na, Sr, Nb, Ni, Pb, Rb, Sc, Se, Sn, Ta, Th, Ti, U, V, W, Y, Zn and Zr | - | - | - | LDA | [45] | ||
| VOO | 36 | Italy | HNO3 | ICP-MS | Be, Mg, Ca, Sc, Cr, Mn, Fe, Co, Ni, As, Se, Sr, Y, Cd, Sb, Sm, Eu and Gd | LOQ: 0.12; 118; 1250; 9.7; 16.3; 9.2; 152; 0.11; 21.2; 0.62; 10.2; 9.6; 0.12; 0.16; 0.14; 0.012; 0.009 and 0.012 (µg kg−1) | - | - | LDA | [36] | ||
| Olive oil (OO) | 21 | Tunisia | HNO3 and H2O2 | ICP-MS | Na, Mg, Fe, Zn, V, Mn, As, Rb, Sr, Ba and Pb | 0.35; 0.47; 0.12; 0.11 (mg kg−1) 1.7; 6; 0.73; 0.3; 5.1; 4.6 and 6.9 (µg kg−1) | Multi-element oil standard S23-100Y | ACC: 66–102% | PCA | [50] | ||
| VOO | 49 | Turkey | HNO3 and H2O2 | ICP-MS | Fe, Ca, K, Na, Mg, As, Ba, Co, Cr, Cu, Mn, Ni, Pb, V, Zn | - | - | - | PCA and HCA | [61] | ||
| EVOO | 125 | Spain | HNO3, H2O2 and HCl | ICP-MS for minor elements and ICP-OES for major elements | Al, Ca, Fe, Mg, Mn, K, Na, Ti, Li, Be, B, V, Cr, Co, Ni, Cu, Zn, Ga, Ge, As, Se, Rb, Sr, Zr, Nb, Mo, Cd, Sn, Sb, Cs, Ba, Hf, Ta, W, Tl, Pb, Bi, Th, U and REE | - | Spike with a multi-element standard solution | R: 82–110% | PCA and LDA | [62] | ||
| EVOO and olive-pomace | 1 6 EVOO/10 olive-pomace | Croatia | HNO3 and milli-Q water | HR ICP-MS | Li, Rb, Mo, Cd, Sn, Cs, Tl, Pb, Na, Mg, P, S, Ca, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Sr, Y, Sb, Ba, La, Ce; and K | - | - | - | - | - | [18] | |
| VOO, pomace-olive, corn, sunflower and soybean oils | 50 | Spain | HNO3 | ICP-MS | Ag, As, Ba, Be, Cd, Co, Cr, Cu, Fe, Hg, Mn, Mo, Ni, Pb, Sb, Ti, Tl and V | 0.8; 3.0; 0.5; 1.5; 1.5; 1.5; 8.0; 1.5; 40; 12; 1.5; 1.5; 15; 0.8; 1.5; 15; 1.5 and 2.0 (µg kg−1) | 109469 Multi-element Standard II Oil Dissolved | R: 85–115% | PCA | Quality identification of oils | [51] | |
| Discrimination between oils of different types | ||||||||||||
| VOO | - | Italy | HNO3 and H2O2 | ICP-OES | Pb, Zn, Cd and Cu | - | - | - | - | The influence of olive cultivars and period of harvest on the contents of some elements, | [63] | |
| OO | 90 | Tunisia | HNO3 | ICP-MS | Li, B, Na, Mg, Al, K, Ca, Sc, Cr, Mn, Fe, Co, Ni, Cu, Sr, Mo, Ba and La | 0.005; 0.051; 0.104; 5.118; 0.953; 0.319; 0.587; 0.000; 0.000; 0.012; 0.294; 0.005; 0.011; 0.000; 0.006; 0.028; 0.007 and 0.017 (mg kg−1) | Spike with standard solutions | R: 69–120% | PCA, LDA and ANOVA | The influence of the irrigation with treated waste water on the multi-elemental profile of olive oils | [30] | |
| Dry ashing | OO | 17 | Croatia | HCl | ElectroThermal Atomic Absorption Spectroscopy (ETAAS) | Cu, Ni, Pb and Fe | - | - | - | - | Comparison between sample preparation procedures | [46] |
| Acid extraction | EVOO | 539 | Italy | 1% HNO3/ 0.2% HCl | ICP-MS | Li, Na, Mg, K, Ca, Mn, Co, Cu, Rb, Sr, Cs, Ba, La, Ce, Sm, Eu, Yb, Pb and U | 0.005; 40; 14; 60; 30; 0.01; 0.004; 0.13; 0.03; 0.04; 0.003; 0.29; 0.0017; 0.0027; 0.0009; 0.0002; 0.0004; 0.02 and 0.001 (µg kg−1) | Spike with NIST 2387 (peanut butter) | R: 82–101% | - | Investigation of mineral composition of authentic PDO Italian olive oils | [41] |
| EVOO | 267 | Italy, France, Spain, Greece and Portugal | 6.7% H2O2 /1% HNO3/0,2% HCl | ICP-MS | Li, B, Na, Mg, Al, K, Ca, V, Mn and Co | 0.008; 0.17;20; 4; 3; 20; 25; 0.007; 0.2; 0.002 and 0.0006 (µg kg−1) | Spike with NIST 2387 (peanut butter) and SPEX s-23 100z | R (NIST): 82–101% R (SPEX standard): 53–92% | Canonical discriminant analysis | Authentication, traceability | [15] | |
| OO, sunflower, soybean, grape and sesame | - | - | 3% HNO3 | FAAS | Cu, Cd, Ni, Pb and Zn | 0.7; 0.3; 0.5; 1.5 and 0.5 (µg kg−1) | - | - | - | Development of analytical method | [64] | |
| EVOO, VOO, ROO, soybean and sunflower oils | - | - | 10% HNO3 | GF-AAS | Cu and Fe | - | Spike with standard | ACC: 94% ± 23–97% ± 12 | - | [65] | ||
| Extraction employing an extraction agent | Sunflower oil, OO, rapeseed oil and salmon oil | - | - | 1% Lipase solution at pH 3 | ICP-MS | Al, Ba, Cd, Cu, Fe, Mn, Mo, Ni, Ti, V and Zn | 0.46, 0.03, 0.007, 0.028, 0.67, 0.038, 0.022, 0.14, 0.17, 0.05 and 0.07 (µg kg−1) | EnviroMAT HU-1 Used oil diluted in sunflower oil | R: 83.3–117.8% | - | [66] | |
| Sunflower oil, OO, rapeseed oil | - | - | 0.01 M EDTA solution at pH8 | - | Al, Ca, Cd, Cu, Mg, Mn, Ni, Ti, V and Zn, | 2.47, 2.81, 0.013, 0.037, 1.37, 0.050, 0.049, 0.47, 0.032 and 0.087 (µg kg−1) | Spike of sunflower seed oil with EnviroMAT HU-1 | - | - | [67] | ||
| Mustard oil, sun flower oil, sesame oil, ground nut oil, coconut oil, rice bran oil and corn oil | - | - | TMAH and 2% EDTA at pH 12 | GF-AAS | Pb, Cd, Cr, Mn, Fe, Cu and Zn | 0.6; 0.4; 3.1; 0.3; 0.1; 2.3 and 1.5 (µg kg−1) | Spike with analytes | R: 92–97% | - | [68] | ||
| Emulsification | VOO | 5 | - | 2% Triton X-100 | ICP-MS | Al, Ba, Bi, Cd, Co, Cu, Mn, Ni, Pb, Sn and V | 5.31; 2.27; 0.98; 0.69; 1.09; 0.33; 0.44; 0.15; 0.02; 0.06 and 3.08 (µg kg−1) | Spike with analytes | R: 49.6–176.2% | - | [56] | |
| Sunflower, hazelnut, canola, corn and OO | 50 | Turkey | Acidic Triton X-114 solution | ICP-OES | Cd, Cr, Cu, Fe, Mn, Ni, Pb and Zn | - | Spike with analytes | R: 96–109% | ANOVA | Comparison between sample preparation procedures | [52] | |
| Solubilization with strong alkaline reagent | Almond, corn, sunflower oils and OO | 17 | - | TMAH and 1% HNO3 | ICP-MS | Cu, Ge, Mn, Mo, Ni, Sb, Sr, Ti, V | 0.02; 0.05; 0.004; 0.008; 0.012; 0.32; 0.004; 0.28 and 0.02 (µg g−1) | - | - | - | Development of analytical method | [69] |
| Dilution with organic solvent | EVOO | 50 | Spain | Methyl-isobutylketone (MIBK) | Electro-Thermal atomic absorption spectroscopy (ETAAS) | Cu, Cr, Fe, Mn and Ni | 25; 1.5; 80; 2 and 10 (pg) | 109469 Multi-element Standard II Oil Dissolved | ACC: 97.9–98.75% | Multivariate discriminant analysis | Authentication, traceability | [70] |
| Vegetable oils and fats | 11 | - | Xylene | ICP-OES equipped with hTISIS | Al, Ba, Ca, Cd, Cr, Cu, Fe, Mg, Mn, Mo, Ni, Si, Ti and V | 1.6; 0.35; 0.6; 2.6; 0.59; 0.94; 0.86; 0.16; 0.2; 4.1; 2.7; 0.91; 0.21 and 0.81 (µg kg−1) | Spike with the Conostan multi-elemental solution | Around 100% | - | Development of analytical method | [53] |
- (ii)
- Ashing in a furnace
- (iii)
- Methods of extraction
- Acid extraction (AE)
- Extraction using various organic/inorganic agents: chelating and emulsification
- (iv)
- Dilution with organic solvent
- (v)
- Emulsification
- (vi)
- Chelation of the analytes
- (vii)
- Comparative studies of different sample preparation methods
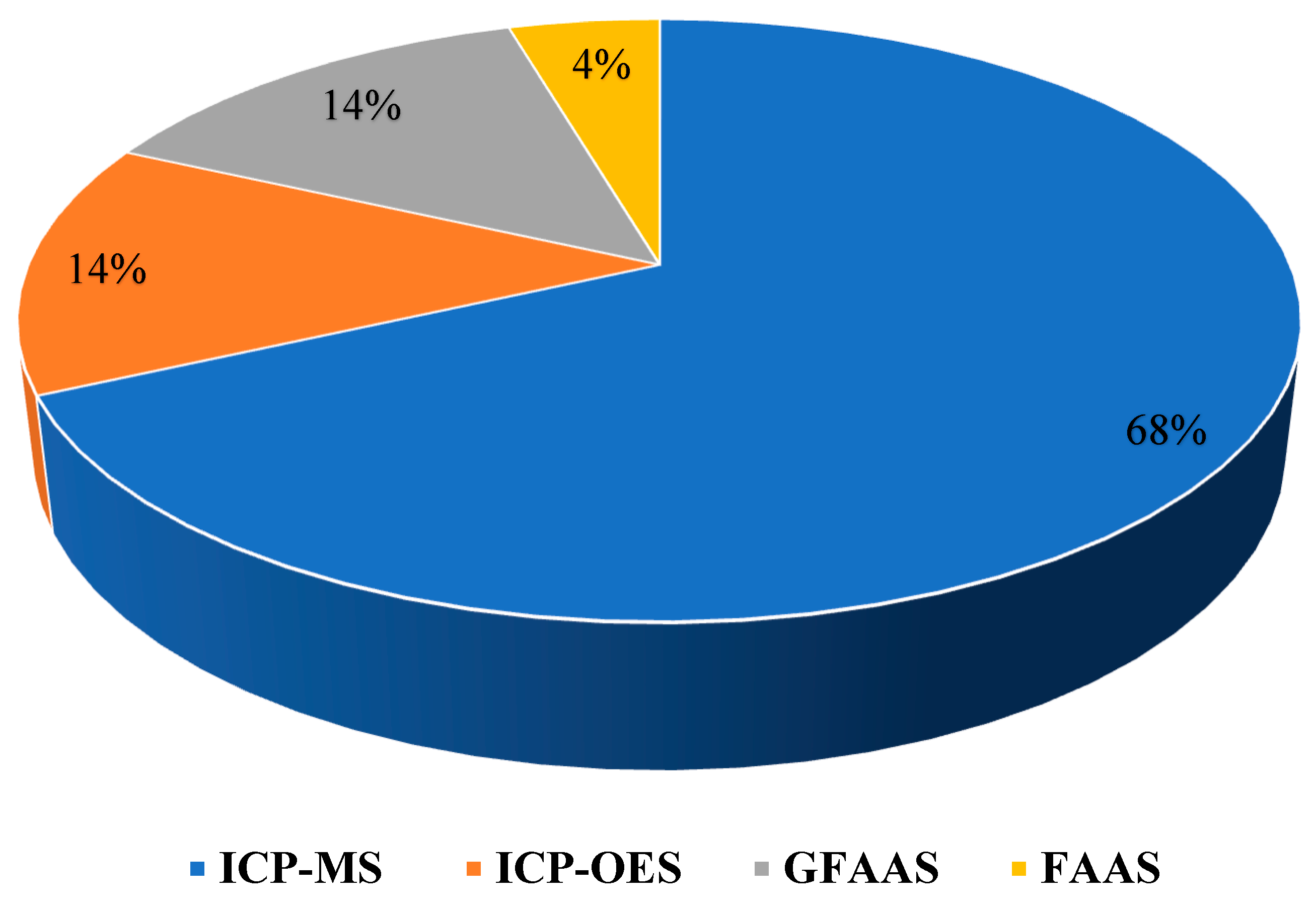
2.2.2. Detection Techniques
2.2.3. Analytical Method Validation
2.3. Limits of Olive Oil Authentication by Means of Trace Elements
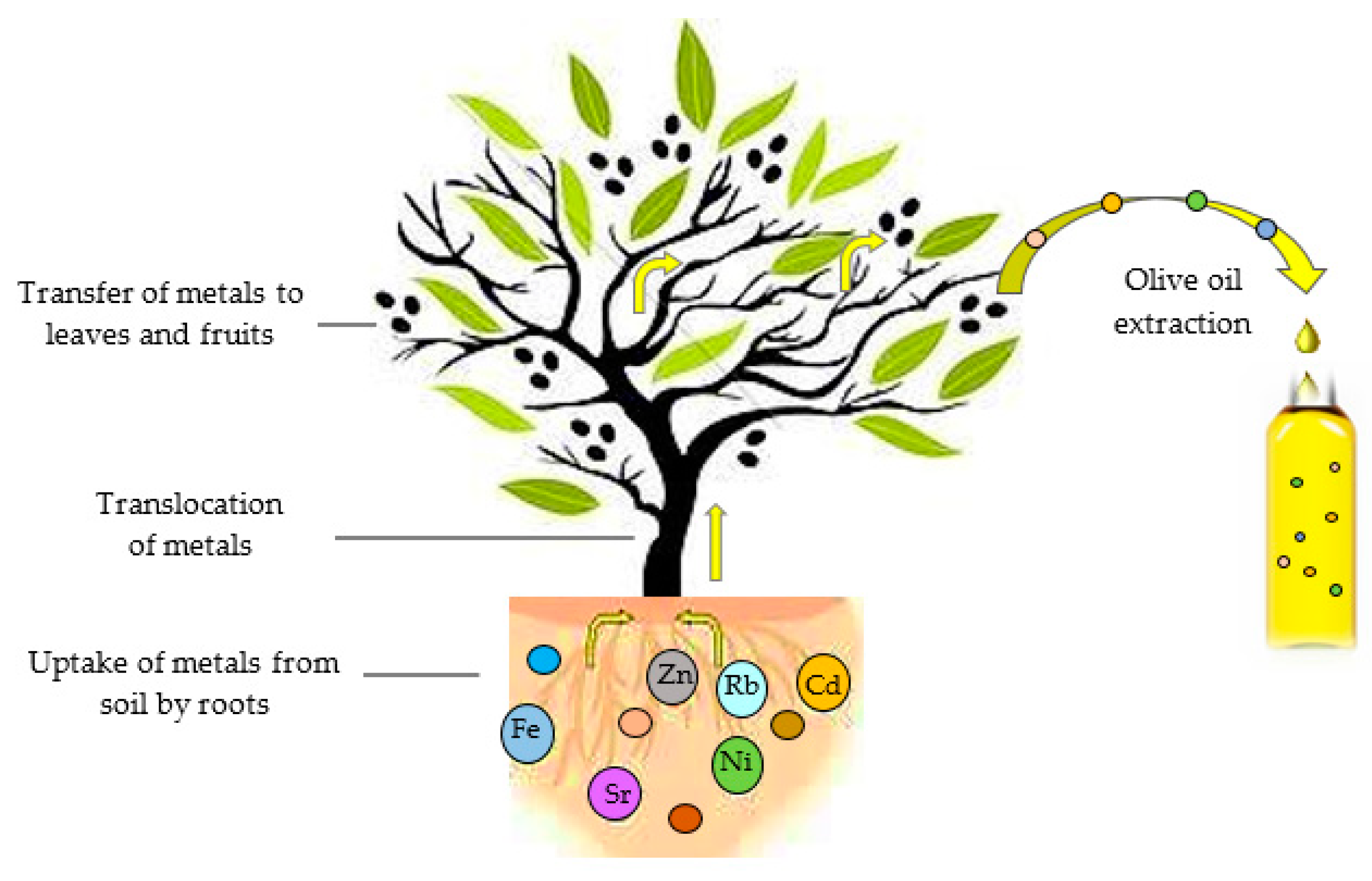
2.3.1. Influence of Agro-Climate Conditions
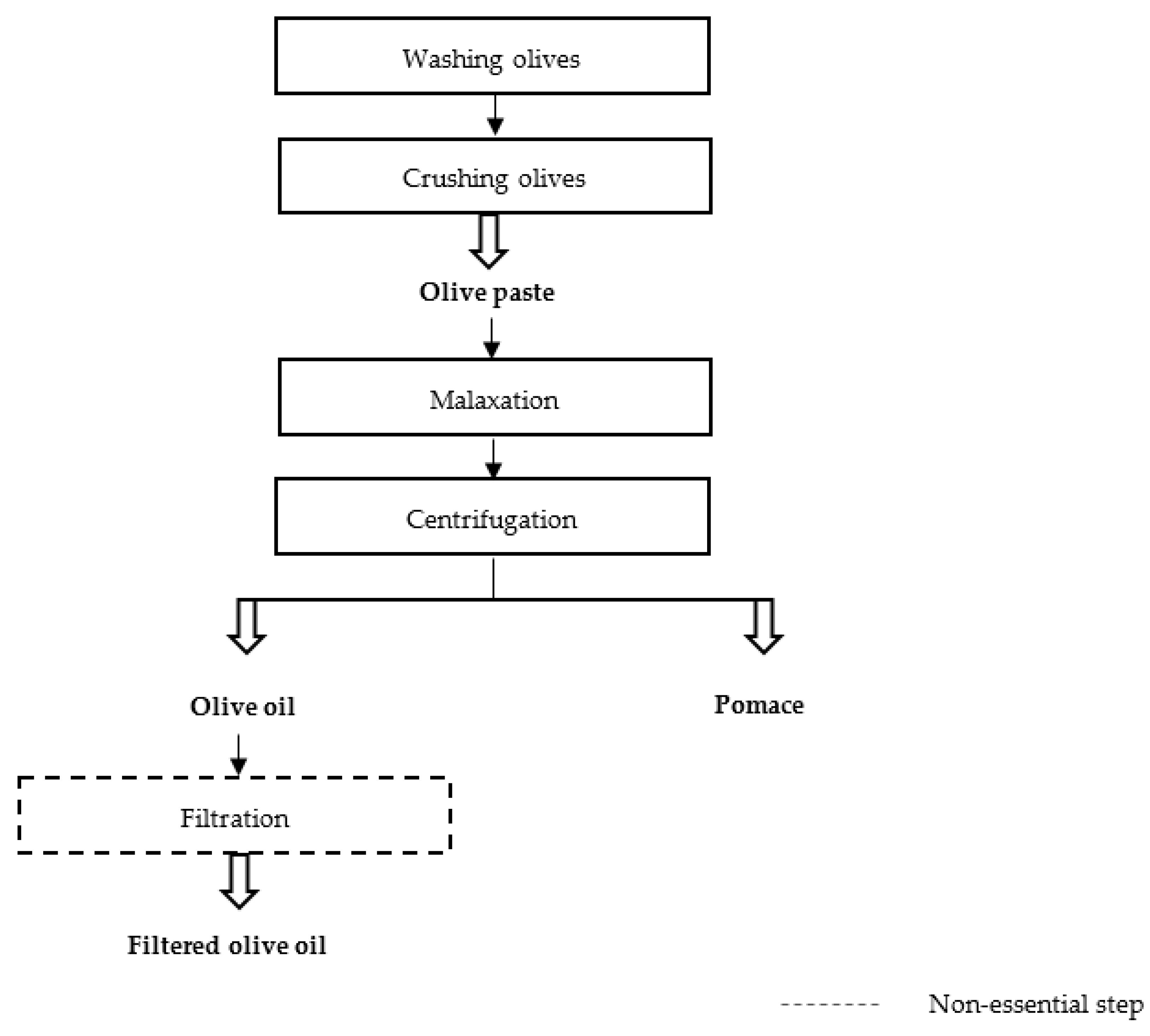
2.3.2. Influence of the Olive Oil Extraction Process
3. Olive Oil Geographical Traceability by Means of Isotopic Analysis
3.1. Stable Isotopes of Light Elements (C, H and O)
- (i)
- δ13C measurements as a tracer of physiological processes of the plant
- (ii)
- δ2H and δ18O measurements as a tracer of water dynamics
| Samples | Number of Samples | Origin | Isotopes | Detection Technique (Manufacturer) | Statistical Evaluation | Complementary Analysis | Purpose of Using Isotopes | References |
|---|---|---|---|---|---|---|---|---|
| EVOO | 539 | Italy | 18O/16O, 2H/1H and 13C/12C | IRMS (Finnigan DELTA XP, Thermo Scientific, Bremen, Germany) | The non-parametric test of Kruskall–Wallis | Multi-elemental analysis |
| [41] |
| EVOO | 267 | Italy, France, Spain, Greece and Portugal | 18O/16O, 2H/1H and 13C/12C | IRMS (Delta plus XL, Delta Plus XP, Delta V, Delta S, Thermo- Finnigan, Bremen, Gremany; Isoprime, AP2003, GV Instruments Ltd., Manchester, U.K.; Optima Micromass) | Kolmogorov-Smirnov test | Finding correlation between H, C and O isotope ratios in olive oil and climatic and geographical characteristics of the provenance locations. | [15] | |
| VOO | 49 | Turkey | 13C/12C | IRMS (Micro- mass, IsoPrime) | PCA and HCA | Combination of trace element concentrations and 13C/12C isotope ratio for better resolution in geographical discrimination of olive oils. | [61] | |
| VOO | 47 | Greece | 18O/16O and 13C/12C | IRMS (Finnigan Delta V Advantage, Thermo Fisher Scientific, Bremen, Germany) | Multivariate analysis | Multi-elemental analysis/ Determination of chlorophyll and carotenoid pigments | Combination of 18O/16O and 13C/12C isotope ratios and physicochemical parameters for geographical classification of olive oils from regions in proximity. | [97] |
| VOO | 387 | Italy | 18O/16O and 13C/12C | IRMS (Isoprime, Cheadle, UK) | Pearson coefficient and ANOVA | - | Use of stable isotope ratios as tracers for environmental conditions and geographic coordinates for olive oil geographical authentication. | [32] |
| Edible oils and sweeteners | 43 | Italy, Greece and Spain | 18O/16O, 2H/1H and 13C/12C | IRMS (Thermo-Finnigan Delta plus XP, Thermo Fisher Scientific Inc., Waltham, MA, USA) | - | Multi-elemental analysis |
| [80] |
| OO | 180 | Italy | 18O/16O, 2H/1H and 13C/12C | IRMS (Finnigan DELTA XP, Thermo Scientific, Bremen, Germany) | - | The acidity values, UV spectrophotometric indices (K232, K270, DK) and fatty acid composition | Measurement of Stable isotope ratios in legal applications for geographical origin of food (olive oil). | [31] |
| OO | - | Italy | 18O/16O and 13C/12C | IRMS (Isoprime, GV, Cheadle, UK) | Factorial analysis of-variance (ANOVA) and Post Hoc Fisher multiple comparison test | Fatty acid composition | Effect of the cultivar and the ripening stage of olives on C and O isotope composition for traceability studies. | [89] |
| EVOO | 53 | Italy and Croatia | 18O/16O and 13C/12C | IRMS (Delta plus XP; ThermoFinnigan, Bremen, German) | Linear discriminant analysis | Major chemical component determination (triacylglycerol and fatty acids)/Thermal properties | Comparison between conventional techniques, stable isotope ratio analysis and thermal properties for olive oil traceability resolution. | [98] |
| OO | 100 | Greece | 18O/16O, 2H/1H and 13C/12C | IRMS (Nu Instruments Limited, Wrexham, UK) | PCA/OPLS-DA | - | Creation of OPLS-DA model, using stable isotope ratios of C, H and O in olive oil, able to discriminate and predict origin of samples from different origin. | [90] |
| EVOO | 210 | Greece | 18O/16O and 13C/12C | IRMS (not mentioned) | - | - |
| [88] |
3.2. Strontium Isotope Ratio Determination for Olive Oil Geographical Traceability
4. Statistical Analysis for Geographical Authentication of Olive Oil
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Fausto, L. Introduction. In Handbook of Olive Oil; Harwood, J., Aparicio, R., Eds.; Aspen Publishers, Inc.: Riverwoods, IL, USA, 2000; ISBN 978-1-4419-5194-6. [Google Scholar]
- Flori, L.; Donnini, S.; Calderone, V.; Zinnai, A.; Taglieri, I.; Venturi, F.; Testai, L. The Nutraceutical Value of Olive Oil and Its Bioactive Constituents on the Cardiovascular System. Focusing on Main Strategies to Slow Down Its Quality Decay during Production and Storage. Nutrients 2019, 11, 1962. [Google Scholar] [CrossRef] [Green Version]
- Viola, P.; Viola, M.; Academy, O.N.; Libertà, P.; Pg, S. Virgin Olive Oil as a Fundamental Nutritional Component and Skin Protector. Clin. Dermatol. 2009, 27, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Poljšak, N.; Kreft, S.; Kočevar Glavač, N. Vegetable Butters and Oils in Skin Wound Healing: Scientific Evidence for New Opportunities in Dermatology. Phytother. Res. 2020, 34, 254–269. [Google Scholar] [CrossRef]
- Matthäus, B.; Willenberg, I.; Engert, S.; Steinberg, P. The German National Reference Centre for Authentic Food (NRZ-Authent). OCL-Oilseeds Fats Crops Lipids 2019, 26, 11. [Google Scholar] [CrossRef]
- Yan, J.; Erasmus, S.W.; Aguilera Toro, M.; Huang, H.; van Ruth, S.M. Food Fraud: Assessing Fraud Vulnerability in the Extra Virgin Olive Oil Supply Chain. Food Control 2020, 111, 107081. [Google Scholar] [CrossRef]
- Bimbo, F.; Bonanno, A.; Viscecchia, R. An Empirical Framework to Study Food Labelling Fraud: An Application to the Italian Extra-virgin Olive Oil Market. Aust. J. Agric. Resour. 2019, 63, 701–725. [Google Scholar] [CrossRef]
- Shears, P. Food Fraud–a Current Issue but an Old Problem. Br. Food J. 2010, 112, 198–213. [Google Scholar] [CrossRef] [Green Version]
- Gonzálvez, A.; de la Guardia, M. Mineral Profile. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; Volume 60, pp. 51–76. [Google Scholar] [CrossRef]
- Dekhili, S.; Sirieix, L.; Cohen, E. How Consumers Choose Olive Oil: The Importance of Origin Cues. Food Qual. Prefer. 2011, 22, 757–762. [Google Scholar] [CrossRef]
- Menapace, L.; Colson, G.; Grebitus, C.; Facendola, M. Consumers’ Preferences for Geographical Origin Labels: Evidence from the Canadian Olive Oil Market. Eur. Rev. Agric. Econ. 2011, 38, 193–212. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Aalizadeh, R.; Dasenaki, M.E.; Thomaidis, N.S. Application of High Resolution Mass Spectrometric Methods Coupled with Chemometric Techniques in Olive Oil Authenticity Studies—A Review. Anal. Chim. Acta 2020, 1134, 150–173. [Google Scholar] [CrossRef]
- Maléchaux, A.; Le Dréau, Y.; Artaud, J.; Dupuy, N. Exploring the Scientific Interest for Olive Oil Origin: A Bibliometric Study from 1991 to 2018. Foods 2020, 9, 556. [Google Scholar] [CrossRef]
- Yorulmaz, H.O.; Konuskan, D.B. Antioxidant Activity, Sterol and Fatty Acid Compositions of Turkish Olive Oils as an Indicator of Variety and Ripening Degree. J. Food Sci. Technol. 2017, 54, 4067–4077. [Google Scholar] [CrossRef]
- Camin, F.; Larcher, R.; Nicolini, G.; Bontempo, L.; Bertoldi, D.; Perini, M.; Schlicht, C.; Schellenberg, A.; Thomas, F.; Heinrich, K.; et al. Isotopic and Elemental Data for Tracing the Origin of European Olive Oils. J. Agric. Food Chem. 2010, 58, 570–577. [Google Scholar] [CrossRef]
- Safarzadeh Markhali, F. Effect of Processing on Phenolic Composition of Olive Oil Products and Olive Mill By-Products and Possibilities for Enhancement of Sustainable Processes. Processes 2021, 9, 953. [Google Scholar] [CrossRef]
- García-González, D.L.; Aparicio, R. Classification of Different Quality Virgin Olive Oils by Metal-Oxide Sensors. Eur. Food Res. Technol. 2004, 218, 484–487. [Google Scholar] [CrossRef]
- Pošćić, F.; Furdek Turk, M.; Bačić, N.; Mikac, N.; Bertoldi, D.; Camin, F.; Jukić Špika, M.; Žanetić, M.; Rengel, Z.; Perica, S. Removal of Pomace Residues Is Critical in Quantification of Element Concentrations in Extra Virgin Olive Oil. J. Food Compos. Anal. 2019, 77, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Frankel, E.N. Chemistry of Extra Virgin Olive Oil: Adulteration, Oxidative Stability, and Antioxidants. J. Agric. Food Chem. 2010, 58, 5991–6006. [Google Scholar] [CrossRef]
- Dabbou, S.; Brahmi, F.; Taamali, A.; Issaoui, M.; Ouni, Y.; Braham, M.; Zarrouk, M.; Hammami, M. Extra Virgin Olive Oil Components and Oxidative Stability from Olives Grown in Tunisia. J. Am. Oil Chem. Soc. 2010, 87, 1199–1209. [Google Scholar] [CrossRef]
- Tres, A.; van der Veer, G.; van Ruth, S.M. Vegetable Oils. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; Volume 60, pp. 543–572. ISBN 978-0-444-59562-1. [Google Scholar]
- Gertz, C.; Gertz, A.; Matthäus, B.; Willenberg, I. A Systematic Chemometric Approach to Identify the Geographical Origin of Olive Oils. Eur. J. Lipid Sci. Technol. 2019, 121, 1900281. [Google Scholar] [CrossRef] [Green Version]
- Bajoub, A.; Hurtado-Fernández, E.; Ajal, E.A.; Ouazzani, N.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Comprehensive 3-Year Study of the Phenolic Profile of Moroccan Monovarietal Virgin Olive Oils from the Meknès Region. J. Agric. Food Chem. 2015, 63, 4376–4385. [Google Scholar] [CrossRef]
- Quintanilla-Casas, B.; Bertin, S.; Leik, K.; Bustamante, J.; Guardiola, F.; Valli, E.; Bendini, A.; Toschi, T.G.; Tres, A.; Vichi, S. Profiling versus Fingerprinting Analysis of Sesquiterpene Hydrocarbons for the Geographical Authentication of Extra Virgin Olive Oils. Food Chem. 2020, 307, 125556. [Google Scholar] [CrossRef] [Green Version]
- Youseff, R.; Soubh, L.; Alassaf, Z. Detection of Vegetable Oils Adulteration Using Desmethylsterols Composition. Int. J. Pharm. Sci. 2014, 28, 229–233. [Google Scholar]
- Noorali, M.; Barzegar, M.; Sahari, M.A. Sterol and fatty acid compositions of olive oil as an indicator of cultivar and growing area. J. Am. Oil Chem. Soc. 2014, 91, 1571–1581. [Google Scholar] [CrossRef]
- Danezis, G.P.; Tsagkaris, A.S.; Camin, F.; Brusic, V.; Georgiou, C.A. Food Authentication: Techniques, Trends & Emerging Approaches. Trends Anal. Chem. 2016, 85, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Yadav, K.K.; Kumar, V.; Kumar, S.; Chadd, R.P.; Kumar, A. Trace Elements in Soil-Vegetables Interface: Translocation, Bioaccumulation, Toxicity and Amelioration—A Review. Sci. Total Environ. 2019, 651, 2927–2942. [Google Scholar] [CrossRef]
- Wadood, S.A.; Boli, G.; Xiaowen, Z.; Hussain, I.; Yimin, W. Recent Development in the Application of Analytical Techniques for the Traceability and Authenticity of Food of Plant Origin. Microchem. J. 2020, 152, 104295. [Google Scholar] [CrossRef]
- Benincasa, C.; Gharsallaoui, M.; Perri, E.; Briccoli Bati, C.; Ayadi, M.; Khlif, M.; Gabsi, S. Quality and Trace Element Profile of Tunisian Olive Oils Obtained from Plants Irrigated with Treated Wastewater. Sci. World J. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Camin, F.; Pavone, A.; Bontempo, L.; Wehrens, R.; Paolini, M.; Faberi, A.; Maria, R.; Capitani, D.; Vista, S.; Mannina, L. The Use of IRMS, 1 H NMR and Chemical Analysis to Characterise Italian and Imported Tunisian Olive Oils. Food Chem. 2016, 196, 98–105. [Google Scholar] [CrossRef]
- Portarena, S.; Gavrichkova, O.; Lauteri, M.; Brugnoli, E. Authentication and Traceability of Italian Extra-Virgin Olive Oils by Means of Stable Isotopes Techniques. Food Chem. 2014, 164, 12–14. [Google Scholar] [CrossRef]
- Voerkelius, S.; Lorenz, G.D.; Rummel, S.; Quétel, C.R.; Heiss, G.; Baxter, M.; Brach-Papa, C.; Deters-Itzelsberger, P.; Hoelzl, S.; Hoogewerff, J.; et al. Strontium Isotopic Signatures of Natural Mineral Waters, the Reference to a Simple Geological Map and Its Potential for Authentication of Food. Food Chem. 2010, 118, 933–940. [Google Scholar] [CrossRef]
- Capo, R.C.; Stewart, B.W.; Chadwick, O.A. Strontium Isotopes as Tracers of Ecosystem Processes: Theory and Methods. Geoderma 1998, 82, 197–225. [Google Scholar] [CrossRef]
- Medini, S.; Janin, M.; Verdoux, P.; Techer, I. Methodological Development for 87Sr/86Sr Measurement in Olive Oil and Preliminary Discussion of Its Use for Geographical Traceability of PDO Nîmes (France). Food Chem. 2015, 171, 78–83. [Google Scholar] [CrossRef]
- Benincasa, C.; Lewis, J.; Perri, E.; Sindona, G.; Tagarelli, A. Determination of Trace Element in Italian Virgin Olive Oils and Their Characterization According to Geographical Origin by Statistical Analysis. Anal. Chim. Acta 2007, 585, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Carini, F.; Bengtsson, G. Post-Deposition Transport of Radionuclides in Fruit. J. Environ. Radioact. 2001, 52, 215–236. [Google Scholar] [CrossRef]
- Alloway, B.J.; Davies, B.E. Trace Element Content of Soils Affected by Base Metal Mining in Wales. Geoderma 1971, 5, 197–208. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z.; Waqas, M. The Uptake and Bioaccumulation of Heavy Metals by Food Plants, Their Effects on Plants Nutrients, and Associated Health Risk: A Review. Environ. Sci. Pollut. Res. 2015, 22, 13772–13799. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, A.; Baize, D.; Raoul, C.; Daniau, C. Proposition de Référentiels Régionaux En Éléments Traces Métalliques Dans Les Sols: Leur Utilisation Dans Les Évaluations Des Risques Sanitaires. Environ. Risques St. 2008, 7, 112–122. [Google Scholar] [CrossRef]
- Camin, F.; Larcher, R.; Perini, M.; Bontempo, L.; Bertoldi, D.; Gagliano, G.; Nicolini, G.; Versini, G. Characterisation of Authentic Italian Extra-Virgin Olive Oils by Stable Isotope Ratios of C, O and H and Mineral Composition. Food Chem. 2010, 118, 901–909. [Google Scholar] [CrossRef]
- Shahzad, B.; Mughal, M.N.; Tanveer, M.; Gupta, D.; Abbas, G. Is Lithium Biologically an Important or Toxic Element to Living Organisms? An Overview. Environ. Sci. Pollut. Res. 2017, 24, 103–115. [Google Scholar] [CrossRef]
- Medini, S. Traçage géographique des Huiles D’olive par les Isotopes du Sr: Développement Analytique et Application aux Huiles AOP de Nîmes. Ph.D. Thesis, Université d’Aix-Marseille, Marseille, France, 2015. [Google Scholar]
- Skrzydlewska, E.; Balcerzak, M.; Vanhaecke, F. Determination of Chromium, Cadmium and Lead in Food-Packaging Materials by Axial Inductively Coupled Plasma Time-of-Flight Mass Spectrometry. Anal. Chim. Acta 2003, 479, 191–202. [Google Scholar] [CrossRef]
- Beltrán, M.; Sánchez-Astudillo, M.; Aparicio, R.; García-González, D.L. Geographical Traceability of Virgin Olive Oils from South-Western Spain by Their Multi-Elemental Composition. Food Chem. 2015, 169, 350–357. [Google Scholar] [CrossRef]
- Brkljača, M.; Giljanović, J.; Prkić, A. Determination of Metals in Olive Oil by Electrothermal Atomic Absorption Spectrometry: Validation and Uncertainty Measurements. Anal. Lett. 2013, 46, 2912–2926. [Google Scholar] [CrossRef]
- Nasr, E.G.; Epova, E.N.; De Diego, A.; Souissi, R.; Hammami, M.; Abderrazak, H.; Donard, O.F.X. Trace Elements Analysis of Tunisian and European Extra Virgin Olive Oils by ICP-MS and Chemometrics for Geographical Discrimination. Foods 2022, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Zeiner, M.; Steffan, I.; Cindric, I.J. Determination of Trace Elements in Olive Oil by ICP-AES and ETA-AAS: A Pilot Study on the Geographical Characterization. Microchem. J. 2005, 81, 171–176. [Google Scholar] [CrossRef]
- Cindric, I.J.; Zeiner, M.; Steffan, I. Trace Elemental Characterization of Edible Oils by ICP–AES and GFAAS. Microchem. J. 2007, 85, 136–139. [Google Scholar] [CrossRef]
- Damak, F.; Asano, M.; Baba, K.; Suda, A.; Araoka, D.; Wali, A.; Isoda, H.; Nakajima, M.; Ksibi, M.; Tamura, K. Interregional Traceability of Tunisian Olive Oils to the Provenance Soil by Multielemental Fingerprinting and Chemometrics. Food Chem. 2019, 283, 656–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llorent-Martínez, E.J.; Ortega-Barrales, P.; Fernández-De Córdova, M.L.; Domínguez-Vidal, A.; Ruiz-Medina, A. Investigation by ICP-MS of Trace Element Levels in Vegetable Edible Oils Produced in Spain. Food Chem. 2011, 127, 1257–1262. [Google Scholar] [CrossRef]
- Bakircioglu, D.; Kurtulus, Y.B.; Yurtsever, S. Comparison of Extraction Induced by Emulsion Breaking, Ultrasonic Extraction and Wet Digestion Procedures for Determination of Metals in Edible Oil Samples in Turkey Using ICP-OES. Food Chem. 2013, 138, 770–775. [Google Scholar] [CrossRef]
- Martínez, S.; Sánchez, R.; Lefevre, J.; Todolí, J.L. Multielemental Analysis of Vegetable Oils and Fats by Means of ICP-OES Following a Dilution and Shot Methodology. J. Anal. At. Spectrom. 2020, 35, 1897–1909. [Google Scholar] [CrossRef]
- Woods, G.D.; Fryer, F.I. Direct Elemental Analysis of Biodiesel by Inductively Coupled Plasma–Mass Spectrometry. Anal. Bioanal. Chem. 2007, 389, 753–761. [Google Scholar] [CrossRef]
- dos Santos, E.J.; Herrmann, A.B.; Chaves, E.S.; Vechiatto, W.W.D.; Schoemberger, A.C.; Frescura, V.L.A.; Curtius, A.J. Simultaneous Determination of Ca, P, Mg, K and Na in Biodiesel by Axial View Inductively Coupled Plasma Optical Emission Spectrometry with Internal Standardization after Multivariate Optimization. J. Anal. At. Spectrom. 2007, 22, 1300. [Google Scholar] [CrossRef]
- Jimenez, M.S.; Velarte, R.; Castillo, J.R. On-Line Emulsions of Olive Oil Samples and ICP-MS Multi-Elemental Determination. J. Anal. At. Spectrom. 2003, 18, 1154–1162. [Google Scholar] [CrossRef]
- Mdluli, N.S.; Nomngongo, P.N.; Mketo, N. A Critical Review on Application of Extraction Methods Prior to Spectrometric Determination of Trace-Metals in Oily Matrices. Crit. Rev. Anal. Chem. 2020, 52, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lepri, F.G.; Chaves, E.S.; Vieira, M.A. Determination of Trace Elements in Vegetable Oils and Biodiesel by Atomic Spectrometric Techniques: A Review. Appl. Spectrosc. Rev. 2011, 46, 175–206. [Google Scholar] [CrossRef]
- Ekezie, F.-G.C.; Sun, D.-W.; Cheng, J.-H. Acceleration of Microwave-Assisted Extraction Processes of Food Components by Integrating Technologies and Applying Emerging Solvents: A Review of Latest Developments. Trends Food Sci. Technol. 2017, 67, 160–172. [Google Scholar] [CrossRef]
- Aceto, M.; Calà, E.; Musso, D.; Regalli, N.; Oddone, M. A Preliminary Study on the Authentication and Traceability of Extra Virgin Olive Oil Made from Taggiasca Olives by Means of Trace and Ultra-Trace Elements Distribution. Food Chem. 2019, 298, 125047. [Google Scholar] [CrossRef]
- Gumus, Z.P.; Celenk, V.U.; Tekin, S.; Yurdakul, O.; Ertas, H. Determination of Trace Elements and Stable Carbon Isotope Ratios in Virgin Olive Oils from Western Turkey to Authenticate Geographical Origin with a Chemometric Approach. Eur. Food Res. Technol. 2017, 243, 1719–1727. [Google Scholar] [CrossRef]
- Sayago, A.; González-Domínguez, R.; Beltrán, R.; Fernández-Recamales, Á. Combination of Complementary Data Mining Methods for Geographical Characterization of Extra Virgin Olive Oils Based on Mineral Composition. Food Chem. 2018, 261, 42–50. [Google Scholar] [CrossRef]
- Angioni, A.; Cabitza, M.; Russo, M.T.; Caboni, P. Influence of Olive Cultivars and Period of Harvest on the Contents of Cu, Cd, Pb, and Zn in Virgin Olive Oils. Food Chem. 2006, 99, 525–529. [Google Scholar] [CrossRef]
- Mohebbi, M.; Heydari, R.; Ramezani, M. Determination of Cu, Cd, Ni, Pb and Zn in Edible Oils Using Reversed-Phase Ultrasonic Assisted Liquid–Liquid Microextraction and Flame Atomic Absorption Spectrometry. J. Anal. Chem. 2018, 73, 30–35. [Google Scholar] [CrossRef]
- De Leonardis, A.; Macciola, V.; De Felice, M. Copper and Iron Determination in Edible Vegetable Oils by Graphite Furnace Atomic Absorption Spectrometry after Extraction with Diluted Nitric Acid. Int. J. Food Sci. 2000, 35, 371–375. [Google Scholar] [CrossRef]
- Kara, D.; Fisher, A.; Hill, S. Extraction of Trace Elements by Ultrasound-Assisted Emulsification from Edible Oils Producing Detergentless Microemulsions. Food Chem. 2015, 188, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Kara, D.; Fisher, A.; Hill, S. Detergentless Ultrasound-Assisted Extraction of Trace Elements from Edible Oils Using Lipase as an Extractant. Talanta 2015, 144, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Manjusha, R.; Shekhar, R.; Kumar, S.J. Ultrasound-Assisted Extraction of Pb, Cd, Cr, Mn, Fe, Cu, Zn from Edible Oils with Tetramethylammonium Hydroxide and EDTA Followed by Determination Using Graphite Furnace Atomic Absorption Spectrometer. Food Chem. 2019, 294, 384–389. [Google Scholar] [CrossRef]
- Savio, M.; Ortiz, M.S.; Almeida, C.A.; Olsina, R.A.; Martinez, L.D.; Gil, R.A. Multielemental Analysis in Vegetable Edible Oils by Inductively Coupled Plasma Mass Spectrometry after Solubilisation with Tetramethylammonium Hydroxide. Food Chem. 2014, 159, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Vique, C.; Bouzas, P.R.; Oliveras-López, M.J. Determination of Trace Elements in Extra Virgin Olive Oils: A Pilot Study on the Geographical Characterisation. Food Chem. 2012, 134, 434–439. [Google Scholar] [CrossRef]
- Damak, F.; Asano, M.; Baba, K.; Ksibi, M.; Tamura, K. Comparison of Sample Preparation Methods for Multielements Analysis of Olive Oil by ICP-MS. Methods Protoc. 2019, 2, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valasques, G.S.; dos Santos, A.M.P.; Teixeira, L.S.G.; da Mata Cerqueira, U.M.F.; de Souza, V.S.; Bezerra, M.A. Applications of Emulsified Systems in Elemental Analysis by Spectroanalytical Techniques. Appl. Spectrosc. Rev. 2017, 52, 729–753. [Google Scholar] [CrossRef]
- Goodarzi, F.; Zendehboudi, S. A Comprehensive Review on Emulsions and Emulsion Stability in Chemical and Energy Industries. Can. J. Chem. Eng. 2019, 97, 281–309. [Google Scholar] [CrossRef] [Green Version]
- Rutkowska, M.; Namieśnik, J.; Konieczka, P. Ultrasound-Assisted Extraction. In The Application of Green Solvents in Separation Processes; Pena-Pereira, F., Tobiszewski, M., Eds.; Elsevier: Amsterdam, The Netherlans, 2017; pp. 301–324. [Google Scholar] [CrossRef]
- Costa, L.M.; Silva, F.V.; Gouveia, S.T.; Nogueira, A.R.A.; Nobrega, J.A. Focused Microwave-Assisted Acid Digestion of Oils: An Evaluation of the Residual Carbon Content. Spectrochim. Acta B 2001, 56, 1981–1985. [Google Scholar] [CrossRef]
- Cesur, H.; Bati, B. Solid-Phase Extraction of Copper with Lead 4-Benzylpiperidinedithiocarbamate on Microcrystalline Naphthalene and Its Spectrophotometric Determination. Turk. J. Chem. 2002, 26, 599–605. [Google Scholar]
- Khan, M.A.; Khan, S.; Khan, A.; Alam, M. Soil Contamination with Cadmium, Consequences and Remediation Using Organic Amendments. Sci. Total Environ. 2017, 601–602, 1591–1605. [Google Scholar] [CrossRef]
- Greenough, J.D.; Fryer, B.J.; Mallory-Greenough, L. Trace Element Geochemistry of Nova Scotia (Canada) Maple Syrup. Can. J. Earth Sci. 2010, 47, 1093–1110. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Domínguez-Perles, R.; Machado, N.; Carvalho, T.; Matos, C.; Barros, A.I.R.N.A. Effect of Agro-Environmental Factors on the Mineral Content of Olive Oils: Categorization of the Three Major Portuguese Cultivars. J. Am. Oil Chem. Soc. 2016, 93, 813–822. [Google Scholar] [CrossRef]
- Banerjee, S.; Kurtis Kyser, T.; Vuletich, A.; Leduc, E. Elemental and Stable Isotopic Study of Sweeteners and Edible Oils: Constraints on Food Authentication. J. Food Compost. Anal. 2015, 42, 98–116. [Google Scholar] [CrossRef]
- Goidanich, P. Filtration Process of Extra Virgin Olive Oil: Effect on Minor Components, Oxidative Stability and Sensorial and Physicochemical Characteristics. Trends Food Sci. Technol. 2010, 21, 201–211. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Mattei, A.; Rovellini, P.; Zunin, P. Effect of Membrane Filtration on the Flavor of Virgin Olive Oil. Eur. J. Lipid Sci. Technol. 2008, 110, 1109–1115. [Google Scholar] [CrossRef]
- Rossmann, A. Determination Of Stable Isotope Ratios In Food Analysis. Food Rev. Int. 2001, 17, 347–381. [Google Scholar] [CrossRef]
- Kelly, S.D. Using Stable Isotope Ratio Mass Spectrometry (IRMS) in Food Authentication and Traceability. In Food Authenticity and Traceability; Elsevier: Amsterdam, The Netherlands, 2003; pp. 156–183. ISBN 978-1-85573-526-2. [Google Scholar]
- Camin, F.; Boner, M.; Bontempo, L.; Fauhl-Hassek, C.; Kelly, S.D.; Riedl, J.; Rossmann, A. Stable Isotope Techniques for Verifying the Declared Geographical Origin of Food in Legal Cases. Trends Food Sci. Technol. 2017, 61, 176–187. [Google Scholar] [CrossRef]
- Angerosa, F.; Bréas, O.; Contento, S.; Guillou, C.; Reniero, F.; Sada, E. Application of Stable Isotope Ratio Analysis to the Characterization of the Geographical Origin of Olive Oils. J. Agric. Food Chem. 1999, 47, 1013–1017. [Google Scholar] [CrossRef]
- Chiocchini, F.; Portarena, S.; Ciolfi, M.; Brugnoli, E.; Lauteri, M. Isoscapes of Carbon and Oxygen Stable Isotope Compositions in Tracing Authenticity and Geographical Origin of Italian Extra-Virgin Olive Oils. Food Chem. 2016, 202, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Karalis, P.; Poutouki, A.E.; Nikou, T.; Halabalaki, M.; Proestos, C.; Tsakalidou, E.; Gougoura, S.; Diamantopoulos, G.; Tassi, M.; Dotsika, E. Isotopic Traceability (13C and 18O) of Greek Olive Oil. Molecules 2020, 25, 5816. [Google Scholar] [CrossRef] [PubMed]
- Portarena, S.; Farinelli, D.; Lauteri, M.; Famiani, F.; Esti, M.; Brugnoli, E. Stable Isotope and Fatty Acid Compositions of Monovarietal Olive Oils: Implications of Ripening Stage and Climate Effects as Determinants in Traceability Studies. Food Control 2015, 57, 129–135. [Google Scholar] [CrossRef]
- Tarapoulouzi, M.; Skiada, V.; Agriopoulou, S.; Psomiadis, D.; Rébufa, C.; Roussos, S.; Theocharis, C.R.; Katsaris, P.; Varzakas, T. Chemometric Discrimination of the Geographical Origin of Three Greek Cultivars of Olive Oils by Stable Isotope Ratio Analysis. Foods 2021, 10, 336. [Google Scholar] [CrossRef]
- Schimmelmann, A.; Qi, H.; Dunn, P.J.H.; Camin, F.; Bontempo, L.; Potočnik, D.; Ogrinc, N.; Kelly, S.; Carter, J.F.; Abrahim, A.; et al. Food Matrix Reference Materials for Hydrogen, Carbon, Nitrogen, Oxygen, and Sulfur Stable Isotope-Ratio Measurements: Collagens, Flours, Honeys, and Vegetable Oils. J. Agric. Food Chem. 2020, 68, 10852–10864. [Google Scholar] [CrossRef] [PubMed]
- Cernusak, L.A.; Ubierna, N.; Winter, K.; Holtum, J.A.M.; Marshall, J.D.; Farquhar, G.D. Environmental and Physiological Determinants of Carbon Isotope Discrimination in Terrestrial Plants. New Phytol. 2013, 200, 950–965. [Google Scholar] [CrossRef]
- Winkler, F.J.; Schmidt, H.-L. Einsatzm6glichkeiten der 13C-Isotopen-Massenspektrometrie in dec Lebensmitteluntersuchung. Z. Lebensm. Unters. Forsch. 1980, 171, 85–94. [Google Scholar] [CrossRef]
- Higgins, P. Isotope ecology from biominerals. In Methods in Paleoecology: Reconstructing Cenozoic Terrestrial Environments and Ecological Communities; Croft, D.A., Su, D.F., Simpson, S.W., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2018; pp. 99–120. [Google Scholar]
- Elflein, L.; Raezke, K.-P. Improved Detection of Honey Adulteration by Measuring Differences between 13C/12C Stable Carbon Isotope Ratios of Protein and Sugar Compounds with a Combination of Elemental Analyzer—Isotope Ratio Mass Spectrometry and Liquid Chromatography—Isotope Ratio Mass Spectrometry (δ13C—EA/LC-IRMS). Apidologie 2008, 39, 574–587. [Google Scholar] [CrossRef] [Green Version]
- Lauteri, M.; Pliura, A.; Monteverdi, M.C.; Brugnoli, E.; Villani, F.; Eriksson, G. Genetic Variation in Carbon Isotope Discrimination in Six European Populations of Castanea Sativa Mill. Originating from Contrasting Localities. J. Evol. Biol. 2004, 17, 1286–1296. [Google Scholar] [CrossRef]
- Karabagias, I.; Michos, C.; Badeka, A.; Kontakos, S.; Stratis, I.; Kontominas, M.G. Classification of Western Greek Virgin Olive Oils According to Geographical Origin Based on Chromatographic, Spectroscopic, Conventional and Chemometric Analyses. Int. Food Res. J. 2013, 54, 1950–1958. [Google Scholar] [CrossRef]
- Chiavaro, E.; Cerretani, L.; Di Matteo, A.; Barnaba, C.; Bendini, A.; Iacumin, P. Application of a Multidisciplinary Approach for the Evaluation of Traceability of Extra Virgin Olive Oil. Eur. J. Lipid Sci. Technol. 2011, 113, 1509–1519. [Google Scholar] [CrossRef]
- Coelho, I.; Castanheira, I.; Bordado, J.M.; Donard, O.; Silva, J.A.L. Recent Developments and Trends in the Application of Strontium and Its Isotopes in Biological Related Fields. TrAC-Trends Anal. Chem. 2017, 90, 45–61. [Google Scholar] [CrossRef]
- Stewart, B.W.; Capo, R.C.; Chadwick, O.A. Quantitative Strontium Isotope Models for Weathering, Pedogenesis and Biogeochemical Cycling. Geoderma 1998, 82, 173–195. [Google Scholar] [CrossRef]
- Liu, H.; Wei, Y.; Lu, H.; Wei, S.; Jiang, T.; Zhang, Y.; Guo, B. Combination of the 87Sr/86Sr Ratio and Light Stable Isotopic Values (Δ13C, Δ15N and ΔD) for Identifying the Geographical Origin of Winter Wheat in China. Food Chem. 2016, 212, 367–373. [Google Scholar] [CrossRef]
- Epova, E.N.; Bérail, S.; Séby, F.; Vacchina, V.; Bareille, G.; Médina, B.; Sarthou, L.; Donard, O.F.X. Strontium Elemental and Isotopic Signatures of Bordeaux Wines for Authenticity and Geographical Origin Assessment. Food Chem. 2019, 294, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-C.; You, C.-F.; Chen, C.-Y.; Liu, Y.-C.; Chung, M.-T. Geographic Determination of Coffee Beans Using Multi-Element Analysis and Isotope Ratios of Boron and Strontium. Food Chem. 2014, 142, 439–445. [Google Scholar] [CrossRef]
- Rummel, S.; Hoelzl, S.; Horn, P.; Rossmann, A.; Schlicht, C. The Combination of Stable Isotope Abundance Ratios of H, C, N and S with 87Sr/86Sr for Geographical Origin Assignment of Orange Juices. Food Chem. 2010, 118, 890–900. [Google Scholar] [CrossRef]
- Pin, C.; Joannon, S.; Bosq, C.; Le Fèvre, B.; Gauthier, P.-J. Precise Determination of Rb, Sr, Ba, and Pb in Geological Materials by Isotope Dilution and ICP-Quadrupole Mass Spectrometry Following Selective Separation of the Analytes. J. Anal. At. Spectrom. 2003, 18, 135–141. [Google Scholar] [CrossRef]
- Techer, I.; Medini, S.; Janin, M.; Arregui, M. Impact of Agricultural Practice on the Sr Isotopic Composition of Food Products: Application to Discriminate the Geographic Origin of Olives and Olive Oil. Appl. Geochem. 2017, 82, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Granato, D.; Putnik, P.; Kovačević, D.B.; Santos, J.S.; Calado, V.; Rocha, R.S.; Cruz, A.G.D.; Jarvis, B.; Rodionova, O.Y.; Pomerantsev, A. Trends in Chemometrics: Food Authentication, Microbiology, and Effects of Processing: Trends in Chemometrics. Compr. Rev. Food Sci. Food Saf. 2018, 17, 663–677. [Google Scholar] [CrossRef] [Green Version]
- Abdi, H.; Williams, L.J. Principal Component Analysis: Principal Component Analysis. WIREs Comp. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasr, E.G.; Epova, E.N.; Sebilo, M.; Larivière, D.; Hammami, M.; Souissi, R.; Abderrazak, H.; Donard, O.F.X. Olive Oil Traceability Studies Using Inorganic and Isotopic Signatures: A Review. Molecules 2022, 27, 2014. https://doi.org/10.3390/molecules27062014
Nasr EG, Epova EN, Sebilo M, Larivière D, Hammami M, Souissi R, Abderrazak H, Donard OFX. Olive Oil Traceability Studies Using Inorganic and Isotopic Signatures: A Review. Molecules. 2022; 27(6):2014. https://doi.org/10.3390/molecules27062014
Chicago/Turabian StyleNasr, Emna G., Ekaterina N. Epova, Mathieu Sebilo, Dominic Larivière, Mohamed Hammami, Radhia Souissi, Houyem Abderrazak, and Olivier F. X. Donard. 2022. "Olive Oil Traceability Studies Using Inorganic and Isotopic Signatures: A Review" Molecules 27, no. 6: 2014. https://doi.org/10.3390/molecules27062014
APA StyleNasr, E. G., Epova, E. N., Sebilo, M., Larivière, D., Hammami, M., Souissi, R., Abderrazak, H., & Donard, O. F. X. (2022). Olive Oil Traceability Studies Using Inorganic and Isotopic Signatures: A Review. Molecules, 27(6), 2014. https://doi.org/10.3390/molecules27062014






