Tea Seed Kaempferol Triglycoside Attenuates LPS-Induced Systemic Inflammation and Ameliorates Cognitive Impairments in a Mouse Model
Abstract
:1. Introduction
2. Results
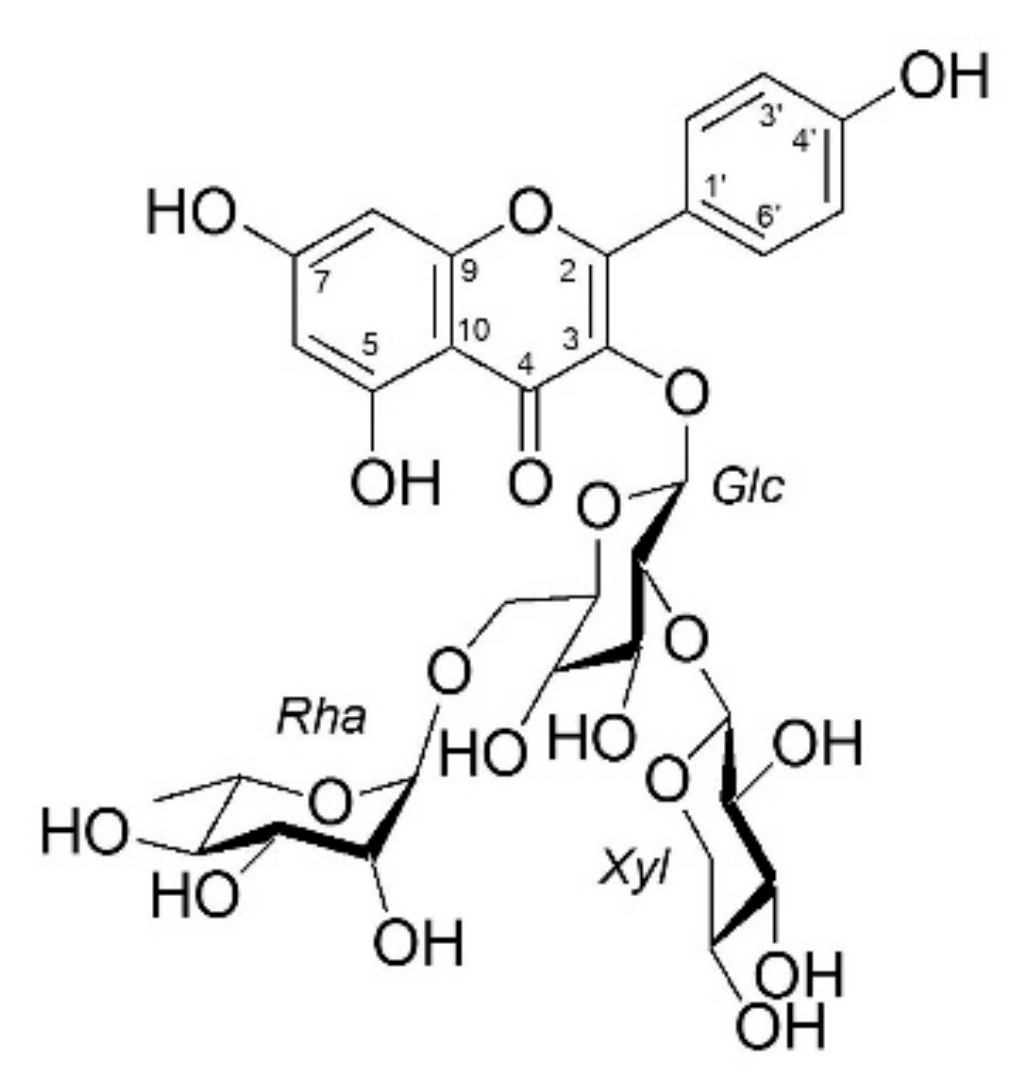
2.1. Structural Identification of KXRG
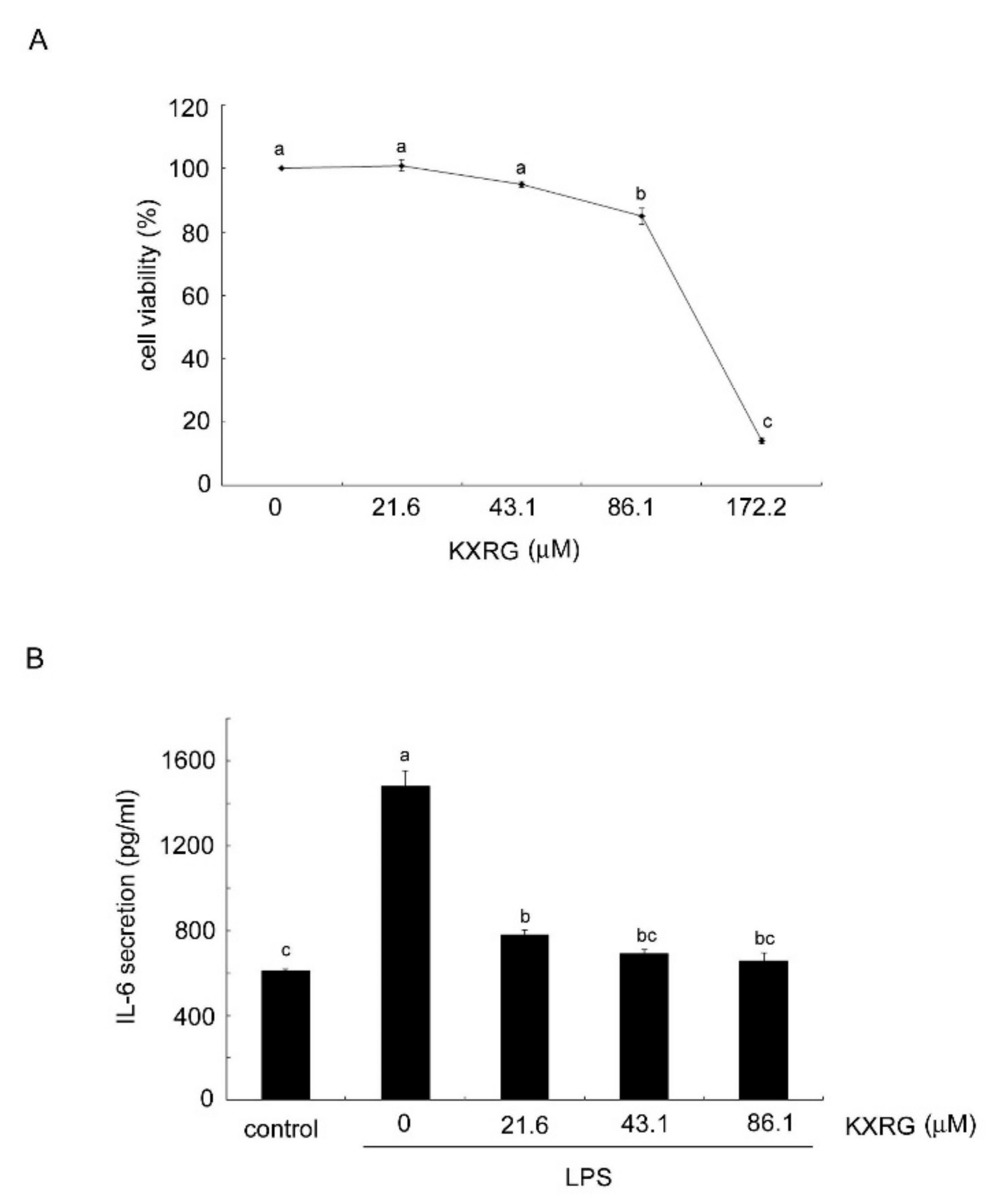
2.2. Anti-Inflammatory Activity of KXRG in Cultured RAW264.7 Cells
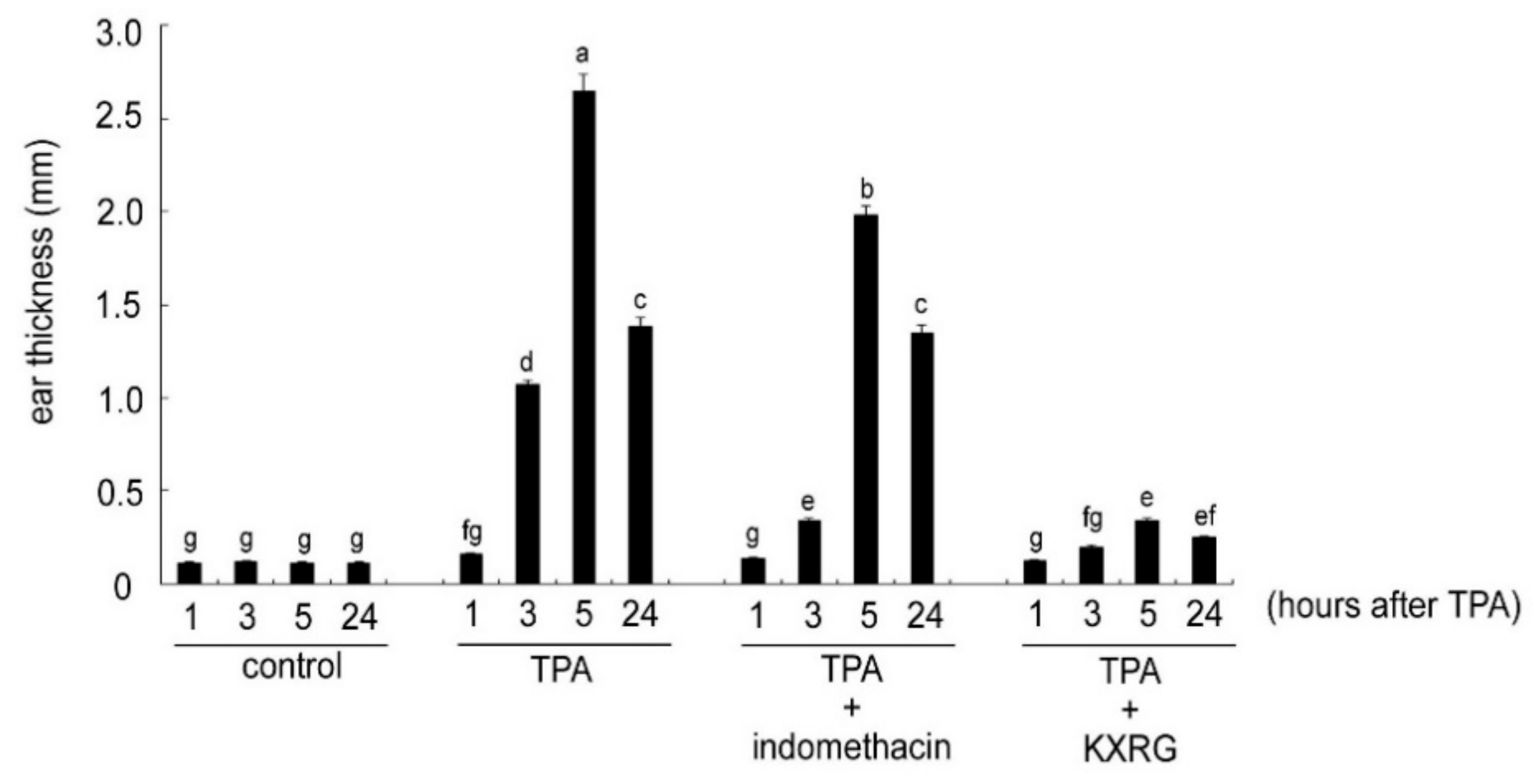
2.3. KXRG Reduced TPA-Induced Local Inflammation
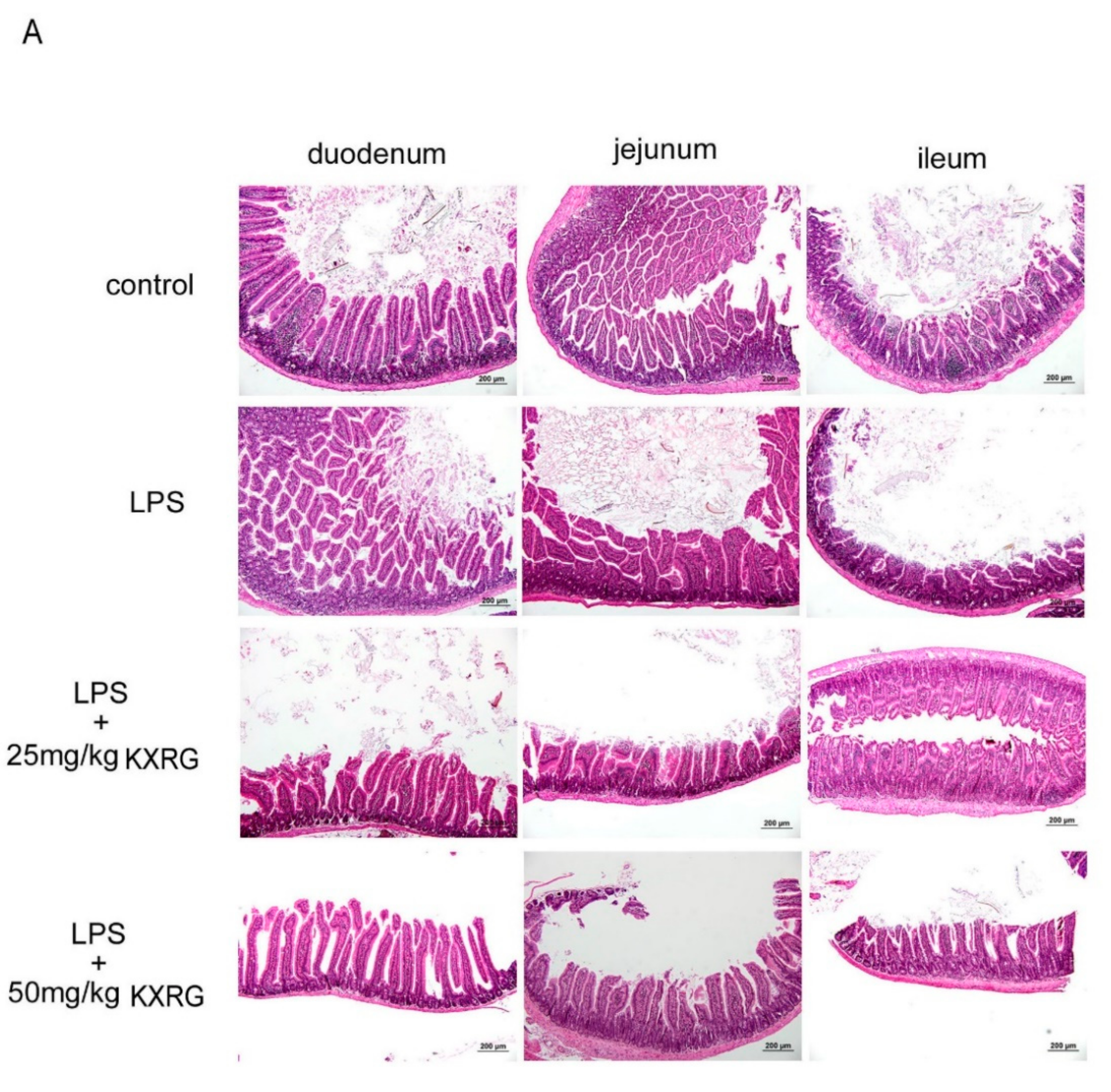
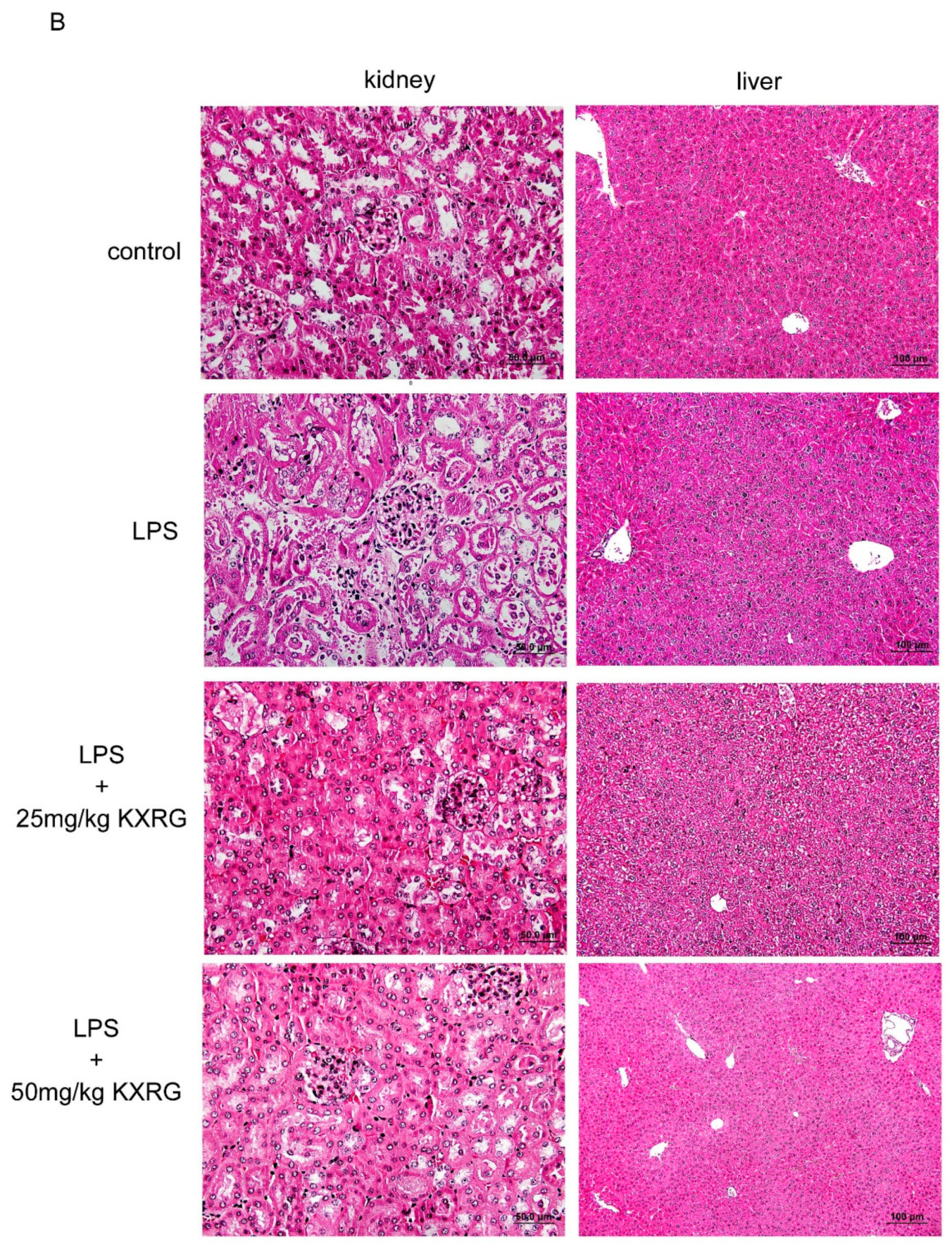
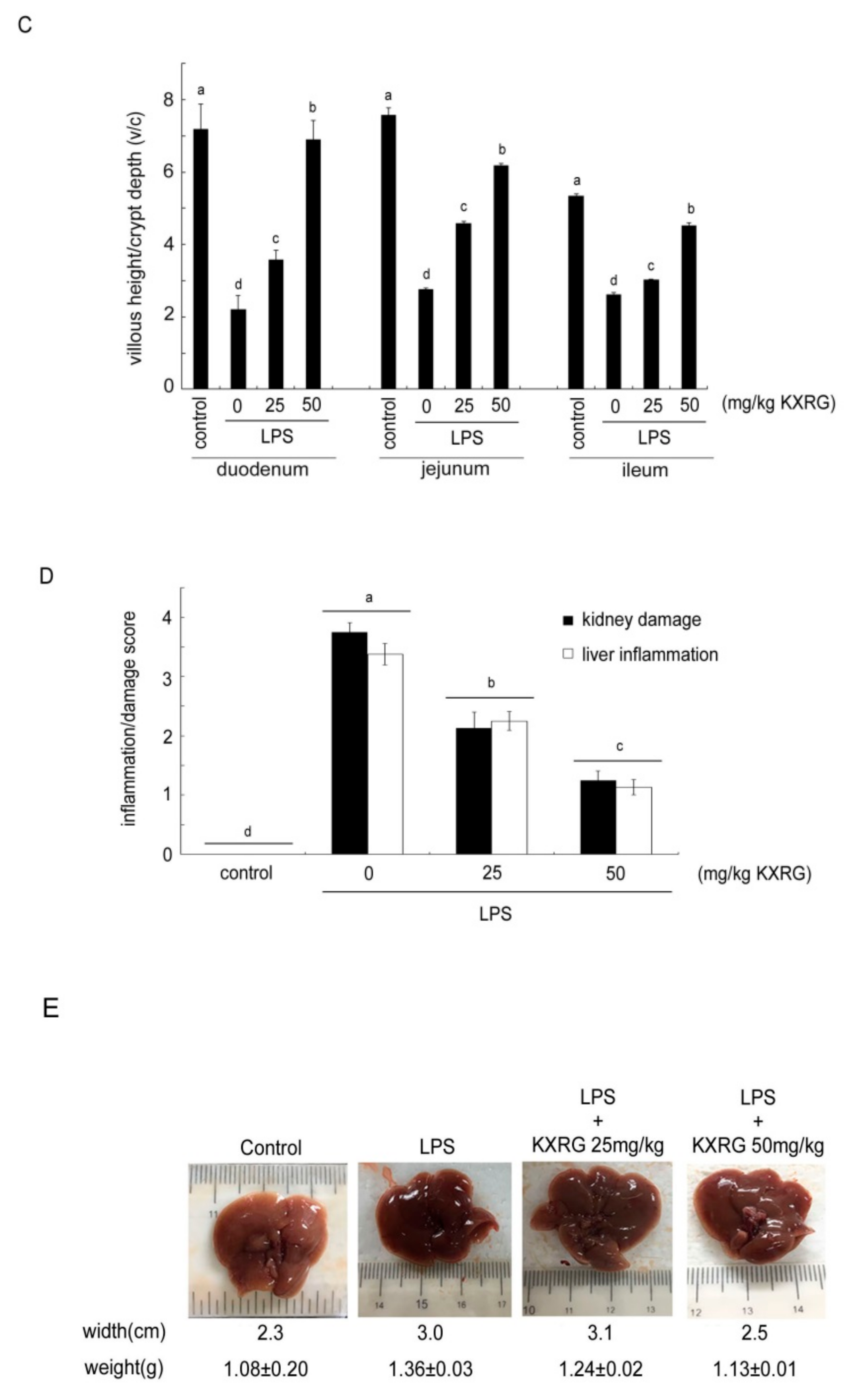
2.4. KXRG Prevented LPS-Induced Systemic Inflammation
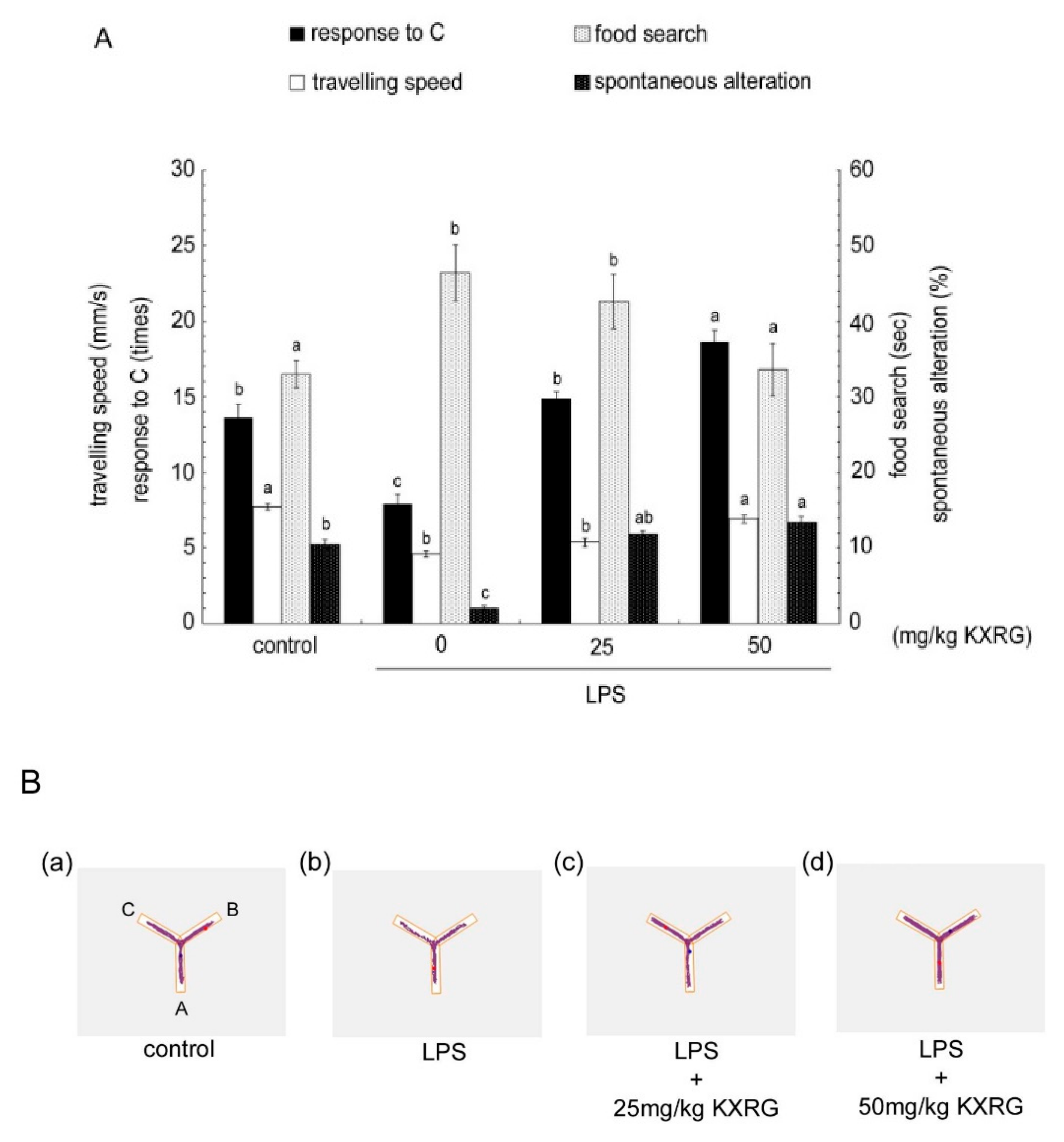
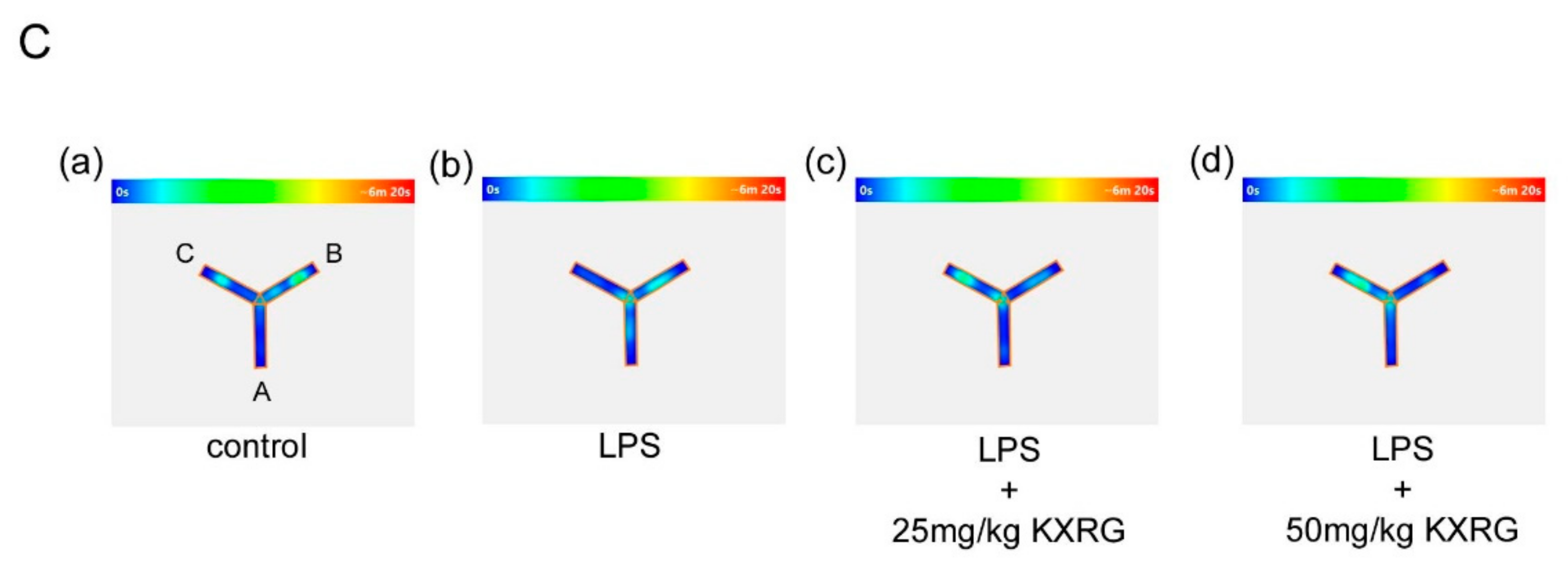
2.5. KXRG Attenuated LPS-Induced Brain Cognitive Impairments
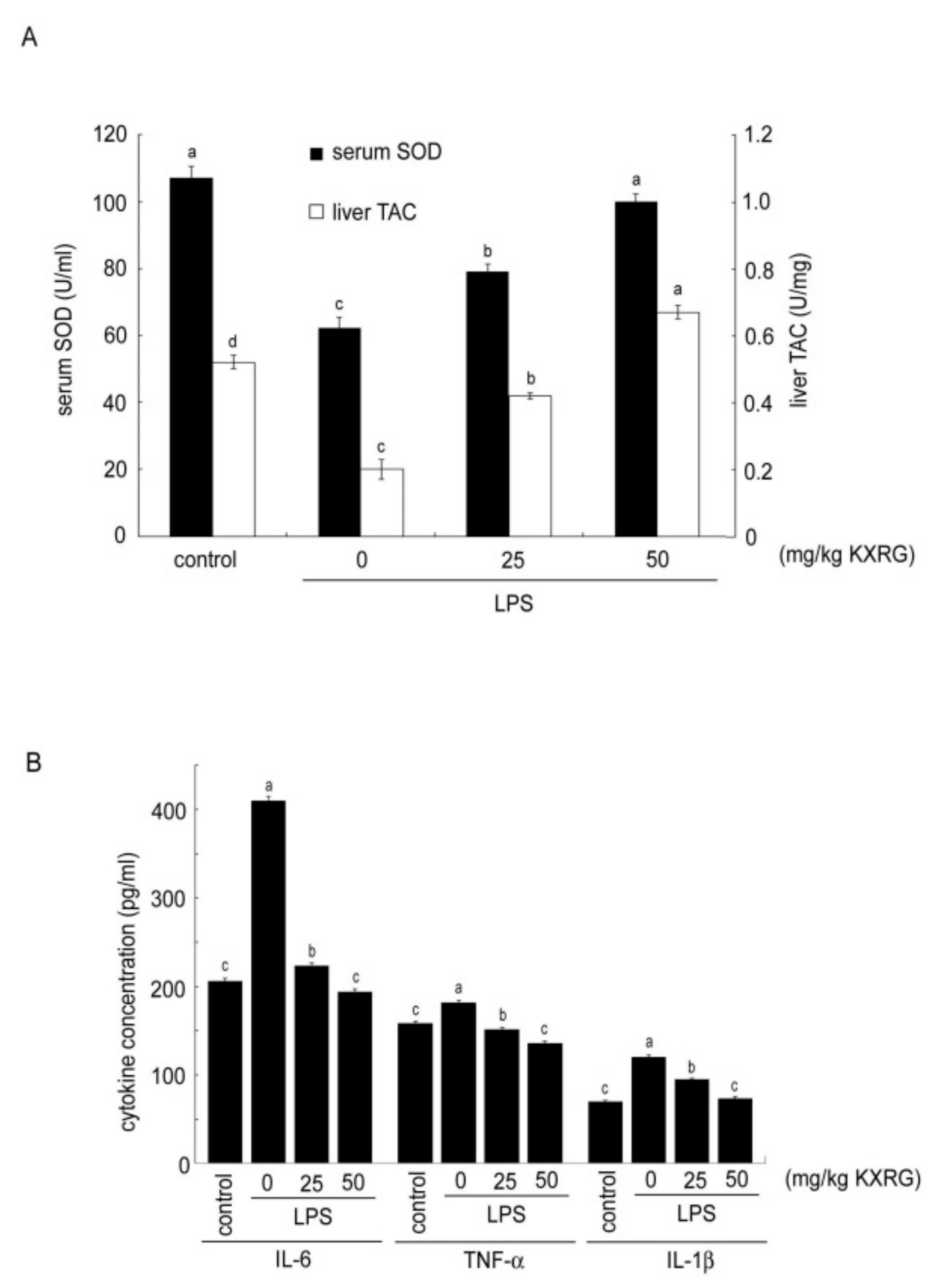
2.6. KXRG Strengthened the Antioxidant Function and Reduced Proinflammatory Cytokines in Mice
2.7. KXRG Reduced the LPS-Induced COX-2 Expression in Mouse Tissue
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction, Isolation, and Identification of Kaempferol-3-O-[2-O-β-d-xylopyranosyl-6-O-α-L-rhanmopyranosyl]-β-d-glucopyranoside (KXRG)
4.3. Chemicals and Reagents
4.4. RAW264.7 Cell Culture, Cytotoxicity, and Treatment
4.5. 12-O-Tetradecanoylphorbol Acetate (TPA)-Induced Mouse Ear Edema
4.6. LPS-Induced Systemic Inflammation in Mice
4.7. Histopathological Analysis
4.8. Biochemical Analysis
4.9. Y-Maze Analysis
4.10. Western Blotting Analysis
4.11. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rees, A.; Dodd, G.F.; Spencer, J.P.E. The Effects of Flavonoids on Cardiovascular Health: A Review of Human Intervention Trials and Implications for Cerebrovascular Function. Nutrients 2018, 10, 1852. [Google Scholar] [CrossRef] [Green Version]
- Leeuwis, A.E.; Smith, L.A.; Melbourne, A.; Hughes, A.D.; Richards, M.; Prins, N.D.; Sokolska, M.; Atkinson, D.; Tillin, T.; Jager, H.R.; et al. Cerebral Blood Flow and Cognitive Functioning in a Community-Based, Multi-Ethnic Cohort: The SABRE Study. Front. Aging Neurosci. 2018, 10, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [Green Version]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Choy, K.W.; Murugan, D.; Leong, X.F.; Abas, R.; Alias, A.; Mustafa, M.R. Flavonoids as Natural Anti-Inflammatory Agents Targeting Nuclear Factor-Kappa B (NFkappaB) Signaling in Cardiovascular Diseases: A Mini Review. Front. Pharmacol. 2019, 10, 1295. [Google Scholar] [CrossRef] [Green Version]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Artero, N.A.; Rasquel-Oliveira, F.S.; Fattori, V.; Casagrande, R.; Verri, W.A., Jr. Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.; Heo, M.Y.; Kim, H.P. Flavonoids: Broad Spectrum Agents on Chronic Inflammation. Biomol. Ther. 2019, 27, 241–253. [Google Scholar] [CrossRef]
- Hamalainen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 45673. [Google Scholar]
- Ohishi, T.; Goto, S.; Monira, P.; Isemura, M.; Nakamura, Y. Anti-inflammatory Action of Green Tea. Antiinflamm Antiallergy Agents. Med. Chem. 2016, 15, 74–90. [Google Scholar]
- Maiti, S.; Nazmeenac, A.; Medda, N.; Patra, R.; Ghoshb, T.K. Flavonoids green tea against oxidant stress and inflammation with related human diseases. Clin. Nutr. Exp. 2019, 24, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, A.M.; Sousa, C. A Review on the Biological Activity of Camellia Species. Molecules 2021, 26, 2178. [Google Scholar] [CrossRef]
- Liu, X.; Jia, L.; Gao, Y.; Li, B.; Tu, Y. Anti-inflammatory activity of total flavonoids from seeds of Camellia oleifera Abel. Acta. Biochim. Biophys. Sin. 2014, 46, 920–922. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.C.; Shen, K.P.; Ke, L.Y.; Lin, H.L.; Wu, C.C.; Shaw, S.Y. Flavonoids from Camellia sinensis (L.) O. Kuntze seed ameliorates TNF-alpha induced insulin resistance in HepG2 cells. Saudi Pharm. J. 2019, 27, 507–516. [Google Scholar] [CrossRef]
- Wang, R.-Y.; Tung, Y.-T.; Chen, S.-Y.; Lee, Y.-L.; Yen, G.-C. Protective effects of camellia oil (Camellia brevistyla) against indomethacin-induced gastrointestinal mucosal damage in vitro and in vivo. J. Funct. Foods 2019, 62, 103539. [Google Scholar] [CrossRef]
- Su, M.H.; Shih, M.C.; Lin, K.H. Chemical composition of seed oils in native Taiwanese Camellia species. Food. Chem. 2014, 156, 369–373. [Google Scholar] [CrossRef]
- Chiang, S.S.; Hsu, F.L.; Hsu, C.K.; Liu, C.F.; Chu, C.Y. Role of Camellia brevistyla (Hayata) Coh. Stuart Seed Pomace Extract on Hypertension and Vascular Function in L-NAME-Treated Mice. J. Food Sci. 2019, 84, 3555–3564. [Google Scholar] [CrossRef]
- Liu, W.; Huang, S.; Li, Y.; Zheng, X.; Zhang, K. Synergistic effect of tolfenamic acid and glycyrrhizic acid on TPA-induced skin inflammation in mice. Med. Chem. Comm. 2019, 10, 1819–1827. [Google Scholar] [CrossRef]
- Hamesch, K.; Borkham-Kamphorst, E.; Strnad, P.; Weiskirchen, R. Lipopolysaccharide-induced inflammatory liver injury in mice. Lab. Anim. 2015, 49, 37–46. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Q.; Sun, R.; Guo, L.; Wang, M.; Jia, J.; Xu, C.; Wu, R. Astragalus membranaceus (Fisch.) Bunge repairs intestinal mucosal injury induced by LPS in mice. BMC Complement. Altern. Med. 2018, 18, 230. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bi, W.; Xiao, S.; Lan, X.; Cheng, X.; Zhang, J.; Lu, D.; Wei, W.; Wang, Y.; Li, H.; et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 2019, 9, 5790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Li, Q.; Hou, R.; Sun, H.; Tang, Q.; Wang, H.; Hao, Z.; Kang, S.; Xu, T.; Wu, S. Kaempferol-3-O-glucorhamnoside inhibits inflammatory responses via MAPK and NF-κB pathways in vitro and in vivo. Toxicol. Appl. Pharmacol. 2019, 364, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Rho, H.S.; Ghimeray, A.K.; Yoo, D.S.; Ahn, S.M.; Kwon, S.S.; Lee, K.H.; Cho, D.H.; Cho, J.Y. Kaempferol and kaempferol rhamnosides with depigmenting and anti-inflammatory properties. Molecules 2011, 16, 3338–3344. [Google Scholar] [CrossRef] [Green Version]
- Pandurangan, A.K.; Esa, N.M. Luteolin, a bioflavonoid inhibits colorectal cancer through modulation of multiple signaling pathways: A review. Asian Pac. J. Cancer. Prev. 2014, 15, 5501–5508. [Google Scholar] [CrossRef]
- Tsoyi, K.; Nizamutdinova, I.T.; Jang, H.J.; Mun, L.; Kim, H.J.; Seo, H.G.; Lee, J.H.; Chang, K.C. Carbon monoxide from CORM-2 reduces HMGB1 release through regulation of IFN-β/JAK2/STAT-1/INOS/NO signaling but not COX-2 in TLR-activated macrophages. Shock 2010, 34, 608–614. [Google Scholar] [CrossRef]
- Tanabe, T.; Tohnai, N. Cyclooxygenase isozymes and their gene structures and expression. Prostaglandins Other Lipid Mediat. 2022, 68–69, 95–114. [Google Scholar] [CrossRef]
- Cho, H.; Yun, C.W.; Park, W.K.; Kong, J.Y.; Kim, K.S.; Park, Y.; Lee, S.; Kim, B.K. Modulation of the activity of pro-inflammatory enzymes, COX-2 and iNOS, by chrysin derivatives. Pharmacol. Res. 2004, 49, 37–43. [Google Scholar] [CrossRef]
- Lee, J.A.; Song, H.Y.; Ju, S.M.; Lee, J.S.; Kwon, H.J.; Eum, W.S.; Jang, S.H.; Choi, S.Y.; Park, J.S. Differential regulation of inducible nitric oxide synthase production in bovine and caprine macrophages. Exp. Mol. Med. 2009, 41, 629–637. [Google Scholar] [CrossRef]
- Durum, S.K.; Schmidt, J.A.; Oppenheim, J.J. Interleukin 1: An immunological perspective. Annu. Rev. Immunol. 1985, 3, 263–287. [Google Scholar] [CrossRef]
- Akira, S.; Hirano, T.; Taga, T.; Kishimoto, T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J. 1990, 4, 2860–2867. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, J.X.; Vilcek, J. Synthesis of interleukin 6 (interferon-beta 2/B cell stimulatory factor 2) in human fibroblasts is triggered by an increase in intracellular cyclic AMP. J. Biol. Chem. 1988, 263, 6177–6182. [Google Scholar] [CrossRef]
- Tanabe, O.; Akira, S.; Kamiya, T.; Wong, G.G.; Hirano, T.; Kishimoto, T. Genomic structure of the murine IL-6 gene. High degree conservation of potential regulatory sequences between mouse and human. J. Immunol. 1988, 141, 3875–3881. [Google Scholar]
- Nedwin, G.E.; Naylor, S.L.; Sakaguchi, A.Y.; Smith, D.; Jarrett-Nedwin, J.; Pennica, D.; Goeddel, D.V.; Gray, P.W. Human Lymphotoxin and tumor necrosis factor genes: Structure, homology and chromosomal localization. Nucleic Acids Res. 1985, 13, 6361–6373. [Google Scholar] [CrossRef] [Green Version]
- Iwai, T.; Iinuma, Y.; Kodani, R.; Oka, J. Neuromedin U inhibits inflammation-mediated memory impairment and neuronal cell-death in rodents. Neurosci. Res. 2008, 61, 113–119. [Google Scholar] [CrossRef]
- Yang, C.E.; Yeh, T.M.; Chang, C.D.; Shih, W.L. Chinese Soft-Shelled Turtle Oil in Combination with Swimming Training Improves Spatial Memory and Sports Performance of Aging Rats. Front. Physiol. 2021, 12, 660552. [Google Scholar] [CrossRef]
- Bliss, T.V.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef]
- Szczepanik, A.M.; Ringheim, G.E. IL-10 and glucocorticoids inhibit Abeta(1-42)- and lipopolysaccharide-induced pro-inflammatory cytokine and chemokine induction in the central nervous system. J. Alzheimers Dis. 2003, 5, 105–117. [Google Scholar] [CrossRef]
- Sarkar, D.; Saha, P.; Gamre, S.; Bhattacharjee, S.; Hariharan, C.; Ganguly, S.; Sen, R.; Mandal, G.; Chattopadhyay, S.; Majumdar, S.; et al. Anti-inflammatory effect of allylpyrocatechol in LPS-induced macrophages is mediated by suppression of iNOS and COX-2 via the NF-kappaB pathway. Int. Immunopharmacol. 2008, 8, 1264–1271. [Google Scholar] [CrossRef]
- Arthur, M.J.; Kowalski-Saunders, P.; Wright, R. Effect of endotoxin on release of reactive oxygen intermediates by rat hepatic macrophages. Gastroenterology 1988, 95, 1588–1594. [Google Scholar] [CrossRef]
- Bautista, A.P.; Spitzer, J.J. Acute endotoxin tolerance downregulates superoxide anion release by the perfused liver and isolated hepatic nonparenchymal cells. Hepatology 1995, 21, 855–862. [Google Scholar]
- Yoshikawa, T.; Takano, H.; Takahashi, S.; Ichikawa, H.; Kondo, M. Changes in tissue antioxidant enzyme activities and lipid peroxides in endotoxin-induced multiple organ failure. Circ. Shock 1994, 42, 53–58. [Google Scholar] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.; Cho, W.C.; Upadhyay, G. Drug-Induced Liver Toxicity and Prevention by Herbal Antioxidants: An Overview. Front. Physiol. 2015, 6, 363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akachi, T.; Shiina, Y.; Ohishi, Y.; Kawaguchi, T.; Kawagishi, H.; Morita, T.; Mori, M.; Sugiyama, K. Hepatoprotective effects of flavonoids from shekwasha (Citrus depressa) against D-galactosamine-induced liver injury in rats. J. Nutr. Sci. Vitaminol. 2010, 56, 60–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharrhan, S.; Chopra, K.; Arora, S.K.; Toor, J.S.; Rishi, P. Down-regulation of NF-kappaB signalling by polyphenolic compounds prevents endotoxin-induced liver injury in a rat model. Innate Immun. 2012, 18, 70–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.C.; Yeh, H.Y.; Shih, W.L. Extraction Procedure, Characteristics, and Feasibility of Caulerpa microphysa (Chlorophyta) Polysaccharide Extract as a Cosmetic Ingredient. Mar. Drugs 2021, 19, 524. [Google Scholar] [CrossRef]
- Lin, P.Y.; Liu, H.J.; Liao, M.H.; Chang, C.D.; Chang, C.I.; Cheng, H.L.; Lee, J.W.; Shih, W.L. Activation of PI 3-kinase/Akt/NF-κB and Stat3 signaling by avian reovirus S1133 in the early stages of infection results in an inflammatory response anddelayed apoptosis. Virology 2010, 400, 104–114. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, T.-M.; Chang, C.-D.; Liu, S.-S.; Chang, C.-I.; Shih, W.-L. Tea Seed Kaempferol Triglycoside Attenuates LPS-Induced Systemic Inflammation and Ameliorates Cognitive Impairments in a Mouse Model. Molecules 2022, 27, 2055. https://doi.org/10.3390/molecules27072055
Yeh T-M, Chang C-D, Liu S-S, Chang C-I, Shih W-L. Tea Seed Kaempferol Triglycoside Attenuates LPS-Induced Systemic Inflammation and Ameliorates Cognitive Impairments in a Mouse Model. Molecules. 2022; 27(7):2055. https://doi.org/10.3390/molecules27072055
Chicago/Turabian StyleYeh, Tsung-Ming, Ching-Dong Chang, Shyh-Shyan Liu, Chi-I Chang, and Wen-Ling Shih. 2022. "Tea Seed Kaempferol Triglycoside Attenuates LPS-Induced Systemic Inflammation and Ameliorates Cognitive Impairments in a Mouse Model" Molecules 27, no. 7: 2055. https://doi.org/10.3390/molecules27072055
APA StyleYeh, T. -M., Chang, C. -D., Liu, S. -S., Chang, C. -I., & Shih, W. -L. (2022). Tea Seed Kaempferol Triglycoside Attenuates LPS-Induced Systemic Inflammation and Ameliorates Cognitive Impairments in a Mouse Model. Molecules, 27(7), 2055. https://doi.org/10.3390/molecules27072055




