Pilot-Scale Optimization of Supercritical CO2 Extraction of Dry Paprika Capsicum annuum: Influence of Operational Conditions and Storage on Extract Composition
Abstract
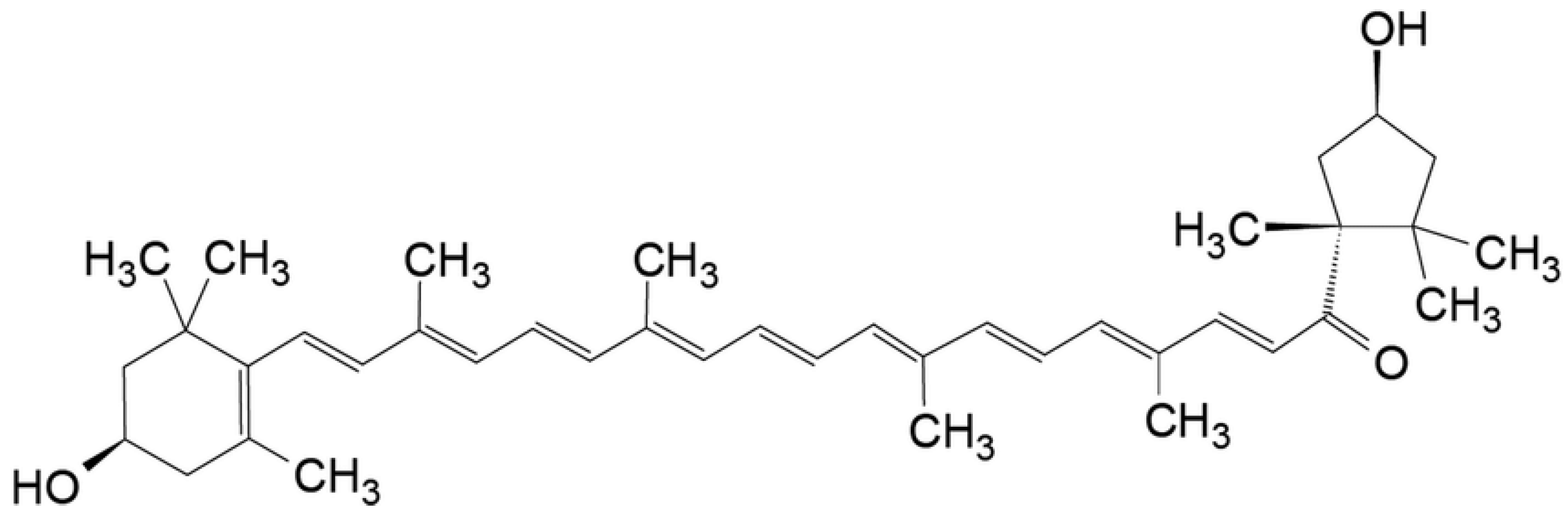
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solvent Extraction (SOX)
2.3. Supercritical Carbon Dioxide (SC-CO2) Extraction
2.4. Design of Experiments
2.5. Characteristics of Raw Material and Paprika Extract
2.6. Determination of Fatty Acids
3. Results and Discussion
3.1. Extraction Kinetics
3.2. Extraction Yield and Carotenoid Content in the Extract
3.3. Process Optimization and Verification of Predicted Model
3.4. Content of Fatty Acids
3.5. Stability of Carotenoids in Paprika Extract
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arimboor, R.; Natarajan, R.B.; Menon, K.R.; Chandrasekhar, L.P.; Moorkoth, V. Red pepper (Capsicum annuum) carotenoids as a source of natural food colors: Analysis and stability—A review. J. Food Sci. Technol. 2014, 52, 1258–1271. [Google Scholar] [CrossRef] [Green Version]
- Warsi, W.; Guntarti, A. Antioxidant activity of methanolic extract from yellow paprika (Capsicum annuum, L.) by DPPH radical scavenging method. Pharmaciana 2017, 7, 123–132. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, W.-E.; Hu, L.; Zhao, L.; Huang, J. Carotenoids inhibit proliferation and regulate expression of peroxisome proliferators-activated receptor gamma (PPARγ) in K562 cancer cells. Arch. Biochem. Biophys. 2011, 512, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Molnár, J.; Serly, J.; Pusztai, R.; Vincze, I.; Molnár, P.; Horváth, G.; Deli, J.; Maoka, T.; Zalatnai, A.; Tokuda, H.; et al. Putative supramolecular complexes formed by carotenoids and xanthophylls with ascorbic acid to reverse multidrug resistance in cancer cells. Anticancer Res. 2012, 32, 507–517. [Google Scholar]
- Han, B.-H.; Kim, J.-W.; Jo, S.-J.; Choi, H.-O.; Kim, J.-H.; Kim, M.-K.; Park, I.-K.; Lee, S.-H. Functional Food and Pharmaceutical Compositions for Anti-Obesity Comprising Capsanthin and Its Fatty Acylester Derivatives Having Anti-Adipogenic Activity. EP12004136.3, 12 December 2012. [Google Scholar]
- MarketsandMarkets. Market Reports: Carotenoid Market. 2021. Available online: https://www.marketsandmarkets.com/Market-Reports/carotenoid-market-158421566.html (accessed on 19 November 2021).
- Kultys, E.; Kurek, M.A. Green extraction of carotenoids from fruit and vegetable byproducts: A review. Molecules 2022, 27, 518. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Reboredo, F.H.; Lidon, F.C. Food colour additives: A synoptical overview on their chemical properties, applications in food products, and health side effects. Foods 2022, 11, 379. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; la Mora, Z.V.-D.; Vázquez-Paulino, O.; Ascencio, F.; Villarruel-López, A. Bell Peppers (Capsicum annum L.) Losses and Wastes: Source for Food and Pharmaceutical Applications. Molecules 2021, 26, 5341. [Google Scholar] [CrossRef] [PubMed]
- Lokaewmanee, K.; Yamauchi, K.; Okuda, N. Effects of dietary red pepper on egg yolk colour and histological intestinal morphology in laying hens. J. Anim. Physiol. Anim. Nutr. 2012, 97, 986–995. [Google Scholar] [CrossRef]
- Maoka, T.; Mochida, K.; Kozuka, M.; Ito, Y.; Fujiwara, Y.; Hashimoto, K.; Enjo, F.; Ogata, M.; Nobukuni, Y.; Tokuda, H.; et al. Cancer chemopreventive activity of carotenoids in the fruits of red paprika Capsicum annuum L. Cancer Lett. 2001, 172, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, K.; Inakuma, T. Dietary capsanthin, the main carotenoid in paprika (Capsicum annuum), alters plasma high-density lipoprotein-cholesterol levels and hepatic gene expression in rats. Br. J. Nutr. 2009, 102, 1760–1766. [Google Scholar] [CrossRef] [Green Version]
- Cervantes-Paz, B.; Yahia, E.M.; de Jesús Ornelas-Paz, J.D.; Victoria-Campos, C.I.; Ibarra-Junquera, V.; Pérez-Martínez, J.D.; Escalante-Minakata, P. Antioxidant activity and content of chlorophylls and carotenoids in raw and heat-processed Jalapeño peppers at intermediate stages of ripening. Food Chem. 2014, 146, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Ha, T.-Y.; Kim, S.; Lee, S.-J.; Ahn, J. Red paprika (Capsicum annuum L.) and its main carotenoid capsanthin ameliorate impaired lipid metabolism in the liver and adipose tissue of high-fat diet-induced obese mice. J. Funct. Foods 2017, 31, 131–140. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2005, 1740, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Maoka, T. Recent progress in structural studies of carotenoids in animals and plants. Arch. Biochem. Biophys. 2009, 483, 191–195. [Google Scholar] [CrossRef]
- Márkus, F.; Daood, H.G.; Kapitány, J.; Biacs, P.A. Change in the carotenoid and antioxidant content of spice red pepper (Paprika) as a function of ripening and some technological factors. J. Agric. Food Chem. 1998, 47, 100–107. [Google Scholar] [CrossRef]
- Gnayfeed, M.H.; Daood, H.G.; Biacs, P.A.; Alcaraz, C.F. Content of bioactive compounds in pungent spice red pepper (paprika) as affected by ripening and genotype. J. Sci. Food Agric. 2001, 81, 1580–1585. [Google Scholar] [CrossRef]
- Matsufuji, H.; Nakamura, H.; Chino, M.; Takeda, M. Antioxidant Activity of Capsanthin and the Fatty Acid Esters in Paprika (Capsicum annuum). J. Agric. Food Chem. 1998, 46, 3468–3472. [Google Scholar] [CrossRef]
- Li, G.; Song, C.; You, J.; Sun, Z.; Xia, L.; Suo, Y. Optimisation of red pepper seed oil extraction using supercritical CO2 and analysis of the composition by reversed-phase HPLC-FLD-MS/MS. Int. J. Food Sci. Technol. 2010, 46, 44–51. [Google Scholar] [CrossRef]
- Fernández-Ronco, M.P.; Gracia, I.; de Lucas, A.; Rodríguez, J.F. Extraction of Capsicum annuum Oleoresin by Maceration and Ultrasound-Assisted Extraction: Influence of Parameters and Process Modeling. J. Food Process Eng. 2012, 36, 343–352. [Google Scholar] [CrossRef]
- Hornero-Méndez, D.; Mínguez-Mosquera, M.I. Rapid Spectrophotometric Determination of Red and Yellow Isochromic Carotenoid Fractions in Paprika and Red Pepper Oleoresins. J. Agric. Food Chem. 2001, 49, 3584–3588. [Google Scholar] [CrossRef]
- Gögus, F.; Ozel, M.Z.; Keskin, H.; Yanık, D.K.; Lewis, A.C. Volatiles of Fresh and Commercial Sweet Red Pepper Pastes: Processing Methods and Microwave Assisted Extraction. Int. J. Food Prop. 2014, 18, 1625–1634. [Google Scholar] [CrossRef]
- Jarén-Galán, M.; Nienaber, U.; Schwartz, S.J. Paprika (Capsicum annuum) Oleoresin Extraction with Supercritical Carbon Dioxide. J. Agric. Food Chem. 1999, 47, 3558–3564. [Google Scholar] [CrossRef] [PubMed]
- Daood, H.; Illés, V.; Gnayfeed, M.; Mészáros, B.; Horváth, G.; Biacs, P. Extraction of pungent spice paprika by supercritical carbon dioxide and subcritical propane. J. Supercrit. Fluids 2002, 23, 143–152. [Google Scholar] [CrossRef]
- Gnayfeed, M.H.; Daood, H.G.; Illés, V.; Biacs, P.A. Supercritical CO2 and Subcritical Propane Extraction of Pungent Paprika and Quantification of Carotenoids, Tocopherols, and Capsaicinoids. J. Agric. Food Chem. 2001, 49, 2761–2766. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.; Moldão-Martins, M.; Gouveia, A.F.; da Costa, S.B.; Leitão, A.; Bernardo-Gil, M. Supercritical fluid extraction of red pepper (Capsicum frutescens L.). J. Supercrit. Fluids 2004, 30, 155–161. [Google Scholar] [CrossRef]
- Perva-Uzunalić, A.; Škerget, M.; Weinreich, B.; Knez, Ž. Extraction of chilli pepper (var. Byedige) with supercritical CO2: Effect of pressure and temperature on capsaicinoid and colour extraction efficiency. Food Chem. 2004, 87, 51–58. [Google Scholar] [CrossRef]
- de Aguiar, A.C.; Sales, L.P.; Coutinho, J.P.; Barbero, G.F.; Godoy, H.T.; Martínez, J. Supercritical carbon dioxide extraction of Capsicum peppers: Global yield and capsaicinoid content. J. Supercrit. Fluids 2013, 81, 210–216. [Google Scholar] [CrossRef]
- Shah, N.A.; Prasad, R.; Patel, B.B. Optimization of Supercritical Fluid Extraction of Paprika (cv. Reshampatti) Oil, Capsaicin and Pigments. Flavour Fragr. J. 2020, 35, 469–477. [Google Scholar] [CrossRef]
- del Valle, J.; Jiménez, M.; de la Fuente, J. Extraction kinetics of pre-pelletized Jalapeño peppers with supercritical CO2. J. Supercrit. Fluids 2003, 25, 33–44. [Google Scholar] [CrossRef]
- Uquiche, E.; del Valle, J.M.; Ortiz, J. Supercritical carbon dioxide extraction of red pepper (Capsicum annuum L.) oleoresin. J. Food Eng. 2004, 65, 55–66. [Google Scholar] [CrossRef]
- Illes, V.; Daood, H.G.; Biacs, P.A.; Gnayfeed, M.H.; Meszaros, B. Supercritical CO2 and Subcritical Propane Extraction of Spice Red Pepper Oil with Special Regard to Carotenoid and Tocopherol Content. J. Chromatogr. Sci. 1999, 37, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Kostrzewa, D.; Dobrzyńska-Inger, A.; Reszczyński, R. Pilot scale supercritical CO2 extraction of carotenoids from sweet paprika (Capsicum annuum L.): Influence of particle size and moisture content of plant material. LWT 2020, 136, 110345. [Google Scholar] [CrossRef]
- FAO. Compendium of Food Additive Specifications, 69rd Meeting Fao Jecfa Monographs 5; FAO: Rome, Italy, 2008; ISBN 978-92-5-106065-0. [Google Scholar]
- Datta, P.R. Official Analytical Methods of the American Spice Trade Association, 3rd ed.; ASTA: Englewood Cliffs, NJ, USA, 1985; pp. 41–42. [Google Scholar]
- Fernández-Ronco, M.; Ortega-Noblejas, C.; Gracia, I.; De Lucas, A.; García, M.; Rodríguez, J. Supercritical fluid fractionation of liquid oleoresin capsicum: Statistical analysis and solubility parameters. J. Supercrit. Fluids 2010, 54, 22–29. [Google Scholar] [CrossRef]
- Kostrzewa, D.; Dobrzyńska-Inger, A.; Turczyn, A. Optimization of supercritical carbon dioxide extraction of sweet paprika (Capsicum annuum L.) using response surface methodology. Chem. Eng. Res. Des. 2020, 160, 39–51. [Google Scholar] [CrossRef]
- Kostrzewa, D.; Dobrzyńska-Inger, A.; Turczyn, A. Experimental Data and Modelling of the Solubility of High-Carotenoid Paprika Extract in Supercritical Carbon Dioxide. Molecules 2019, 24, 4174. [Google Scholar] [CrossRef] [Green Version]
- Ambrogi, A.; Cardarelli, D.A.; Eggers, R. Separation of Natural Colorants Using a Combined High Pressure Extraction-Adsorption Process. Lat. Am. Appl. Res. 2003, 33, 323–326. [Google Scholar]
- Andrich, G.; Balzini, S.; Zinnai, A.; De Vitis, V.; Silvestri, S.; Venturi, F.; Fiorentini, R. Supercritical Fluid Extraction in Sunflower Seed Technology. Eur. J. Lipid Sci. Technol. 2001, 103, 151–157. [Google Scholar]
- Tepić, A.; Zeković, Z.; Kravić, S.; Mandic, A. Pigment content and fatty acid composition of paprika oleoresins obtained by conventional and supercritical carbon dioxide extraction. CyTA—J. Food 2009, 7, 95–102. [Google Scholar] [CrossRef]
- El-Adawy, T.A.; Taha, K.M. Characteristics and Composition of Watermelon, Pumpkin, and Paprika Seed Oils and Flours. J. Agric. Food Chem. 2001, 49, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Asilbekova, D.T. Lipids from Capsicum annuum Seeds. Chem. Nat. Compd. 2003, 39, 528–530. [Google Scholar] [CrossRef]
- Abdulkarim, S.; Long, K.; Lai, O.-M.; Muhammad, K.; Ghazali, H. Frying quality and stability of high-oleic Moringa oleifera seed oil in comparison with other vegetable oils. Food Chem. 2007, 105, 1382–1389. [Google Scholar] [CrossRef]
- Chen, H.E.; Peng, H.Y.; Chen, B.H. Stability of carotenoids and vitamin A during storage of carrot juice. Food Chem. 1996, 57, 497–503. [Google Scholar] [CrossRef]



| Run Order | Independent Variables | Dependent Variables | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pressure | Temperature | Time | Ye (%) | Be (ASTA) | Ck (%) | Cr (mg/g) | Cy (mg/g) | Bp (ASTA) | ||||
| X1 | p (MPa) | X2 | T (°C) | X3 | t (min) | |||||||
| 1 | 1 | 45 | 0 | 50 | −1 | 10 | 8.43 | 508.4 | 1.49 | 6.33 | 6.84 | 124.12 |
| 2 | −1 | 25 | −1 | 40 | 0 | 60 | 8.93 | 743.2 | 2.18 | 8.97 | 10.25 | 85.00 |
| 3 | −1 | 25 | 0 | 50 | −1 | 10 | 5.10 | 130.4 | 0.38 | 1.07 | 2.21 | 165.14 |
| 4 | 1 | 45 | 1 | 60 | 0 | 60 | 10.23 | 1400.2 | 4.11 | 22.53 | 14.02 | 15.42 |
| 5 | 0 | 35 | 1 | 60 | 1 | 110 | 10.12 | 1442.1 | 4.20 | 22.80 | 14.55 | 12.15 |
| 6 | 0 | 35 | −1 | 40 | 1 | 110 | 9.88 | 1478.7 | 4.34 | 24.27 | 14.46 | 16.63 |
| 7 | 0 | 35 | 0 | 50 | 0 | 60 | 9.63 | 1149.0 | 3.37 | 16.74 | 13.06 | 22.00 |
| 8 | 1 | 45 | 0 | 50 | 1 | 110 | 10.00 | 1496.8 | 4.39 | 25.08 | 14.06 | 13.00 |
| 9 | 0 | 35 | 0 | 50 | 0 | 60 | 9.84 | 1222.8 | 3.59 | 18.70 | 13.27 | 36.93 |
| 10 | −1 | 25 | 0 | 50 | 1 | 110 | 9.57 | 931.2 | 2.73 | 12.30 | 11.78 | 49.00 |
| 11 | −1 | 25 | 1 | 60 | 0 | 60 | 9.17 | 602.1 | 1.77 | 6.32 | 9.15 | 87.00 |
| 12 | 0 | 35 | 0 | 50 | 0 | 60 | 9.85 | 1180.3 | 3.46 | 17.95 | 12.85 | 28.00 |
| 13 | 0 | 35 | 1 | 60 | −1 | 10 | 7.69 | 324.8 | 0.95 | 3.41 | 4.85 | 129.34 |
| 14 | 0 | 35 | −1 | 40 | −1 | 10 | 6.81 | 325.0 | 0.95 | 3.74 | 4.62 | 86.63 |
| 15 | 1 | 45 | −1 | 40 | 0 | 60 | 9.57 | 1350.3 | 3.96 | 21.77 | 13.48 | 23.04 |
| Source | Ye (%) | Be (ASTA) | Ck (%) | Cr (mg/g) | Cy (mg/g) | Bp (ASTA) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p-Value | β | p-Value | β | p-Value | β | p-Value | β | p-Value | β | p-Value | |
| Model | 0.0011 | 0.0008 | 0.0008 | 0.0025 | <0.0001 | 0.0022 | ||||||
| Lack of Fit | 0.0757 | 0.0692 | 0.0731 | 0.0983 | 0.0995 | 0.1723 | ||||||
| β0 | 9.79 | 1184.03 | 3.47 | 17.80 | 13.06 | 28.98 | ||||||
| X1 | 0.68 | 0.0026 | 293.60 | 0.0006 | 0.86 | 0.0006 | 5.88 | 0.0010 | 1.88 | 0.0001 | −26.32 | 0.0029 |
| X2 | 0.25 | 0.0950 | −16.00 | 0.6937 | −0.05 | 0.6721 | −0.46 | 0.6134 | −0.03 | 0.8747 | 4.08 | 0.4418 |
| X3 | 1.44 | <0.0001 | 507.53 | <0.0001 | 1.49 | <0.0001 | 8.74 | 0.0002 | 4.54 | <0.0001 | −51.81 | 0.0001 |
| X1X2 | 0.11 | 0.5720 | 47.75 | 0.4187 | 0.14 | 0.4145 | 0.85 | 0.5130 | 0.41 | 0.1697 | −2.41 | 0.7418 |
| X1X3 | −0.73 | 0.0087 | 46.90 | 0.4265 | 0.14 | 0.4224 | 1.88 | 0.1814 | −0.59 | 0.0699 | 1.26 | 0.8629 |
| X2X3 | −0.16 | 0.3994 | −9.10 | 0.8733 | −0.04 | 0.8328 | −0.29 | 0.8233 | −0.04 | 0.8965 | −11.80 | 0.1482 |
| X12 | −0.33 | 0.1330 | −143.02 | 0.0522 | −0.42 | 0.0522 | −2.63 | 0.0914 | −1.12 | 0.0085 | 25.13 | 0.0173 |
| X22 | 0.03 | 0.8920 | −17.07 | 0.7745 | −0.05 | 0.7598 | −0.27 | 0.8391 | −0.22 | 0.4485 | −1.49 | 0.8434 |
| X32 | −1.17 | <0.0013 | −274.32 | 0.0046 | −0.81 | 0.0043 | −3.97 | 0.0254 | −3.22 | <0.0001 | 33.71 | 0.0054 |
| R2 | 0.9792 | 0.9814 | 0.9818 | 0.9711 | 0.9945 | 0.9727 | ||||||
| Adjusted R2 | 0.9419 | 0.9480 | 0.9489 | 0.9190 | 0.9846 | 0.9235 | ||||||
| Predicted R2 | 0.6825 | 0.7147 | 0.7206 | 0.5636 | 0.9171 | 0.6073 | ||||||
| Fatty Acids (%) | Method of Extraction | |
|---|---|---|
| SC-CO2 (45 MPa, 50 °C) a | SOX a | |
| Lauric acid (C12:0) | 0.43 ± 0.11 | 0.83 ± 0.18 |
| Palmitic acid (C16:0) | 10.68 ± 0.23 | 10.46 ± 0.21 |
| Stearic acid (C18:0) | 1.86 ± 0.01 | 1.84 ± 0.03 |
| Oleic acid (C18:1) | 8.23 ± 0.10 | 7.86 ± 0.20 |
| Linoleic acid (C18:2) | 44.87 ± 0.33 | 42.91 ± 0.32 |
| Arachidic acid (C20:0) | 0.26 ± 0.01 | 0.28 ± 0.01 |
| α-Linolenic acid (C18:3) | 2.86 ± 0.18 | 3.54 ± 0.15 |
| Behenic acid (C22:0) | 0.16 ± 0.01 | 0.18 ± 0.01 |
| Total saturated fatty acids | 13.39 | 13.59 |
| Total unsaturated fatty acids | 55.96 | 54.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostrzewa, D.; Dobrzyńska-Inger, A.; Mazurek, B.; Kostrzewa, M. Pilot-Scale Optimization of Supercritical CO2 Extraction of Dry Paprika Capsicum annuum: Influence of Operational Conditions and Storage on Extract Composition. Molecules 2022, 27, 2090. https://doi.org/10.3390/molecules27072090
Kostrzewa D, Dobrzyńska-Inger A, Mazurek B, Kostrzewa M. Pilot-Scale Optimization of Supercritical CO2 Extraction of Dry Paprika Capsicum annuum: Influence of Operational Conditions and Storage on Extract Composition. Molecules. 2022; 27(7):2090. https://doi.org/10.3390/molecules27072090
Chicago/Turabian StyleKostrzewa, Dorota, Agnieszka Dobrzyńska-Inger, Barbara Mazurek, and Marcin Kostrzewa. 2022. "Pilot-Scale Optimization of Supercritical CO2 Extraction of Dry Paprika Capsicum annuum: Influence of Operational Conditions and Storage on Extract Composition" Molecules 27, no. 7: 2090. https://doi.org/10.3390/molecules27072090






