Cardiorenal Protective Effect of Costunolide against Doxorubicin-Induced Toxicity in Rats by Modulating Oxidative Stress, Inflammation and Apoptosis
Abstract
:1. Introduction
2. Results
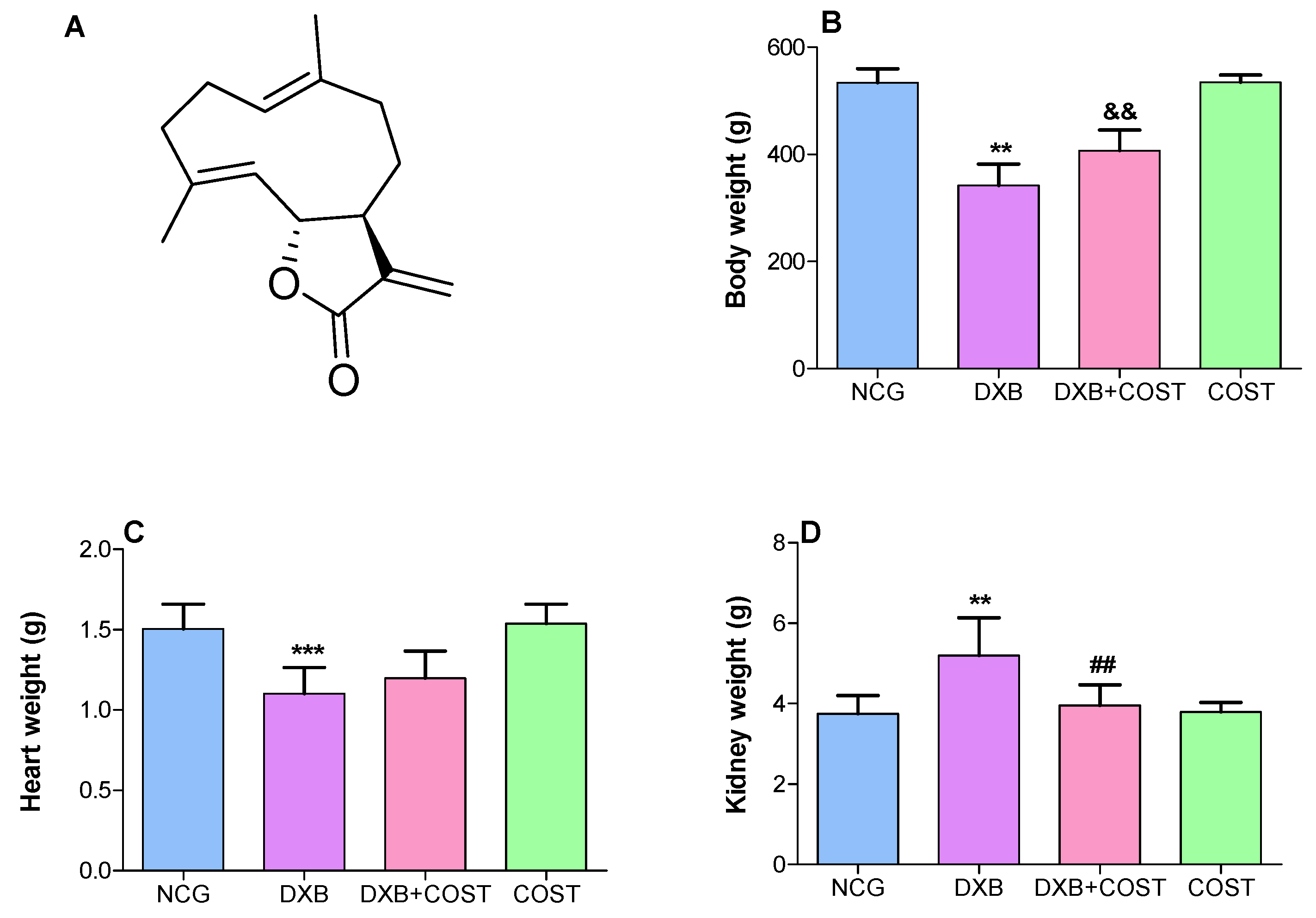
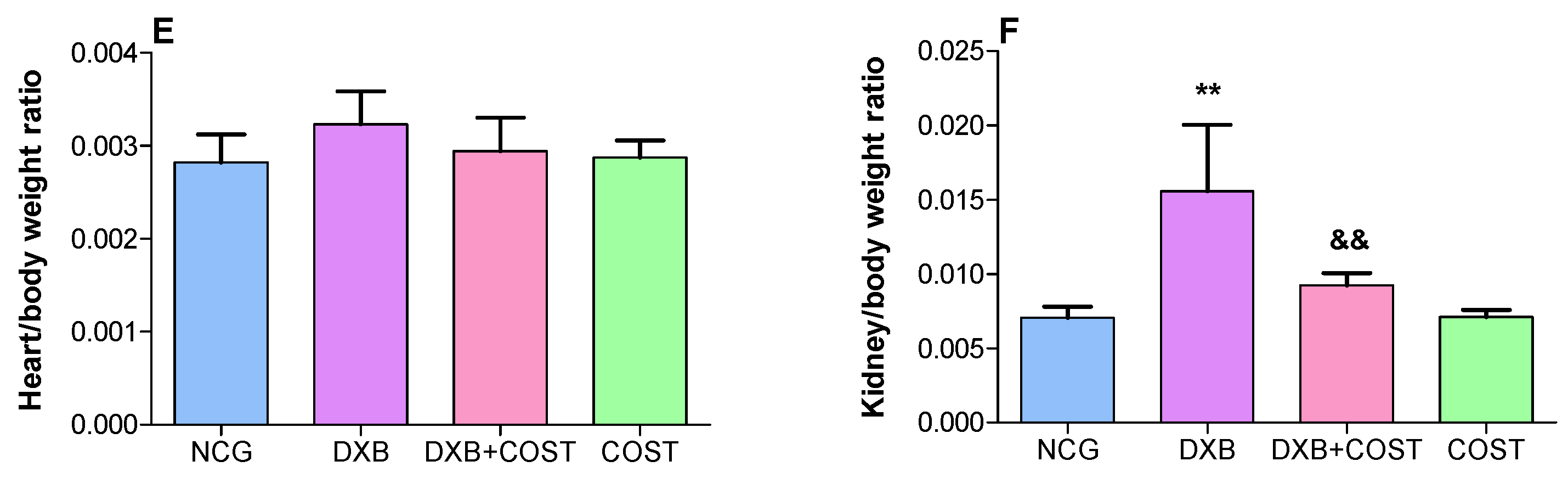
2.1. Effect of COST on Body, Heart and Kidney Weights
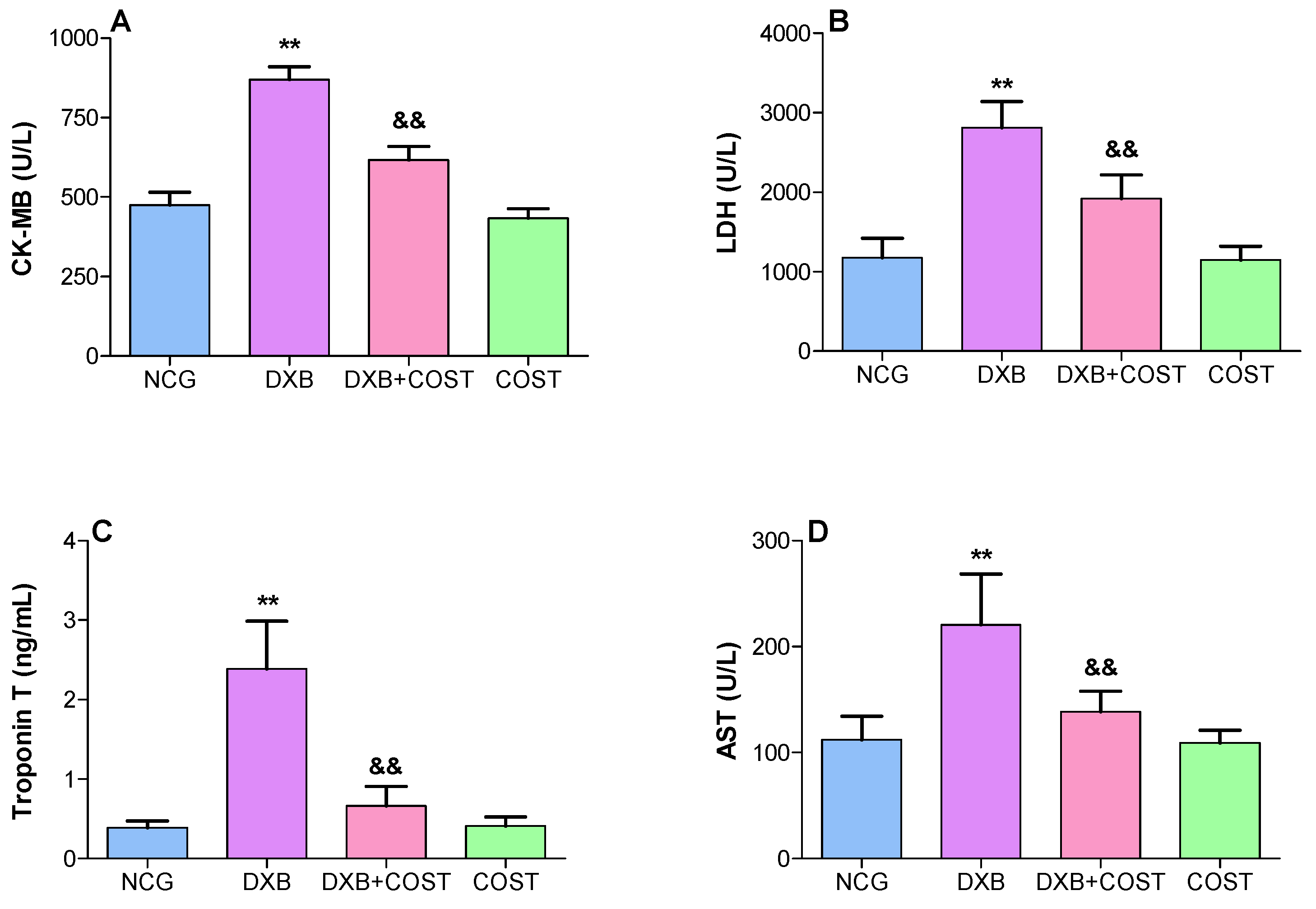
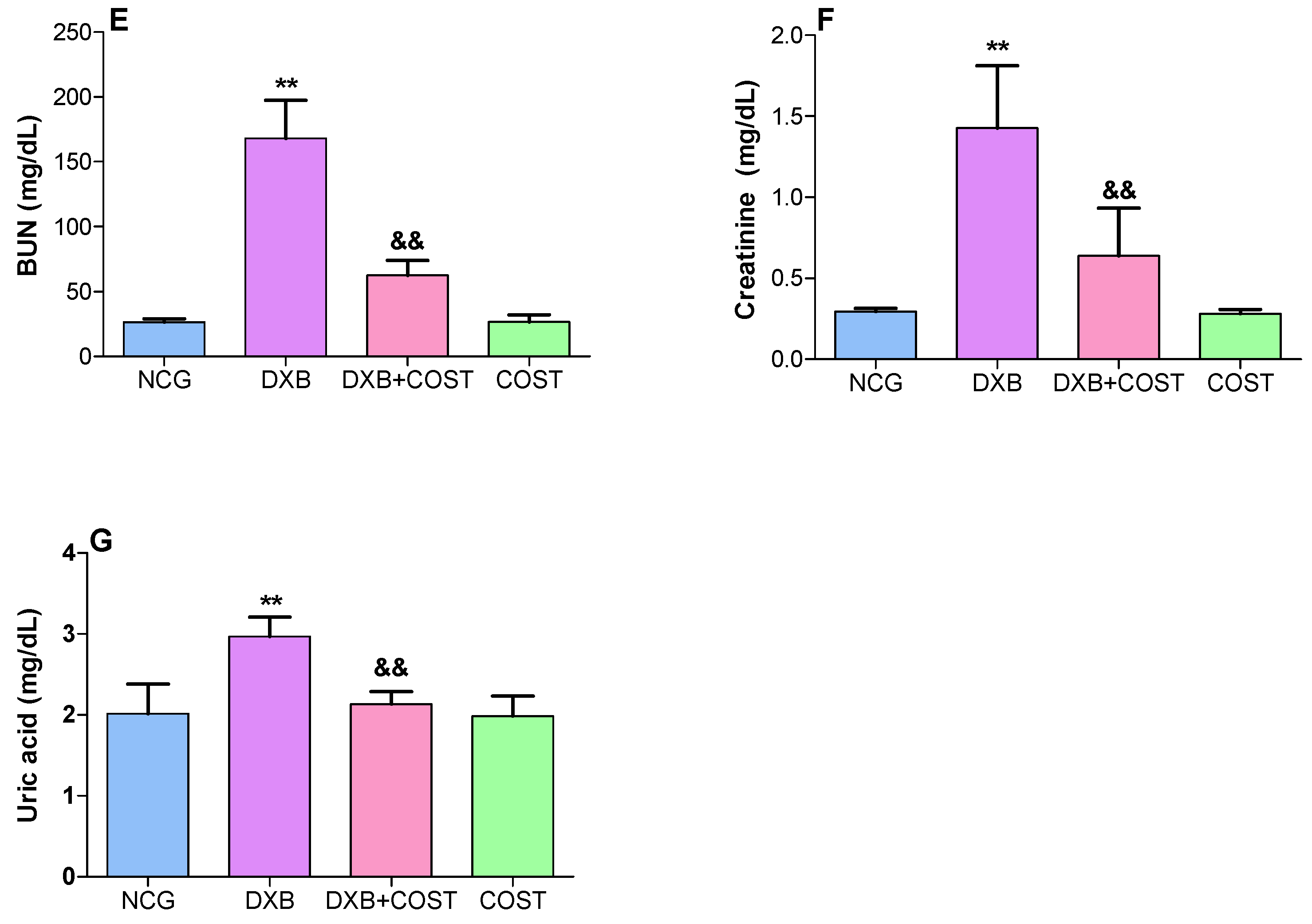
2.2. Effect of COST on Cardiorenal Function Markers
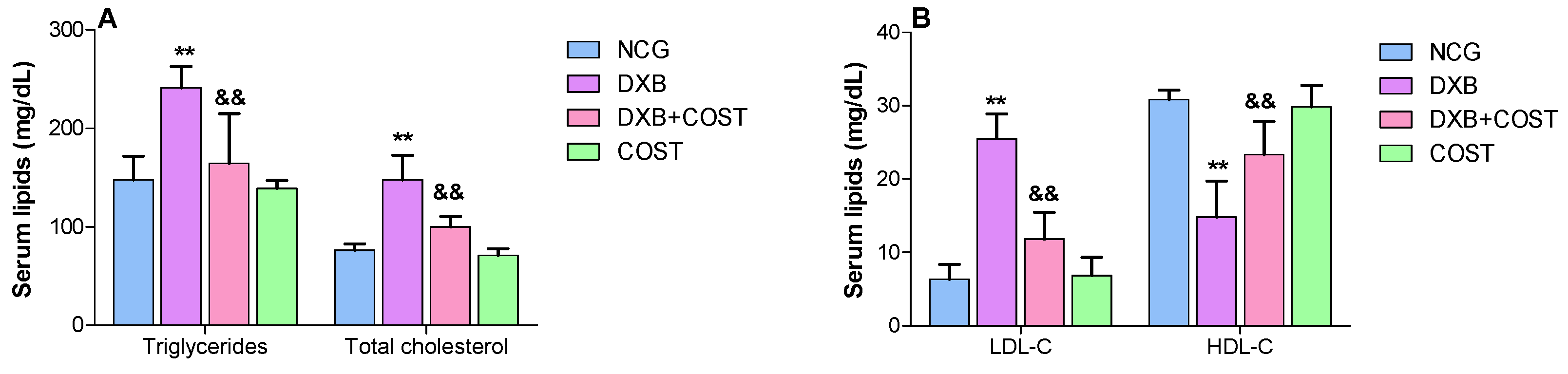
2.3. Effects of COST on Serum Lipid Profiles
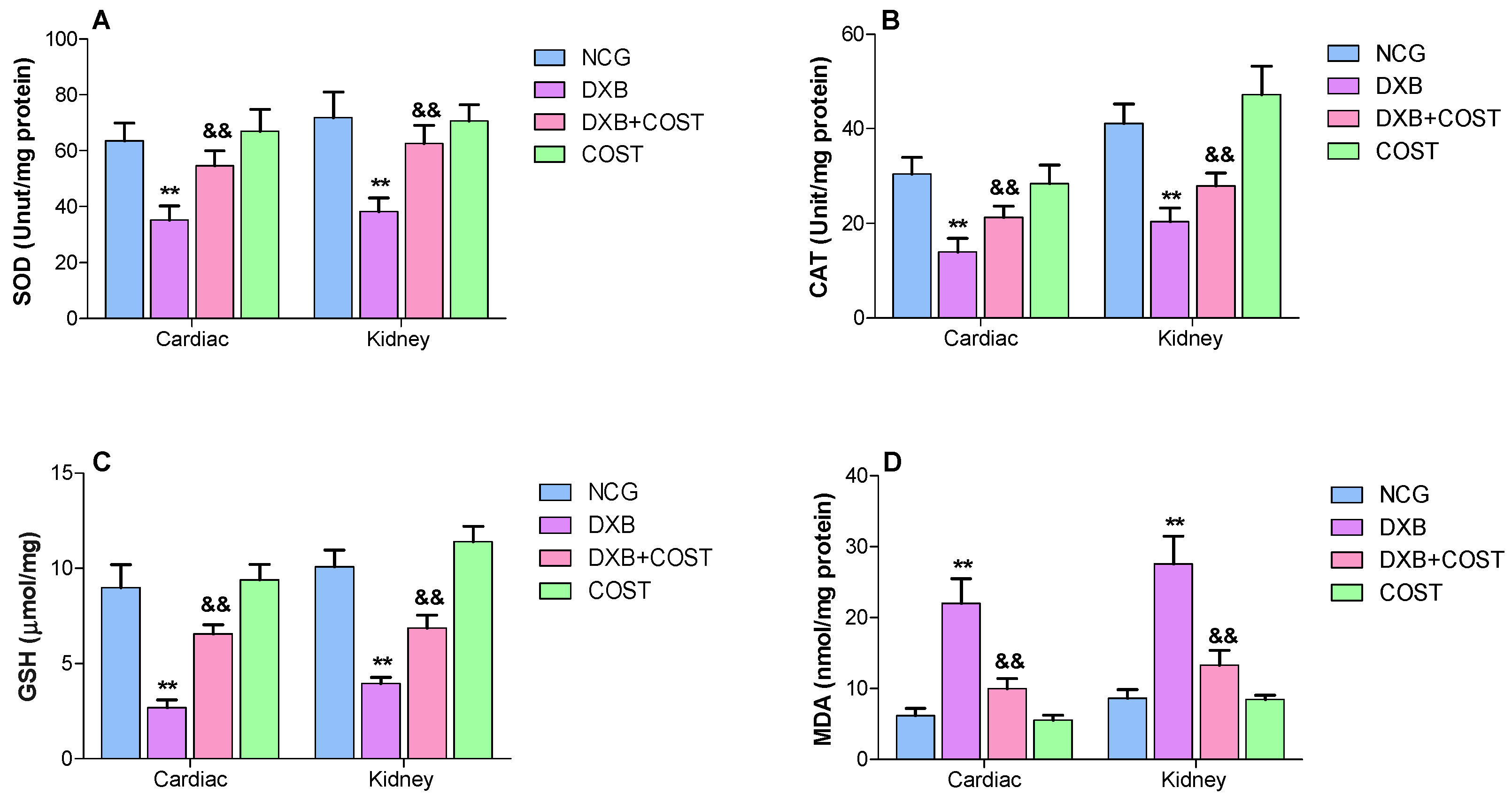
2.4. Effect of COST Treatment on Cardiorenal Antioxidants Activities
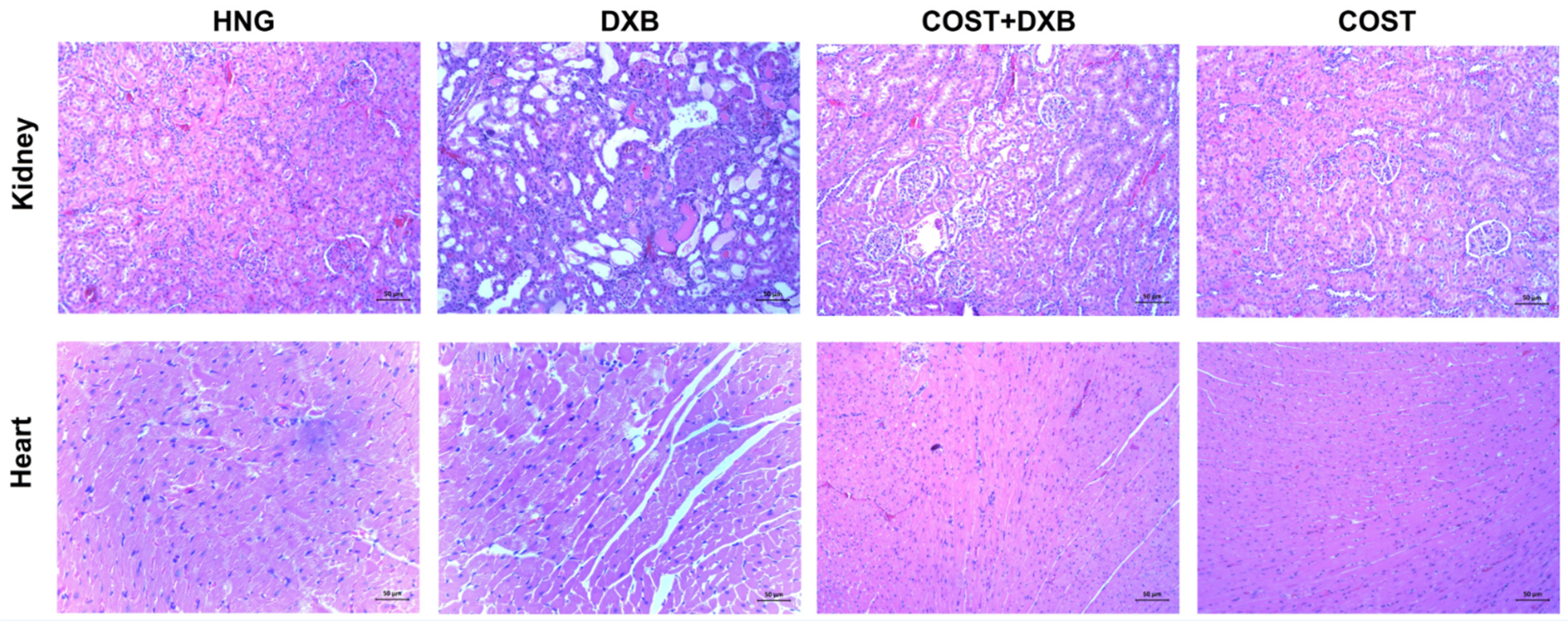
2.5. Effect of COST Treatment on Cardiorenal Histopathological Analysis
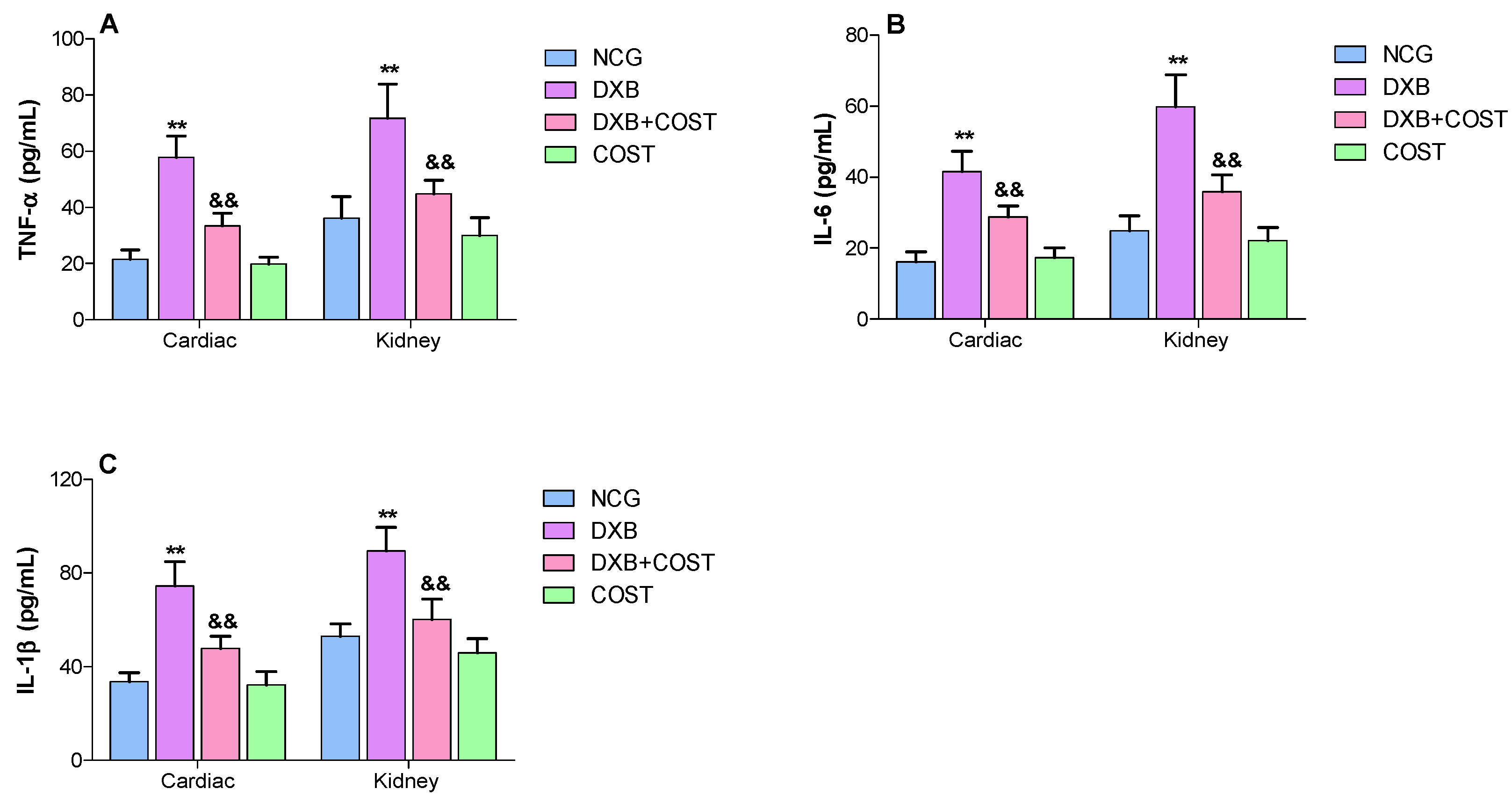
2.6. Effects of COST Treatment of Cardiorenal Proinflammatory Cytokines
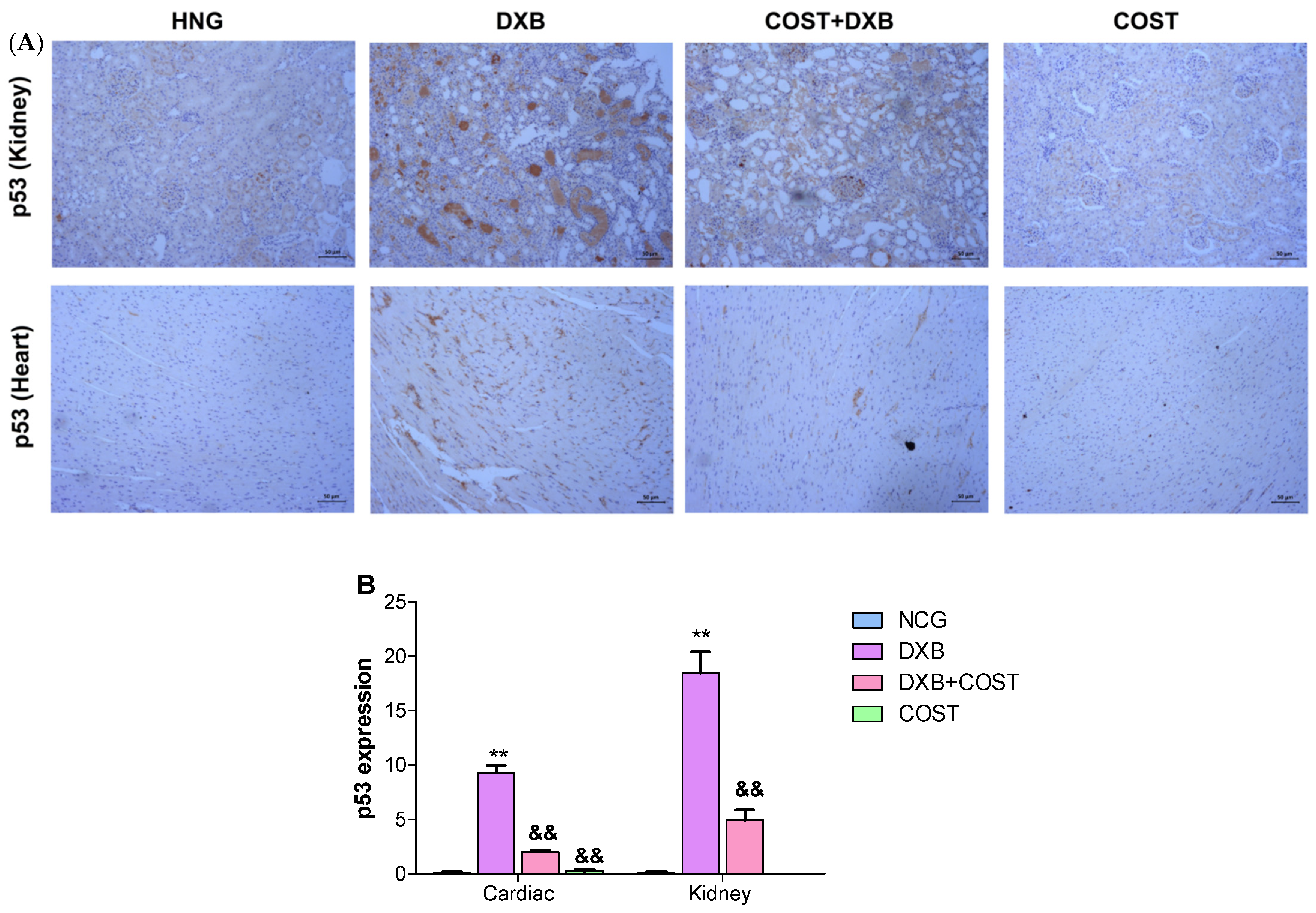
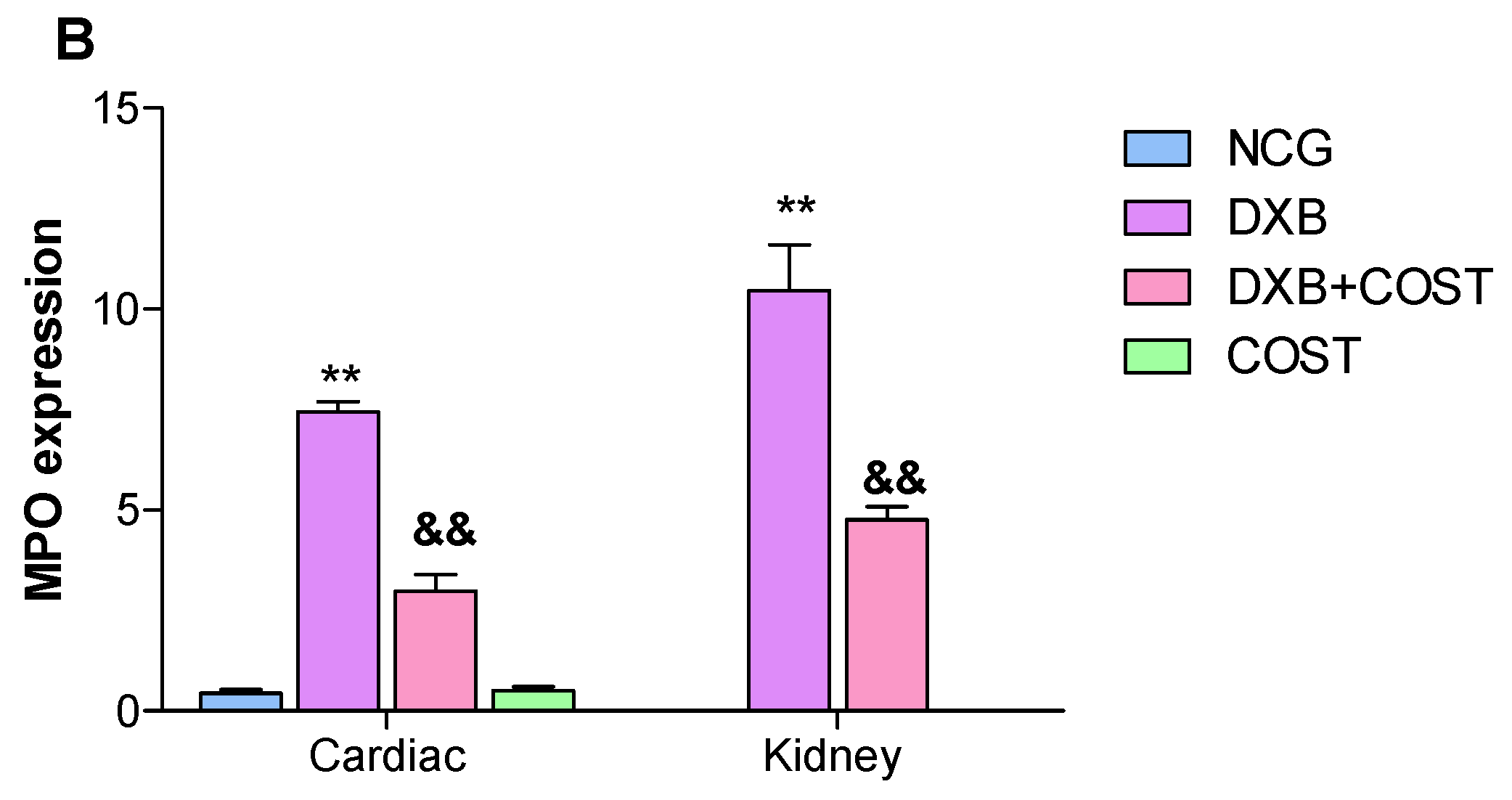
2.7. Effects of COST Treatment of Cardiorenal p53 and Myeloperoxidase
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals and Experimental Treatment
- Group 1: Healthy control group (HCG), received 5% DMSO orally for 4 weeks
- Group 2: DXB control group (DXB), received 5% DMSO orally for 4 weeks
- Group 3: COST + DXB group, received COST (50 mg/kg) orally for 4 weeks.
- Group 4: COST group (COST), received COST (50 mg/kg body weight) orally for 4 weeks.
4.3. Biochemical Assays
4.4. Antioxidant and Proinflammatory Cytokines Parameters
4.5. Hematoxylin and Eosin Staining
4.6. Immunohistochemical Expression of p53 and Myeloperoxidase
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Akindele, A.J.; Oludadepo, G.O.; Amagon, K.I.; Singh, D.; Osiagwu, D.D. Protective effect of carvedilol alone and co-administered with diltiazem and prednisolone on doxorubicin and 5-fluorouracil-induced hepatotoxicity and nephrotoxicity in rats. Pharmacol. Res. Perspect. 2018, 6, e00381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, K.M.; Mantawy, E.M.; Elanany, M.M.; Abdelgawad, H.S.; Khalifa, N.M.; Hussien, R.H.; El-Agroudy, N.N.; El-Demerdash, E. Protection from doxorubicin-induced nephrotoxicity by clindamycin: Novel antioxidant, anti-inflammatory and anti-apoptotic roles. Naunyn-Schmiedeb. Arch. Pharmacol. 2020, 393, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Altınkaynak, Y.; Kural, B.; Akcan, B.A.; Bodur, A.; Özer, S.; Yuluğ, E.; Munğan, S.; Kaya, C.; Örem, A. Protective effects of L-theanine against doxorubicin-induced nephrotoxicity in rats. Biomed. Pharmacother. 2018, 108, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Cappetta, D.; De Angelis, A.; Sapio, L.; Prezioso, L.; Illiano, M.; Quaini, F.; Rossi, F.; Berrino, L.; Naviglio, S.; Urbanek, K. Oxidative stress and cellular response to doxorubicin: A common factor in the complex milieu of anthracycline cardiotoxicity. Oxidative Med. Cell. Longev. 2017, 2017, 1521020. [Google Scholar] [CrossRef]
- Farías, J.G.; Molina, V.M.; Carrasco, R.A.; Zepeda, A.B.; Figueroa, E.; Letelier, P.; Castillo, R. Antioxidant therapeutic strategies for cardiovascular conditions associated with oxidative stress. Nutrients 2017, 9, 966. [Google Scholar] [CrossRef]
- Afsar, T.; Razak, S.; Almajwal, A.; Al-Disi, D. Doxorubicin-induced alterations in kidney functioning, oxidative stress, DNA damage, and renal tissue morphology; Improvement by Acacia hydaspica tannin-rich ethyl acetate fraction. Saudi J. Biol. Sci. 2020, 7, 2251–2260. [Google Scholar] [CrossRef]
- Sheibani, M.; Nezamoleslami, S.; Faghir-Ghanesefat, H.; Emami, A.H.; Dehpour, A.R. Cardioprotective effects of dapsone against doxorubicin-induced cardiotoxicity in rats. Cancer Chemother. Pharmacol. 2020, 85, 563–571. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, K.J.; Kim, J.H.; Kwak, J.H.; Song, H.; Cho, J.Y.; Hwang, D.Y.; Kim, K.S.; Jung, Y.S. Comparison of doxorubicin induced cardiotoxicity in the ICR mice of diferent sources. Lab. Anim. Res. 2017, 33, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Ikewuchi, J.C.; Ikewuchi, C.C.; Ifeanacho, M.O.; Jaja, V.S.; Okezue, E.C.; Jamabo, C.N.; Adeku, K.A. Attenuation of doxorubicin-induced cardiotoxicity in Wistar rats by aqueous leaf-extracts of Chromolaena odorata and Tridax procumbens. J. Ethnopharmacol. 2021, 274, 114004. [Google Scholar] [CrossRef]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.L.; Sanchez-Rovira, P.; Ramirez-Tortosa, M.C. New advances in molecular mechanisms and the prevention of adriamycin toxicity by antioxidant nutrients. Food Chem. Toxicol. 2010, 48, 1425–1438. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Xie, Y.; Hu, H. Antitumor activity and mechanism of costunolide and dehydrocostus lactone: Two natural sesquiterpene lactones from the Asteraceae family. Biomed. Pharmacother. 2020, 125, 109955. [Google Scholar] [CrossRef]
- Jin, X.; Wang, C.; Wang, L. Costunolide inhibits osteosarcoma growth and metastasis via suppressing STAT3 signal pathway. Biomed. Pharmacother. 2022, 121, 109659. [Google Scholar] [CrossRef]
- Kim, D.Y.; Choi, B.Y. Costunolide—A bioactive sesquiterpene lactone with diverse therapeutic potential. Int. J. Mol. Sci. 2019, 20, 2926. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Chen, Y.; Zhang, J.; Wang, L.; Jin, Z.; Huang, H.; Man, S.; Gao, W. Evaluation of protective effects of costunolide and dehydrocostuslactone on ethanol-induced gastric ulcer in mice based on multi-pathway regulation. Chem.-Biol. Interact. 2016, 250, 68–77. [Google Scholar] [CrossRef]
- Peng, S.; Hou, Y.; Yao, J.; Fang, J. Activation of Nrf2 by costunolide provides neuroprotective effect in PC12 cells. Food Funct. 2019, 10, 4143–4152. [Google Scholar] [CrossRef]
- Monzel, J.V.; Budde, T.; Meyer Zu Schwabedissen, H.E.; Schwebe, M.; Bien-Möller, S.; Lütjohann, D.; Kroemer, H.K.; Jedlitschky, G.; Grube, M. Doxorubicin enhances oxysterol levels resulting in a LXR-mediated upregulation of cardiac cholesterol transporters. Biochem. Pharmacol. 2017, 144, 108–119. [Google Scholar] [CrossRef]
- Sergazy, S.; Shulgau, Z.; Fedotovskikh, G.; Chulenbayeva, L.; Nurgozhina, A.; Nurgaziyev, M.; Krivyh, E.; Kamyshanskiy, Y.; Kushugulova, A.; Gulyayev, A.; et al. Cardioprotective effect of grape polyphenol extract against doxorubicin induced cardiotoxicity. Sci. Rep. 2020, 10, 14720. [Google Scholar] [CrossRef]
- Xu, H.; Yu, W.; Sun, S.; Li, C.; Zhang, Y.; Ren, J. Luteolin attenuates doxorubicin-induced cardiotoxicity through promoting mitochondrial autophagy. Front. Physiol. 2020, 11, 113. [Google Scholar] [CrossRef]
- Du, J.; Zhang, A.; Li, J.; Liu, X.; Wu, S.; Wang, B.; Wang, Y.; Jia, H. Doxorubicin-induced cognitive impairment: The mechanistic insights. Front. Oncol. 2021, 11, 673340. [Google Scholar] [CrossRef]
- El-Maddawy, Z.K.; Abd El Naby, W.S.H. Protective effects of zinc oxide nanoparticles against doxorubicin induced testicular toxicity and DNA damage in male rats. Toxicol. Res. 2019, 8, 654–662. [Google Scholar] [CrossRef]
- Galal, A.; El Bakly, W.M.; Al Haleem, E.N.A.; El-Demerdash, E. Selective A 3 adenosine receptor agonist protects against doxorubicin-induced cardiotoxicity. Cancer Chemother. Pharmacol. 2016, 77, 309–322. [Google Scholar] [CrossRef]
- Xiang, C.; Yan, Y.; Zhang, D. Alleviation of the doxorubicin-induced nephrotoxicity by fasudil in vivo and in vitro. J. Pharmacol. Sci. 2021, 145, 6–15. [Google Scholar] [CrossRef]
- Sindhu, E.R.; Nithya, T.R.; Binitha, P.P.; Kuttan, R. Amelioration of doxorubicin-induced cardiac and renal toxicity by oxycarotenoid lutein and its mechanism of action. J. Environ. Pathol. Toxicol. Oncol. 2016, 35, 237–247. [Google Scholar] [CrossRef]
- Aziz, T.A. Cardioprotective effect of quercetin and sitagliptin in doxorubicin-induced cardiac toxicity in rats. Cancer Manag. Res. 2021, 13, 2349–2357. [Google Scholar]
- Haybar, H.; Goudarzi, M.; Mehrzadi, S.; Aminzadeh, A.; Khodayar, M.J.; Kalantar, M.; Fatemi, I. Effect of gemfibrozil on cardiotoxicity induced by doxorubicin in male experimental rats. Biomed. Pharmacother. 2019, 109, 530–535. [Google Scholar] [CrossRef]
- Iliskovic, N.; Singal, P.K. Lipid lowering: An important factor in preventing adriamycin-induced heart failure. Am. J. Pathol. 1997, 150, 727–734. [Google Scholar]
- Riad, A.; Bien, S.; Westermann, D.; Becher, P.M.; Loya, K.; Landmesser, U.; Kroemer, H.K.; Schultheiss, H.P.; Tschope, C. Pretreatment with statin attenuates the cardiotoxicity of doxorubicin in mice. Cancer Res. 2009, 69, 695–699. [Google Scholar] [CrossRef] [Green Version]
- Khames, A.; Khalaf, M.M.; Gad, A.M.; Abd El-Raouf, O.M.; Kandeil, M.A. Nicorandil combats doxorubicin-induced nephrotoxicity via amendment of TLR4/P38 MAPK/NFκ-B signaling pathway. Chem. Biol. Interact. 2019, 311, 108777. [Google Scholar]
- Molehin, O.R.; Adeyanju, A.A.; Adefegha, S.A.; Oyeyemi, A.O.; Idowu, K.A. Protective mechanisms of protocatechuic acid against doxorubicin-induced nephrotoxicity in rat model. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 4. [Google Scholar] [CrossRef]
- Qi, W.; Boliang, W.; Xiaoxi, T.; Guoqiang, F.; Jianbo, X.; Gang, W. Cardamonin protects against doxorubicin-induced cardiotoxicity in mice by restraining oxidative stress and inflammation associated with Nrf2 signaling. Biomed. Pharmacother. 2020, 122, 109547. [Google Scholar] [CrossRef]
- Li, Z.; Chinnathambi, A.; Ali Alharbi, S.; Yin, F. Plumbagin protects the myocardial damage by modulating the cardiac biomarkers, antioxidants, and apoptosis signaling in the doxorubicin-induced cardiotoxicity in rats. Environ. Toxicol. 2020, 35, 1374–1385. [Google Scholar] [CrossRef] [PubMed]
- Akolkar, G.; da Silva, D.D.; Ayyappan, P.; Bagchi, A.K.; Jassal, D.S.; Salemi, V.M.C.; Irigoyen, M.C.; De Angelis, K.; Singal, P.K. Vitamin C mitigates oxidative/nitrosative stress and infammation in doxorubicin-induced cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H795–H809. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, P.L.; Renu, K.; Valsala Gopalakrishnan, A. New molecular and biochemical insights of doxorubicin-induced hepatotoxicity. Life Sci. 2020, 250, 117599. [Google Scholar] [CrossRef] [PubMed]
- Mantawy, E.M.; Esmat, A.; El-Bakly, W.M.; Salah ElD, R.A.; El-Demerdash, E. Mechanistic clues to the protective effect of chrysin against doxorubicin-induced cardiomyopathy: Plausible roles of p53, MAPK and AKT pathways. Sci. Rep. 2017, 7, 4795. [Google Scholar] [CrossRef] [Green Version]
- Xie, F.; Zhang, H.; Zheng, C.; Shen, X.F. Costunolide improved dextran sulfate sodium-induced acute ulcerative colitis in mice through NF-κB, STAT1/3, and Akt signaling pathways. Int. Immunopharmacol. 2020, 84, 106567. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, D.; Li, M.; Wang, B. Costunolide ameliorates lipoteichoic acid-induced acute lung injury via attenuating MAPK signaling pathway. Int. Immunopharmacol. 2018, 61, 283–289. [Google Scholar] [CrossRef]
- Alkreathy, H.M.; Damanhouri, Z.A.; Ahmed, N.; Slevin, M.; Osman, A.M. Mechanisms of cardioprotective effect of aged garlic extract against Doxorubicin-induced cardiotoxicity. Integr. Cancer Ther. 2012, 11, 364–370. [Google Scholar] [CrossRef]
- Mantawy, E.M.; Bakly, W.M.; Esmat, A.; Badr, A.M.; Demerdash, E. Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, infammation and apoptosis. Eur. J. Pharmacol. 2014, 728, 107–118. [Google Scholar] [CrossRef]
- Soltani Hekmat, A.; Chenari, A.; Alipanah, H.; Javanmardi, K. Protective effect of alamandine on doxorubicin-induced nephrotoxicity in rats. BMC Pharmacol. Toxicol. 2021, 22, 31. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Liu, T.J.; Wang, L.C.; Lee, H.W.; Ting, C.T.; Lee, W.L.; Hung, C.J.; Wang, K.Y.; Lai, H.C.; Lai, H.C. A standardized extract of Ginkgo biloba suppresses doxorubicin-induced oxidative stress and p53-mediated mitochondrial apoptosis in rat testes. Br. J. Pharmacol. 2009, 156, 48–61. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Lu, W.; Lin, N.; Lin, H.; Zhang, J.; Ni, T.; Meng, L.; Zhang, C.; Guo, H. Dihydromyricetin alleviates doxorubicin-induced cardiotoxicity by inhibiting NLRP3 inflammasome through activation of SIRT1. Biochem. Pharmacol. 2020, 175, 113888. [Google Scholar] [CrossRef]
- Mao, J.; Yi, M.; Wang, R.; Huang, Y.; Chen, M. Protective effects of costunolide against D-galactosamine and lipopolysaccharide-induced acute liver injury in mice. Front. Pharmacol. 2018, 9, 1469. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, W.; Wen, C.; Wang, D.; Shao, H.; Liu, C.; He, C.; Olatunji, O.J. Cardiorenal Protective Effect of Costunolide against Doxorubicin-Induced Toxicity in Rats by Modulating Oxidative Stress, Inflammation and Apoptosis. Molecules 2022, 27, 2122. https://doi.org/10.3390/molecules27072122
Xing W, Wen C, Wang D, Shao H, Liu C, He C, Olatunji OJ. Cardiorenal Protective Effect of Costunolide against Doxorubicin-Induced Toxicity in Rats by Modulating Oxidative Stress, Inflammation and Apoptosis. Molecules. 2022; 27(7):2122. https://doi.org/10.3390/molecules27072122
Chicago/Turabian StyleXing, Wen, Chaoling Wen, Deguo Wang, Hui Shao, Chunhong Liu, Chunling He, and Opeyemi Joshua Olatunji. 2022. "Cardiorenal Protective Effect of Costunolide against Doxorubicin-Induced Toxicity in Rats by Modulating Oxidative Stress, Inflammation and Apoptosis" Molecules 27, no. 7: 2122. https://doi.org/10.3390/molecules27072122
APA StyleXing, W., Wen, C., Wang, D., Shao, H., Liu, C., He, C., & Olatunji, O. J. (2022). Cardiorenal Protective Effect of Costunolide against Doxorubicin-Induced Toxicity in Rats by Modulating Oxidative Stress, Inflammation and Apoptosis. Molecules, 27(7), 2122. https://doi.org/10.3390/molecules27072122






