Insights on the Synthesis of N-Heterocycles Containing Macrocycles and Their Complexion and Biological Properties
Abstract
:1. Introduction
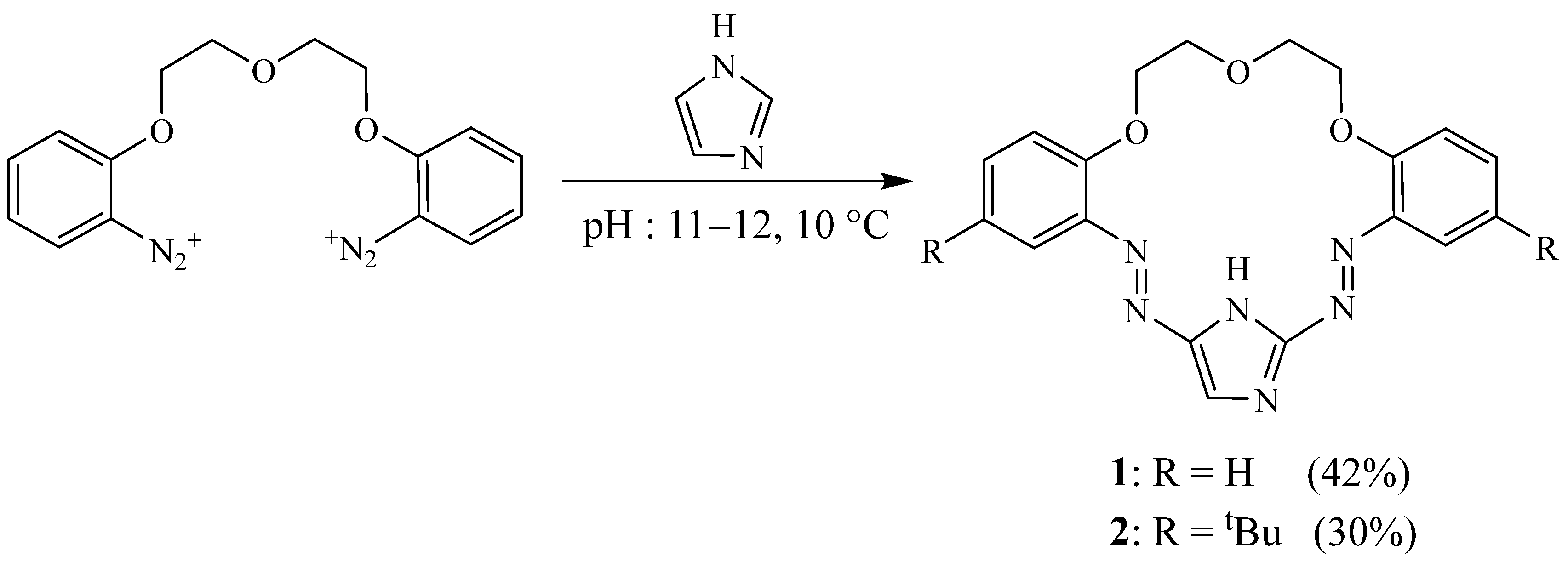
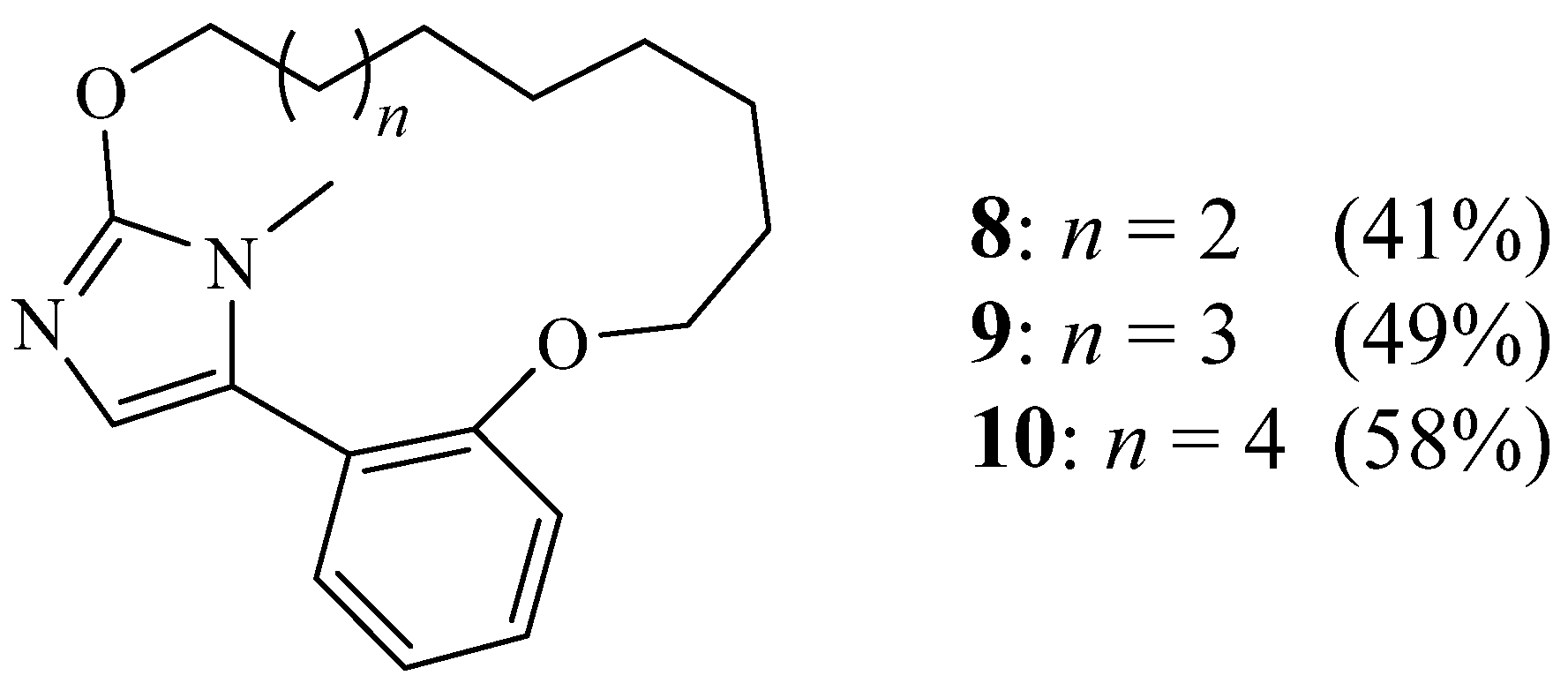
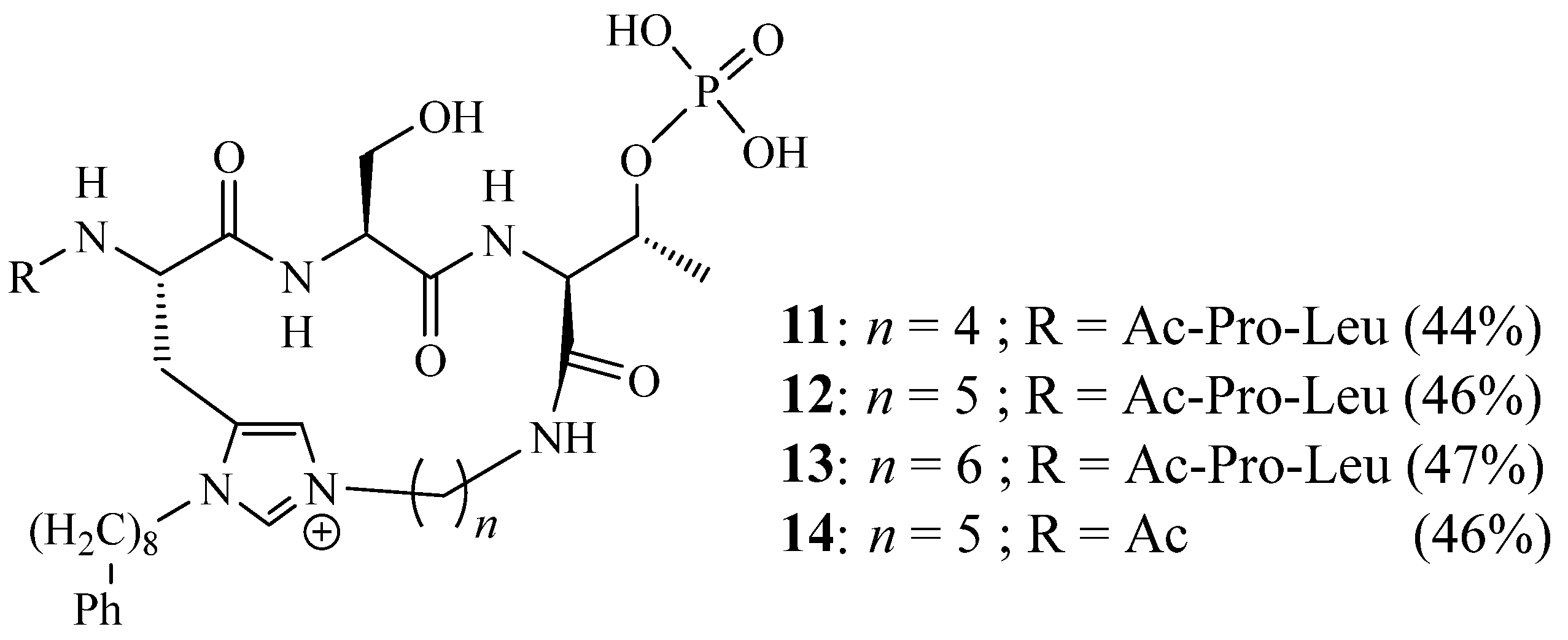
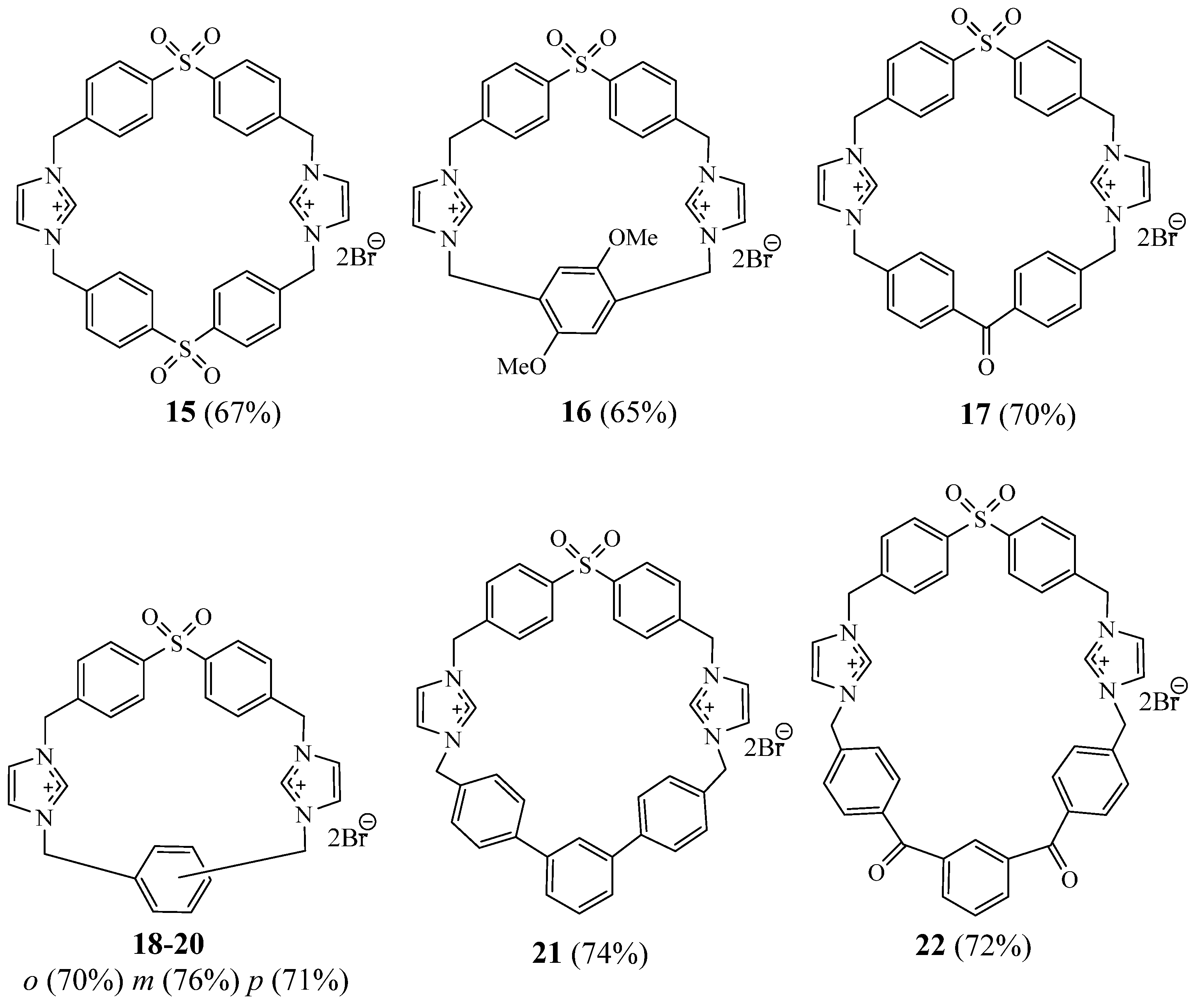
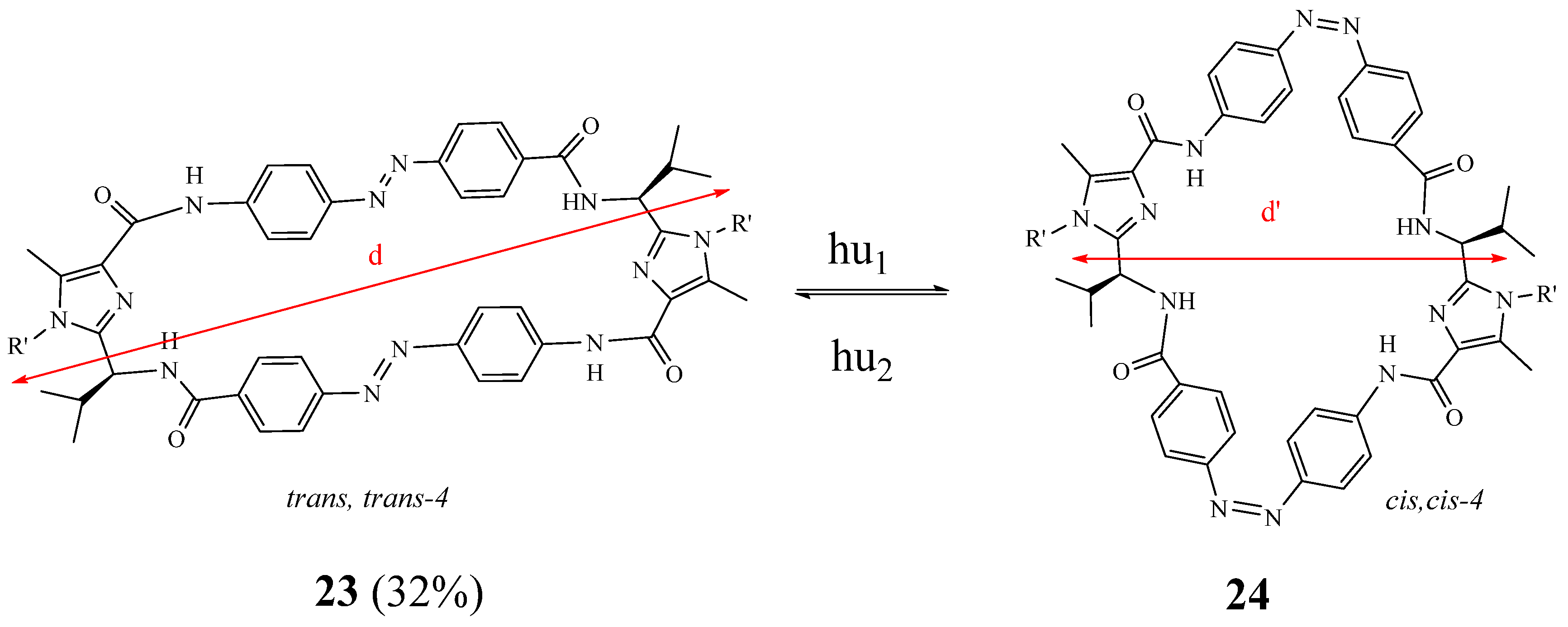
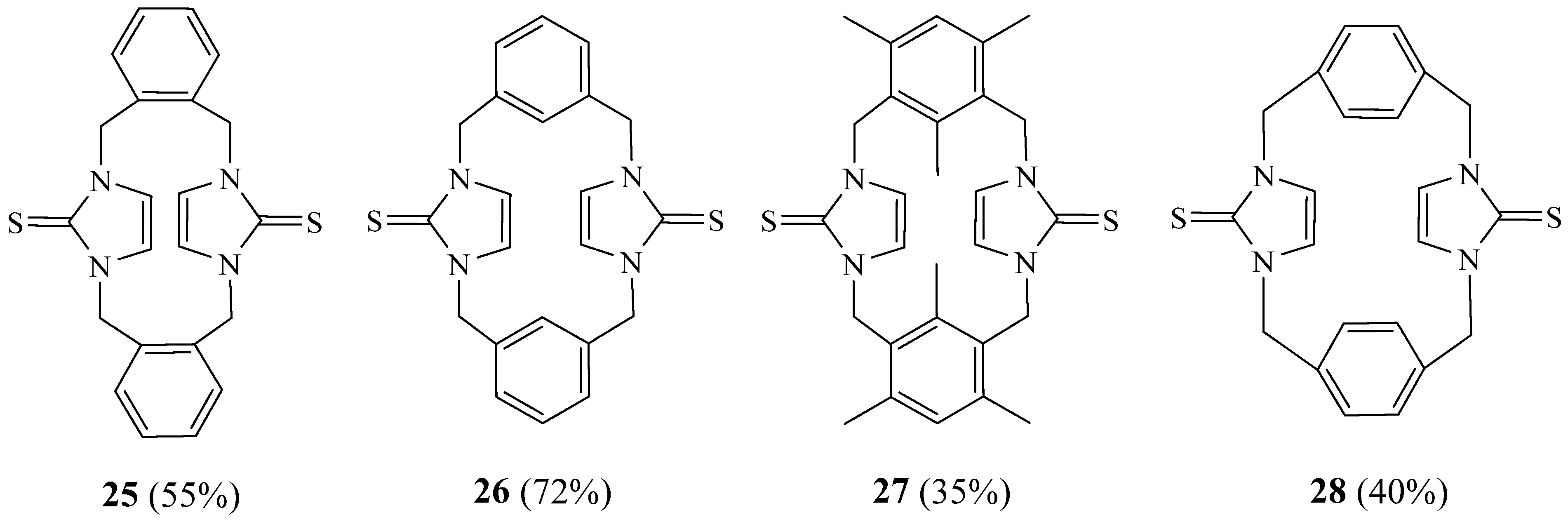
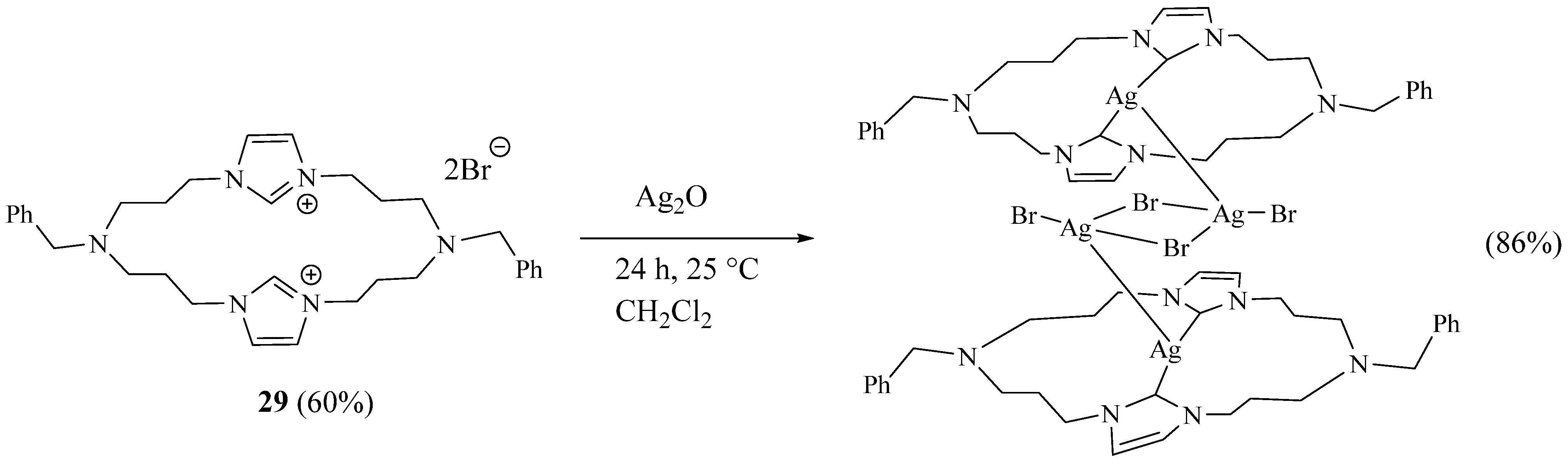
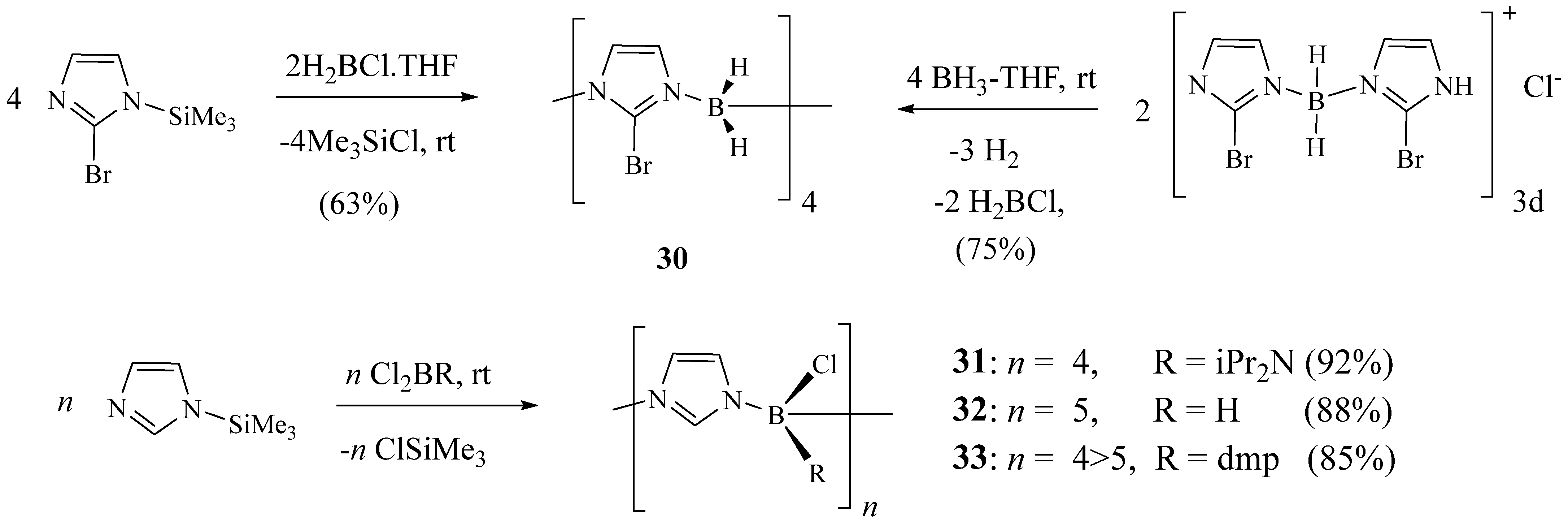
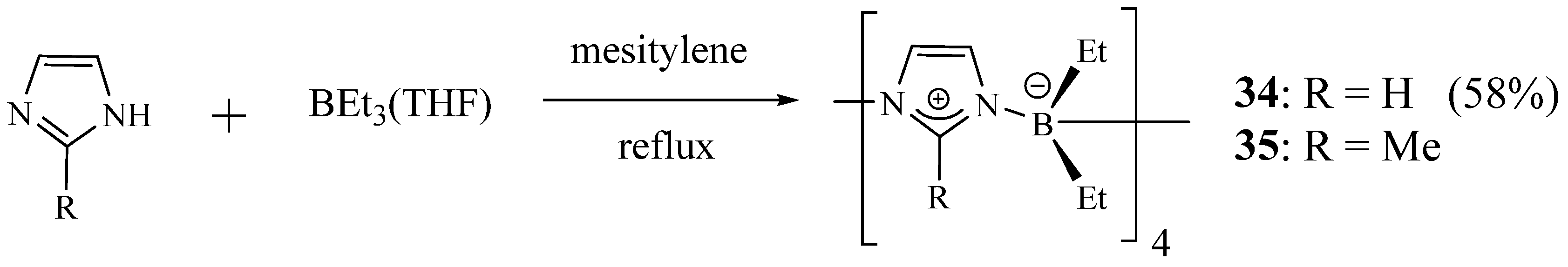
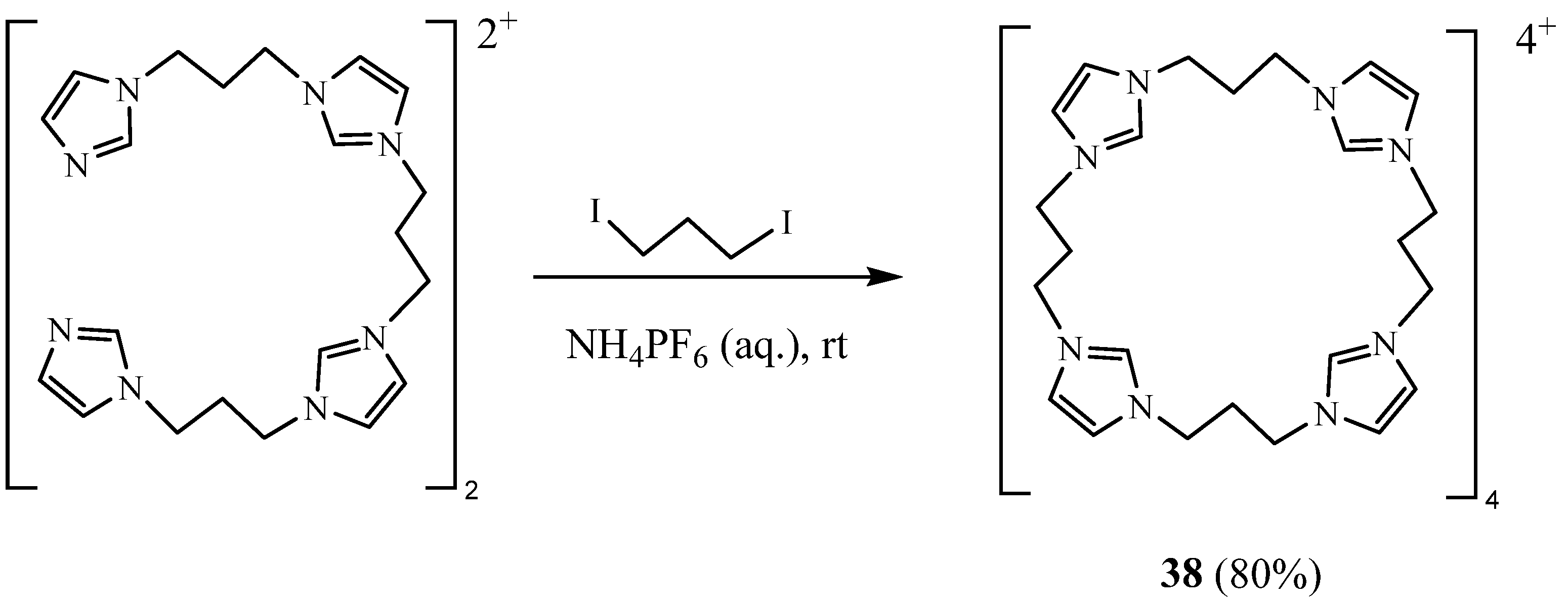
2. Synthesis of Imidazolic Macrocycles
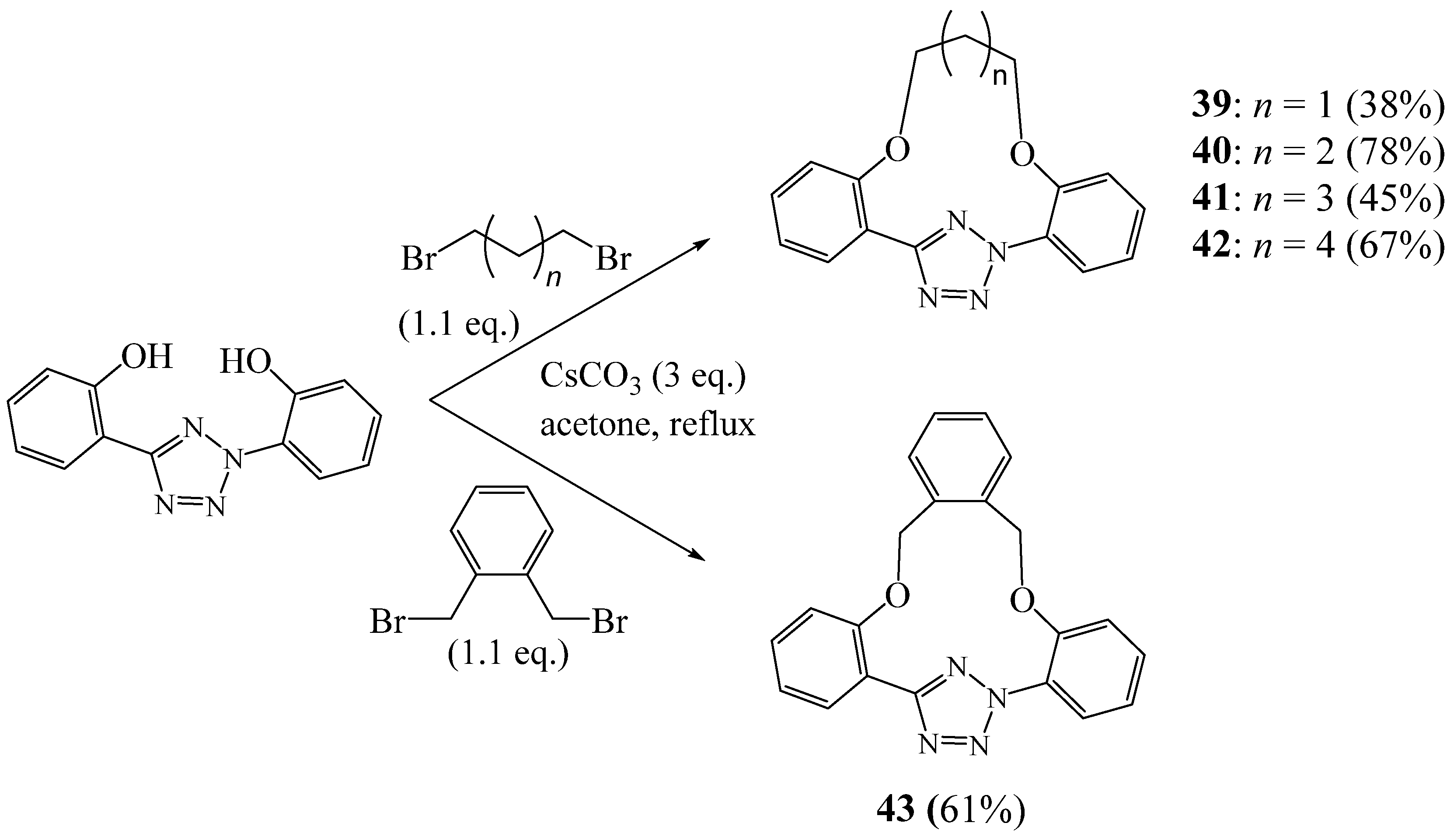
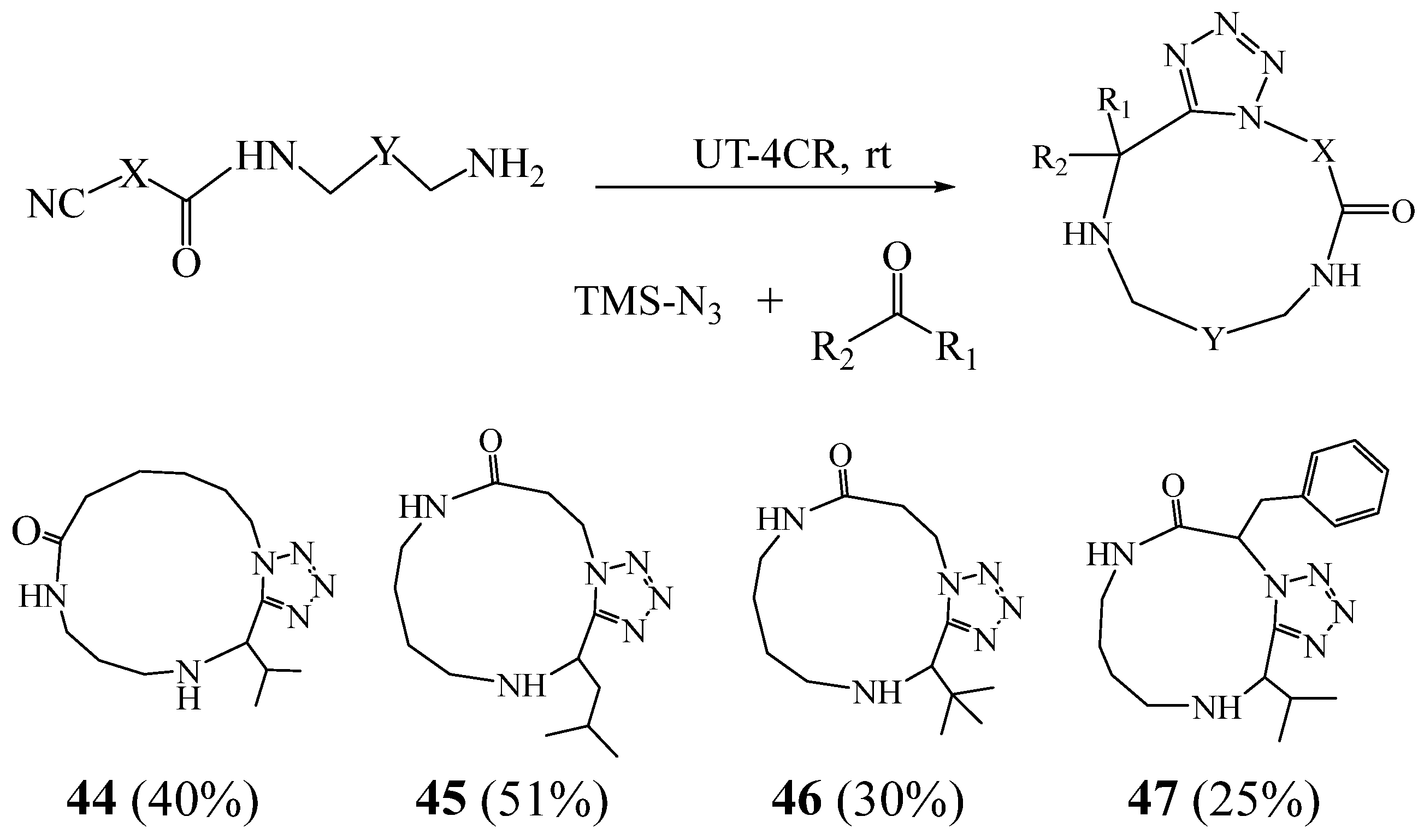
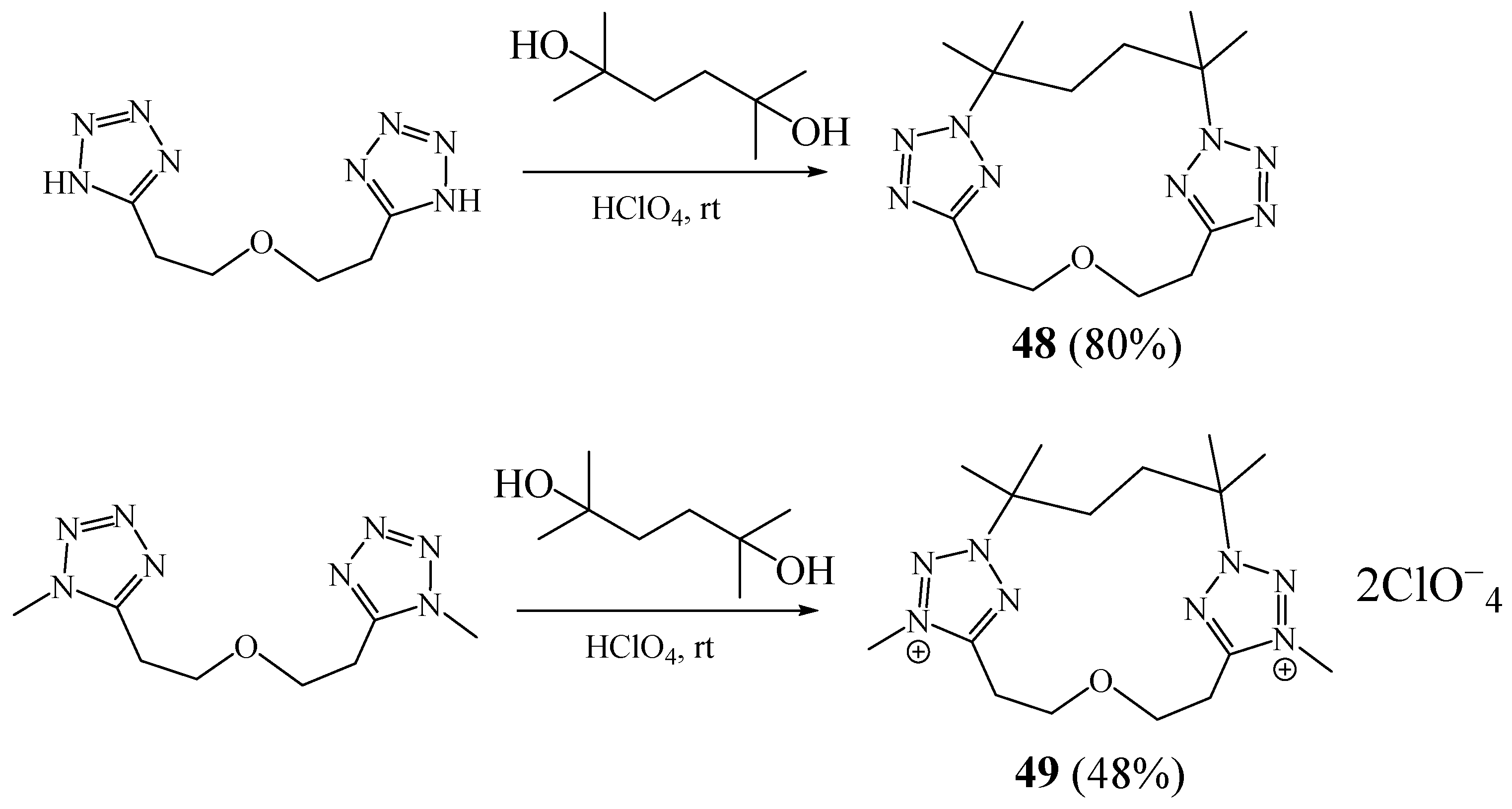
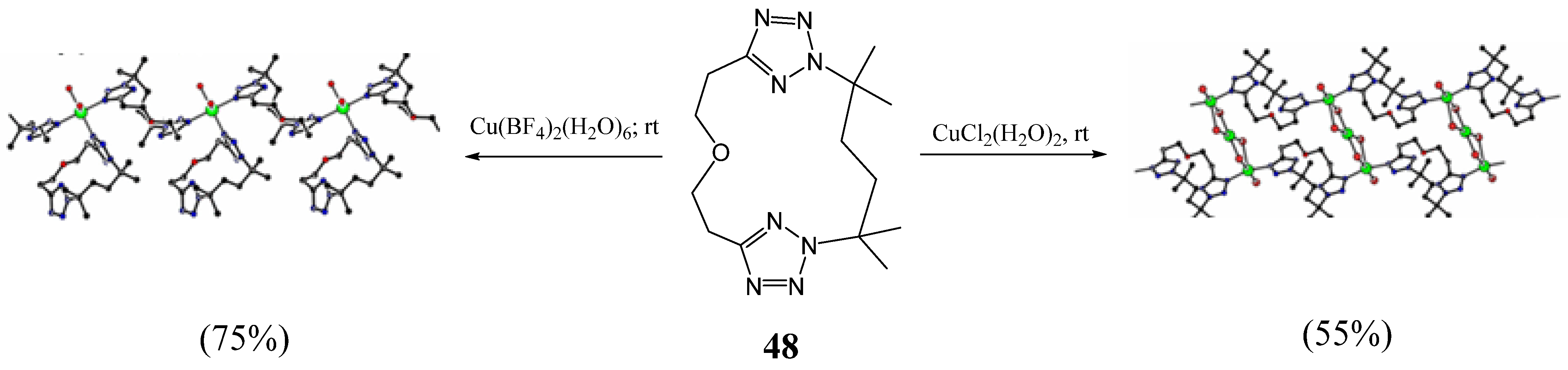
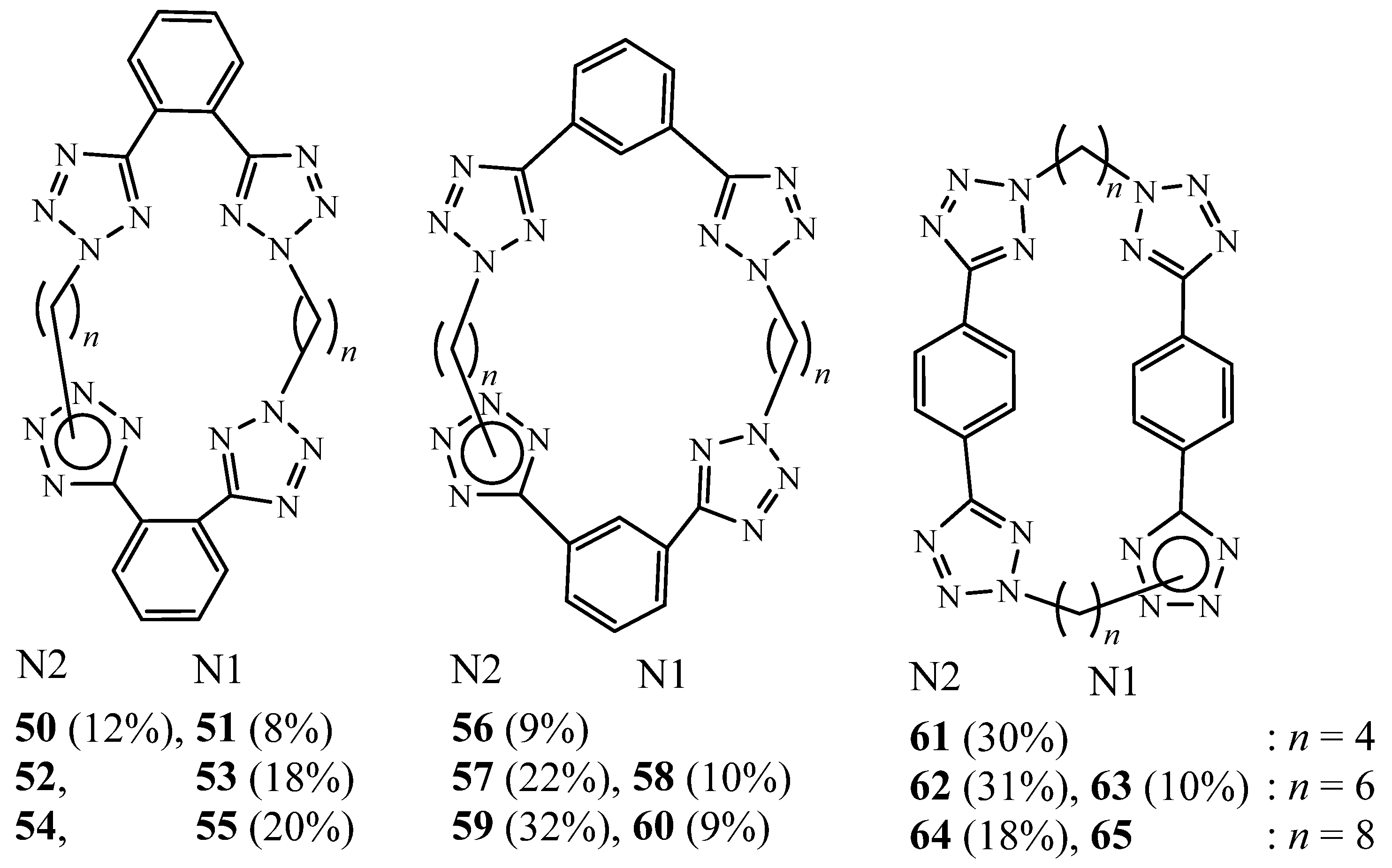
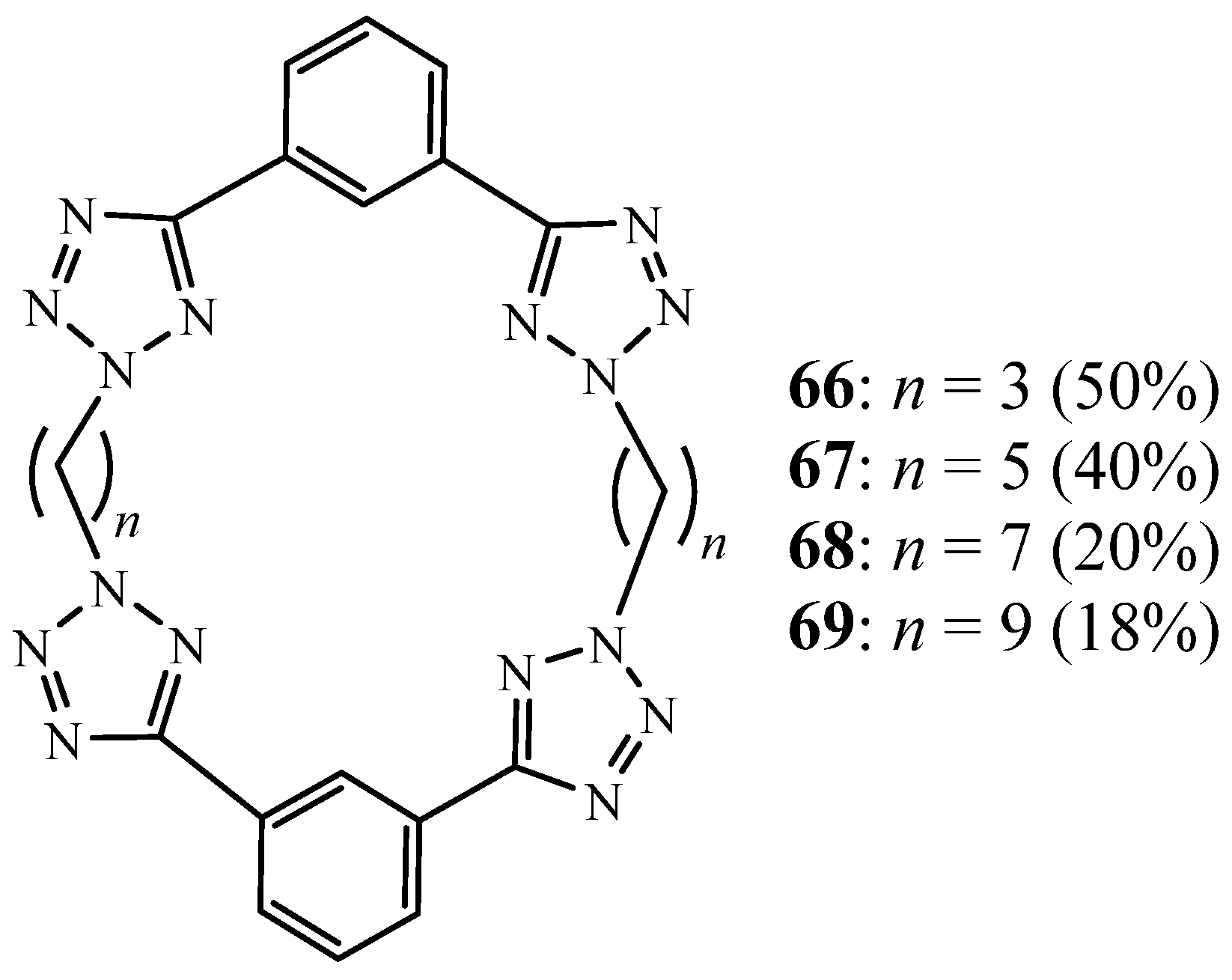
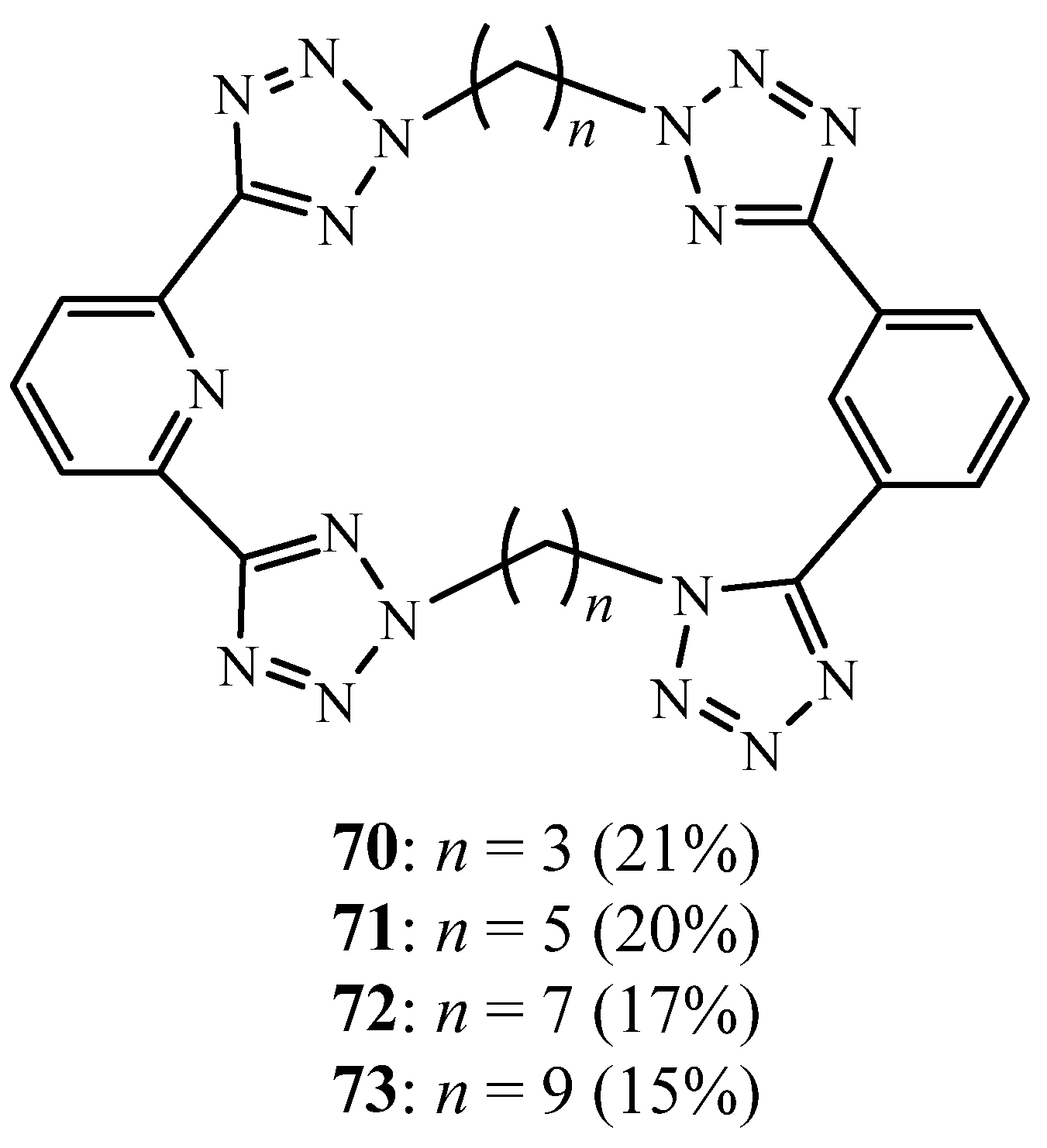
3. Synthesis of Tetrazolic Macrocycles
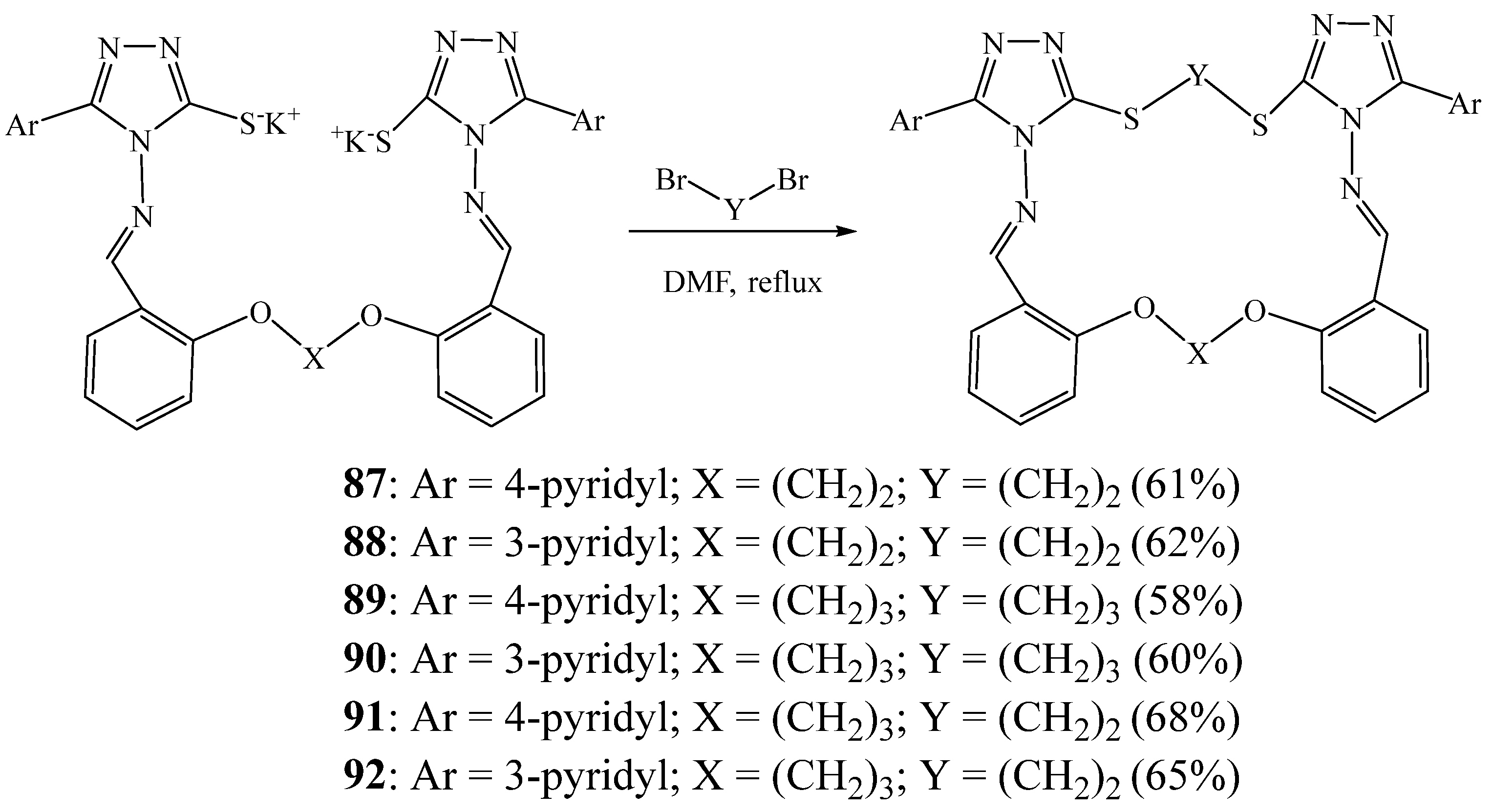
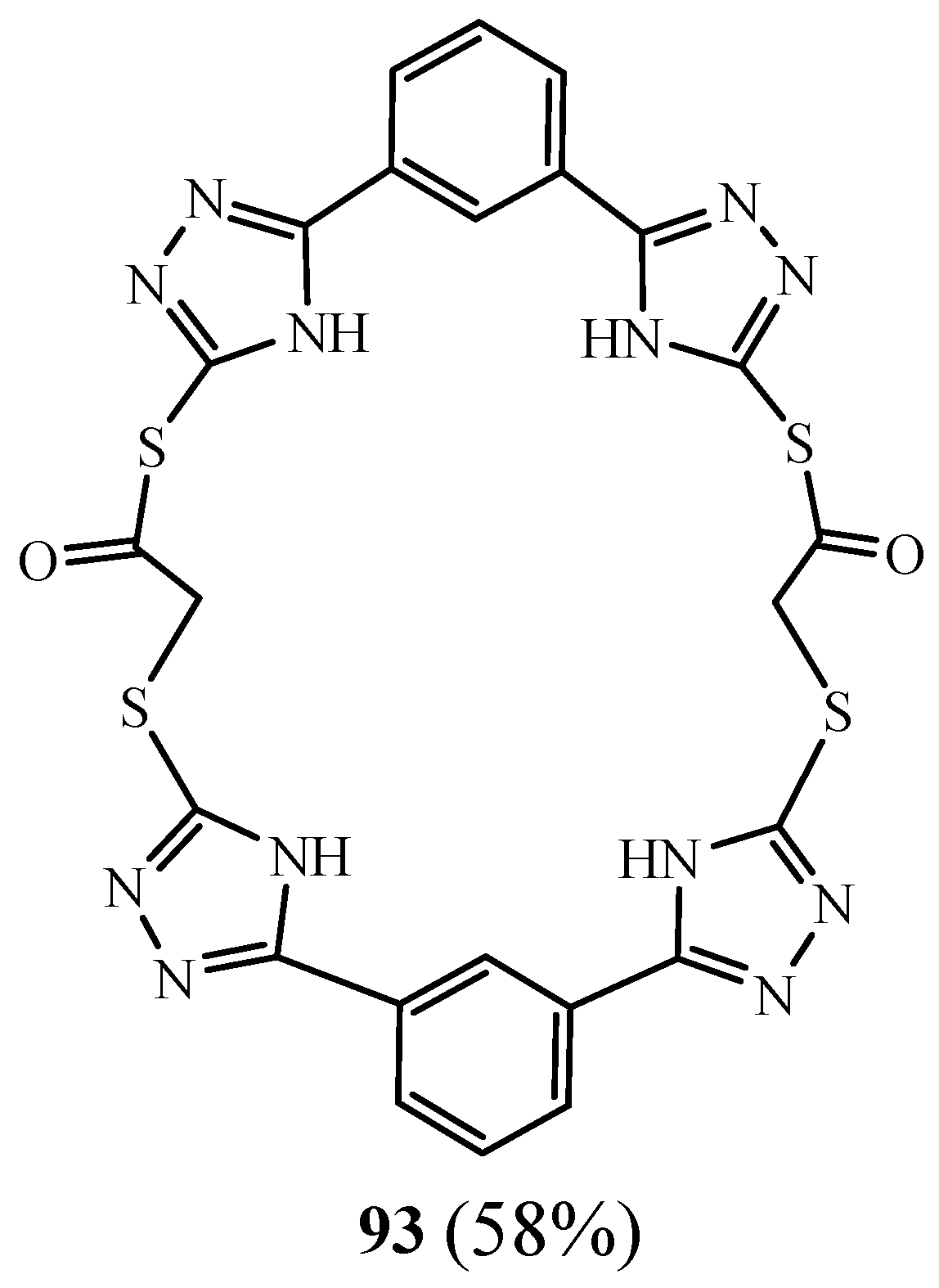
4. Synthesis of 1,2,4 Triazolic Macrocycles
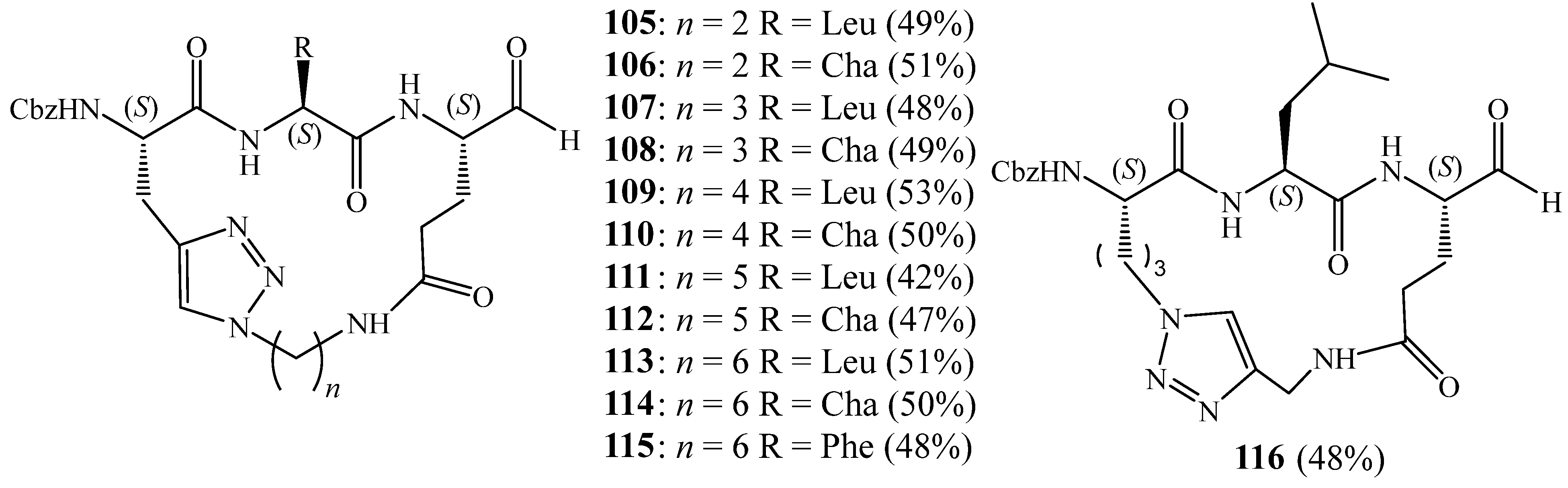
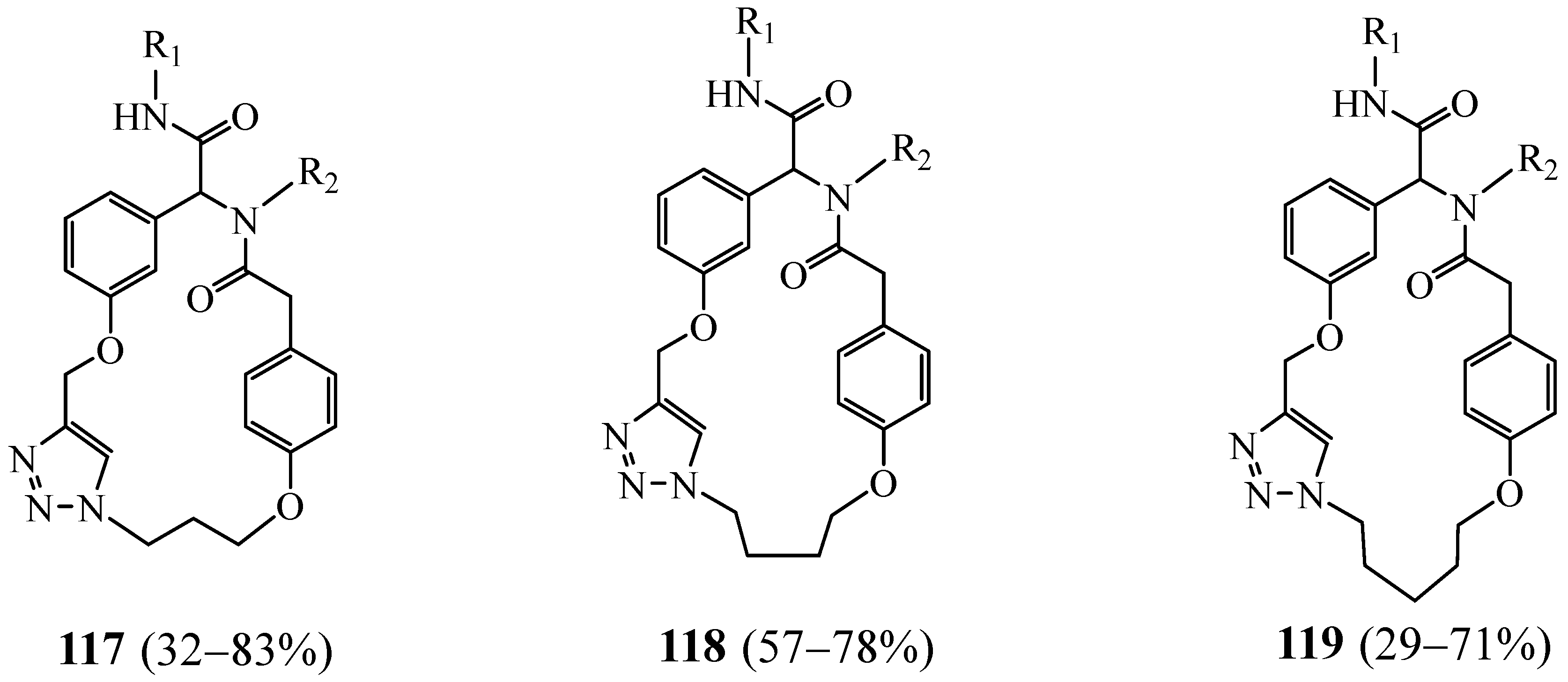
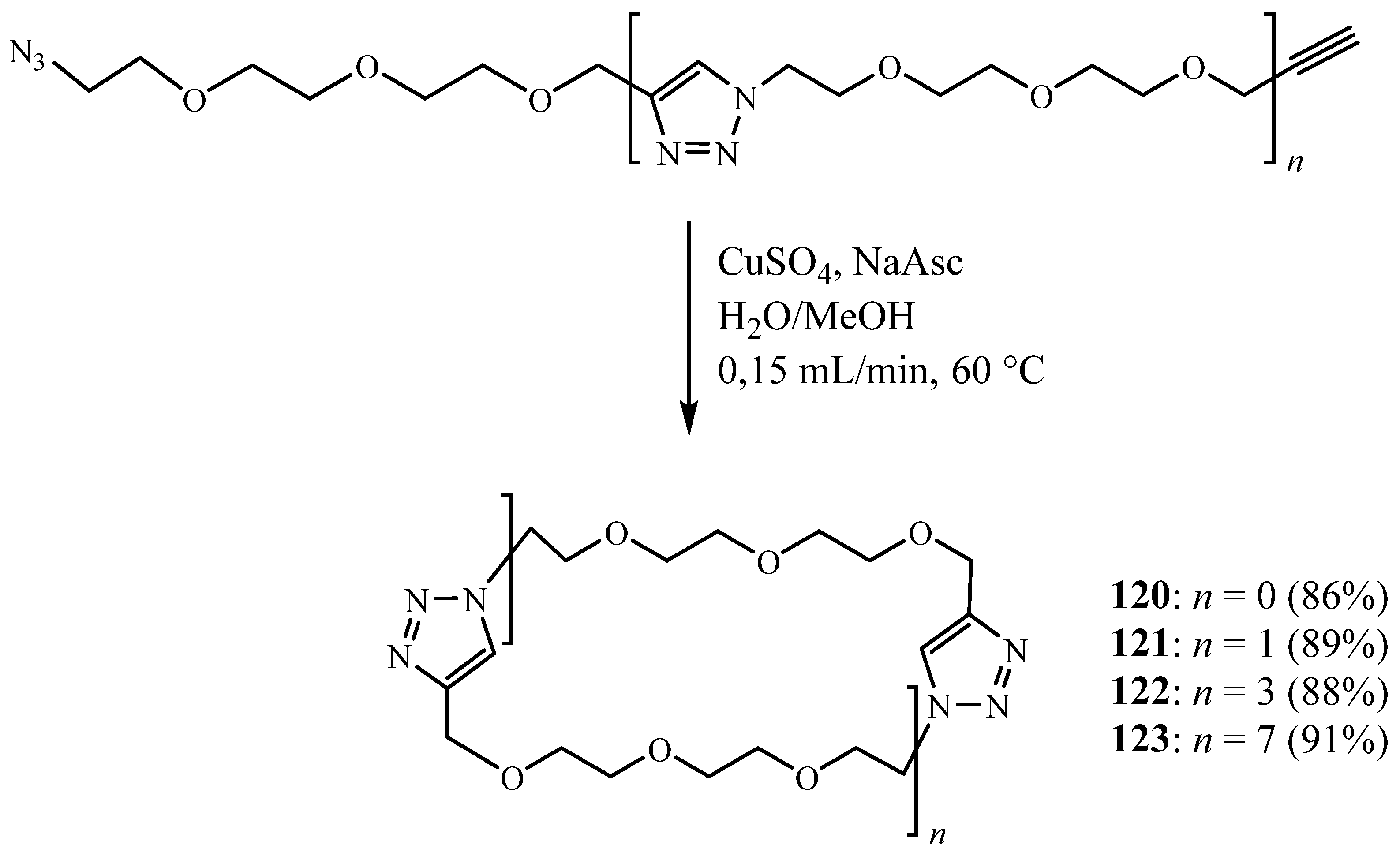
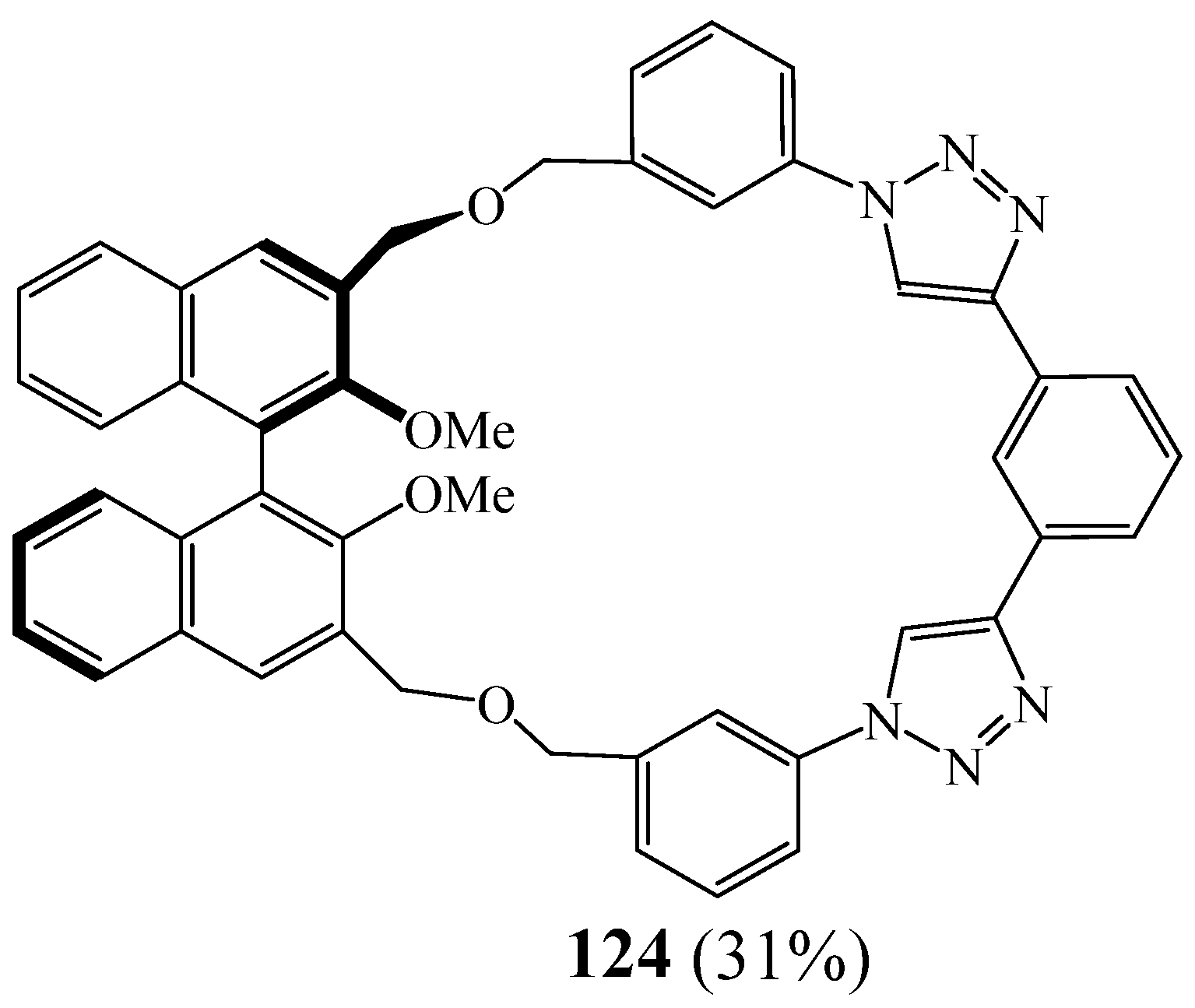
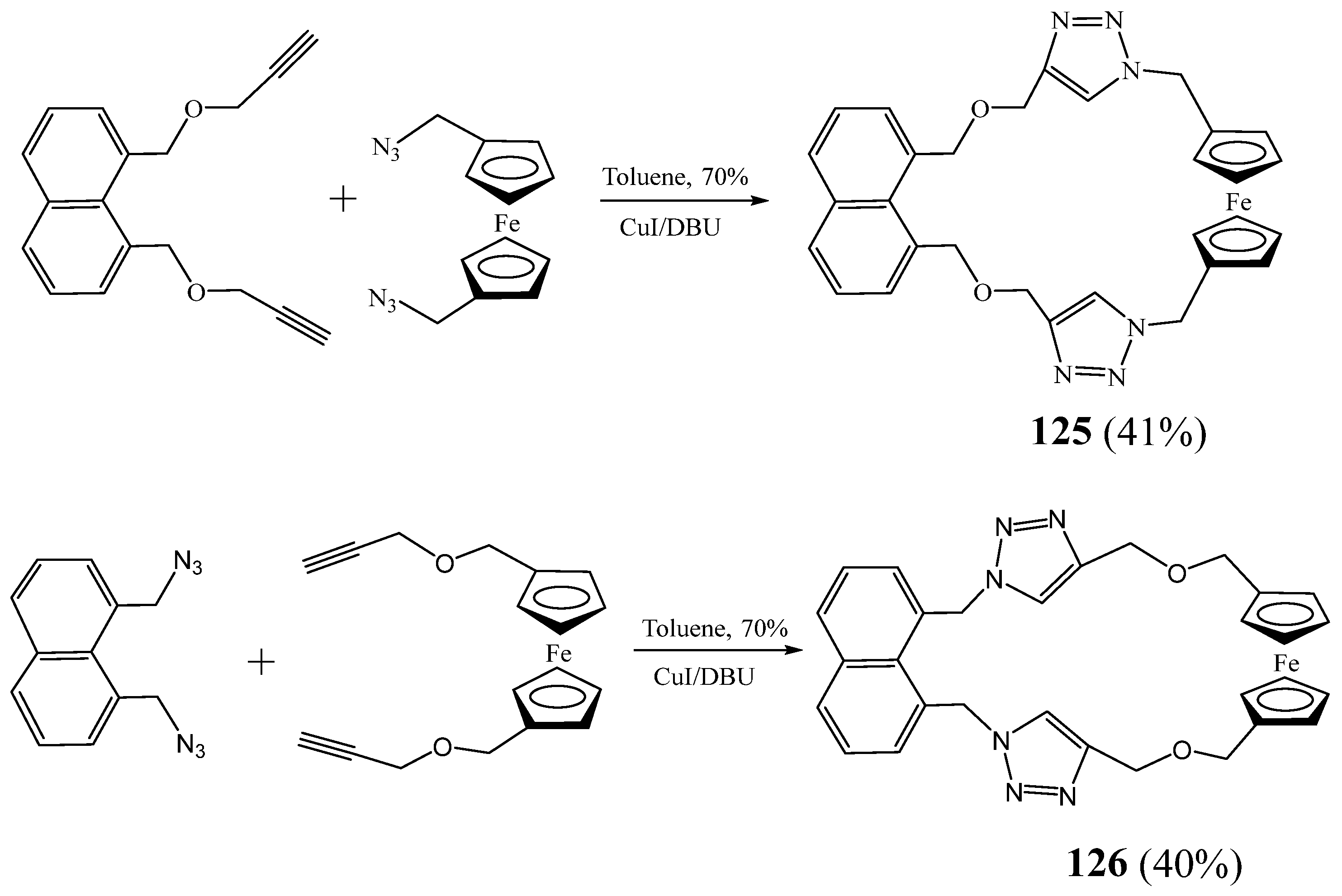
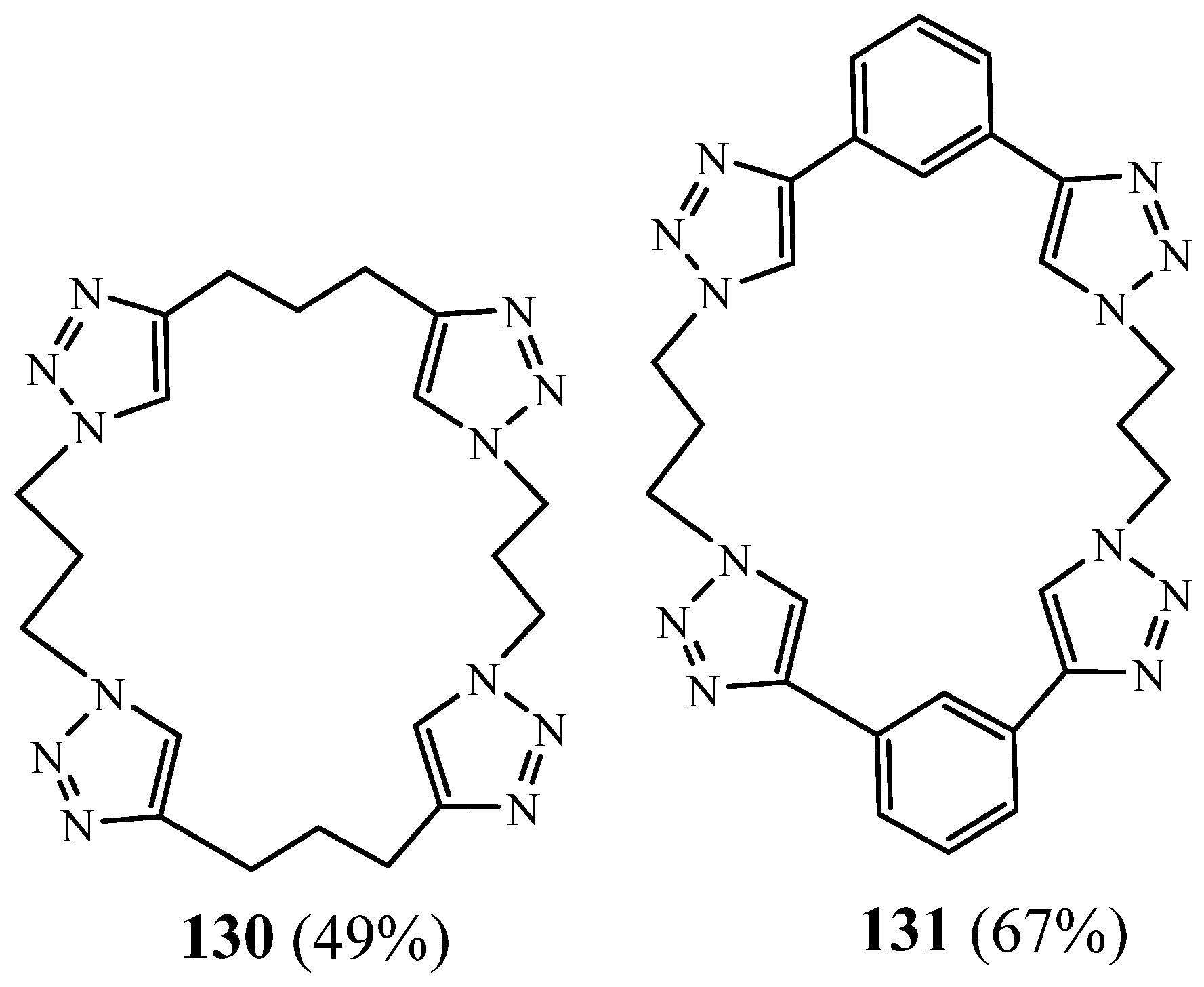
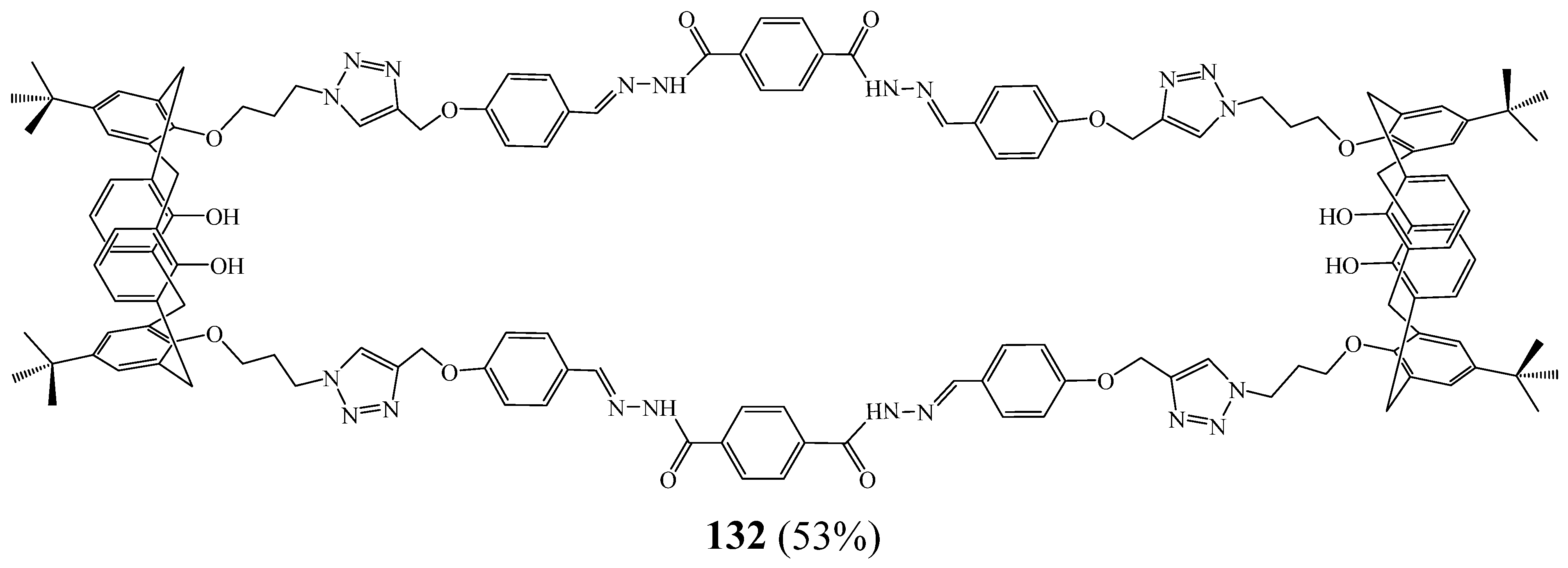
5. Synthesis of 1,2,3 Triazolic Macrocycles
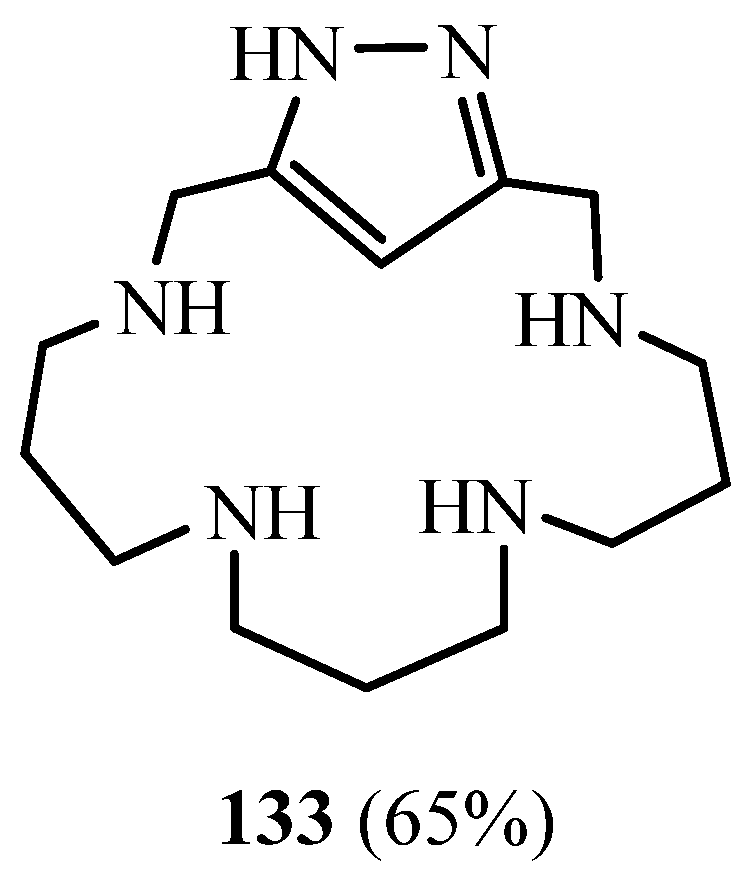
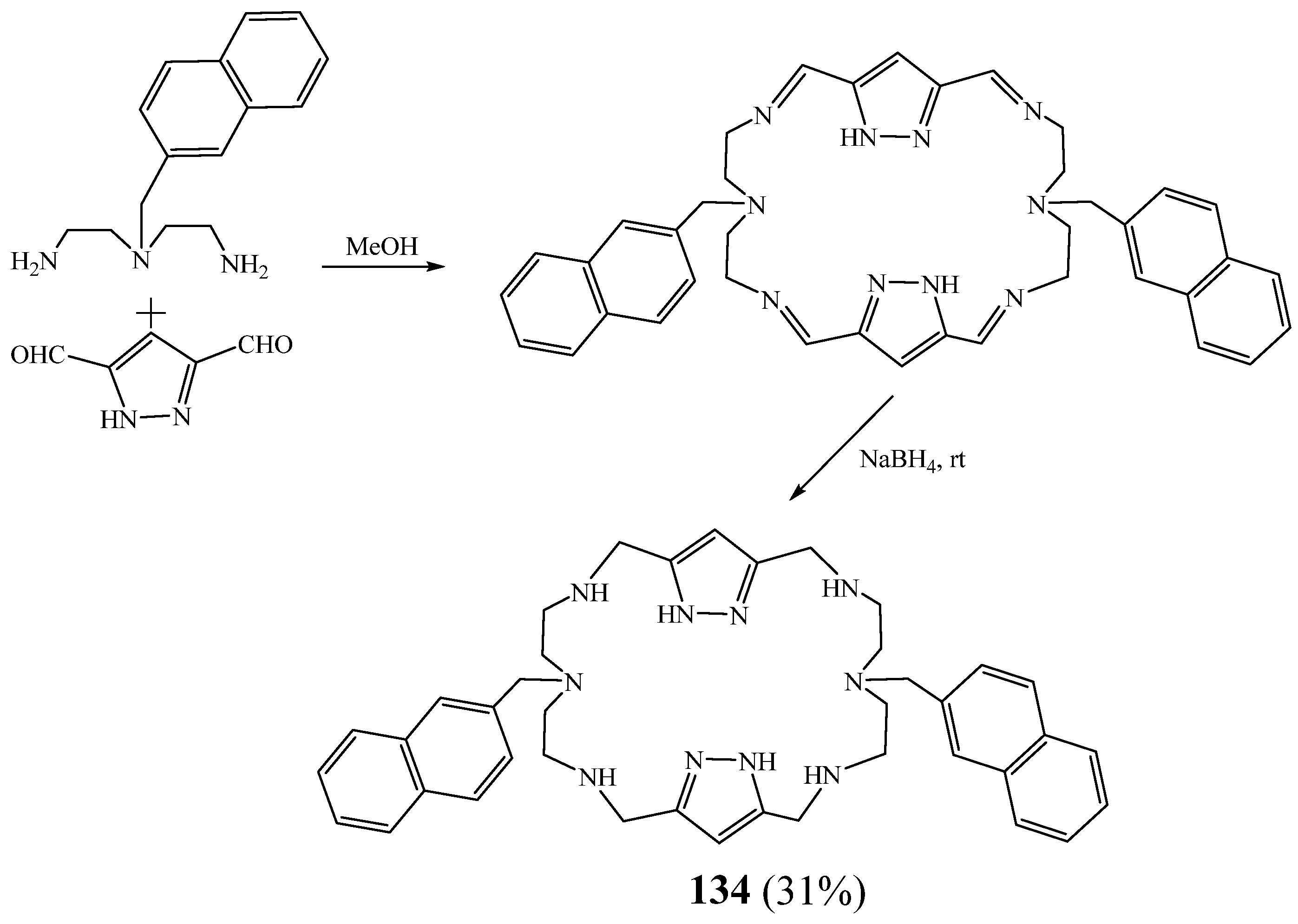
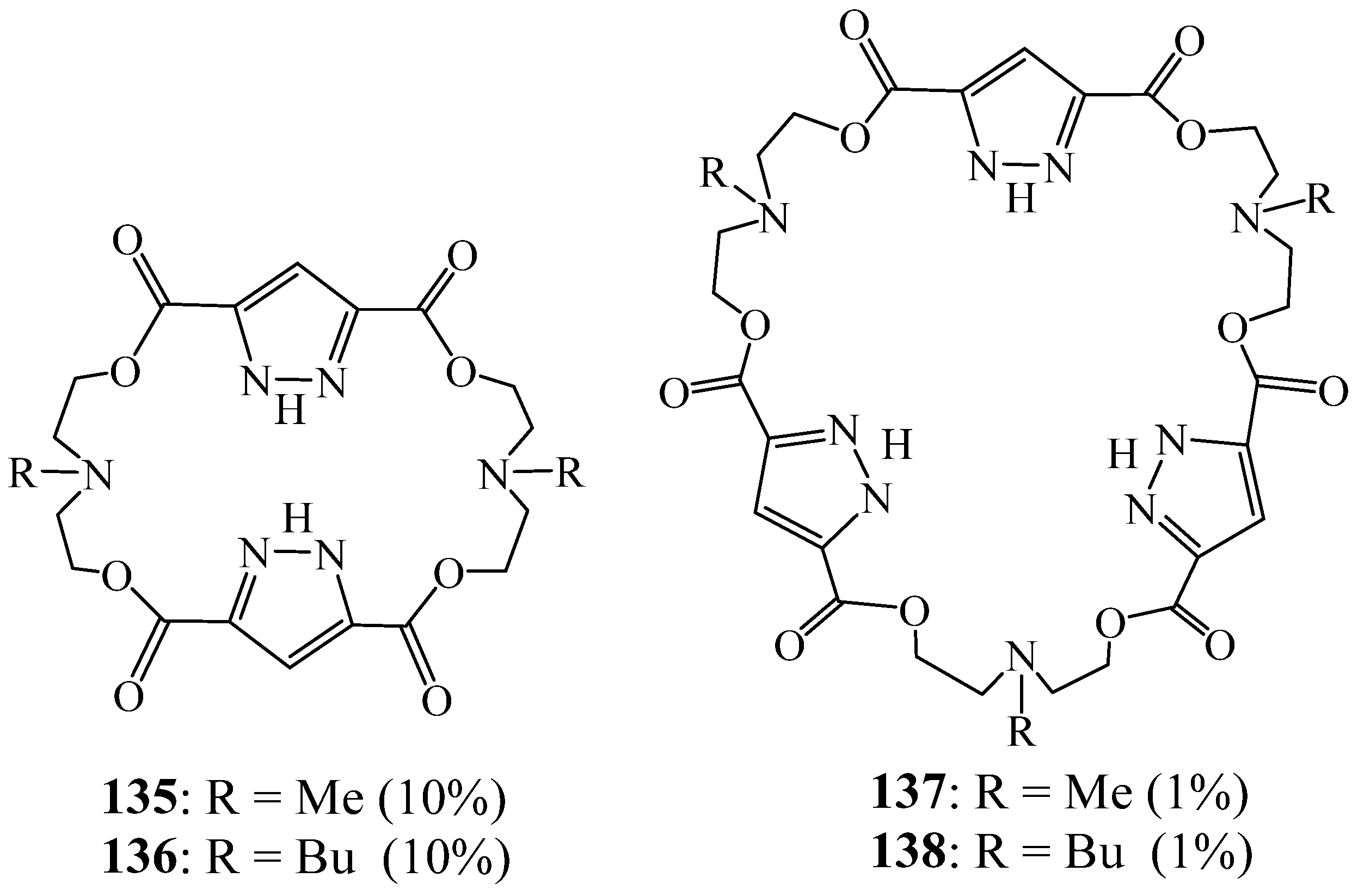
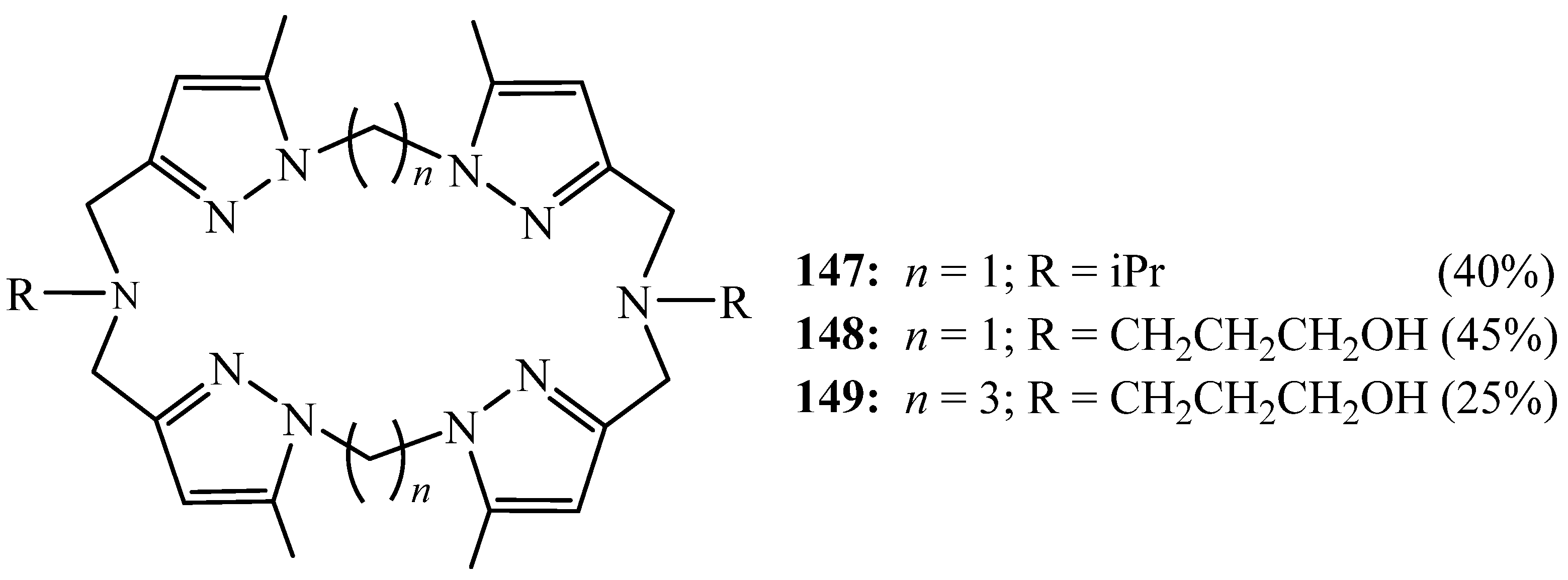
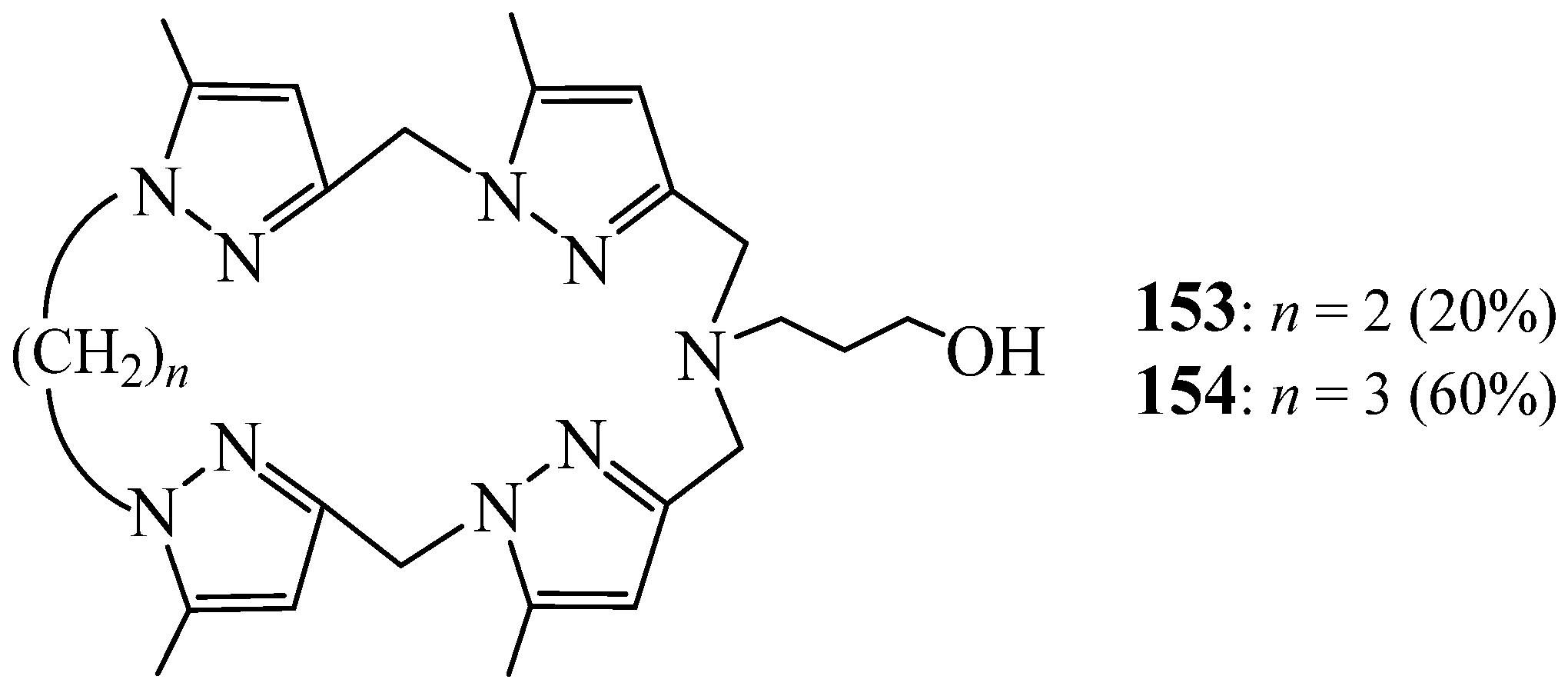
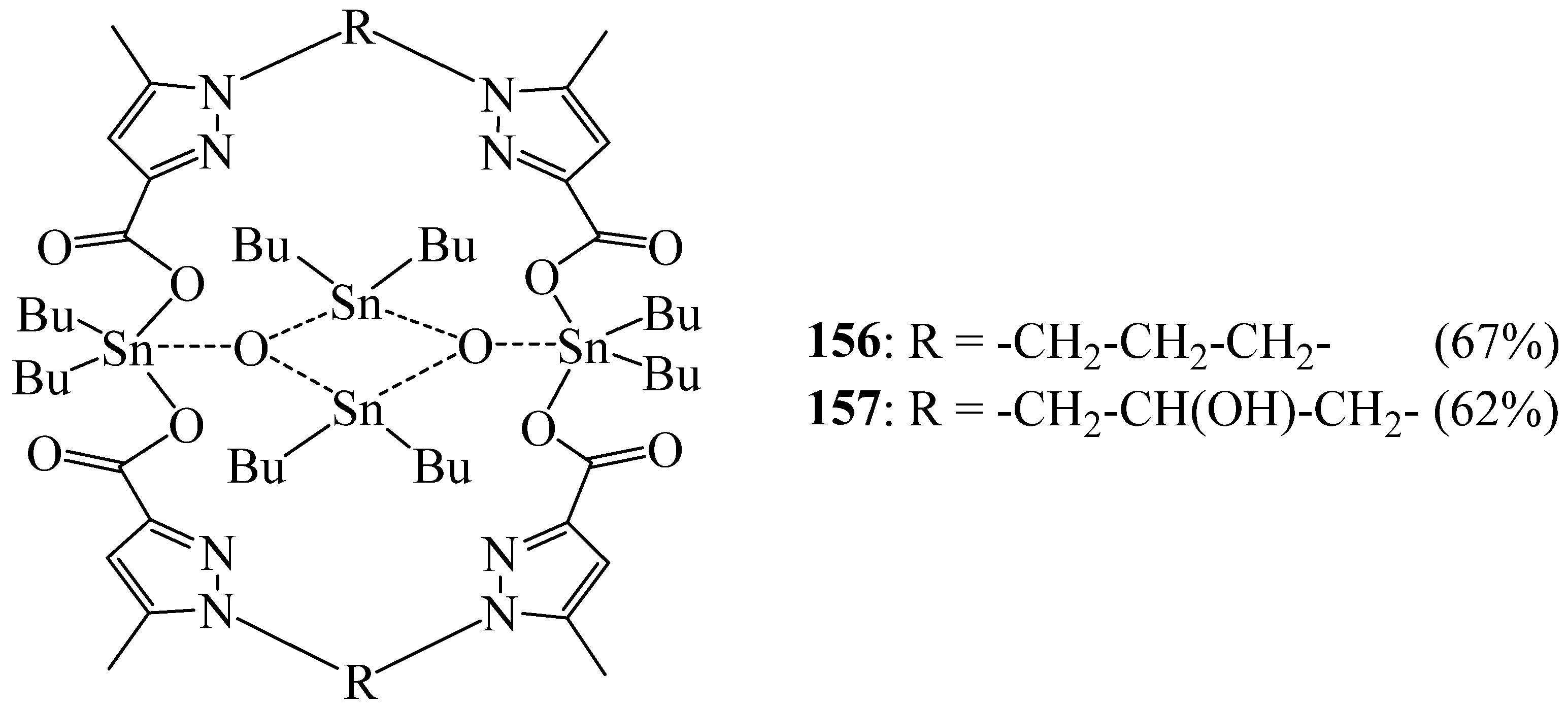
6. Synthesis of Pyrazolic Macrocycles
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vinodh, M.; Alipour, F.H.; Mohamod, A.A.; Al-Azemi, T.F. Molecular assemblies of porphyrins and macrocyclic receptors: Recent developments in their synthesis and applications. Molecules 2012, 17, 11763–11799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Della Sala, G.; Nardone, B.; De Riccardis, F.; Izzo, I. Cyclopeptoids: A novel class of phase-transfer catalysts. Org. Biomol. Chem. 2013, 11, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Schühle, D.T.; Peters, J.A.; Schatz, J. Metal binding calixarenes with potential biomimetic and biomedical applications. Coord. Chem. Rev. 2011, 255, 2727–2745. [Google Scholar] [CrossRef]
- Doebler, J.A. Effects of neutral ionophores on membrane electrical characteristics of NG108-15 cells. Toxicol. Lett. 2000, 114, 27–38. [Google Scholar] [CrossRef]
- Makrlík, E.; Vaňura, P. Experimental and theoretical study on interaction of the silver cation with nonactin. Mol. Phys. 2015, 113, 3712–3716. [Google Scholar] [CrossRef]
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 89, 7017–7036. [Google Scholar] [CrossRef]
- Lehn, J.M.; Sauvage, J.P.; Dietrich, B. Cryptates. Cation exchange rates. J. Am. Chem. Soc. 1970, 92, 2916–2918. [Google Scholar] [CrossRef]
- Cram, D.J.; Helgeson, R.C.; Sousa, L.R.; Timko, J.M.; Newcomb, M.; Moreau, P.; de Jong, F.; Gokel, G.W.; Hoffman, D.H.; Domeier, L.A.; et al. Chiral recognition in complexation of guests by designed host molecules. Pure Appl. Chem. 1975, 43, 327–349. [Google Scholar] [CrossRef] [Green Version]
- James, L.K.; Laylin, J.K. Nobel Laureates in Chemistry, 1901–1992; Chemical Heritage Foundation: Philadelphia, PA, USA, 1993. [Google Scholar]
- Liu, Z.; Nalluri, S.K.M.; Stoddart, J.F. Surveying macrocyclic chemistry: From flexible crown ethers to rigid cyclophanes. Chem. Soc. Rev. 2017, 46, 2459–2478. [Google Scholar] [CrossRef]
- Agrawal, M.; Jain, S.; Agarwal, A.; Dwivedi, J.; Sharma, S.; Kishore, D. Application of novel precursors derived from carbazolo condensed azepinones to the direct single step synthesis of corresponding isoxazole and pyrazole annulated analogues of medicinal importance. Orient. Pharm. Exp. Med. 2012, 12, 141–150. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- McGinley, J.; Fleming, A. Synthesis of macrocycles containing tetrazole units-potential metal complexation sites. J. Incl. Phenom. Macrocycl. Chem. 2008, 61, 1–10. [Google Scholar] [CrossRef]
- Yang, J.; Liu, J.; Wang, Y.; Wang, J. Synthesis, structure and catalysis/applications of N-heterocyclic carbene based on macrocycles. J. Incl. Phenom. Macrocycl. Chem. 2018, 90, 15–37. [Google Scholar] [CrossRef]
- Wagner-Wysiecka, E.; Luboch, E.; Kowalczyk, M.; Biernat, J.F. Chromogenic macrocyclic derivatives of azoles—Synthesis and properties. Tetrahedron 2003, 59, 4415–4420. [Google Scholar] [CrossRef]
- Nshimyumukiza, P.; Van Den Berge, E.; Delest, B.; Mijatovic, T.; Kiss, R.; Marchand-Brynaert, J.; Robiette, R. Synthesis and biological evaluation of novel imidazole-containing macrocycles. Tetrahedron 2010, 66, 4515–4520. [Google Scholar] [CrossRef]
- Grubbs, R.H.; O’Leary, D.J. (Eds.) Handbook of Metathesis: Applications in Organic Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2015; Volume 2. [Google Scholar]
- Van Den Berge, E.; Pospíšil, J.; Trieu-Van, T.; Collard, L.; Robiette, R. Planar Chirality of Imidazole-Containing Macrocycles—Understanding and Tuning Atropisomerism. Eur. J. Org. Chem. 2011, 33, 6649–6655. [Google Scholar] [CrossRef]
- Hymel, D.; Grant, R.A.; Tsuji, K.; Yaffe, M.B.; Burke, T.R., Jr. Histidine N (τ)-cyclized macrocycles as a new genre of polo-like kinase 1 polo-box domain-binding inhibitors. Bioorg. Med. Chem. Lett. 2018, 28, 3202–3205. [Google Scholar] [CrossRef]
- Rajakumar, P.; Satheeshkumar, C.; Mohanraj, G.; Mathivanan, N. Synthesis and antimicrobial activity of some novel dicationic sulphonophanes. Eur. J. Med. Chem. 2011, 46, 3093–3098. [Google Scholar] [CrossRef]
- Mehrparvar, S.; Adam, A.; Haberhauer, G. Switchable Imidazole Platform–Synthesis and Structural Investigation. Eur. J. Org. Chem. 2018, 2018, 4306–4316. [Google Scholar] [CrossRef]
- Mageed, A.H.; Skelton, B.W.; Sobolev, A.N.; Baker, M.V. Exploring structural and conformational behaviour of cyclophanes incorporating imidazole-2-thiones. Tetrahedron 2018, 74, 2956–2966. [Google Scholar] [CrossRef]
- Thapa, R.; Kilyanek, S.M. Synthesis and structural characterization of 20-membered macrocyclic rings bearing trans-chelating bis (N-heterocyclic carbene) ligands and the catalytic activity of their palladium (II) complexes. Dalton Trans. 2019, 48, 12577–12590. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Barba, V.; Pritzkow, H.; Siebert, W. Synthesis, structures and reactivity of macrocyclic imidazolylboranes. J. Organomet. Chem. 2003, 680, 294–300. [Google Scholar] [CrossRef]
- Iwanek, W.; Iwanek, A.; Woźniak, K.; Malińska, M. A simple and efficient synthesis of boron-imidazole macrocycles and their crystal structures. Tetrahedron Lett. 2012, 53, 4526–4528. [Google Scholar] [CrossRef]
- Sargent, A.L.; Hawkins, I.C.; Allen, W.E.; Liu, H.; Sessler, J.L.; Fowler, C.J. Global versus Local Aromaticity in Porphyrinoid Macrocycles: Experimental and Theoretical Study of “Imidacene”, an Imidazole-Containing Analogue of Porphycene. Chem. Eur. J. 2003, 9, 3065–3072. [Google Scholar] [CrossRef]
- Wong, W.W.; Vickers, M.S.; Cowley, A.R.; Paul, R.L.; Beer, P.D. Tetrakis (imidazolium) macrocyclic receptors for anion binding. Org. Biomol. Chem. 2005, 3, 4201–4208. [Google Scholar] [CrossRef]
- Yu, Z.; Lim, R.K.; Lin, Q. Synthesis of Macrocyclic Tetrazoles for Rapid Photoinduced Bioorthogonal 1,3-Dipolar Cycloaddition Reactions. Chem. Eur. J. 2010, 16, 13325–13329. [Google Scholar] [CrossRef] [Green Version]
- Abdelraheem, E.M.; de Haan, M.P.; Patil, P.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Shaabani, S.; Dömling, A. Concise Synthesis of Tetrazole Macrocycle. Org. Lett. 2017, 19, 5078–5081. [Google Scholar] [CrossRef]
- Voitekhovich, S.V.; Lyakhov, A.S.; Ivashkevich, L.S.; Gaponik, P.N. Facile synthesis of macrocyclic tetrazoles by regioselective cycloalkylation of bistetrazoles with 2,5-dimethylhexane-2,5-diol in perchloric acid. Tetrahedron Lett. 2012, 53, 6111–6114. [Google Scholar] [CrossRef]
- Voitekhovich, S.V.; Lyakhov, A.S.; Ivashkevich, L.S.; Schmorl, S.; Kersting, B.; Ivashkevich, O.A. The First Characterized Coordination Compounds of Macrocyclic Ligands Including Incorporated Tetrazole Rings. Crys. Growth. Des. 2017, 17, 1796–1805. [Google Scholar] [CrossRef]
- Bond, A.D.; Fleming, A.; Kelleher, F.; McGinley, J.; Prajapati, V.; Skovsgaard, S. Synthesis and characterisation of tetra-tetrazole macrocycles. Tetrahedron 2007, 63, 6835–6842. [Google Scholar] [CrossRef] [Green Version]
- Bond, A.D.; Fleming, A.; Gaire, J.; Kelleher, F.; McGinley, J.; McKee, V. First X-ray structural characterisation of host-guest interactions in tetra-tetrazole macrocycles. Tetrahedron 2009, 65, 7942–7947. [Google Scholar] [CrossRef] [Green Version]
- Fleming, A.; Gaire, J.; Kelleher, F.; McGinley, J.; McKee, V. Synthesis and characterisation of macrocycles containing both tetrazole and pyridine functionalities. Tetrahedron 2011, 67, 3260–3266. [Google Scholar] [CrossRef] [Green Version]
- Teng, Y.; Du, J.; Xu, J.; Teng, Q.; Wu, S. Structures and spectra of complexes of tetra-tetrazole macrocycle with organic contaminants. Russ. J. Phys. Chem. 2015, 89, 1041–1046. [Google Scholar] [CrossRef]
- Elwahy, A.H.; Masaret, G.S. Synthesis of novel benzo-substituted macrocyclic schiff bases containing two triazole rings. J. Heterocycl. Chem. 2007, 44, 1475–1484. [Google Scholar] [CrossRef]
- Brandt, C.D.; Kitchen, J.A.; Beckmann, U.; White, N.G.; Jameson, G.B.; Brooker, S. Synthesis and structures of 3,5-disubstituted 1,2,4-triazole head units and incorporation of 3,5-dibenzoyl-1,2,4-triazolate into new [2+2] Schiff-base macrocyclic complexes. Supramol. Chem. 2007, 19, 17–27. [Google Scholar] [CrossRef]
- Foroughifar, N.; Mobinikhaledi, A.; Ebrahimi, S. Synthesis of a novel class of azacrown macrocycles and lariat crown ethers containing two 1,2,4-triazole rings as subunits. Synthesis 2009, 2009, 2557–2560. [Google Scholar] [CrossRef]
- Foroughifar, N.; Mobinikhaledi, A.; Ebrahimi, S.; Moghanian, H.; Fard, M.A.B.; Kalhor, M. Synthesis of a new class of azathia crown macrocycles containing two 1,2,4-triazole or two 1,3,4-thiadiazole rings as subunits. Tetrahedron Lett. 2009, 50, 836–839. [Google Scholar] [CrossRef]
- Avaji, P.G.; Patil, S.A. Synthesis, spectral, thermal, solid state dc electrical conductivity, fluorescence and biological studies of lanthanum (III) and thorium (IV) complexes of 24-membered macrocyclic triazoles. J. Coord. Chem. 2008, 61, 2570–2583. [Google Scholar] [CrossRef]
- Patil, S.A.; Kamble, U.V.; Badami, P.S. Antimicrobial and DNA-cleavage studies of 22-membered N4 tetraaza macrocyclic triazoles: Template synthesis and physicochemical characterization. Nucleosides Nucleotides Nucleic Acids 2010, 29, 658–675. [Google Scholar] [CrossRef]
- Vinay Kumar, B.; Naik, H.B.; Girija, D.; Sharath, N.; Sudeep, H.V.; Joy Hoskeri, H. Synthesis and Biological Evaluation of New Tetra-Aza Macrocyclic Scaffold Constrained Oxadiazole, Thiadiazole and Triazole Rings. Arch. Pharm. 2012, 345, 240–249. [Google Scholar] [CrossRef]
- Zheng, C.; Huang, D.Y.; Li, H.Y.; Huang, F.P.; Bian, H.D. In situ synthesis, characterization, bovine serum albumin (BSA) binding studies of FeII/CoII/NiII complexes derived from a new double bis-triazole macrocyclic ligand. J. Coord. Chem. 2017, 70, 2453–2462. [Google Scholar] [CrossRef]
- Kelly, A.R.; Wei, J.; Kesavan, S.; Marie, J.C.; Windmon, N.; Young, D.W.; Marcaurelle, L.A. Accessing skeletal diversity using catalyst control: Formation of n and n+1 macrocyclic triazole rings. Org. Lett. 2009, 11, 2257–2260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogdan, A.R.; James, K. Efficient Access to New Chemical Space Through Flow—Construction of Druglike Macrocycles Through Copper-Surface-Catalyzed Azide-Alkyne Cycloaddition Reactions. Chem. Eur. J. 2010, 16, 14506–14512. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, A.R.; James, K. Synthesis of 5-iodo-1,2,3-triazole-containing macrocycles using copper flow reactor technology. Org. Lett. 2011, 13, 4060–4063. [Google Scholar] [CrossRef] [PubMed]
- Weerawarna, P.M.; Kim, Y.; Kankanamalage, A.C.G.; Damalanka, V.C.; Lushington, G.H.; Alliston, K.R.; Mehzabeen, N.; Battaile, K.P.; Lovell, S.; Chang, K.O.; et al. Structure-based design and synthesis of triazole-based macrocyclic inhibitors of norovirus protease: Structural, biochemical, spectroscopic, and antiviral studies. Eur. J. Med. Chem. 2016, 119, 300–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Vázquez, E.; Chávez-Riveros, A.; Romo-Pérez, A.; Ramírez-Apán, M.T.; Chávez-Blanco, A.D.; Morales-Bárcenas, R.; Dueñas-González, A.; Miranda, L.D. Cytotoxic Activity and Structure–Activity Relationship of Triazole-Containing Bis (Aryl Ether) Macrocycles. Chem. Med. Chem. 2018, 13, 1193–1209. [Google Scholar] [CrossRef] [Green Version]
- Binauld, S.; Hawker, C.J.; Fleury, E.; Drockenmuller, E. A modular approach to functionalized and expanded crown ether based macrocycles using click chemistry. Angew. Chem. Int. Ed. 2009, 48, 6654–6658. [Google Scholar] [CrossRef]
- Caricato, M.; Olmo, A.; Gargiulli, C.; Gattuso, G.; Pasini, D. A ‘clicked’macrocyclic probe incorporating Binol as the signalling unit for the chiroptical sensing of anions. Tetrahedron 2012, 68, 7861–7866. [Google Scholar] [CrossRef]
- Anandhan, R.; Kannan, A.; Rajakumar, P. Synthesis and anti-inflammatory activity of triazole-based macrocyclic amides through click chemistry. Synth. Commun. 2017, 47, 671–679. [Google Scholar] [CrossRef]
- Li, C.T.; Cao, Q.Y.; Li, J.J.; Wang, Z.W.; Dai, B.N. Ferrocene-containing macrocyclic triazoles for the electrochemical sensing of dihydrogen phosphate anion. Inorg. Chim. Acta 2016, 449, 31–37. [Google Scholar] [CrossRef]
- Hradilová, L.; Grepl, M.; Hlaváč, J.; Lyčka, A.; Hradil, P. Synthesis of Macrocycles Containing 1,2,3-Triazole Motifs. Synthesis 2012, 44, 1398–1404. [Google Scholar] [CrossRef]
- White, N.G.; Carvalho, S.; Félix, V.; Beer, P.D. Anion binding in aqueous media by a tetra-triazolium macrocycle. Org. Biomol. Chem. 2012, 10, 6951–6959. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.; Shah, M.R.; Ahmed, D.; Rabnawaz, M.; Anis, I.; Afridi, S.; Makhmoor, T.; Tahir, M.N. Synthesis, characterization and Cu2+ triggered selective fluorescence quenching of Bis-calix[4]arene tetra-triazole macrocycle. J. Hazard. Mater. 2016, 309, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Belda, R.; Pitarch-Jarque, J.; Soriano, C.; Llinares, J.M.; Blasco, S.; Ferrando-Soria, J.; García-España, E. Intermolecular Binding Modes in a Novel [1+1] Condensation 1 H-Pyrazole Azamacrocycle: A Solution and Solid State Study with Evidence for CO2 Fixation. Inorg. Chem. 2013, 52, 10795–10803. [Google Scholar] [CrossRef] [PubMed]
- Ashok, D.; Devulapally, M.G.; Gundu, S.; Aamate, V.K.; Chintalapally, S. Synthesis and antimicrobial evaluation of novel pyrazole-annulated oxygen-containing macrocycles. Chem. Heterocycl. Compd. 2016, 52, 609–614. [Google Scholar] [CrossRef]
- Pitarch, J.; Clares, M.P.; Belda, R.; Costa, R.D.; Navarro, P.; Ortí, E.; Soriano, C.; García-España, E. Zn (II)-coordination and fluorescence studies of a new polyazamacrocycle incorporating 1H-pyrazole and naphthalene units. Dalton Trans. 2010, 39, 7741–7746. [Google Scholar] [CrossRef]
- Reviriego, F.; Navarro, P.; Arán, V.J.; Jimeno, M.L.; García-España, E.; Latorre, J.; Yunta, M.J. Hydrogen-Bond-Mediated Self-Assembly of 26-Membered Diaza Tetraester Crowns of 3,5-Disubstituted 1 H-Pyrazole. Dimerization Study in the Solid State and in CDCl3 Solution. J. Org. Chem. 2011, 76, 8223–8231. [Google Scholar] [CrossRef]
- Ali, T.E.S.; Abdel-Ghaffar, S.A.A.; El-Mahdy, K.M.; Abdel-Karim, S.M. Synthesis, characterization, and antimicrobial activity of some new phosphorus macrocyclic compounds containing pyrazole rings. Turk. J. Chem. 2013, 37, 160–169. [Google Scholar]
- Sanchez-Moreno, M.; Marín, C.; Navarro, P.; Lamarque, L.; Garcia-Espana, E.; Miranda, C.; Huertas, Ó.; Olmo, F.; Gómez-Contreras, F.; Pitarch, J.; et al. In vitro and in vivo trypanosomicidal activity of pyrazole-containing macrocyclic and macrobicyclic polyamines: Their action on acute and chronic phases of Chagas disease. J. Med. Chem. 2012, 55, 4231–4243. [Google Scholar] [CrossRef]
- Navarro, P.; Sánchez-Moreno, M.; Marãn, C.; Garcãa-España, E.; Ramãrez-Macãas, I.; Olmo, F.; Rosales, M.J.; Gómez-Contreras, F.; Yunta, M.J.R.; Gutierrez-Sánchez, R. In vitro leishmanicidal activity of pyrazole-containing polyamine macrocycles which inhibit the Fe-SOD enzyme of Leishmania infantum and Leishmania braziliensis species. Parasitology 2014, 141, 1031–1043. [Google Scholar] [CrossRef]
- Bol, J.E.; Maase, B.; Gonesh, G.; Driessen, W.L.; Goubitz, K.; Reedijk, J. Novel double tripodal pyrazolyl macrocycles. Synthesis and X-ray structure of hexa-azole ligands. Heterocycles 1997, 8, 1477–1492. [Google Scholar]
- Malek, F.; Persin, M.; Ramdani, A.; Sarrazin, J.; Zidane, I. Elaboration de nouveaux matériaux membranaires incorporant des macrocycles tetrapyrazoliques. Etude du transport facilité des métaux alcalins Li+, Na+ et K+. New J. Chem. 2002, 26, 876–882. [Google Scholar] [CrossRef]
- Cherfi, M.; Harit, T.; Malek, F. Synthesis of new tetrapyrazolic macrocycle and examination of its complexation properties. Mater. Today Proc. 2020, 31, S75–S77. [Google Scholar] [CrossRef]
- Harit, T.; Malek, F.; El Bali, B.; Dusek, M.; Kucerakova, M. Synthesis and characterization of two new tetrapyrazolic macrocycles for the selective extraction of cesium cation. Tetrahedron 2016, 72, 3966–3973. [Google Scholar] [CrossRef]
- Harit, T.; Malek, F. New polymeric membrane incorporating a tetrapyrazolic macrocycle for the selective transport of cesium cation. Sep. Purif. Technol. 2017, 176, 8–14. [Google Scholar] [CrossRef]
- Harit, T.; Bellaouchi, R.; Mokhtari, C.; El Bali, B.; Asehraou, A.; Malek, F. New generation of tetrapyrazolic macrocycles: Synthesis and examination of their complexation properties and antibacterial activity. Tetrahedron 2017, 73, 5138–5143. [Google Scholar] [CrossRef]
- Harit, T.; Cherfi, M.; Abouloifa, H.; Isaad, J.; Bouabdallah, I.; Rahal, M.; Asehraou, A.; Malek, F. Synthesis, Characterization, Antibacterial Properties and DFT Studies of Two New Polypyrazolic Macrocycles. Polycycl. Aromat. Compd. 2020, 40, 1459–1469. [Google Scholar] [CrossRef]
- Radi, S.; Yahyi, A.; Ramdani, A.; Zidane, I.; Hacht, B. A new tetrapyrazolic macrocycle. Synthesis and its use in extraction and transport of K+, Na+ and Li+. Tetrahedron 2006, 62, 9153–9155. [Google Scholar] [CrossRef]
- Radi, S.; Ramdani, A.; Lekchiri, Y.; Morcellet, M.; Crini, G.; Janus, L. New tetrapyrazolic macrocycle. Synthesis and preliminary use in metal ion extraction. Tetrahedron 2004, 60, 939–942. [Google Scholar] [CrossRef]
- Tarrago, G.; Zidane, I.; Marzin, C.; Tep, A. Synthesis and ionophore properties of a series of new tetrapyrazolic macrocycles. Tetrahedron 1988, 44, 91–100. [Google Scholar] [CrossRef]
- Harit, T.; Isaad, J.; Malek, F. Novel efficient functionalized tetrapyrazolic macrocycle for the selective extraction of lithium cations. Tetrahedron 2016, 72, 2227–2232. [Google Scholar] [CrossRef]
- Harit, T.; Malek, F. Elaboration of new thin solid membrane bearing a tetrapyrazolic macrocycle for the selective transport of lithium cation. Sep. Purif. Technol. 2017, 188, 394–398. [Google Scholar] [CrossRef]
- Dahmani, M.; Harit, T.; Et-Touhami, A.; Yahyi, A.; Eddike, D.; Tillard, M.; Benabbes, R. Two novel macrocyclic organotin (IV) carboxylates based on bipyrazoledicarboxylic acid derivatives: Syntheses, crystal structures and antifungal activities. J. Organomet. Chem. 2021, 948, 121913. [Google Scholar] [CrossRef]

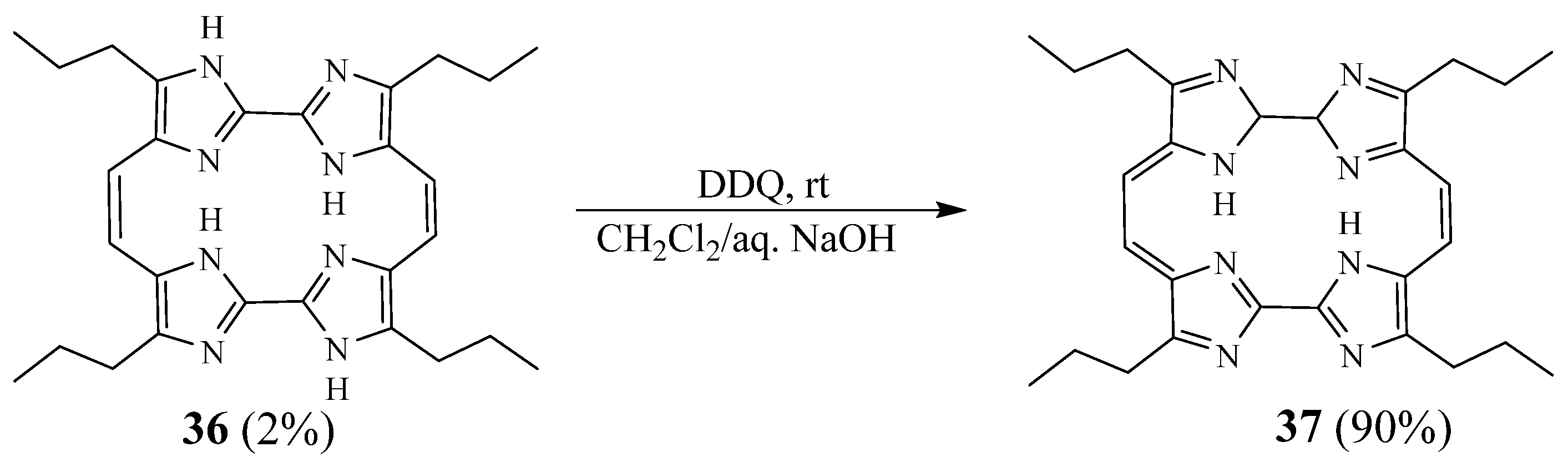
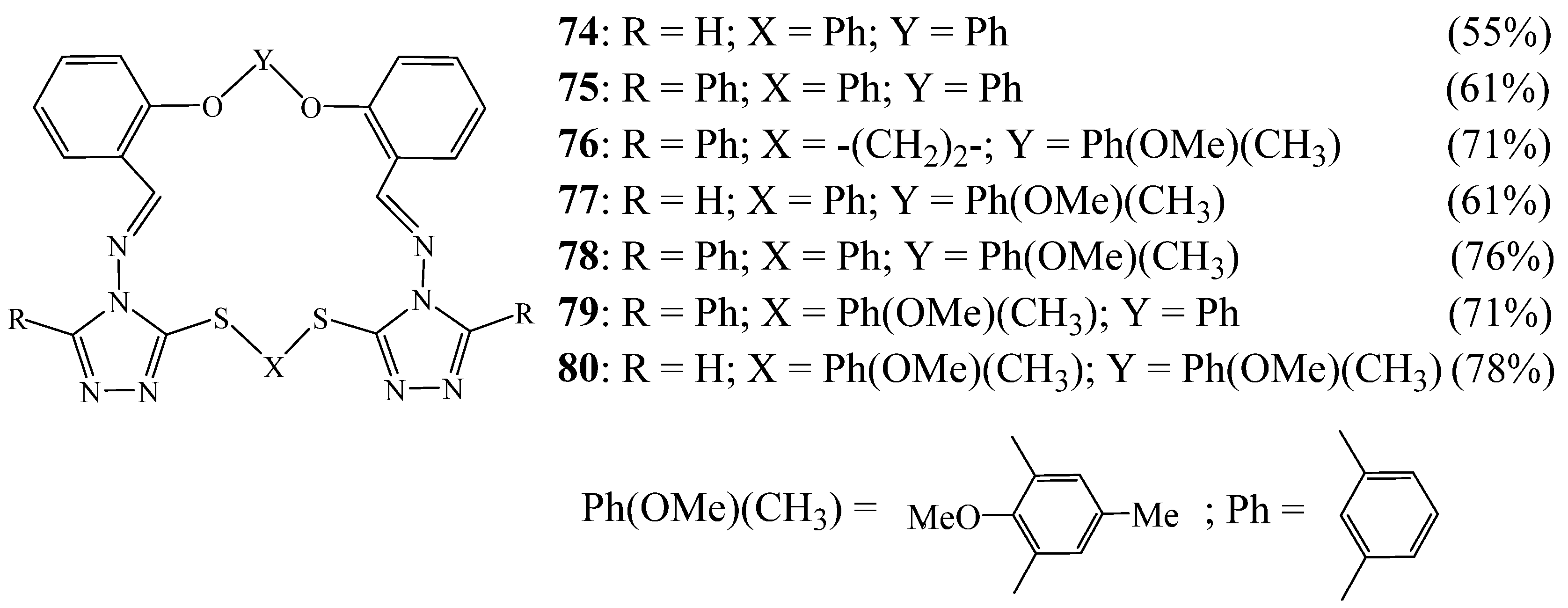







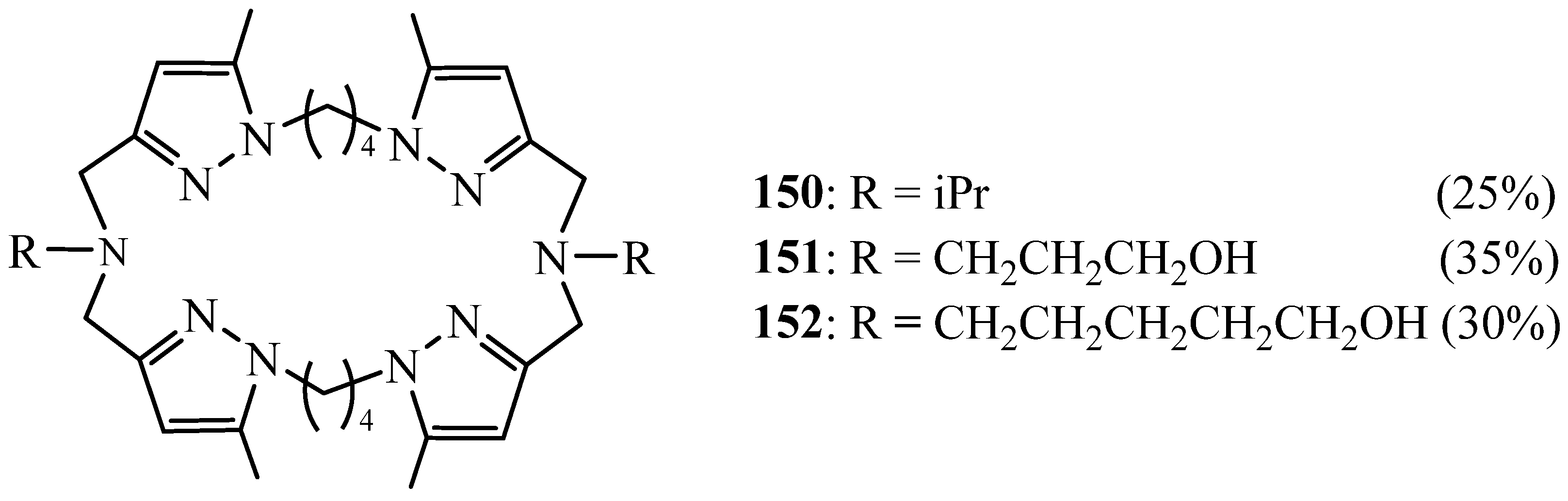

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malek, F.; Harit, T.; Cherfi, M.; Kim, B. Insights on the Synthesis of N-Heterocycles Containing Macrocycles and Their Complexion and Biological Properties. Molecules 2022, 27, 2123. https://doi.org/10.3390/molecules27072123
Malek F, Harit T, Cherfi M, Kim B. Insights on the Synthesis of N-Heterocycles Containing Macrocycles and Their Complexion and Biological Properties. Molecules. 2022; 27(7):2123. https://doi.org/10.3390/molecules27072123
Chicago/Turabian StyleMalek, Fouad, Tarik Harit, Mounir Cherfi, and Bonglee Kim. 2022. "Insights on the Synthesis of N-Heterocycles Containing Macrocycles and Their Complexion and Biological Properties" Molecules 27, no. 7: 2123. https://doi.org/10.3390/molecules27072123
APA StyleMalek, F., Harit, T., Cherfi, M., & Kim, B. (2022). Insights on the Synthesis of N-Heterocycles Containing Macrocycles and Their Complexion and Biological Properties. Molecules, 27(7), 2123. https://doi.org/10.3390/molecules27072123






