Polydopamine-Coated Poly-Lactic Acid Aerogels as Scaffolds for Tissue Engineering Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. PLLA Gels and Aerogels Preparation
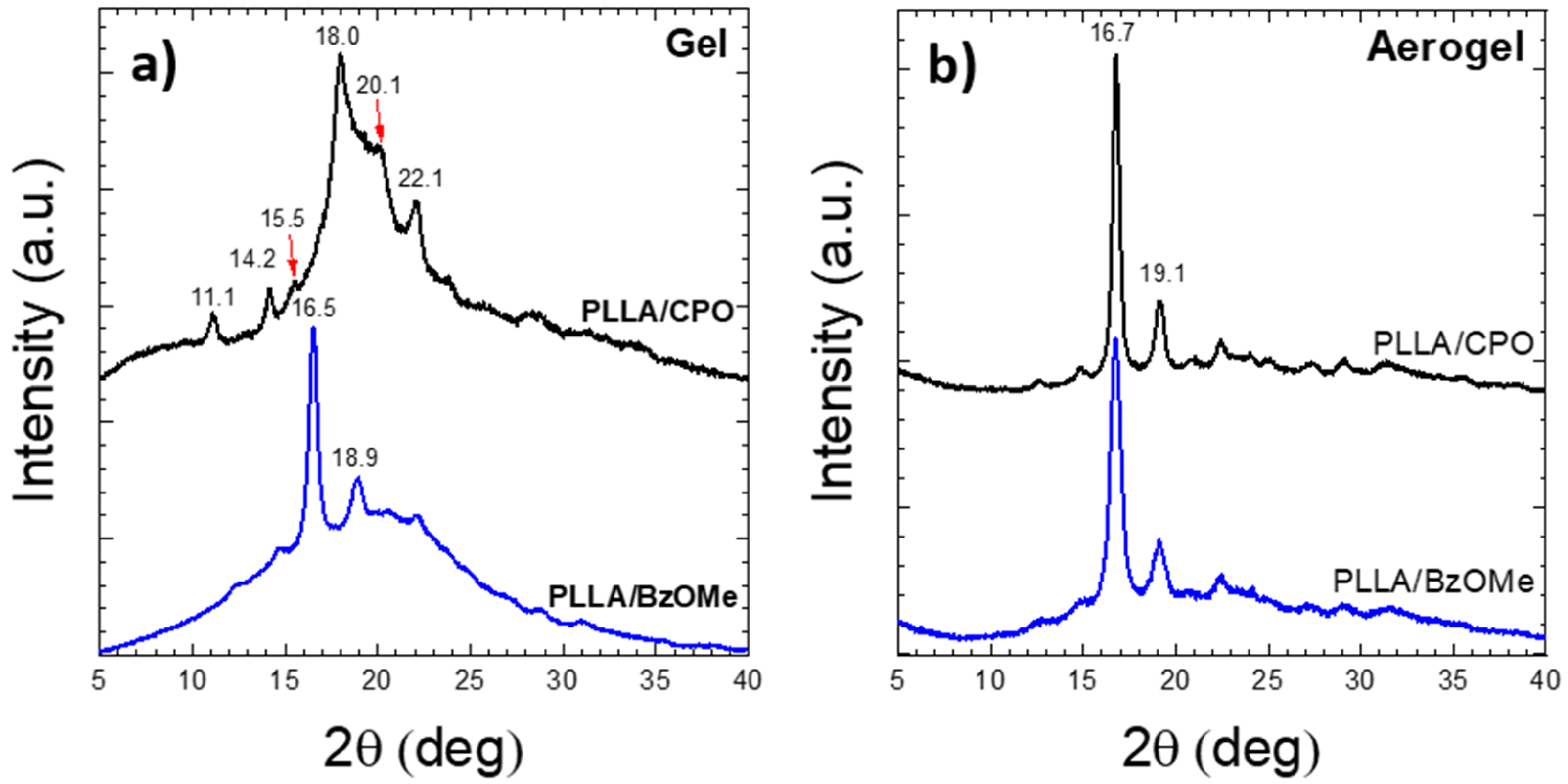
2.2. WAXD Analysis of PLLA Gels and Aerogels
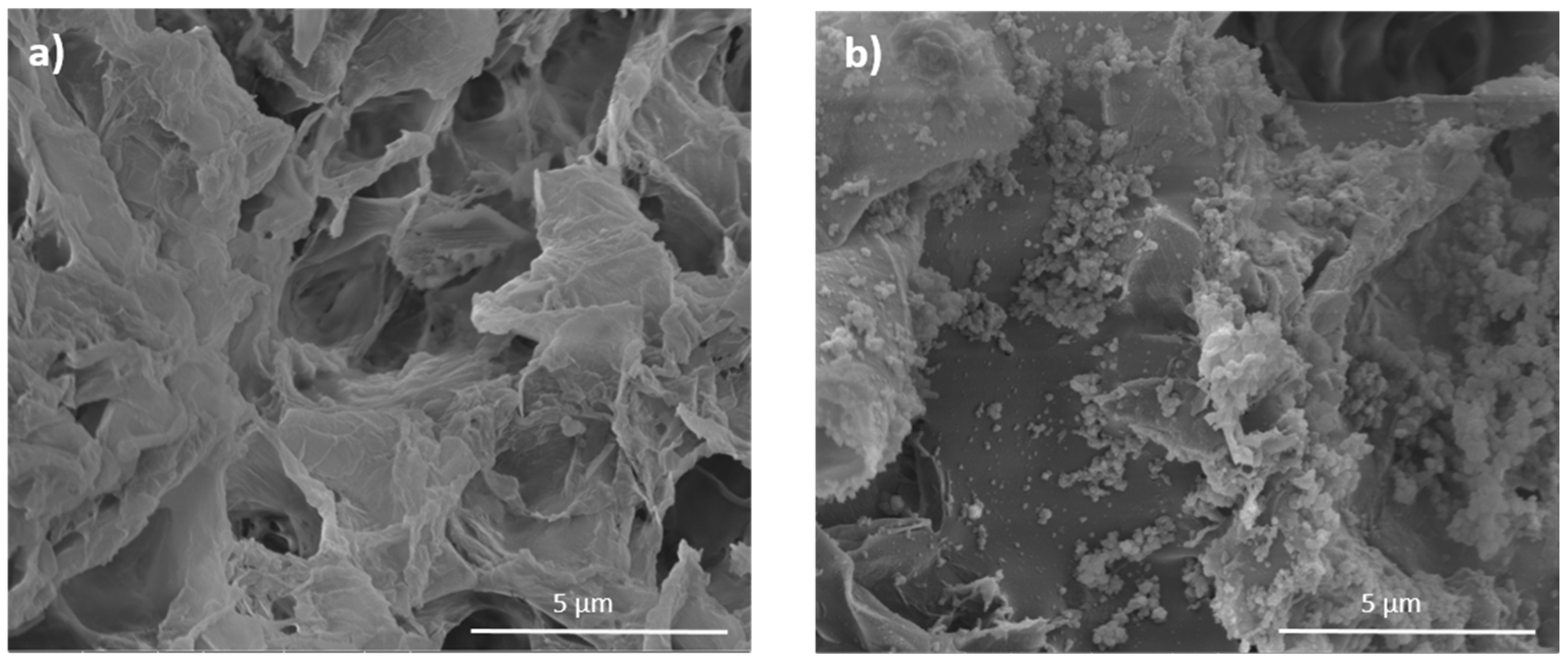
2.3. Morphological Analysis
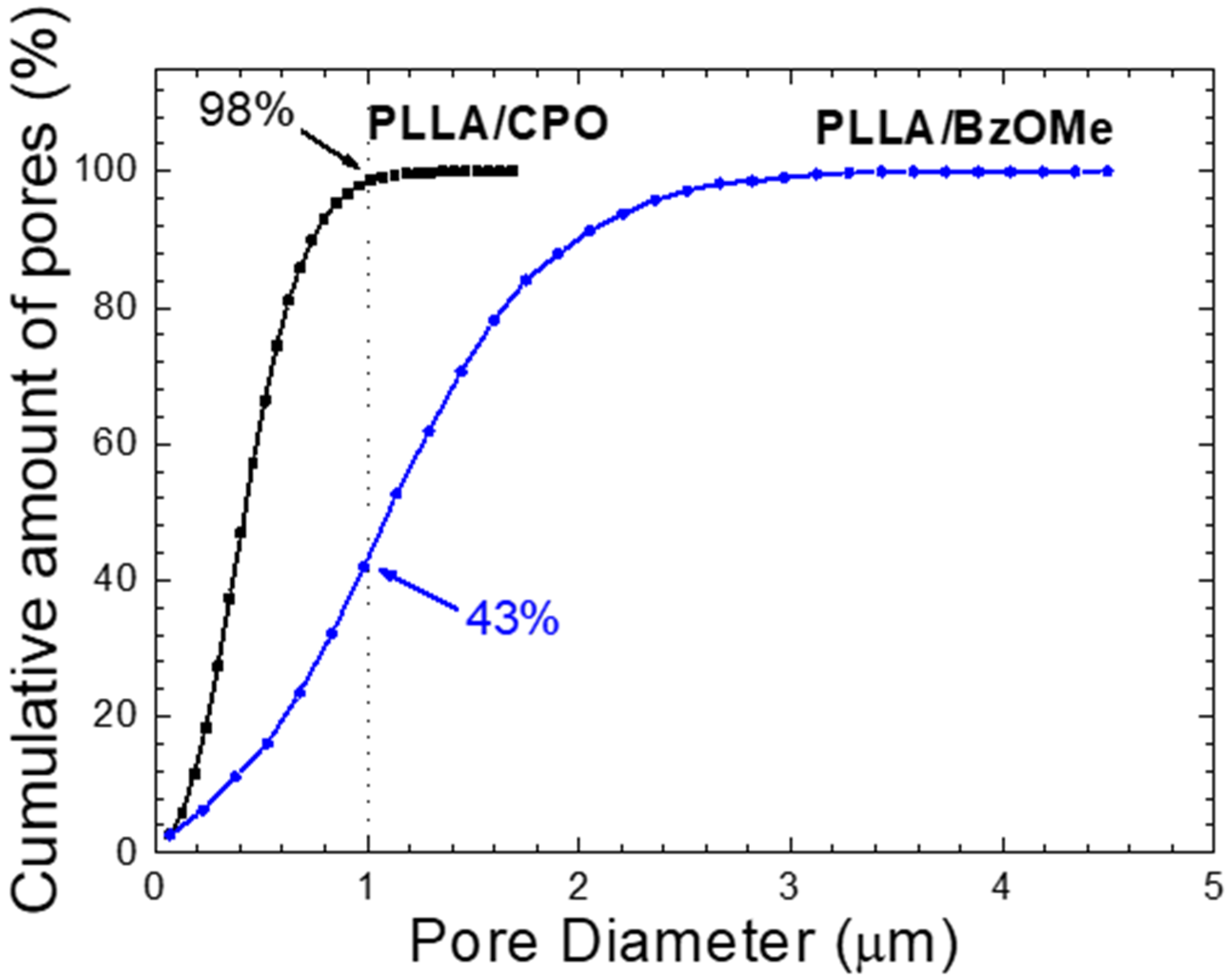
2.4. Characterization of Aerogel Porosities
2.4.1. Analysis of Pore Size Distribution by SEM
2.4.2. Analysis of Nitrogen Adsorption–Desorption Isotherms
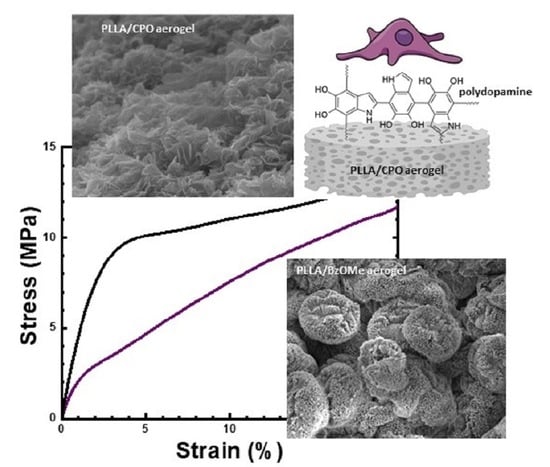
2.5. Mechanical Analysis
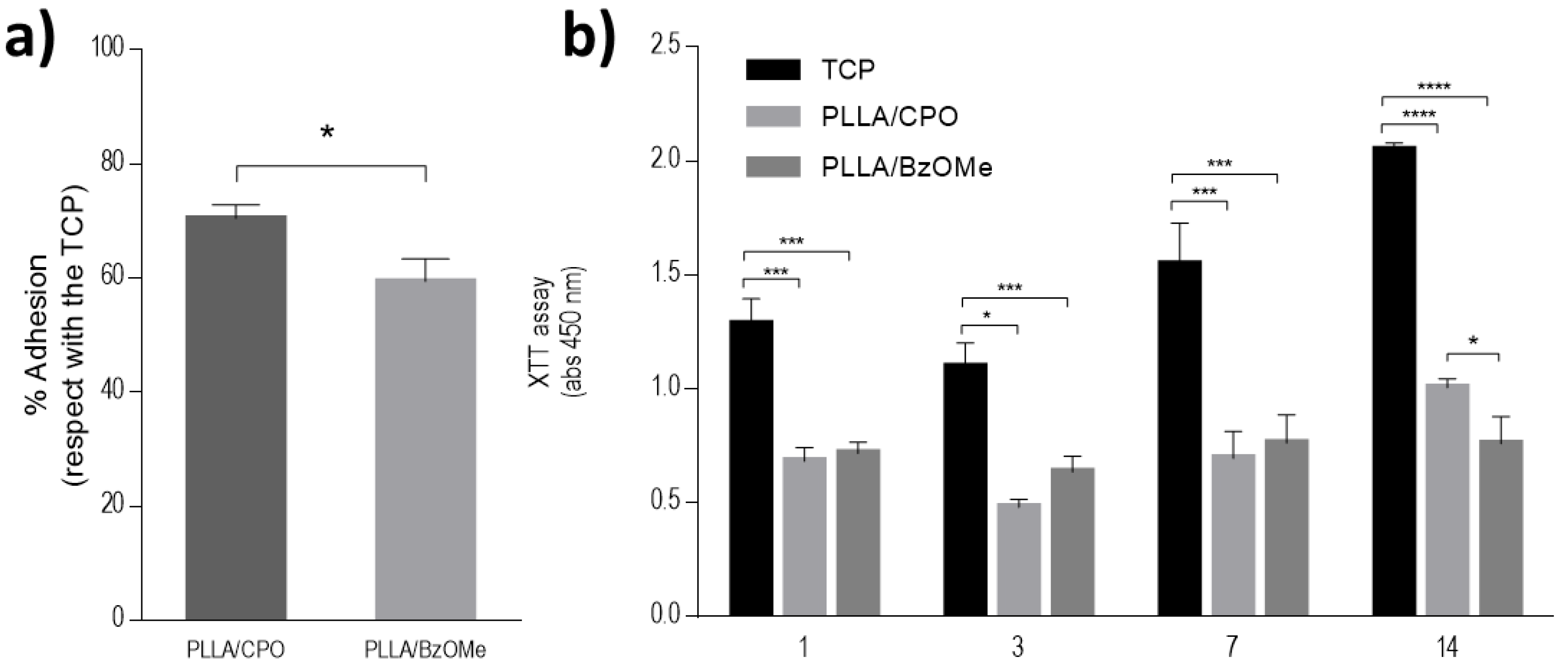
2.6. Preliminary In Vitro Viability Tests on Bare Aerogels
2.7. PDA Coated Aerogels: Surface Morphology and In Vitro Validation
3. Materials and Methods
3.1. Chemicals
3.2. PLLA Gels and Aerogels Preparation
3.3. WAXD Analysis of PLLA Gels and Aerogels
3.4. Morphological Analysis
3.5. Aerogel Porosity Characterization
3.6. Mechanical Analysis
3.7. Preparation and Characterization of PDA-Coated Aerogel
3.8. In Vitro Viability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matsumura, G.; Hibino, N.; Ikada, Y.; Kurosawa, H.; Shin’oka, T. Successful application of tissue engineered vascular autografts: Clinical experience. Biomaterials 2003, 24, 2303–2308. [Google Scholar] [CrossRef]
- Khan, Y.; Yaszemski, M.J.; Mikos, A.G.; Laurencin, C.T. Tissue Engineering of Bone: Material and Matrix Considerations. J. Bone Jt. Surg. 2008, 90, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; de Wijn, J.R.; van Blitterswijk, C.A. 3D fiber-deposited scaffolds for tissue engineering: Influence of pores geometry and architecture on dynamic mechanical properties. Biomaterials 2006, 27, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Carletti, E.; Motta, A.; Migliaresi, C. Scaffolds for Tissue Engineering and 3D Cell Culture. In 3D Cell Culture; Methods in Molecular Biology; Haycock, J.W., Ed.; Humana Press: Totowa, NJ, USA, 2011; Volume 695, pp. 17–39. ISBN 978-1-60761-983-3. [Google Scholar]
- Salerno, A.; Fernández-Gutiérrez, M.; San Román del Barrio, J.; Domingo, C. Bio-safe fabrication of PLA scaffolds for bone tissue engineering by combining phase separation, porogen leaching and scCO2 drying. J. Supercrit. Fluids 2015, 97, 238–246. [Google Scholar] [CrossRef]
- Carfì Pavia, F.; La Carrubba, V.; Brucato, V. Polymeric scaffolds based on blends of poly-l-lactic acid (PLLA) with poly-d-l-lactic acid (PLA) prepared via thermally induced phase separation (TIPS): Demixing conditions and morphology. Polym. Bull. 2013, 70, 563–578. [Google Scholar] [CrossRef]
- Guaccio, A.; Guarino, V.; Perez, M.A.A.; Cirillo, V.; Netti, P.A.; Ambrosio, L. Influence of electrospun fiber mesh size on hMSC oxygen metabolism in 3D collagen matrices: Experimental and theoretical evidences. Biotechnol. Bioeng. 2011, 108, 1965–1976. [Google Scholar] [CrossRef]
- Cestari, F.; Petretta, M.; Yang, Y.; Motta, A.; Grigolo, B.; Sglavo, V.M. 3D printing of PCL/nano-hydroxyapatite scaffolds derived from biogenic sources for bone tissue engineering. Sustain. Mater. Technol. 2021, 29, e00318. [Google Scholar] [CrossRef]
- Torricelli, P.; Gioffrè, M.; Fiorani, A.; Panzavolta, S.; Gualandi, C.; Fini, M.; Focarete, M.L.; Bigi, M. Co-electrospun gelatin-poly(l-lactic acid) scaffolds: Modulation of mechanical properties and chondrocyte response as a function of composition. Mater. Sci. Eng. C 2014, 36, 130–138. [Google Scholar] [CrossRef]
- Baldino, L.; Zuppolini, S.; Cardea, S.; Diodato, L.; Borriello, A.; Reverchon, E.; Nicolais, L. Production of biodegradable superabsorbent aerogels using a supercritical CO2 assisted drying. J. Supercrit. Fluids 2020, 156, 104681. [Google Scholar] [CrossRef]
- Guarino, V.; Ambrosio, L. Temperature-driven processing techniques for manufacturing fully interconnected porous scaffolds in bone tissue engineering. Proc. Inst. Mech. Eng. H 2010, 224, 1389–1400. [Google Scholar] [CrossRef]
- Guarino, V.; Causa, F.; Salerno, A.; Ambrosio, L.; Netti, P.A. Design and manufacture of microporous polymeric materials with hierarchal complex structure for biomedical application. Mater. Sci. Technol. 2008, 24, 1111–1117. [Google Scholar] [CrossRef]
- Guarino, V.; Altobelli, R.; Cirillo, V.; Cummaro, A.; Ambrosio, L. Additive electrospraying: A route to process electrospun scaffolds for controlled molecular release: Electrospraying/Electrospinning Integrated Platforms. Polym. Adv. Technol. 2015, 26, 1359–1369. [Google Scholar] [CrossRef]
- Bueno, A.; Luebbert, C.; Enders, S.; Sadowski, G.; Smirnova, I. Production of polylactic acid aerogels via phase separation and supercritical CO2 drying: Thermodynamic analysis of the gelation and drying process. J. Mater. Sci. 2021, 56, 18926–18945. [Google Scholar] [CrossRef]
- Tsuji, H. Hydrolytic Degradation. In Poly (Lactic Acid); Auras, R., Lim, L.-T., Selke, S.E.M., Tsuji, H., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 343–381. ISBN 978-0-470-64984-8. [Google Scholar]
- Elsawy, M.A.; Kim, K.-H.; Park, J.-W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, M.L.; Cocca, M.; Malinconico, M. Crystal polymorphism of poly(l-lactic acid) and its influence on thermal properties. Thermochim. Acta 2011, 522, 110–117. [Google Scholar] [CrossRef]
- Pan, P.; Inoue, Y. Polymorphism and isomorphism in biodegradable polyesters. Prog. Polym. Sci. 2009, 34, 605–640. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Androsch, R. Influence of α’-/α-crystal polymorphism on properties of poly (l-lactic acid): α’-/α-crystal polymorphism of PLLA. Polym. Int. 2019, 68, 320–334. [Google Scholar] [CrossRef]
- Wasanasuk, K.; Tashiro, K. Crystal structure and disorder in Poly (L-lactic acid) δ form (α’ form) and the phase transition mechanism to the ordered α form. Polymer 2011, 52, 6097–6109. [Google Scholar] [CrossRef]
- Cartier, L.; Okihara, T.; Ikada, Y.; Tsuji, H.; Puiggali, J.; Lotz, B. Epitaxial crystallization and crystalline polymorphism of polylactides. Polymer 2000, 41, 8909–8919. [Google Scholar] [CrossRef]
- Puiggali, J.; Ikada, Y.; Tsuji, H.; Cartier, L.; Okihara, T.; Lotz, B. The frustrated structure of poly (L-lactide). Polymer 2000, 41, 8921–8930. [Google Scholar] [CrossRef]
- Marubayashi, H.; Asai, S.; Sumita, M. Complex Crystal Formation of Poly (L-lactide) with Solvent Molecules. Macromolecules 2012, 45, 1384–1397. [Google Scholar] [CrossRef]
- Rizzo, P.; Ianniello, G.; Venditto, V.; Tarallo, O.; Guerra, G. Poly (l-lactic acid): Uniplanar Orientation in Cocrystalline Films and Structure of the Cocrystalline Form with Cyclopentanone. Macromolecules 2015, 48, 7513–7520. [Google Scholar] [CrossRef]
- Shaiju, P.; Murthy, N.S.; Gowd, E.B. Molecular, Crystalline, and Lamellar Length-Scale Changes in the Poly (l-lactide) (PLLA) during Cyclopentanone (CPO) Desorption in PLLA/CPO Cocrystals. Macromolecules 2016, 49, 224–233. [Google Scholar] [CrossRef]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Klimek, K.; Ginalska, G. Proteins and Peptides as Important Modifiers of the Polymer Scaffolds for Tissue Engineering Applications—A Review. Polymers 2020, 12, 844. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Maya, I.; Guarino, V.; Antonio Alvarez-Perez, M. Protein Based Devices for Oral Tissue Repair and Regeneration. AIMS Mater. Sci. 2018, 5, 156–170. [Google Scholar] [CrossRef]
- Zuppolini, S.; Cruz-Maya, I.; Guarino, V.; Borriello, A. Optimization of Polydopamine Coatings onto Poly-ε-Caprolactone Electrospun Fibers for the Fabrication of Bio-Electroconductive Interfaces. JFB 2020, 11, 19. [Google Scholar] [CrossRef] [Green Version]
- Lynge, M.E.; van der Westen, R.; Postma, A.; Städler, B. Polydopamine—A nature-inspired polymer coating for biomedical science. Nanoscale 2011, 3, 4916. [Google Scholar] [CrossRef]
- Tsai, W.-B.; Chen, W.-T.; Chien, H.-W.; Kuo, W.-H.; Wang, M.-J. Poly (dopamine) coating to biodegradable polymers for bone tissue engineering. J. Biomater. Appl. 2014, 28, 837–848. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Elucidating the Structure of Poly (dopamine). Langmuir 2012, 28, 6428–6435. [Google Scholar] [CrossRef] [PubMed]
- Salazar, P.; Martín, M.; González-Mora, J.L. Polydopamine-modified surfaces in biosensor applications. Polym. Sci. Res. Adv. Pract. Appl. Educ. Aspects. Formatex Res. Cent. SL Spain 2016, 385–396. [Google Scholar]
- Matsuda, Y.; Fukatsu, A.; Tasaka, S. Solvent Exchange and Gelation Mechanism of Poly (L-lactic acid) Gel Formed by Complex Crystallization with Solvents. Chem. Lett. 2013, 42, 1046–1047. [Google Scholar] [CrossRef]
- Daniel, C.; Alfano, D.; Venditto, V.; Cardea, S.; Reverchon, E.; Larobina, D.; Mensitieri, G.; Guerra, G. Aerogels with a Microporous Crystalline Host Phase. Adv. Mater. 2005, 17, 1515–1518. [Google Scholar] [CrossRef]
- Tarallo, O.; Petraccone, V.; Venditto, V.; Guerra, G. Crystalline structures of intercalate molecular complexes of syndiotactic polystyrene with two fluorescent guests: 1,3,5-Trimethyl-benzene and 1,4-dimethyl-naphthalene. Polymer 2006, 47, 2402–2410. [Google Scholar] [CrossRef]
- Daniel, C.; Longo, S.; Ricciardi, R.; Reverchon, E.; Guerra, G. Monolithic Nanoporous Crystalline Aerogels. Macromol. Rapid Commun. 2013, 34, 1194–1207. [Google Scholar] [CrossRef]
- Cardea, S.; Baldino, L.; Pisanti, P.; Reverchon, E. 3-D PLLA scaffolds formation by a supercritical freeze extraction assisted process. J. Mater. Sci. Mater. Med. 2014, 25, 355–362. [Google Scholar] [CrossRef]
- Pan, P.; Zhu, B.; Kai, W.; Dong, T.; Inoue, Y. Polymorphic Transition in Disordered Poly (l-lactide) Crystals Induced by Annealing at Elevated Temperatures. Macromolecules 2008, 41, 4296–4304. [Google Scholar] [CrossRef]
- Zdravkov, B.; Čermák, J.; Šefara, M.; Janků, J. Pore classification in the characterization of porous materials: A perspective. Open Chem. 2007, 5, 385–395. [Google Scholar] [CrossRef]
- Guarino, V.; Causa, F.; Ambrosio, L. Porosity and Mechanical Properties Relationship in PCL Porous Scaffolds. J. Appl. Biomater. Biomech. 2007, 5, 149–157. [Google Scholar]
- Sahmani, S.; Fattahi, A.M. Development of efficient size-dependent plate models for axial buckling of single-layered graphene nanosheets using molecular dynamics simulation. Microsyst. Technol. 2018, 24, 1265–1277. [Google Scholar] [CrossRef]
- Sahmani, S.; Fattahi, A.M.; Ahmed, N.A. Develop a refined truncated cubic lattice structure for nonlinear large-amplitude vibrations of micro/nano-beams made of nanoporous materials. Eng. Comput. 2020, 36, 359–375. [Google Scholar] [CrossRef]
- Quraishi, S.; Martins, M.; Barros, A.A.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Novel non-cytotoxic alginate–lignin hybrid aerogels as scaffolds for tissue engineering. J. Supercrit. Fluids 2015, 105, 1–8. [Google Scholar] [CrossRef]
- Ball, V. Polydopamine Nanomaterials: Recent Advances in Synthesis Methods and Applications. Front. Bioeng. Biotechnol. 2018, 6, 109. [Google Scholar] [CrossRef] [Green Version]
- Davidsen, M.B.; Teixeira, J.F.L.; Dehli, J.; Karlsson, C.; Kraft, D.; Souza, P.P.C.; Foss, M. Post-treatments of polydopamine coatings influence cellular response. Colloids Surf. B Biointerfaces 2021, 207, 111972. [Google Scholar] [CrossRef]
- Nashchekina, Y.; Yudintceva, N.; Nikonov, P.; Smagina, L.; Yudin, V.; Blinova, M.; Voronkina, I. Protein expression by bone mesenchymal stem cells cultivated in PLLA scaffolds with different pore geometry. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 248–257. [Google Scholar] [CrossRef]
- Khan, Z.; Shanker, R.; Um, D.; Jaiswal, A.; Ko, H. Bioinspired Polydopamine and Composites for Biomedical Applications. In Electrically Conductive Polymer and Polymer Composites; Khan, A., Jawaid, M., Parwaz Khan, A.A., Asiri, A.M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; pp. 1–29. ISBN 978-3-527-80791-8. [Google Scholar]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]





| Sample | Area Mesopores (m2/g) | Volume Mesopores (cc/g) | Area Micropores (m2/g) | Volume Micropores (cc/g) |
|---|---|---|---|---|
| PLLA/CPO | 35 | 0.088 | 50 | 0.018 |
| PLLA/BzOMe | 81 | 0.25 | 97 | 0.035 |
| Sample | Dissolution Temperature | Dissolution Time | Extraction Time | Extraction Pressure | Extraction Temperature |
|---|---|---|---|---|---|
| PLLA/CPO | 130 °C | 240 min | 180 min | 2900 psi | 40 °C |
| PLLA/BzOMe | 180 °C | 240 min | 240 min | 2900 psi | 40 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlacchio, R.; Zuppolini, S.; Cruz-Maya, I.; Pragliola, S.; Borriello, A.; Guarino, V.; Fittipaldi, R.; Lettieri, M.; Venditto, V. Polydopamine-Coated Poly-Lactic Acid Aerogels as Scaffolds for Tissue Engineering Applications. Molecules 2022, 27, 2137. https://doi.org/10.3390/molecules27072137
Orlacchio R, Zuppolini S, Cruz-Maya I, Pragliola S, Borriello A, Guarino V, Fittipaldi R, Lettieri M, Venditto V. Polydopamine-Coated Poly-Lactic Acid Aerogels as Scaffolds for Tissue Engineering Applications. Molecules. 2022; 27(7):2137. https://doi.org/10.3390/molecules27072137
Chicago/Turabian StyleOrlacchio, Ramona, Simona Zuppolini, Iriczalli Cruz-Maya, Stefania Pragliola, Anna Borriello, Vincenzo Guarino, Rosalba Fittipaldi, Mariateresa Lettieri, and Vincenzo Venditto. 2022. "Polydopamine-Coated Poly-Lactic Acid Aerogels as Scaffolds for Tissue Engineering Applications" Molecules 27, no. 7: 2137. https://doi.org/10.3390/molecules27072137