Intermolecular Vibration Energy Transfer Process in Two CL-20-Based Cocrystals Theoretically Revealed by Two-Dimensional Infrared Spectra
Abstract
:1. Introduction
2. Computational Methods and Theories
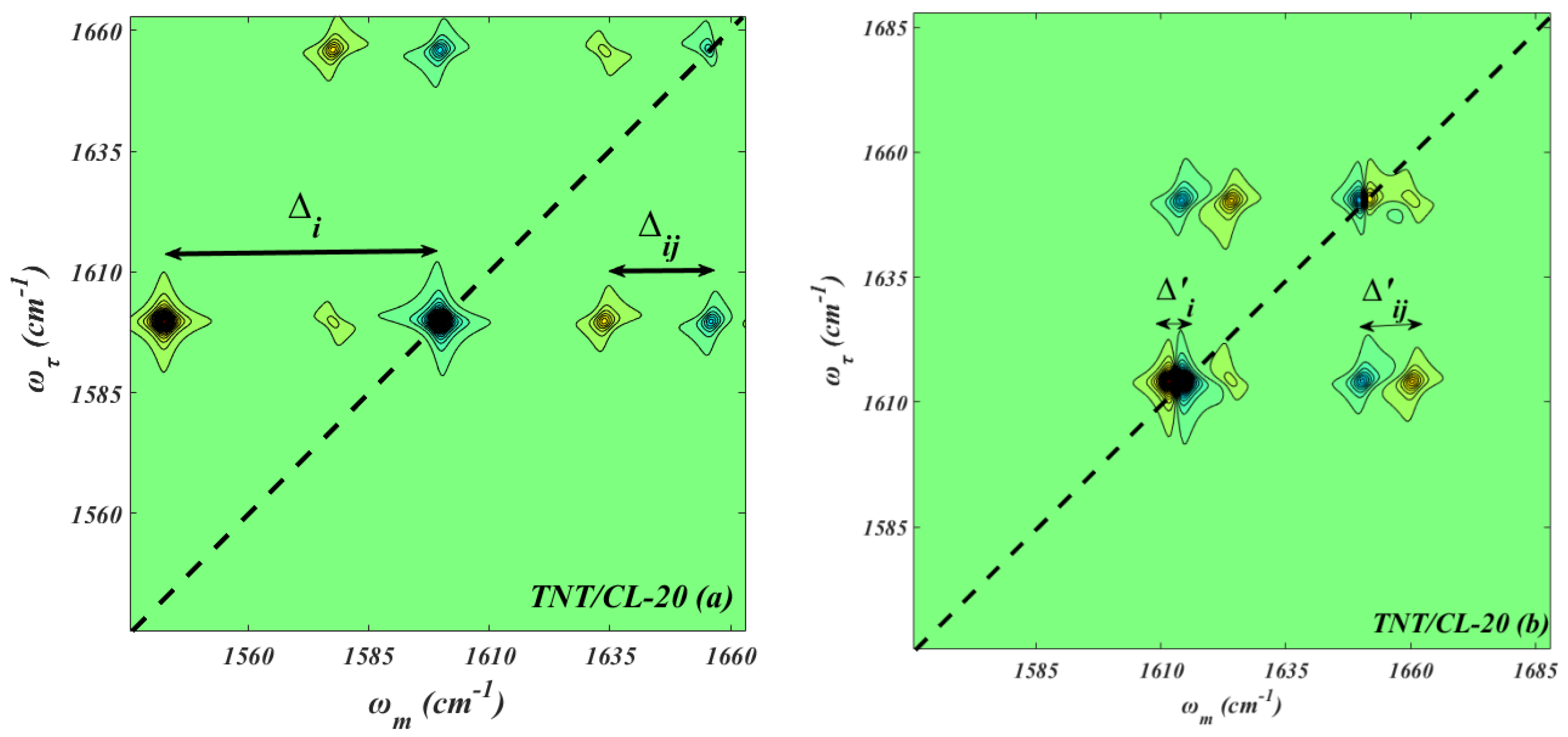
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Badgujar, D.; Talawar, M.; Asthana, S.; Mahulikar, P. Advances in science and technology of modern energetic materials: An overview. J. Hazard. Mater. 2008, 151, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Klapötke, T.M.; Stierstorfer, J. Potassium 1, 1′-Dinitramino-5, 5′-bistetrazolate: A primary explosive with fast detonation and high initiation power. Angew. Chem. Int. Edit. 2014, 53, 8172–8175. [Google Scholar] [CrossRef] [PubMed]
- Sikder, A.; Sikder, N. A review of advanced high performance, insensitive and thermally stable energetic materials emerging for military and space applications. J. Hazard. Mater. 2004, 112, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhao, X.; Long, X.; Dai, X.; Zhang, K.; Li, M.; Guo, F.; Qiao, Z.; Wen, Y. Comparison study of carbon clusters formation during thermal decomposition of 1, 3, 5-triamino-2, 4, 6-trinitrobenzene and benzotrifuroxan: A reaxFF based sequential molecular dynamics simulation. Phys. Chem. Chem. Phys. 2020, 22, 5154–5162. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.; Urtiew, P.; Ornellas, D.; Moody, G.; Scribner, K.; Hoffman, D. CL-20 performance exceeds that of HMX and its sensitivity is moderate. Propellants Explos. Pyrotech. 1997, 22, 249–255. [Google Scholar] [CrossRef]
- Guo, D.; An, Q.; Goddard, W.A., III; Zybin, S.V.; Huang, F. Compressive shear reactive molecular dynamics studies indicating that cocrystals of TNT/CL-20 decrease sensitivity. J. Phys. Chem. C 2014, 118, 30202–30208. [Google Scholar] [CrossRef]
- Varga, R.; Zeman, S. Decomposition of some polynitro arenes initiated by heat and shock: Part I. 2, 4, 6-trinitrotoluene. J. Hazard. Mater. 2006, 132, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.; Zhu, Z.; Madigan, C.F.; Swager, T.M.; Bulović, V. Sensitivity gains in chemosensing by lasing action in organic polymers. Nature 2005, 434, 876–879. [Google Scholar] [CrossRef] [PubMed]
- Cady, H.H.; Larson, A.C.; Cromer, D.T. The crystal structure of α-HMX and a refinement of the structure of β-HMX. Acta Crystallogr. 1963, 16, 617–623. [Google Scholar] [CrossRef]
- Ter Horst, J.; Kramer, H.; Van Rosmalen, G.; Jansens, P. Molecular modelling of the crystallization of polymorphs. Part I: The morphology of HMX polymorphs. J. Cryst. Growth 2002, 237, 2215–2220. [Google Scholar] [CrossRef]
- Liu, G.; Wei, S.-H.; Zhang, C. Review of the intermolecular interactions in energetic molecular cocrystals. Cryst. Growth Des. 2020, 20, 7065–7079. [Google Scholar] [CrossRef]
- Bao, L.; Lv, P.; Fei, T.; Liu, Y.; Sun, C.; Pang, S. Crystal structure and explosive performance of a new CL-20/benzaldehyde cocrystal. J. Mol. Struct. 2020, 1215, 128267. [Google Scholar] [CrossRef]
- Bolton, O.; Matzger, A.J. Improved stability and smart-material functionality realized in an energetic cocrystal. Angew. Chem. Int. Edit. 2011, 123, 9122–9125. [Google Scholar] [CrossRef]
- Bolton, O.; Simke, L.R.; Pagoria, P.F.; Matzger, A.J. High power explosive with good sensitivity: A 2: 1 cocrystal of CL-20: HMX. Cryst. Growth Des. 2012, 12, 4311–4314. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Li, H.; Zhou, X.; Nie, F.; Li, J.; Huang, H. Preparation, structure and properties of CL-20/TNT cocrystal. Chin. J. Energ. Mater. 2012, 20, 674–679. [Google Scholar]
- Hu, Y.; Yuan, S.; Li, X.; Liu, M.; Sun, F.; Yang, Y.; Hao, G.; Jiang, W. Preparation and characterization of nano-CL-20/TNT cocrystal explosives by mechanical ball-milling method. ACS Omega 2020, 5, 17761–17766. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Li, H.; Ye, B.; Wang, J. Nano-CL-20/HMX cocrystal explosive for significantly reduced mechanical sensitivity. J. Nanomater. 2017, 2017, 3791320. [Google Scholar] [CrossRef]
- Ghosh, M.; Sikder, A.K.; Banerjee, S.; Gonnade, R.G. Studies on CL-20/HMX, (2:1) cocrystal: A new preparation method and structural and thermo kinetic analysis. Cryst. Growth Des. 2018, 18, 3781–3793. [Google Scholar] [CrossRef]
- Hübner, J.; Deckert-Gaudig, T.; Glorian, J.; Deckert, V.; Spitzer, D. Surface characterization of nanoscale co-crystals enabled through tip enhanced raman spectroscopy. Nanoscale 2020, 12, 10306–10319. [Google Scholar] [CrossRef]
- Shi, L.; Duan, X.-H.; Zhu, L.-G.; Liu, X.; Pei, C.-H. Directly insight into the inter- and intramolecular interactions of CL-20/TNT energetic cocrystal through the theoretical simulations of THz spectroscopy. J. Phys. Chem. A 2016, 120, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Q.; Yuan, J.-N.; Selvaraj, G.; Ji, G.-F.; Chen, X.-R.; Wei, D.-Q. Towards the low-sensitive and high-energetic co-crystal explosive CL-20/TNT: From intermolecular interactions to structures and properties. Phys. Chem. Chem. Phys. 2018, 20, 17253–17261. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Li, X.; Guo, L. Chemical insight on decreased sensitivity of CL-20/TNT cocrystal revealed by ReaxFF MD simulations. J. Chem. Inf. Model. 2019, 59, 2079–2092. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Ji, J.; Zhu, W. Intermolecular interactions, vibrational spectra, and detonation performance of CL-20/TNT cocrystal. J. Chin. Chem. Soc. 2020, 67, 1742–1752. [Google Scholar] [CrossRef]
- Li, S.; Xiao, J. Molecular dynamics simulations for effects of fluoropolymer binder content in CL-20/TNT based polymer-bonded explosives. Molecules 2021, 26, 4876. [Google Scholar] [CrossRef] [PubMed]
- Landenberger, K.B.; Matzger, A.J. Cocrystal engineering of a prototype energetic material: Supramolecular chemistry of 2, 4, 6-trinitrotoluene. Cryst. Growth Des. 2010, 10, 5341–5347. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Zhou, J.; Li, H.; Huang, H.; Nie, F. Preparation and performance of a BTF/DNB cocrystal explosive. Propellants Explos. Pyrotech. 2014, 39, 9–13. [Google Scholar] [CrossRef]
- Spitzer, D.; Risse, B.; Schnell, F.; Pichot, V.; Klaumünzer, M.; Schaefer, M. Continuous engineering of nano-cocrystals for medical and energetic applications. Sci. Rep. 2014, 4, 6575. [Google Scholar] [CrossRef] [Green Version]
- Tokmakoff, A.; Fayer, M.; Dlott, D.D. Chemical reaction initiation and hot-spot formation in shocked energetic molecular materials. J. Phys. Chem. 1993, 97, 1901–1913. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, M.; Venkatesan, V.; Mandave, S.; Banerjee, S.; Sikder, N.; Sikder, A.K.; Bhattacharya, B. Probing crystal growth of ε-and α-CL-20 polymorphs via metastable phase transition using microscopy and vibrational Spectroscopy. Cryst. Growth Des. 2014, 14, 5053–5063. [Google Scholar] [CrossRef]
- Al-Saidi, W.; Asher, S.A.; Norman, P. Resonance raman spectra of TNT and RDX using vibronic theory, excited-state gradient, and complex polarizability approximations. J. Phys. Chem. A 2012, 116, 7862–7872. [Google Scholar] [CrossRef]
- Aubuchon, C.; Rector, K.; Holmes, W.; Fayer, M. Nitro group asymmetric stretching mode lifetimes of molecules used in energetic materials. Chem. Phys. Lett. 1999, 299, 84–90. [Google Scholar] [CrossRef]
- Ostrander, J.S.; Knepper, R.; Tappan, A.S.; Kay, J.J.; Zanni, M.T.; Farrow, D.A. Energy transfer between coherently delocalized states in thin films of the explosive Pentaerythritol Tetranitrate, (PETN) revealed by two-dimensional infrared spectroscopy. J. Phys. Chem. B 2017, 121, 1352–1361. [Google Scholar] [CrossRef]
- Shi, L.; Yu, P.; Zhao, J.; Wang, J. Ultrafast intermolecular vibrational energy transfer in hexahydro-1, 3, 5-trinitro-1, 3, 5-triazine in molecular crystal by 2D IR spectroscopy. J. Phys. Chem. C 2020, 124, 2388–2398. [Google Scholar] [CrossRef]
- Kraack, J.P. Ultrafast structural molecular dynamics investigated with 2D infrared spectroscopy methods. Multidimens. Time-Resolv. Spectrosc. 2019, 113–205. [Google Scholar] [CrossRef]
- Hamm, P.; Zanni, M. Concepts and Methods of 2D Infrared Spectroscopy; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Ren, H.C.; Yuan, J.N.; Chen, T.N.; Selvaraj, G.; Kaliamurthi, S.; Zhang, X.Q.; Wei, D.Q.; Ji, G.F.; Zhang, Z.M. Computational insights of two-dimensional infrared spectroscopy under electric fields in phosphorylcholine. Int. J. Quantum Chem. 2020, 120, e26169. [Google Scholar] [CrossRef]
- Baiz, C.R.; Błasiak, B.; Bredenbeck, J.; Cho, M.; Choi, J.-H.; Corcelli, S.A.; Dijkstra, A.G.; Feng, C.-J.; Garrett-Roe, S.; Ge, N.-H. Vibrational spectroscopic map, vibrational spectroscopy, and intermolecular interaction. Chem. Rev. 2020, 120, 7152–7218. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.C.; Ji, L.X.; Chen, T.N.; Yuan, J.N.; Huang, Y.Y.; Wei, D.-Q.; Ji, G.F.; Zhang, Z.M. Quasi-static two-dimensional infrared spectra of the carboxyhemoglobin subsystem under electric fields: A theoretical study. J. Phys. Chem. B 2020, 124, 9570–9578. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.-C.; Yuan, J.-N.; Xu, W.-S.; Chen, T.-N.; Ji, G.-F.; Wei, D.-Q. Two-dimensional infrared spectra of cationic dopamine under different electric fields: Theoretical studies from the density function theory anharmonic potential. J. Phys. Chem. C 2018, 122, 17994–18004. [Google Scholar] [CrossRef]
- Ren, H.-C.; Ji, L.-X.; Chen, T.-N.; Liu, Y.-G.; Liu, R.-P.; Wei, D.-Q.; Jia, X.-Z.; Ji, G.-F. Revealing the relationship between electric fields and the conformation of Oxytocin using quasi-static Amide-I two-dimensional infrared spectra. Acs Omega 2022, 7, 3758–3767. [Google Scholar] [CrossRef] [PubMed]
- Le Sueur, A.L.; Horness, R.E.; Thielges, M.C. Applications of two-dimensional infrared spectroscopy. Analyst 2015, 140, 4336–4349. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Chen, Q. Independent gradient model based on Hirshfeld partition: A new method for visula study of interactions in chemical systems. J. Comput. Chem. 2022, 43, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Fuster, F.; Grabowski, S.J. Intramolecular hydrogen bonds: The QTAIM and ELF characteristics. J. Phys. Chem. A 2011, 115, 10078–10086. [Google Scholar] [CrossRef] [PubMed]
- Casals-Sainz, J.L.; Fernández-Alarcón, A.; Francisco, E.; Costales, A.; Martin Pendas, A. Bond order densities in real space. J. Phys. Chem. A 2019, 124, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Fast, P.L.; Corchado, J.; Sanchez, M.L.; Truhlar, D.G. Optimized parameters for scaling correlation energy. J. Phys. Chem. A 1999, 103, 3139–3143. [Google Scholar] [CrossRef]
- Goerigk, L.; Grimme, S. A thorough benchmark of density functional methods for general main group thermochemistry, kinetics, and noncovalent interactions. Phys. Chem. Chem. Phys. 2011, 13, 6670–6688. [Google Scholar] [CrossRef]
- Grimme, S. Density functional theory with London dispersion correction. Wires Comput. Mol. Sci. 2011, 1, 211–228. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuserria, G.E.; Robb, M.A.; Cheeseman, J.R.; Schlmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision A. 03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Osmont, A.; Catoire, L.; Goekalp, I.; Yang, V. Ab Initio quantum chemical predictions of enthalpies of formation, heat capacities, and entropies of gas-phase energetic compounds. Combust. Flame 2007, 151, 262–273. [Google Scholar] [CrossRef]
- Jamróz, M.H. Vibrational energy distribution analysis, (VEDA): Scopes and limitations. Spectrochim. Acta A 2013, 114, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.J.; Zhao, L.; Zhu, W.; Chen, J.; Ji, G.F.; Zhao, F.; Wu, Q.; Xiao, H.M. Molecular dynamics study on the relationships of modeling, structural and energy properties with sensitivity for RDX-based PBXs. Sci. China Chem. 2012, 55, 2587–2594. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavenumber analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
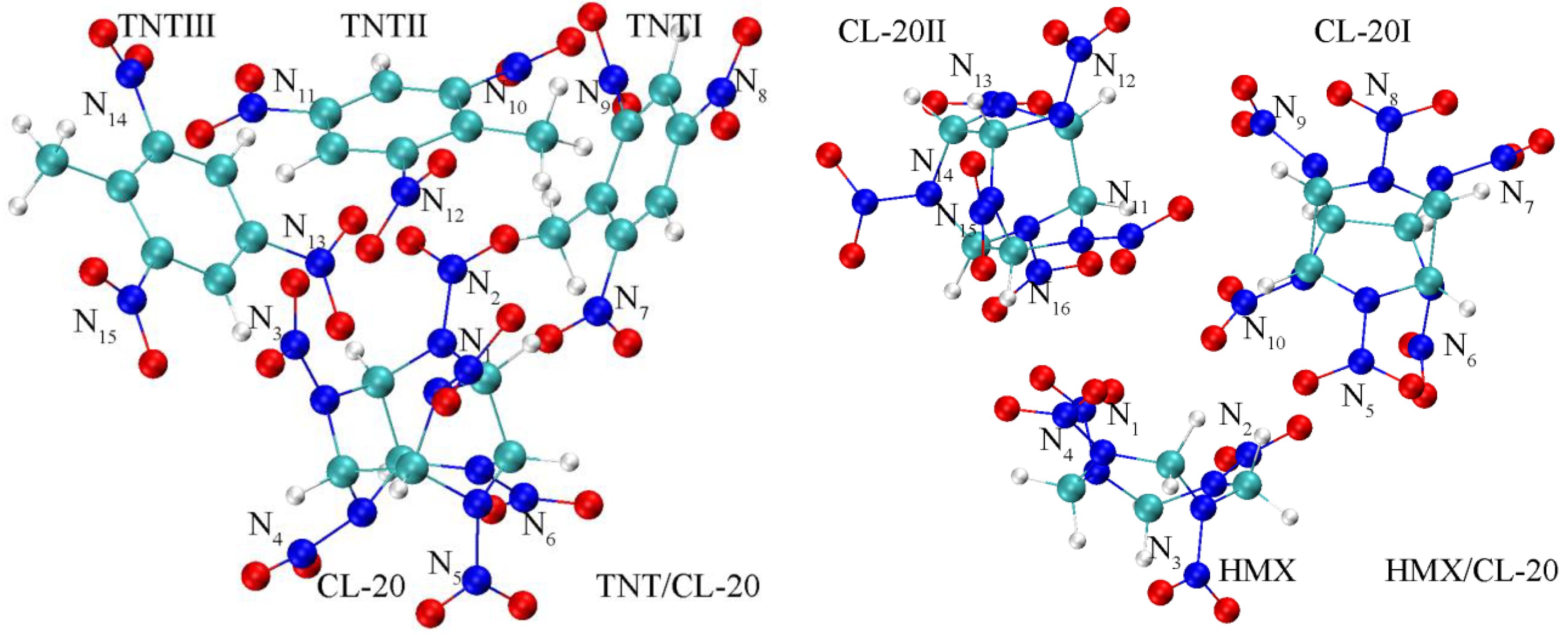


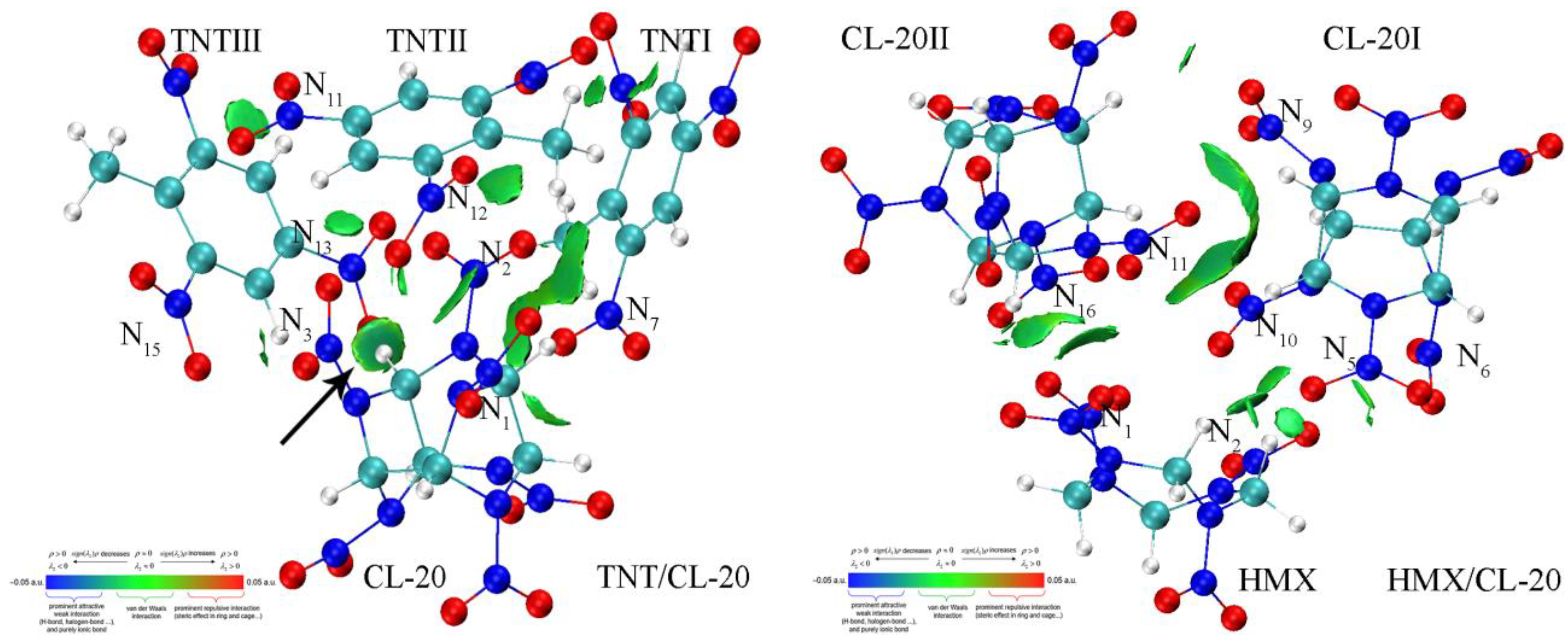
| Bond Length | Calculation | Experiment |
|---|---|---|
| N1-N1’ | 1.440 | |
| N2-N2’ | 1.386 | 1.378 |
| N3-N3’ | 1.384 | 1.383 |
| N4-N4’ | 1.448 | |
| N5-N5’ | 1.399 | 1.400 |
| N6-N6’ | 1.417 | 1.414 |
| N1-O | 1.204 (1.215) | 1.204 |
| N2-O | 1.214 (1.223) | 1.215 |
| N3-O | 1.211 (1.225) | |
| N4-O | 1.208 (1.210) | |
| N5-O | 1.213 (1.217) | |
| N6-O | 1.208 (1.212) | |
| N1’-C | 1.454 (1.475) | 1.455 |
| N2’-C | 1.440 (1.443) | 1.445 |
| N3’-C | 1.464 (1.466) | 1.469 |
| N4’-C | 1.451 (1.481) | 1.483 |
| N5’-C | 1.465 (1.474) | 1.478 |
| N6’-C | 1.427 (1.433) | 1.434 |
| TNT/CL-20 | HMX/CL-20 | ||||
|---|---|---|---|---|---|
| CL-20 | Mayer BOD | CL-20I | Mayer BOD | CL-20II | Mayer BOD |
| N1-N | 0.9518 | N5-N | 0.9255 | N11-N | 1.0176 |
| N2-N | 0.9612 | N6-N | 0.9238 | N12-N | 0.8588 |
| N3-N | 0.8748 | N7-N | 0.8718 | N13-N | 0.9265 |
| N4-N | 0.9240 | N8-N | 0.8821 | N14-N | 0.8984 |
| N5-N | 0.8902 | N9-N | 0.9120 | N15-N | 0.8792 |
| N6-N | 0.8648 | N10-N | 0.9198 | N16-N | 0.8712 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.-C.; Ji, L.-X.; Chen, T.-N.; Jia, X.-Z.; Liu, R.-P.; Zhang, X.-Q.; Wei, D.-Q.; Wang, X.-F.; Ji, G.-F. Intermolecular Vibration Energy Transfer Process in Two CL-20-Based Cocrystals Theoretically Revealed by Two-Dimensional Infrared Spectra. Molecules 2022, 27, 2153. https://doi.org/10.3390/molecules27072153
Ren H-C, Ji L-X, Chen T-N, Jia X-Z, Liu R-P, Zhang X-Q, Wei D-Q, Wang X-F, Ji G-F. Intermolecular Vibration Energy Transfer Process in Two CL-20-Based Cocrystals Theoretically Revealed by Two-Dimensional Infrared Spectra. Molecules. 2022; 27(7):2153. https://doi.org/10.3390/molecules27072153
Chicago/Turabian StyleRen, Hai-Chao, Lin-Xiang Ji, Tu-Nan Chen, Xian-Zhen Jia, Rui-Peng Liu, Xiu-Qing Zhang, Dong-Qing Wei, Xiao-Feng Wang, and Guang-Fu Ji. 2022. "Intermolecular Vibration Energy Transfer Process in Two CL-20-Based Cocrystals Theoretically Revealed by Two-Dimensional Infrared Spectra" Molecules 27, no. 7: 2153. https://doi.org/10.3390/molecules27072153






