Effect of Thermal and Non-Thermal Technologies on Kinetics and the Main Quality Parameters of Red Bell Pepper Dried with Convective and Microwave–Convective Methods
Abstract
:1. Introduction
2. Results and Discussion
2.1. Convective and Microwave–Convective Drying Kinetics
2.2. Quality Parameters of Red Bell Pepper Dried with Convective and Microwave–Convective Methods
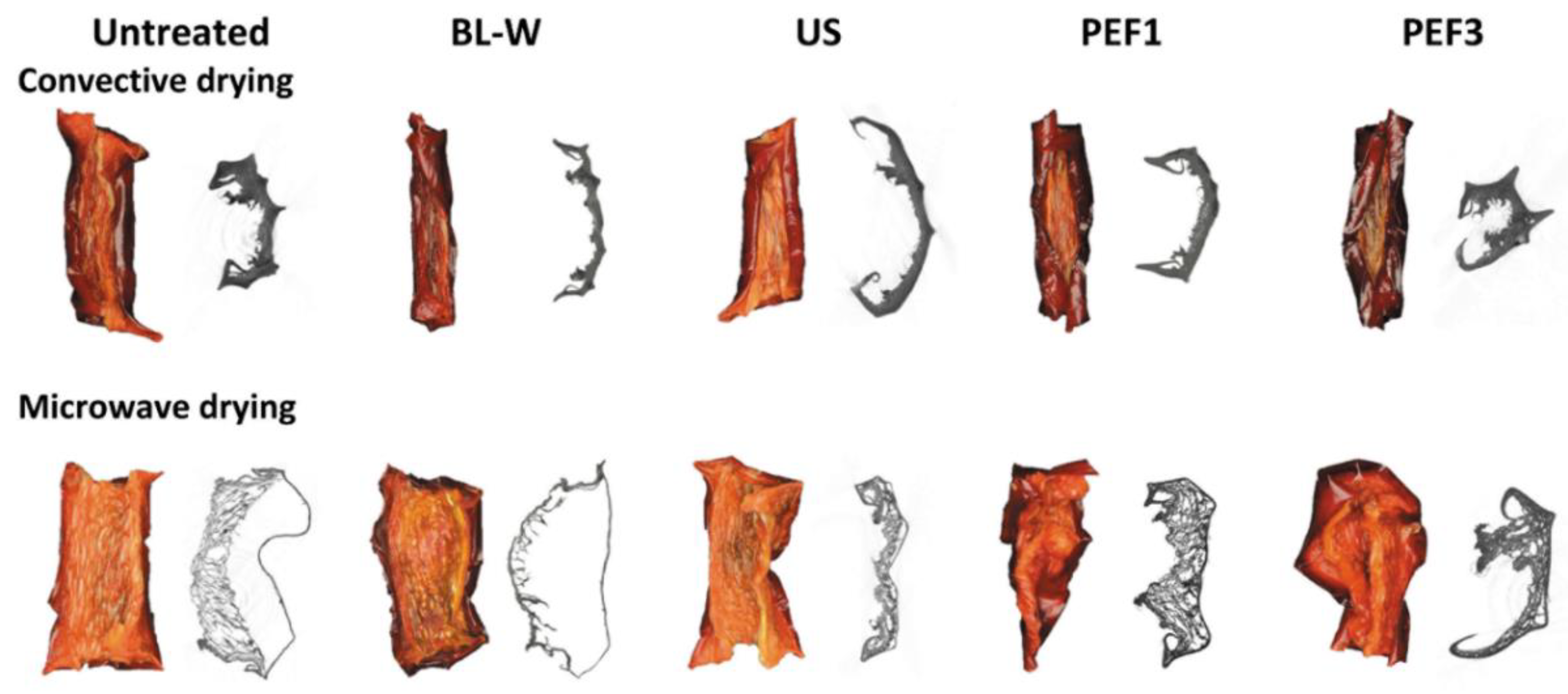
2.2.1. Physical Properties of Dried Red Bell Pepper
Water Activity, Porosity, and Rehydration Rate of Convective and Microwave–Convective Dried Red Bell Pepper
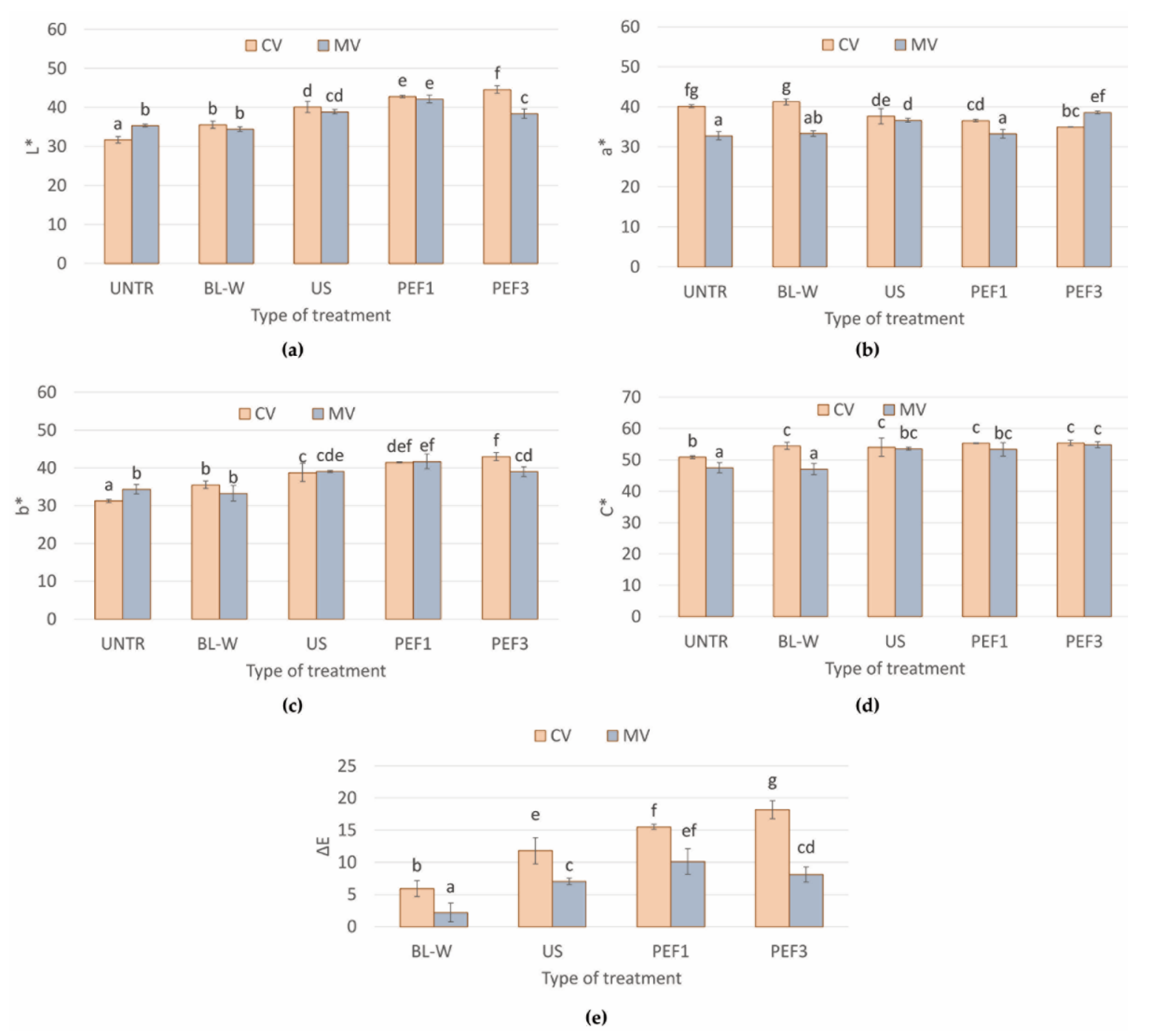
Color of Convective and Microwave–Convective Dried Red Bell Pepper
2.2.2. Chemical Properties of Dried Red Bell Pepper
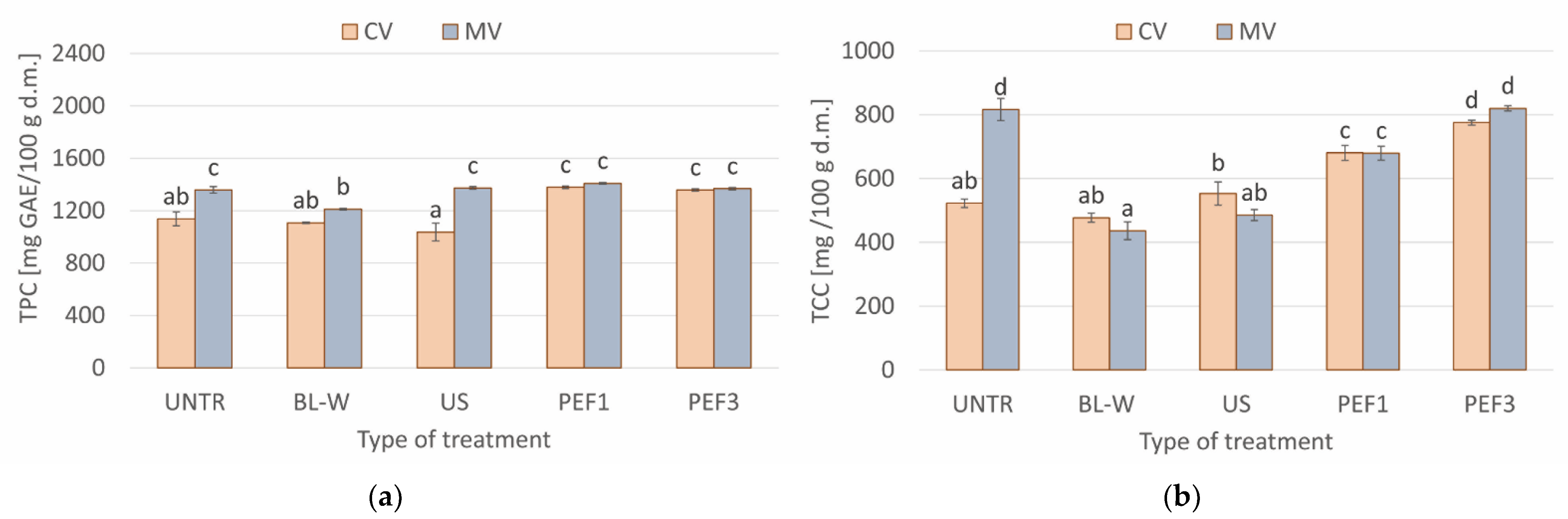
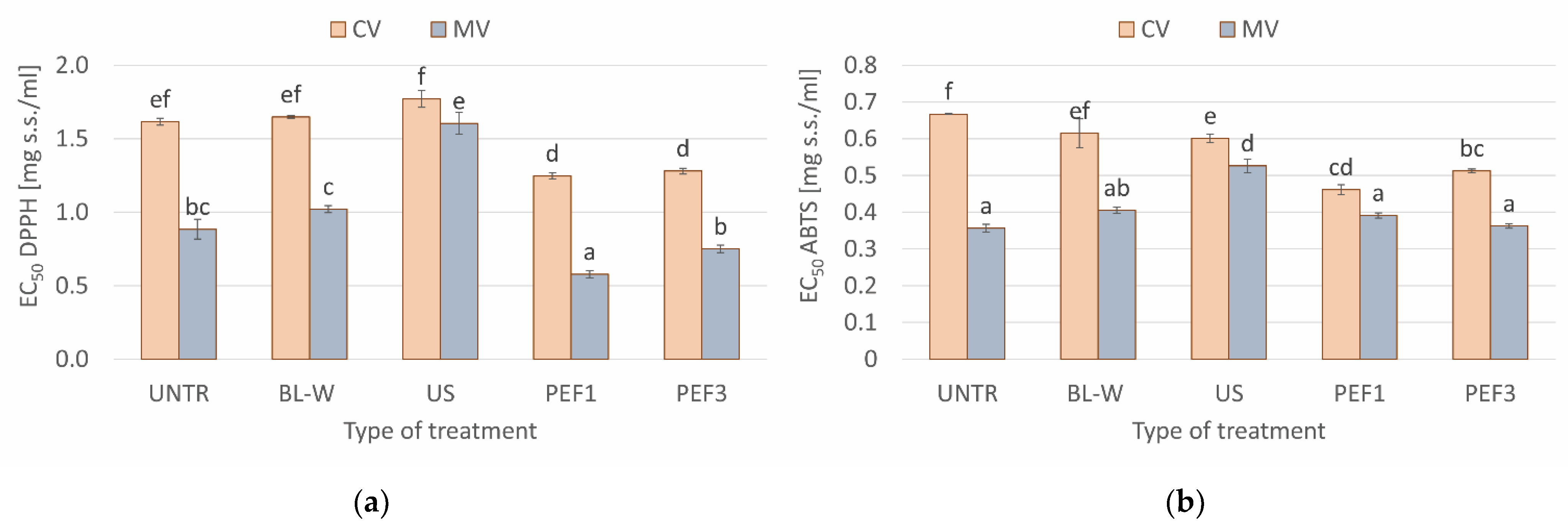
Total Polyphenol Content, Total Carotenoid Content, and Antioxidant Activity (DPPH and ABTS Assay) of Convective and Microwave–Convective Dried Red Bell Pepper
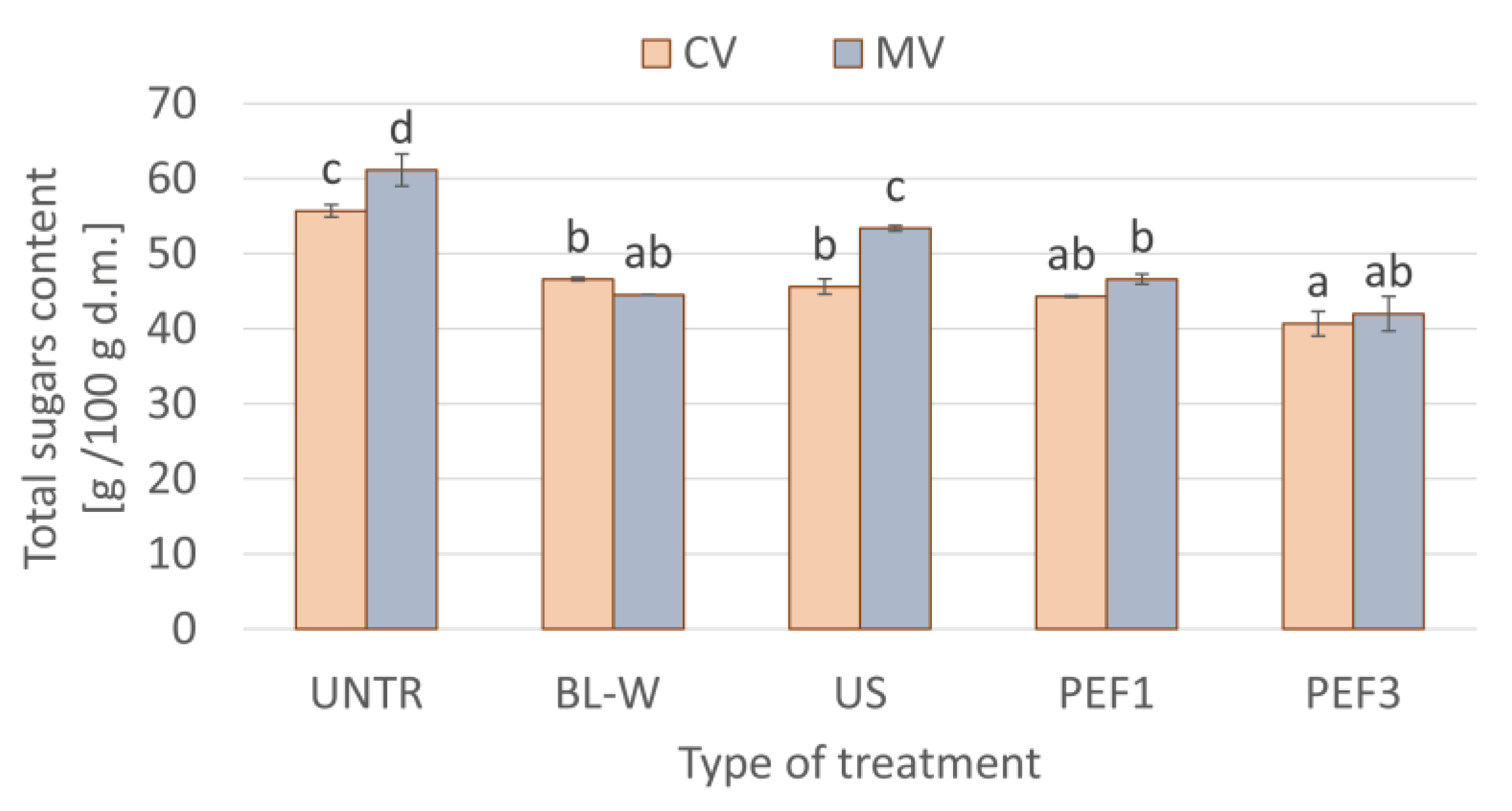
Total Sugars Content of Convective and Microwave–Convective Dried Red Bell Pepper
2.2.3. Cluster Analysis
3. Materials and Methods
3.1. Material
3.2. Experimental Design
3.3. Thermal and Non-Thermal Treatment
3.3.1. Thermal Treatment: Blanching (BL-W)
3.3.2. Non-Thermal Treatment: Ultrasound (US) and Pulsed Electric Field (PEF)
3.4. Drying
3.5. Quality of Dried Red Bell Pepper
3.5.1. Physical Properties of Dried Red Bell Pepper
Water Activity of Dried Red Bell Pepper
Porosity of Dried Red Bell Pepper
Rehydration Rate of Dried Red Bell Pepper
Color of Dried Red Bell Pepper
Photos and X-ray—CT Images of Dried Red Bell Pepper
3.5.2. Chemical Properties of Dried Red Bell Pepper
Extract Preparation
Total Phenolic Content (TPC) of Dried Red Bell Pepper
Total Carotenoids Content (TCC) of Dried Red Bell Pepper
Antioxidant Activity (DPPH and ABTS Assay) of Dried Red Bell Pepper
DPPH Assay
ABTS Assay
Total Sugars Content (TSC) of Dried Red Bell Pepper
Thermogravimetry Analysis (TGA)
Fourier-Transform Infrared Spectroscopy (FTIR)
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hallmann, E.; Marszałek, K.; Lipowski, J.; Jasińska, U.; Kazimierczak, R.; Średnicka-Tober, D.; Rembiałkowska, E. Polyphenols and carotenoids in pickled bell pepper from organic and conventional production. Food Chem. 2019, 278, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Belović, M.; Ilic, N.; Moreno, D.A.; García-Viguera, C. Industrial use of pepper (Capsicum annum L.) derived products: Technological benefits and biological advantages. Food Chem. 2019, 274, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, I.; Khan, M.A. Microwave assisted fluidized bed drying of red bell pepper: Drying kinetics and optimization of process conditions using statistical models and response surface methodology. Sci. Hortic. (Amst.) 2021, 286, 110209. [Google Scholar] [CrossRef]
- Di Cesare, L.F.; Forni, E.; Viscardi, D.; Nani, R.C. Changes in the chemical composition of basil caused by different drying procedures. J. Agric. Food Chem. 2003, 51, 3575–3581. [Google Scholar] [CrossRef]
- Pei, Y.; Li, Z.; Song, C.; Li, J.; Song, F.; Zhu, G.; Liu, M. Effects of combined infrared and hot-air drying on ginsenosides and sensory properties of ginseng root slices (Panax ginseng Meyer). J. Food Process. Preserv. 2020, 44, 1–13. [Google Scholar] [CrossRef]
- Kowalska, H.; Marzec, A.; Kowalska, J.; Trych, U.; Masiarz, E.; Lenart, A. The Use of a Hybrid Drying Method with Pre-Osmotic Treatment in Strawberry Bio-Snack Technology. Int. J. Food Eng. 2020, 16, 1–14. [Google Scholar] [CrossRef]
- Wray, D.; Ramaswamy, H.S. Novel Concepts in Microwave Drying of Foods. Dry. Technol. 2015, 33, 769–783. [Google Scholar] [CrossRef]
- Carvalho, G.R.; Monteiro, R.L.; Laurindo, J.B.; Augusto, P.E.D. Microwave and microwave-vacuum drying as alternatives to convective drying in barley malt processing. Innov. Food Sci. Emerg. Technol. 2021, 73, 102770. [Google Scholar] [CrossRef]
- Macedo, L.L.; Corrêa, J.L.G.; Júnior, I.P.; Da Silva Araújo, C.; Vimercati, W.C. Intermittent microwave drying and heated air drying of fresh and isomaltulose (Palatinose) impregnated strawberry. LWT 2022, 155, 112918. [Google Scholar] [CrossRef]
- Deng, L.Z.; Mujumdar, A.S.; Zhang, Q.; Yang, X.H.; Wang, J.; Zheng, Z.A.; Gao, Z.J.; Xiao, H.W. Chemical and physical pretreatments of fruits and vegetables: Effects on drying characteristics and quality attributes–A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1408–1432. [Google Scholar] [CrossRef]
- Jha, P.; Meghwal, M.; Prabhakar, P.K.; Singh, A. Exploring effects of different pretreatments on drying kinetics, moisture diffusion, physico-functional, and flow properties of banana flower powder. J. Food Process. Preserv. 2021, 45, 1–15. [Google Scholar] [CrossRef]
- Xu, B.; Sylvain Tiliwa, E.; Yan, W.; Roknul Azam, S.M.; Wei, B.; Zhou, C.; Ma, H.; Bhandari, B. Recent Development in High Quality drying of Fruits and Vegetables Assisted by Ultrasound: A Review; Elsevier Ltd.: Amsterdam, The Netherlands, 2022; Volume 152, ISBN 5118878020. [Google Scholar]
- Morales-de la Peña, M.; Rábago-Panduro, L.M.; Soliva-Fortuny, R.; Martín-Belloso, O.; Welti-Chanes, J. Pulsed Electric Fields Technology for Healthy Food Products. Food Eng. Rev. 2021, 13, 509–523. [Google Scholar] [CrossRef]
- Dehghannya, J.; Aghazade-Khoie, E.; Khakbaz Heshmati, M.; Ghanbarzadeh, B. Influence of Ultrasound Intensification on the Continuous and Pulsed Microwave during Convective Drying of Apple. Int. J. Fruit Sci. 2020, 20, S1751–S1764. [Google Scholar] [CrossRef]
- Dellarosa, N.; Frontuto, D.; Laghi, L.; Dalla Rosa, M.; Lyng, J.G. The impact of pulsed electric fields and ultrasound on water distribution and loss in mushrooms stalks. Food Chem. 2017, 236, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.O.; Fu, Q.Q.; Chen, S.J.; Hu, Z.C.; Xie, H.X. Effect of Hot-Water Blanching Pretreatment on Drying Characteristics and Product Qualities for the Novel Integrated Freeze-Drying of Apple Slices. J. Food Qual. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Castro, S.M.; Saraiva, J.A.; Domingues, F.M.J.; Delgadillo, I. Effect of mild pressure treatments and thermal blanching on yellow bell peppers (Capsicum annuum L.). LWT Food Sci. Technol. 2011, 44, 363–369. [Google Scholar] [CrossRef]
- Orikasa, T.; Ono, N.; Watanabe, T.; Ando, Y.; Shiina, T.; Koide, S. Impact of blanching pretreatment on the drying rate and energy consumption during far-infrared drying of Paprika (Capsicum annuum L.). Food Qual. Saf. 2018, 2, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Motevali, A.; Minaei, S.; Banakar, A.; Ghobadian, B.; Khoshtaghaza, M.H. Comparison of energy parameters in various dryers. Energy Convers. Manag. 2014, 87, 711–725. [Google Scholar] [CrossRef]
- Nagvanshi, S.; Venkata, S.K.; Goswami, T.K. Study of color kinetics of banana (Musa cavendish) under microwave drying by application of image analysis. Food Sci. Technol. Int. 2020, 1–14. [Google Scholar] [CrossRef]
- Jha, P.; Meghwal, M.; Prabhakar, P.K. Microwave drying of banana blossoms (Musa acuminata): Mathematical modeling and drying energetics. J. Food Process. Preserv. 2021, 45, 1–14. [Google Scholar] [CrossRef]
- Rahaman, A.; Zeng, X.A.; Kumari, A.; Farooq, M.A.; Siddeeg, A.; Manzoor, M.F. Combined effect of pulsed electric fields and ultrasound on mass energy transfer and diffusion coefficient of plum. Heat Mass Transf. und Stoffuebertragung 2021, 57, 1087–1095. [Google Scholar] [CrossRef]
- Abbaspour-Gilandeh, Y.; Kaveh, M.; Fatemi, H.; Khalife, E.; Witrowa-Rajchert, D.; Nowacka, M. Effect of pretreatments on convective and infrared drying kinetics, energy consumption and quality of terebinth. Appl. Sci. 2021, 11, 7672. [Google Scholar] [CrossRef]
- Rybak, K.; Wiktor, A.; Witrowa-rajchert, D.; Parniakov, O.; Nowacka, M. The Quality of Red Bell Pepper Subjected to Freeze-Drying Preceded by Traditional and Novel Pretreatment. Foods 2021, 10, 226. [Google Scholar] [CrossRef]
- Halder, A.; Datta, A.K.; Spanswick, R.M. Water Transport in Cellular Tissues During Thermal Processing. AIChE J. 2011, 57, 2574–2588. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Kowalska, H.; Kowalska, J.; Galus, S.; Marzec, A.; Domian, E. The Effect of Pre-Treatment (Blanching, Ultrasound and Freezing) on Quality of Freeze-Dried Red Beets. Foods 2021, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zeng, Y.; Hu, X.; Sun, X. Effect of Ultrasonic Power on Water Removal Kinetics and Moisture Migration of Kiwifruit Slices During Contact Ultrasound Intensified Heat Pump Drying. Food Bioprocess Technol. 2020, 13, 430–441. [Google Scholar] [CrossRef]
- Tylewicz, U.; Aganovic, K.; Vannini, M.; Toepfl, S.; Bortolotti, V.; Dalla Rosa, M.; Oey, I.; Heinz, V. Effect of pulsed electric field treatment on water distribution of freeze-dried apple tissue evaluated with DSC and TD-NMR techniques. Innov. Food Sci. Emerg. Technol. 2016, 37, 352–358. [Google Scholar] [CrossRef]
- Fellows, P.J. Dehydration. In Woodhead Publishing Series in Food Science, Technology and Nutrition, Food Processing Technology; P.J. Fellows, Ed.; Woodhead Publishing: Sawston, UK, 2009; pp. 481–524. [Google Scholar]
- Joardder, M.U.H.; Alsbua, R.; Akram, W.; Karim, M.A. Effect of sample rugged surface on energy consumption and quality of plant-based food materials in convective drying. Dry. Technol. 2021, 39, 1339–1348. [Google Scholar] [CrossRef]
- Szadzińska, J.; Mierzwa, D.; Pawłowski, A.; Pashminehazar, R.; Kharaghani, A. Ultrasound- and microwave-assisted intermittent drying of red beetroot. Dry. Technol. 2019, 38, 93–107. [Google Scholar] [CrossRef]
- Tao, Y.; Han, M.; Gao, X.; Han, Y.; Show, P.L.; Liu, C.; Ye, X.; Xie, G. Applications of water blanching, surface contacting ultrasound-assisted air drying, and their combination for dehydration of white cabbage: Drying mechanism, bioactive profile, color and rehydration property. Ultrason. Sonochem. 2019, 53, 192–201. [Google Scholar] [CrossRef]
- Mutuli, G.P.; Gitau, A.N.; Mbuge, D.O. Convective Drying Modeling Approaches: A Review for Herbs, Vegetables, and Fruits. J. Biosyst. Eng. 2020, 45, 197–212. [Google Scholar] [CrossRef]
- Miano, A.C.; Rojas, M.L.; Augusto, P.E.D. Structural changes caused by ultrasound pretreatment: Direct and indirect demonstration in potato cylinders. Ultrason. Sonochem. 2019, 52, 176–183. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Y.; Wang, Q.; Sun, C.; Xi, H. Drying characteristics, microstructure, glass transition temperature, and quality of ultrasound-strengthened hot air drying on pear slices. J. Food Process. Preserv. 2019, 43, e13899. [Google Scholar] [CrossRef]
- Fauster, T.; Giancaterino, M.; Pittia, P.; Jaeger, H. Effect of pulsed electric field pretreatment on shrinkage, rehydration capacity and texture of freeze-dried plant materials. LWT Food Sci. Technol. 2020, 121, 108937. [Google Scholar] [CrossRef]
- Shynkaryk, M.V.; Lebovka, N.I.; Vorobiev, E. Pulsed electric fields and temperature effects on drying and rehydration of red beetroots. Dry. Technol. 2008, 26, 695–704. [Google Scholar] [CrossRef]
- Dorantes-Alvarez, L.; Jaramillo-Flores, E.; González, K.; Martinez, R.; Parada, L. Blanching peppers using microwaves. Procedia Food Sci. 2011, 1, 178–183. [Google Scholar] [CrossRef] [Green Version]
- Rojas, M.L.; Hellmeister Trevilin, J.; Augusto, P.E.D.; Rojas, M.L.; Hellmeister Trevilin, J.; Augusto, D.; Esteves, P. The ultrasound technology for modifying enzyme activity. Sci. Agropecu. 2016, 07, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Ohshima, T.; Tamura, T.; Sato, M. Influence of pulsed electric field on various enzyme activities. J. Electrostat. 2007, 65, 156–161. [Google Scholar] [CrossRef]
- Hiwilepo-van Hal, P.; Bosschaart, C.; van Twisk, C.; Verkerk, R.; Dekker, M. Kinetics of thermal degradation of vitamin C in marula fruit (Sclerocarya birrea subsp. caffra) as compared to other selected tropical fruits. LWT Food Sci. Technol. 2012, 49, 188–191. [Google Scholar] [CrossRef]
- Gamboa-Santos, J.; Megías-Pérez, R.; Soria, A.C.; Olano, A.; Montilla, A.; Villamiel, M. Impact of processing conditions on the kinetic of vitamin C degradation and 2-furoylmethyl amino acid formation in dried strawberries. Food Chem. 2014, 153, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Perera, C.O.; Alzahrani, M.A.J. Ultrasound as a pre-treatment for extraction of bioactive compounds and food safety: A review. LWT Food Sci. Technol. 2021, 142, 111114. [Google Scholar] [CrossRef]
- Segovia, F.J.; Luengo, E.; Corral-Pérez, J.J.; Raso, J.; Almajano, M.P. Improvements in the aqueous extraction of polyphenols from borage (Borago officinalis L.) leaves by pulsed electric fields: Pulsed electric fields (PEF) applications. Ind. Crops Prod. 2015, 65, 390–396. [Google Scholar] [CrossRef]
- Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D.; Parniakov, O.; Nowacka, M. The Effect of Traditional and Non-Thermal Treatments on the Bioactive Compounds and Sugars content of Red Bell Pepper. Molecules 2020, 25, 4287. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiao, H.W.; Ye, J.H.; Wang, J.; Raghavan, V. Ultrasound Pretreatment to Enhance Drying Kinetics of Kiwifruit (Actinidia deliciosa) Slices: Pros and Cons. Food Bioprocess Technol. 2019, 12, 865–876. [Google Scholar] [CrossRef]
- Arslan, D.; Özcan, M.M. Dehydration of red bell-pepper (Capsicum annuum L.): Change in drying behavior, colour and antioxidant content. Food Bioprod. Process. 2011, 89, 504–513. [Google Scholar] [CrossRef]
- Rybak, K.; Samborska, K.; Jedlinska, A.; Parniakov, O.; Nowacka, M.; Witrowa-Rajchert, D.; Wiktor, A. The impact of pulsed electric field pretreatment of bell pepper on the selected properties of spray dried juice. Innov. Food Sci. Emerg. Technol. 2020, 65, 102446. [Google Scholar] [CrossRef]
- Martí-Quijal, F.J.; Ramon-Mascarell, F.; Pallarés, N.; Ferrer, E.; Berrada, H.; Phimolsiripol, Y.; Barba, F.J. Extraction of antioxidant compounds and pigments from spirulina (Arthrospira platensis) assisted by pulsed electric fields and the binary mixture of organic solvents and water. Appl. Sci. 2021, 11, 7629. [Google Scholar] [CrossRef]
- Rawson, A.; Tiwari, B.K.; Tuohy, M.G.; O’Donnell, C.P.; Brunton, N. Effect of ultrasound and blanching pretreatments on polyacetylene and carotenoid content of hot air and freeze dried carrot discs. Ultrason. Sonochem. 2011, 18, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Sancheti, S.V.; Gogate, P.R. A review of engineering aspects of intensification of chemical synthesis using ultrasound. Ultrason. Sonochem. 2017, 36, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Dadan, M.; Rybak, K.; Wiktor, A.; Nowacka, M.; Zubernik, J.; Witrowa-Rajchert, D. Selected chemical composition changes in microwave-convective dried parsley leaves affected by ultrasound and steaming pre-treatments—An optimization approach. Food Chem. 2018, 239, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Paul, I.D.; Das, M. Effect of freeze, microwave-convective hot air, vacuum and dehumidified air drying on total phenolics content, anthocyanin content and antioxidant activity of jamun (Syzygium cumini L.) pulp. J. Food Sci. Technol. 2018, 55, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M.; Al Juhaimi, F.; Ahmed, I.A.M.; Uslu, N.; Babiker, E.E.; Ghafoor, K. Effect of microwave and oven drying processes on antioxidant activity, total phenol and phenolic compounds of kiwi and pepino fruits. J. Food Sci. Technol. 2020, 57, 233–242. [Google Scholar] [CrossRef]
- Galindo, F.G.; Vernier, P.T.; Dejmek, P.; Vicente, A.; Gundersen, M.A. Pulsed electric field reduces the permeability of potato cell wall. Bioelectromagnetics 2008, 29, 296–301. [Google Scholar] [CrossRef] [Green Version]
- Paniwnyk, L.; Beaufoy, E.; Lorimer, J.P.; Mason, T.J. The extraction of rutin from flower buds of Sophora japonica. Ultrason. Sonochem. 2001, 8, 299–301. [Google Scholar] [CrossRef]
- Yadav, D.K.; Kumar, S.; Choi, E.H.; Kim, M.H. Electric-field-induced electroporation and permeation of reactive oxygen species across a skin membrane. J. Biomol. Struct. Dyn. 2021, 39, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Martínez, I.; Meza-Márquez, O.G.; Osorio-Revilla, G.; Proal-Nájera, J.; Gallardo-Velázquez, T. Determination of capsaicin, ascorbic acid, total phenolic compounds and antioxidant activity of Capsicum annuum L. var. serrano by mid infrared spectroscopy (Mid-FTIR) and chemometric analysis. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 133–142. [Google Scholar] [CrossRef]
- Bureau, S.; Cozzolino, D.; Clark, C.J. Contributions of Fourier-transform mid infrared (FT-MIR) spectroscopy to the study of fruit and vegetables: A review. Postharvest Biol. Technol. 2019, 148, 1–14. [Google Scholar] [CrossRef]
- Chhe, C.; Imaizumi, T.; Tanaka, F.; Uchino, T. Effects of hot-water blanching on the biological and physicochemical properties of sweet potato slices. Eng. Agric. Environ. Food 2018, 11, 19–24. [Google Scholar] [CrossRef]
- Pieczywek, P.M.; Kozioł, A.; Konopacka, D.; Cybulska, J.; Zdunek, A. Changes in cell wall stiffness and microstructure in ultrasonically treated apple. J. Food Eng. 2017, 197, 1–8. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Oliveira, F.I.P.; Rodrigues, S. Use of ultrasound for dehydration of papayas. Food Bioprocess Technol. 2008, 1, 339–345. [Google Scholar] [CrossRef]
- Ostermeier, R.; Parniakov, O.; Töpfl, S.; Jäger, H. Applicability of Pulsed Electric Field (PEF) Pre-Treatment for a Convective Two-Step Drying Process. Foods 2020, 9, 512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sotelo, K.A.G.; Hamid, N.; Oey, I.; Pook, C.; Gutierrez-Maddox, N.; Ma, Q.; Ying Leong, S.; Lu, J. Red cherries (Prunus avium var. Stella) processed by pulsed electric field – Physical, chemical and microbiological analyses. Food Chem. 2018, 240, 926–934. [Google Scholar] [CrossRef]
- Zlatanović, S.; Ostojić, S.; Micić, D.; Rankov, S.; Dodevska, M.; Vukosavljević, P.; Gorjanović, S. Thermal behaviour and degradation kinetics of apple pomace flours. Thermochim. Acta 2019, 673, 17–25. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Lim, X.Y.; Ani, F.N.; Jusoh, A. Fruit waste as feedstock for recovery by pyrolysis technique. Int. Biodeterior. Biodegrad. 2016, 113, 325–333. [Google Scholar] [CrossRef]
- Cheng, X.C.; Cui, X.Y.; Qin, Z.; Liu, H.M.; De Wang, X.; Liu, Y.L. Effect of drying pretreatment methods on structural features and antioxidant activities of Brauns native lignin extracted from Chinese quince fruit. Process Biochem. 2021, 106, 70–77. [Google Scholar] [CrossRef]
- Śledź, M.; Nowacka, M.; Wiktor, A.; Witrowa-Rajchert, D. Selected chemical and physico-chemical properties of microwave-convective dried herbs. Food Bioprod. Process. 2013, 91, 421–428. [Google Scholar] [CrossRef]
- Cunniff, P. AOAC International Official Methods of Analysis of AOAC International; AOAC International: Arlington, VA, USA, 2002. [Google Scholar]
- Tylewicz, U.; Mannozzi, C.; Castagnini, J.M.; Genovese, J.; Romani, S.; Rocculi, P.; Rosa, M.D. Application of PEF- and OD-assisted drying for kiwifruit waste valorisation. Innov. Food Sci. Emerg. Technol. 2022, 77, 102952. [Google Scholar] [CrossRef]
- Fijalkowska, A.; Nowacka, M.; Witrowa-Rajchert, D. The physical, optical and reconstitution properties of apples subjected to ultrasound before drying. Ital. J. Food Sci. 2017, 29, 343–356. [Google Scholar]
- Lammerskitten, A.; Wiktor, A.; Mykhailyk, V.; Samborska, K.; Gondek, E.; Witrowa-Rajchert, D.; Parniakov, O. Pulsed electric field pre-treatment improves microstructure and crunchiness of freeze-dried plant materials: Case of strawberry. LWT Food Sci. Technol. 2020, 134, 110266. [Google Scholar] [CrossRef]
- Polish Committee for Standardization. Fruit and vegetable juice—Total carotenoids and carotenoids fraction determination (in Polish) (PN-EN 12136). In Polish Standard (2000); PKN: Warsaw, Poland, 2000. [Google Scholar]
- El Kossori, R.L.; Villaume, C.; El Boustani, E.; Sauvaire, Y.; Méjean, L. Composition of pulp, skin and seeds of prickly pears fruit (Opuntia ficus indica sp.). Plant Foods Hum. Nutr. 1998, 52, 263–270. [Google Scholar] [CrossRef]
- Mikus, M.; Galus, S.; Ciurzyńska, A.; Janowicz, M. Development and characterization of novel composite films based on soy protein isolate and oilseed flours. Molecules 2021, 26, 3738. [Google Scholar] [CrossRef] [PubMed]
- Ríos-González, L.J.; Medina-Morales, M.A.; Rodríguez-De la Garza, J.A.; Romero-Galarza, A.; Medina, D.D.; Morales-Martínez, T.K. Comparison of dilute acid pretreatment of agave assisted by microwave versus ultrasound to enhance enzymatic hydrolysis. Bioresour. Technol. 2021, 319, 124099. [Google Scholar] [CrossRef] [PubMed]





| Drying Time to MR = 0.02 (min) | |||||
|---|---|---|---|---|---|
| Drying Method | Untreated | BL-W | US | PEF1 | PEF3 |
| CV | 810 ± 65 aA | 332 ± 45 cA | 666 ± 45 bA | 554 ± 30 bA | 526 ± 45 bA |
| MV | 190 ± 22 aB | 129 ± 19 bB | 152 ± 16 abB | 153 ± 16 abB | 147 ± 28 abB |
| Dried Red Bell Pepper | aw (−) | Porosity (%) | RR (−) |
|---|---|---|---|
| Convective Dried Red Bell Pepper | |||
| CV_Untr | 0.314 ± 0.004 ef | 4.7 ± 0.7 a | 2.64 ± 0.05 a |
| CV_BL-W | 0.270 ± 0.005 d | 13.6 ± 1.3 bc | 4.21 ± 0.01 cd |
| CV_US | 0.227 ± 0.005 b | 10.8 ± 0.9 b | 2.60 ± 0.19 a |
| CV_PEF1 | 0.258 ± 0.006 cd | 10.4 ± 0.4 b | 3.00 ± 0.08 ab |
| CV_PEF3 | 0.201 ± 0.004 a | 16.4 ± 0.9 c | 2.87 ± 0.08 a |
| Microwave–convective dried red bell pepper | |||
| MV_Untr | 0.393 ± 0.005 g | 10.0 ± 0.9 ab | 3.88 ± 0.33 bcd |
| MV_BL-W | 0.242 ± 0.005 bc | 10.5 ± 1.8 ab | 4.06 ± 0.17 cd |
| MV_US | 0.234 ± 0.006 b | 39.9 ± 1.8 e | 4.68 ± 0.29 d |
| MV_PEF1 | 0.318 ± 0.010 f | 25.7 ± 1.8 d | 4.18 ± 0.44 cd |
| MV_PEF3 | 0.294 ± 0.007 e | 26.4 ± 1.9 d | 3.47 ± 0.20 abc |
| Treatment | Description and Treatment Parameters | ||
|---|---|---|---|
| Thermal treatment | |||
| UNTR | Untreated | - | |
| BL-W | Blanching in water | 98 °C, 3 min | |
| Non-thermal treatment | |||
| US | Ultrasound | Sonication time, 30 min; ultrasonic bath with frequency of 21 kHz; ultrasound intensity equal to 3 W/cm2 | |
| PEF1 | Pulsed electric field | Electric field intensity of 1.07 kV/cm | Specific energy intake: 1.0 kJ/kg |
| PEF3 | Pulsed electric field | Specific energy intake: 3.0 kJ/kg | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybak, K.; Wiktor, A.; Kaveh, M.; Dadan, M.; Witrowa-Rajchert, D.; Nowacka, M. Effect of Thermal and Non-Thermal Technologies on Kinetics and the Main Quality Parameters of Red Bell Pepper Dried with Convective and Microwave–Convective Methods. Molecules 2022, 27, 2164. https://doi.org/10.3390/molecules27072164
Rybak K, Wiktor A, Kaveh M, Dadan M, Witrowa-Rajchert D, Nowacka M. Effect of Thermal and Non-Thermal Technologies on Kinetics and the Main Quality Parameters of Red Bell Pepper Dried with Convective and Microwave–Convective Methods. Molecules. 2022; 27(7):2164. https://doi.org/10.3390/molecules27072164
Chicago/Turabian StyleRybak, Katarzyna, Artur Wiktor, Mohammad Kaveh, Magdalena Dadan, Dorota Witrowa-Rajchert, and Małgorzata Nowacka. 2022. "Effect of Thermal and Non-Thermal Technologies on Kinetics and the Main Quality Parameters of Red Bell Pepper Dried with Convective and Microwave–Convective Methods" Molecules 27, no. 7: 2164. https://doi.org/10.3390/molecules27072164








