Tocotrienols Attenuate White Adipose Tissue Accumulation and Improve Serum Cholesterol Concentration in High-Fat Diet-Treated Mice
Abstract
:1. Introduction
2. Results
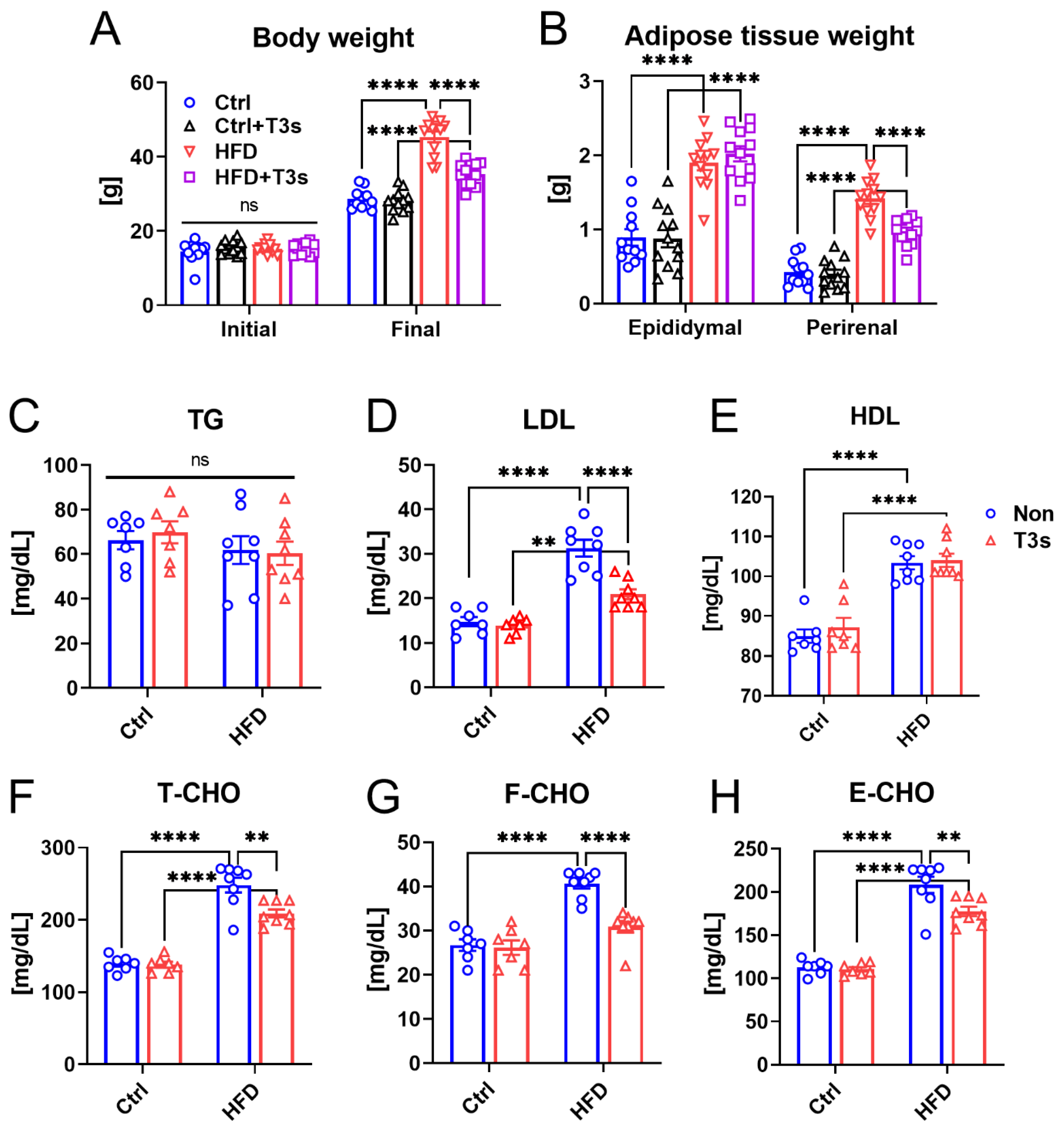
2.1. T3s Attenuated Obesity via Inhibition of Adipose Tissue Accumulation and Lipid Metabolism Dysfunction
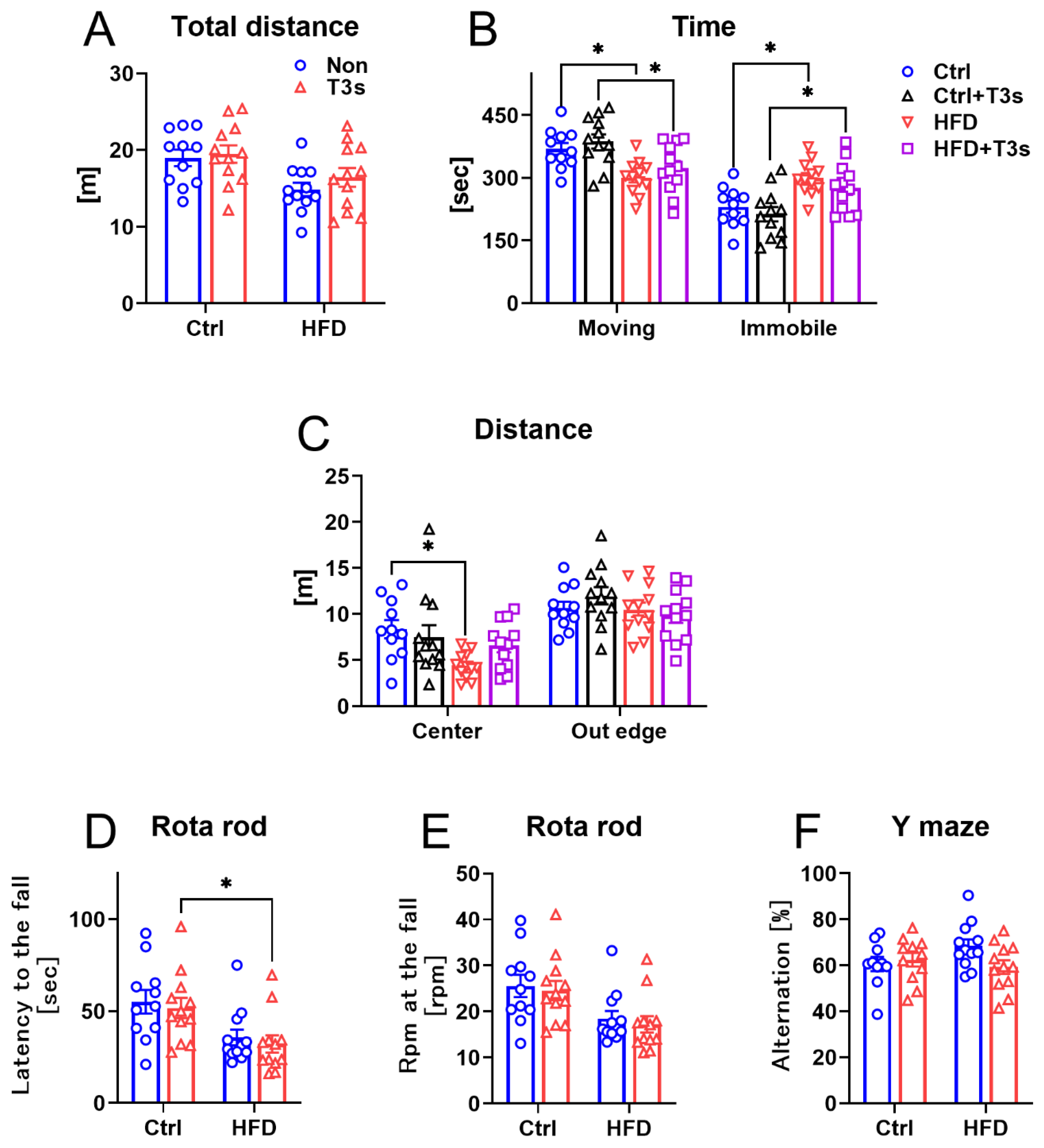
2.2. The Changes in Mice Behavior
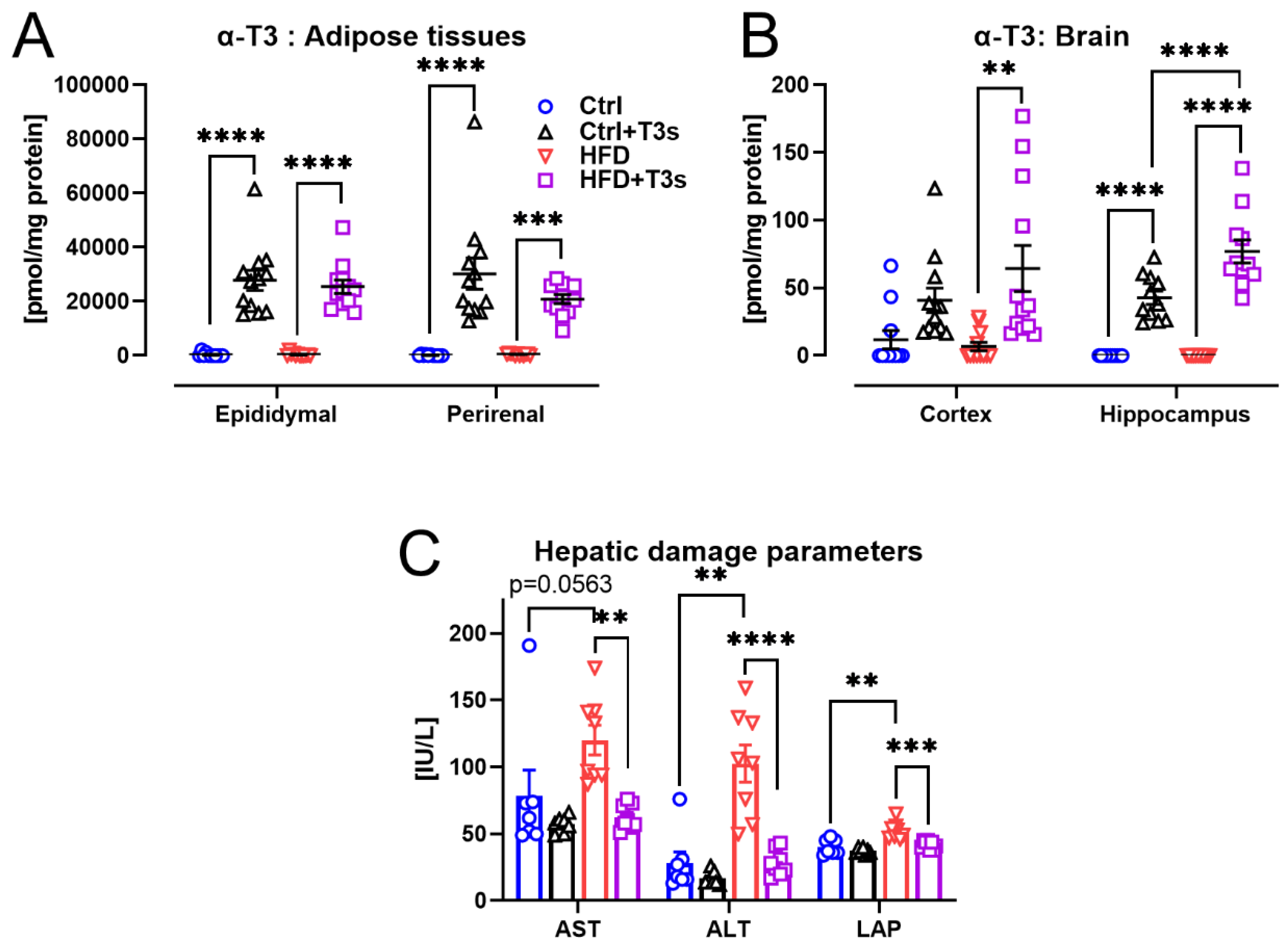
2.3. T3s Protected against HFD-Induced Hepatic Damage
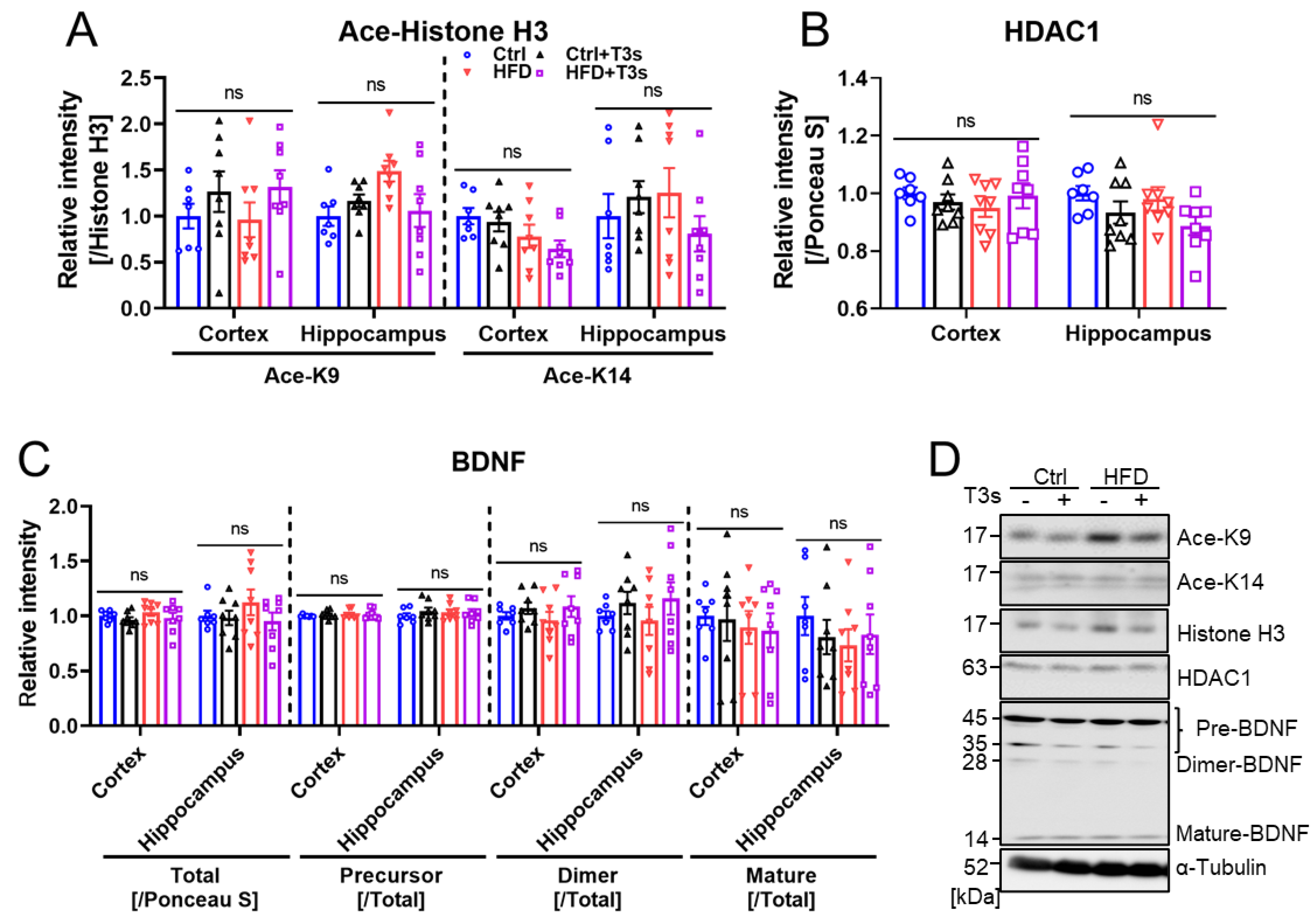
2.4. HFD and T3s Did Not Alter Brain Oxidation Levels
2.5. HFD and T3s Did Not Influence the Expression of BDNF
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Behavioral Assessment
4.2.1. Open Field Test
4.2.2. Rota Rod Test
4.2.3. Y-Maze Test
4.3. VE Measurement
4.4. Reduced (GSH) and Oxidized (GSSG) Glutathione Concentrations
4.5. Serum Parameters
4.6. Western Blotting
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- World Health Organization. Health Topics Obesity. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 8 November 2021).
- Prasenjit, M.; Sushi, J.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar]
- de Mello, A.H.; Costa, A.B.; Engel, J.D.G.; Rezin, G.T. Mitochondrial dysfunction in obesity. Life Sci. 2018, 192, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Letra, L.; Santana, I.; Seica, R. Obesity as a risk factor for Alzheimer’s disease: The role of adipocytokines. Mrtab. Brain Dis. 2014, 29, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, C.A.M.; Vaca, D.M.G.; Echeverria, V.; Gonzalez, J.; Barreto, G.E. Relationship between Obesity, Alzheimer’s Disease, and Parkinson’s Disease: An Astrocentric View. Mol. Neurobiol. 2017, 54, 7096–7115. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Zhang, X.; Chen, W.W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef] [Green Version]
- Fukui, K.; Takatsu, H.; Koike, T.; Urano, S. Hydrogen peroxide induces neurite degeneration: Prevention by tocotrienols. Free Radic. Res. 2011, 45, 681–691. [Google Scholar] [CrossRef]
- Kiyose, C.; Nishikawa, H.; Nagase, M.; Yachi, R.T.; Muto, C.T. δ-Tocopherol Slightly Accumulates in the Adipose Tissue of Mice. J. Oleo Sci. 2021, 70, 247–252. [Google Scholar] [CrossRef]
- Fukui, K.; Shirai, M.; Ninuma, T.; Kato, Y. Anti-Obesity Effects of Tocotrienols and Bran in High-Fat Diet-Treated Mice. Nutrients 2019, 11, 830. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Aoki, Y.; Fukui, K. Tocotrienols Influence Body Weight Gain and Brain Protein Expression in Long-Term High-Fat Diet-Treated Mice. Int. J. Mol. Sci. 2020, 21, 4533. [Google Scholar] [CrossRef]
- Kato, Y.; Uchiumi, H.; Usami, R.; Takatsu, H.; Aoki, Y.; Yanai, S.; Endo, S.; Fukui, K. Tocotrienols reach the brain and play roles in the attenuation of body weight gain and improvement of cognitive function in high-fat diet-treated mice. J. Clin. Biochem. Nutr. 2021, 69, 256–264. [Google Scholar] [CrossRef]
- Wu, S.J.; Huang, G.Y.; Ng, L.T. γ-Tocotrienol induced cell cycle arrest and apoptosis via activating the Bax-mediated mitochondrial and AMPK signaling pathways in 3T3-L1 adipocytes. Food Chem. Toxicol. 2013, 59, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.A.; Pearce, B.C.; Clark, R.W.; Gordon, D.A.; Wright, J.J. Tocotrienols regulate cholesterol production in mammalian cells by post-transcriptional suppression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J. Biol. Chem. 1993, 268, 11230–11238. [Google Scholar] [CrossRef]
- Tobert, J.A. Lovastatin and beyond: The history of the HMG-CoA reductase inhibitors. Nat. Rev. Drug Discov. 2003, 2, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Stine, J.E.; Guo, H.; Sheng, X.; Han, X.; Schointuch, M.N.; Gilliam, T.P.; Gehrig, P.A.; Zhou, C.; Jump, V.L.B. The HMG-CoA reductase inhibitor, simvastatin, exhibits anti-metastatic and anti-tumorigenic effects in ovarian cancer. Oncotarget 2016, 7, 946–960. [Google Scholar] [CrossRef]
- Bouitbir, J.; Sanvee, G.M.; Panajatovic, M.V.; Singh, F.; Krahenbuhl, S. Mechanisms of statin-associated skeletal muscle-associated symptoms. Pharmacol. Res. 2020, 154, 104201. [Google Scholar] [CrossRef]
- Lim, S.; Moon, M.; Oh, H.; Kim, H.G.; Kim, S.Y.; Oh, M.S. Ginger improves cognitive function via NGF-induced ERK/CREB activation in the hippocampus of the mouse. J. Nutr. Biochem. 2014, 25, 1058–1065. [Google Scholar] [CrossRef]
- Bekinschtein, P.; Cammarota, M.; Medina, J.H. BDNF and memory processing. Neuropharmacology 2014, 76, 677–683. [Google Scholar] [CrossRef]
- Ng, T.K.S.; Ho, C.S.H.; Tam, W.W.S.; Kua, E.H.; Ho, R.C.M. Decreased Serum Brain-Derived Neurotrophic Factor (BDNF) Levels in Patients with Alzheimer’s Disease (AD): A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 257. [Google Scholar] [CrossRef] [Green Version]
- Mews, P.; Donahue, G.; Drake, A.M.; Luczak, V.; Abel, T.; Berger, S.L. Acetyl-CoA synthetase regulates histone acetylation and hippocampal memory. Nature 2017, 546, 381–386. [Google Scholar] [CrossRef] [Green Version]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Piche, M.E.; Tchernof, A.; Despres, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.B.; Glass, D.S.; Voloshyna, I.; Glass, A.D.; Kasselman, L.J.; Leon, J.D. Obesity and atherosclerosis: The exosome link. Vessel Plus 2020, 4, 19. [Google Scholar] [CrossRef]
- Song, B.L.; Boyd, R.A.D. Insig-dependent ubiquitination and degradation of 3-hydroxy-3-methylglutaryl coenzyme a reductase stimulated by delta- and gamma-tocotrienols. J. Biol. Chem. 2006, 281, 25054–26061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rios, M.; Fan, G.; Fekete, C.; Kelly, J.; Bates, B.; Kuehn, R.; Lechan, R.M.; Jaenisch, R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol. Endocrinol. 2001, 10, 1748–1757. [Google Scholar] [CrossRef]
- Peng, S.; Li, W.; Lv, L.; Zhang, Z.; Zhan, X. BDNF as a biomarker in diagnosis and evaluation of treatment for schizophrenia and depression. Discov. Med. 2018, 26, 127–136. [Google Scholar]
- Chen, B.; Dowlatshahi, D.; MacQueen, G.M.; Wang, J.F.; Young, L.T. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol. Phychiatry 2001, 50, 260–265. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, J.; Hong, L.; Huang, W.; Dai, X.; Ye, Q.; Chen, X. Metformin ameliorates stress-induced depression-like behaviors via enhancing the expression of BDNF by activating AMPK/CREB-mediated histone acetylation. J. Affect. Disord. 2020, 260, 302–313. [Google Scholar] [CrossRef]
- Sleoman, S.F.; Henry, J.; Haddad, R.A.; Hayek, L.E.; Haidar, E.A.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. eLife 2016, 5, e15092. [Google Scholar] [CrossRef]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W.J.H. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2015, 51, 164–175. [Google Scholar] [CrossRef] [Green Version]
- Vavakova, M.; Durackova, Z.; Trebaticka, J. Markers of Oxidative Stress and Neuroprogression in Depression Disorder. Oxid. Med. Cell Longev. 2015, 2015, 898393. [Google Scholar] [CrossRef] [Green Version]
- Lindqvist, D.; Dhabhar, F.S.; James, S.J.; Hough, C.M.; Jain, F.A.; Bersani, F.S.; Reus, V.; Verhoeven, J.E.; Epel, E.S.; Mahan, L.; et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 2017, 76, 197–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohler, O.; Krogh, J.; Mors, O.; Benros, M.E. Inflammation in Depression and the Potential for Anti-Inflammatory Treatment. Curr. Neuropharmacol. 2016, 14, 732–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolarcayk, E. Adipose tissue inflammation in obesity: A metabolic or immune response? Curr. Opin. Pharmacol. 2017, 37, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef] [PubMed]
- Lach, H.C.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, N.; Xu, Y.; Tan, H.Y.; Li, S.; Feng, Y. Molecular Mechanisms Involved in Oxidative Stress-Associated Liver Injury Induced by Chinese Herbal Medicine: An Experimental Evidence-Based Literature Review and Network Pharmacology Study. Int. J. Mol. Sci. 2018, 19, 2745. [Google Scholar] [CrossRef] [Green Version]
- Mowla, S.J.; Farhadi, H.F.; Pareek, S.; Atwal, J.K.; Morris, S.J.; Seidah, N.G.; Murphy, R.A. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J. Biol. Chem. 2001, 276, 12660–12666. [Google Scholar] [CrossRef] [Green Version]
- Han, K.; Jia, N.; Li, J.; Yang, L.; Min, L.Q. Chronic caffeine treatment reverses memory impairment and the expression of brain BNDF and TrkB in the PS1/APP double transgenic mouse model of Alzheimer’s disease. Mol. Med. Rep. 2013, 8, 737–740. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Zhu, Y.; He, T.; Li, W.; Yan, H.; Miao, Y. Rolipram-induced improvement of cognitive function correlates with changes in hippocampal CREB phosphorylation, BDNF and Arc protein levels. Neurosci. Lett. 2016, 610, 171–176. [Google Scholar] [CrossRef]
- Bengoetxea, H.; Barrio, I.R.; Ortuzar, N.; Goyena, A.M.; Lafuente, J.V. Environmental Enrichment Reverses Tyrosine Kinase Inhibitor-Mediated Impairment Through BDNF-TrkB Pathway. Mol. Neurobiol. 2018, 55, 43–59. [Google Scholar] [CrossRef]
- Sasi, M.; Vignoli, B.; Canossa, M.; Blum, R. Neurobiology of local and intercellular BDNF signaling. Pflugers. Arch. 2017, 469, 593–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Guo, W.; Zhou, H.; Tang, L.; Feng, S.; Zhong, J.H.; Zhou, X.F. proBDNF inhibits the proliferation and migration of OLN-93 oligodendrocytes. Mol. Med. Rep. 2018, 18, 3809–3817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myzak, M.C.; Ho, E.; Dashwood, R.H. Dietary agents as histone deacetylase inhibitors. Mol. Carcinog. 2006, 45, 443–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dashwood, R.H.; Myzak, M.C.; Ho, E. Dietary HDAC inhibitors: Time to rethink weak ligands in cancer chemoprevention? Carcinogenesis 2006, 27, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K.; Kimura, S.; Kato, Y.; Kohno, M. Effects of far infrared light on Alzheimer’s disease-transgenic mice. PLoS ONE 2021, 16, e0253320. [Google Scholar] [CrossRef]
- Fukui, K.; Okihiro, S.; Ohfuchi, Y.; Hashimoto, M.; Kato, Y.; Yoshida, N.; Mochizuki, K.; Tsumoto, H.; Miura, Y. Proteomic study on neurite responses to oxidative stress: Search for differentially, expressed proteins in isolated neurites of N1E-115 cells. J. Clin. Biochem. Nutr. 2019, 64, 36–44. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, Y.; Aoki, Y.; Kiyose, C.; Fukui, K. Tocotrienols Attenuate White Adipose Tissue Accumulation and Improve Serum Cholesterol Concentration in High-Fat Diet-Treated Mice. Molecules 2022, 27, 2188. https://doi.org/10.3390/molecules27072188
Kato Y, Aoki Y, Kiyose C, Fukui K. Tocotrienols Attenuate White Adipose Tissue Accumulation and Improve Serum Cholesterol Concentration in High-Fat Diet-Treated Mice. Molecules. 2022; 27(7):2188. https://doi.org/10.3390/molecules27072188
Chicago/Turabian StyleKato, Yugo, Yoshinori Aoki, Chikako Kiyose, and Koji Fukui. 2022. "Tocotrienols Attenuate White Adipose Tissue Accumulation and Improve Serum Cholesterol Concentration in High-Fat Diet-Treated Mice" Molecules 27, no. 7: 2188. https://doi.org/10.3390/molecules27072188
APA StyleKato, Y., Aoki, Y., Kiyose, C., & Fukui, K. (2022). Tocotrienols Attenuate White Adipose Tissue Accumulation and Improve Serum Cholesterol Concentration in High-Fat Diet-Treated Mice. Molecules, 27(7), 2188. https://doi.org/10.3390/molecules27072188





