Preparation and Performance of Thickened Liquids for Patients with Konjac Glucomannan-Mediated Dysphagia
Abstract
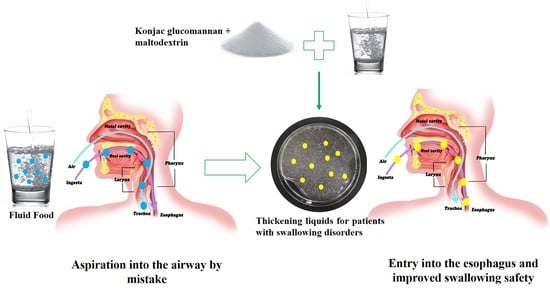
:1. Introduction
2. Results and Discussion
2.1. Performance Studies of KGM
2.1.1. Rheological Measurements
2.1.2. Swelling Property
2.2. Formulation Optimization of Thickening Component Based on KGM for Special Medical Use
2.2.1. Thickening Properties of Thickeners
2.2.2. Effect of Maltodextrin on Thickening Component Based on KGM for Special Medical Use
2.3. Process Optimization of Thickening Component Based on KGM for Special Medical Use
2.4. Performance Studies of Thickening Component Based on KGM for Special Medical Use
2.4.1. Rheological Properties
2.4.2. Particle Shape and Particle Size Distribution
2.4.3. Crystallization Performance
2.4.4. FT-IR Analysis
3. Materials and Methods
3.1. Materials
3.2. Performance Studies of KGM
3.2.1. Rheological Properties
3.2.2. Swelling Characteristics
3.3. Formulation Optimization of Thickening Component Based on KGM for Special Medical Use
3.3.1. Thickening Properties of Thickeners
3.3.2. Effect of Maltodextrin on Thickening Component Based on KGM for Special Medical Use
3.4. Process Optimization of Thickening Component Based on KGM for Special Medical Use
3.5. Performance of Thickening Component Based on KGM for Special Medical Use
3.5.1. Rheological Properties
3.5.2. Particle Shape and Particle Size Distribution Analysis
3.5.3. Crystallization Performance
3.5.4. FT-IR Analysis
3.6. Data Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kaiser, M.; Bandinelli, S.; Lunenfeld, B. Frailty and the role of nutrition in older people. A review of the current literature. Acta Bio-Med. Atenei Parm. 2010, 81, 37–45. [Google Scholar]
- Kikawada, M.; Iwamoto, T.; Takasaki, M. Aspiration and infection in the elderly: Epidemiology, diagnosis and management. Drugs Aging 2005, 22, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Cichero, J.A.Y.; Steele, C.; Duivestein, J.; Clavé, P.; Chen, J.; Kayashita, J. The Need for International Terminology and Definitions for Texture-Modified Foods and Thickened Liquids Used in Dysphagia Management: Foundations of a Global Initiative. Arch. Phys. Med. Rehabil. 2013, 1, 280–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Kuhn, A.; Medina, Y.; Pérez-García, M.; Martínez-Sola, M.; De Haro, P.; Flores, P. Neurorehabilitation treatment of dysphagia after-stroke with transcranial direct current stimulation: A clinical case. Brain Stimul. 2017, 10, 431. [Google Scholar] [CrossRef]
- Wei, Y.; Guo, Y.; Li, R.; Ma, A.; Zhang, H. Rheological characterization of polysaccharide thickeners oriented for dysphagia management: Carboxymethylated curdlan, konjac glucomannan and their mixtures compared to xanthan gum. Food Hydrocoll. 2021, 110, 106198. [Google Scholar] [CrossRef]
- Cichero, J.A.Y.; Lam, P. Thickened liquids for children and adults with oropharyngeal dysphagia: The complexity of rheological considerations. J. Gastroenterol. Hepatol. Res. 2014, 3, 1073–1079. [Google Scholar] [CrossRef]
- Garcia, J.M.; Chambers, E.T.; Molander, M. Thickened liquids: Practice patterns of speech-language pathologists. Am. J. Speech Lang. Pathol. 2005, 14, 4–13. [Google Scholar] [CrossRef]
- Clavé, P.; De Kraa, M.; Arreola, V.; Girvent, M.; Farré, R.; Palomera, E. The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Aliment. Pharmacol. Ther. 2006, 24, 1385–1394. [Google Scholar] [CrossRef]
- National Dysphagia Diet: Standardization for Optimal Care; American Dietetic Association: Chicago, IL, USA, 2002.
- Cichero, J.A.Y.; Lam, P.; Steele, C.M.; Hanson, B.; Chen, J.; Dantas, R.O. Development of international terminology and definitions for texture-modified foods and thickened fluids used in dysphagia management: The IDDSI framework. Dysphagia 2017, 32, 293–314. [Google Scholar] [CrossRef] [Green Version]
- Zargaraan, A.; Rastmanesh, R.; Fadavi, G.; Zayeri, F.; Mohammadifar, M.A. Rheological aspects of dysphagia-oriented food products: A mini review. Food Sci. Hum. Wellness 2013, 2, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Abu-Zarim, N.; Zainul-Abidin, S.; Ariffin, F. Rheological studies on the effect of different thickeners in texture-modified chicken rendang for individuals with dysphagia. Food Sci. Technol. 2018, 55, 4522–4529. [Google Scholar] [CrossRef] [PubMed]
- Moret-Tatay, A.; Rodríguez-García, J.; Martí-Bonmatí, E.; Hernando, I.; Hernándeza, M.J. Commercial thickeners used by patients with dysphagia: Rheological and structural behaviour in different food matrices. Food Hydrocoll. 2015, 51, 318–326. [Google Scholar] [CrossRef]
- Talens, P.; Castells, M.L.; Verdú, S.; Barata, J.M.; Graua, R. Flow, viscoelastic and masticatory properties of tailor made thickened pea cream for people with swallowing problems. J. Food Eng. 2021, 292, 110265. [Google Scholar] [CrossRef]
- Yang, X.; Gong, T.; Li, D.; Li, A.; Sun, L.; Guo, Y. Preparation of high viscoelastic emulsion gels based on the synergistic gelation mechanism of xanthan and konjac glucomannan. Carbohydr. Polym. 2019, 226, 115278. [Google Scholar] [CrossRef]
- Paradossi, G.; Chiessi, E.; Barbiroli, A.; Fessas, D. Xanthan and glucomannan mixtures: Synergistic interactions and gelation. Biomacrololecules 2002, 3, 498–504. [Google Scholar] [CrossRef]
- Yang, X.; Li, A.; Li, D.; Li, X.; Li, P.; Sun, L.; Guo, Y. Improved physical properties of konjac glucomannan gels by co-incubating composite konjac glucomannan/xanthan systems under alkaline conditions. Food Hydrocoll. 2020, 106, 105870. [Google Scholar] [CrossRef]
- Brenner, T.; Tuvikene, R.; Fang, Y.; Matsukawaa, S.; Nishinaribe, K. Rheology of highly elastic iota-carrageenan/kappa-carrageenan/xanthan/konjac glucomannan gels. Food Hydrocoll. 2015, 44, 136–144. [Google Scholar] [CrossRef]
- Hu, Y.; Tian, J.; Zou, J.; Yuan, X.; Li, J.; Liang, H.; Zhan, F.; Li, B. Partial removal of acetyl groups in konjac glucomannan significantly improved the rheological properties and texture of konjac glucomannan and κ-carrageenan blends. Int. J. Biol. Macromol. 2019, 123, 1165–1171. [Google Scholar] [CrossRef]
- Guo, J.; Liu, F.; Gan, C.; Wang, Y.; Wang, P.; Li, X.; Hao, J. Effects of Konjac glucomannan with different viscosities on the rheological and microstructural properties of dough and the performance of steamed bread. Food Chem. 2022, 368, 130853. [Google Scholar] [CrossRef]
- Charoenrein, S.; Tatirat, O.; Rengsutthi, K.; Thongngam, M. Effect of konjac glucomannan on syneresis, textural properties and the microstructure of frozen rice starch gels. Carbohydr. Polym. 2011, 83, 291–296. [Google Scholar] [CrossRef]
- Shahidi, F.; Han, X.Q. Encapsulation of food ingredients. Crit. Rev. Food Sci. Nutr. 1993, 33, 501–547. [Google Scholar] [CrossRef]
- Klinkesorn, U.; Sophanodora, P.; Chinachoti, P.; McClementsb, D.J. Stability and rheology of corn oil-in-water emulsions containing maltodextrin. Food Res. Int. 2004, 37, 851–859. [Google Scholar] [CrossRef]
- Dokic-Baucal, L.; Dokic, P.; Jakovljevic, J. Influence of different maltodextrins on properties of O/W emulsions. Food Hydrocoll. 2004, 18, 233–239. [Google Scholar] [CrossRef]
- Moore, G.R.P.; Canto, L.R.; Amante, E.R.; Soldi, V. Cassava and corn starch in maltodextrin production. Química Nova 2005, 28, 596–600. [Google Scholar] [CrossRef] [Green Version]
- Nickerson, M.T.; Paulson, A.T.; Wagar, E.; Farnworth, R.; Hodge, S.M.; Rousseau, D. Some physical properties of crosslinked gelatin–maltodextrin hydrogels. Food Hydrocoll. 2006, 20, 1072–1079. [Google Scholar] [CrossRef]
- Hill, E.K.; Yalin, W.; Dunstan, D.E. Direct measurement of polymer segment orientation and distortion in shear: Semi-dilute solution behavior of a conjugated system. AIP Conf. Proc. 2004, 708, 209–212. [Google Scholar] [CrossRef]
- Yang, X.H.; Zhu, W.L. Viscosity properties of sodium carboxymethylcellulose solutions. Cellulose 2007, 14, 409–417. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhang, W.; Peng, F.B.; Chen, H.; Shu, G.W. Effects and mechanisms of bacterial cellulose on glucose metabolism in an in vitro chyme model and its rheological evaluation. Int. J. Food Sci. Technol. 2021, 56, 6100–6112. [Google Scholar] [CrossRef]
- Gong, J.; Wang, L.; Wu, J.; Yuan, Y.; Mu, R.J.; Du, Y.; Wu, C.; Pang, J. The rheological and physicochemical properties of a novel thermosensitive hydrogel based on konjac glucomannan/gum tragacanth. LWT-Food Sci. Technol. 2019, 100, 271–277. [Google Scholar] [CrossRef]
- Stokes, J.R.; Xu, Y. Rheology of Food Materials: Impact on and Relevance in Food Processing. Ref. Modul. Food Sci. 2019, 1–9. [Google Scholar] [CrossRef]
- Wu, D.; Yu, S.; Liang, H.; He, C.; Li, J.; Li, B. The influence of deacetylation degree of konjac glucomannan on rheological and gel properties of konjac glucomannan/κ-carrageenan mixed system. Food Hydrocoll. 2020, 101, 105523. [Google Scholar] [CrossRef]
- Jian, W.; Siu, K.C.; Wu, J.Y. Effects of pH and temperature on colloidal properties and molecular characteristics of Konjac glucomannan. Carbohydr. Polym. 2015, 134, 285–292. [Google Scholar] [CrossRef]
- Koroskenyi, B.; Mccarthy, S.P. Synthesis of acetlylated konjac glucomannan and effect of degree of acetylation on water absorbency. Biomacromolecules 2001, 2, 824–826. [Google Scholar] [CrossRef] [PubMed]
- Yui, T.; Ogawa, K.; Sarko, A. Molecular and crystal structure of konjac glucomannan in the mannan II polymorphic form. Carbohydr. Res. 1992, 229, 41–55. [Google Scholar] [CrossRef]
- Kato, K.; Watanabe, T.; Matsuda, K. Studies on the Chemical Structure of Konjac Mannan: Part II. Isolation and Characterization of Oligosaccharides from the Enzymatic Hydrolyzate of the Mannan†. Agric. Biol. Chem. 1970, 34, 532–539. [Google Scholar] [CrossRef]
- Liu, F.; Luo, X.; Lin, X. Adsorption of tannin from aqueous solution by deacetylated konjac glucomannan. J. Hazard. Mater. 2010, 172, 844–850. [Google Scholar] [CrossRef]
- Bradley, T.D.; Ball, A.; Harding, S.E.; Mitchell, J.R. Thermal degradation of guar gum. Carbohydr. Polym. 1989, 10, 205–214. [Google Scholar] [CrossRef]
- Alves, M.M.; Antonov, Y.A.; Goncalves, M.P. Phase equilibria and mechanical properties of gel-like water–gelatin–locust bean gum systems. Int. J. Biol. Macromol. 2000, 21, 41–47. [Google Scholar] [CrossRef]
- The Chinese Dietary Guidelines; Chinese Nutrition Society: Beijing, China. 2016. Available online: http://dg.cnsoc.org/article/04/8a2389fd5520b4f30155be1475e02741.html (accessed on 10 January 2022).
- Qiao, L.; Li, Y.; Chi, Y.; Ji, Y.; Gao, Y.; Hwang, H.; Aker, W.G.; Wang, P. Rheological properties, gelling behavior and texture characteristics of polysaccharide from Enteromorpha prolifera. Carbohydr. Polym. 2016, 136, 1307–1314. [Google Scholar] [CrossRef]
- Eratte, D.; McKnight, S.; Gengenbach, T.R.; Dowling, K.; Barrow, C.J.; Adhikari, B.P. Co-encapsulation and characterisation of omega-3 fatty acids and probiotic bacteria in whey protein isolate–gum Arabic complex coacervates. J. Funct. Foods 2015, 19, 882–892. [Google Scholar] [CrossRef]
- Chang, X.L.; Feng, Y.M.; Wang, W.H. Comparison of the polysaccharides isolated from skin juice, gel juice and flower of Aloe arborescens tissues. J. Taiwan Inst. Chem. Eng. 2011, 42, 13–19. [Google Scholar] [CrossRef]
- Li, B.; Li, J.; Xia, J.; Kennedy, J.F.; Yie, X.; Liu, T.G. Effect of gamma irradiation on the condensed state structure and mechanical properties of konjac glucomannan/chitosan blend films. Carbohydr. Polym. 2011, 83, 44–51. [Google Scholar] [CrossRef]
- Oakenfull, D.; Naden, J.; Paterson, J. Solvent structure and the influence of anions on the gelation of κ-carrageenan and its synergistic interaction with locust bean gum. In Gums and Stabilisers for the Food Industry 10; Woodhead Publishing: Cambridge, UK, 2000; pp. 221–228. ISBN 978-1-85573-788-4. (In English) [Google Scholar]
- Rohindra, D.R.; Nand, A.V.; Khurma, J.R. Swelling properties of chitosan hydrogels. South Pac. J. Nat. Sci. 2004, 22, 32–35. [Google Scholar] [CrossRef]
- ISO 5492:2008; Sensory Analysis-Vocabulary. International Organization for Standardization (ISO): China, Beijing, 2008.
- Zhou, Y.; Cao, H.; Hou, M.; Nirasawa, S.; Tatsumi, E.; Foster, T.F.; Cheng, Y. Effect of konjac glucomannan on physical and sensory properties of noodles made from low-protein wheat flour. Food Res. Int. 2013, 51, 879–885. [Google Scholar] [CrossRef]
- Chai, Y.; Zhao, M. Purification, characterization and anti-proliferation activities of polysaccharides extracted from Viscum coloratum (Kom.) Nakai. Carbohydr. Polym. 2016, 149, 121–130. [Google Scholar] [CrossRef]


















| Sensory Indicators | 14–20 Points | 7–13 Points | 6 Points |
|---|---|---|---|
| Color (20 points) | Aggregate score | Normal color, no gloss | Uneven, impure color |
| Scent (20 points) | Unique smell of KGM | No strange smell | Other peculiar smell |
| Taste (20 points) | Delicate and smooth taste | Grainy taste | Rough taste with dry powder |
| Dissolvability (20 points) | Dissolve quickly and evenly | Slightly agglomerated, dissolve after stirring | More agglomerates, not easy to dissolve |
| Structural state (20 points) | Suitable viscosity without impurities | High viscosity with a small amount of impurities | Low viscosity with obvious impurities |
| Aggregate score | 100 points (full score) | ||
| Number | 1# | 2# | 3# |
|---|---|---|---|
| Mass fraction of compound thickener/% | 33.3 | 37.5 | 41.2 |
| Mass fraction of maltodextrin/% | 66.7 | 62.5 | 58.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Ren, X.; Zhang, L.; Chen, J. Preparation and Performance of Thickened Liquids for Patients with Konjac Glucomannan-Mediated Dysphagia. Molecules 2022, 27, 2194. https://doi.org/10.3390/molecules27072194
Zhang W, Ren X, Zhang L, Chen J. Preparation and Performance of Thickened Liquids for Patients with Konjac Glucomannan-Mediated Dysphagia. Molecules. 2022; 27(7):2194. https://doi.org/10.3390/molecules27072194
Chicago/Turabian StyleZhang, Wen, Xuening Ren, Lele Zhang, and Jianghu Chen. 2022. "Preparation and Performance of Thickened Liquids for Patients with Konjac Glucomannan-Mediated Dysphagia" Molecules 27, no. 7: 2194. https://doi.org/10.3390/molecules27072194
APA StyleZhang, W., Ren, X., Zhang, L., & Chen, J. (2022). Preparation and Performance of Thickened Liquids for Patients with Konjac Glucomannan-Mediated Dysphagia. Molecules, 27(7), 2194. https://doi.org/10.3390/molecules27072194