A Platform Approach to Protein Encapsulates with Controllable Surface Chemistry
Abstract
:1. Introduction
1.1. Core-Shell Particles for Protein Encapsulation
1.2. Evaporation-Induced Self Assembly (EISA) of Core Shell Particles by Spray Drying
1.3. EISA of Core Shell Protein Encapsulates by Spray Drying
1.4. Study Aims
2. Results and Discussion
2.1. System Design
2.2. Characterisation of Synthesised Nanoparticles
2.3. Characterisation of Functionalised Nanoparticles
2.4. Characterisation of Colloidal Feed Solution
2.5. Characterisation of Spray Dried Particles
2.5.1. General Morphology
2.5.2. Core-Shell Structure
2.6. Investigation of Predictive Parameters
3. Conclusions and Outlook
4. Materials and Methods
4.1. Nanoparticle (NP) Functionalisation
4.1.1. Seed-Growth Synthesis of Silica NP
4.1.2. Solvent Exchange of Ludox Silica
4.1.3. Aminopropyl Functionalised Silica NP
4.1.4. Octyl Functionalised Silica NP
4.2. Spray Drying
4.3. X-ray Photoelectron Spectroscopy
4.4. Elemental Analysis
4.5. Field Emission Gun Scanning Electron Microscopy
4.6. Dynamic Light Scattering and Zeta Potential
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Supplementary Information
Appendix A.1. Nanoparticle Excipient Characterisation
Appendix A.1.1. Commercial Nanoparticle Characterisation

Appendix A.1.2. Synthesised Nanoparticle Characterisation

Appendix A.1.3. Functionalised Nanoparticle Characterisation


Appendix A.2. Morphology of Spray Dried Particles





| Sample | Particle Size (m) | Shape | Structure |
|---|---|---|---|
| 5-0[small-OH] | 3.51 ± 1.92 | Corrugated spheres | Agglomerated structure |
| 5-0[med-OH] | 3.3 ± 1.82 | Corrugated spheres | Flaky agglomerated surface |
| 5-0[large-OH] | 3.51 ± 1.72 | Corrugated spheres | Smooth surface |
| 5-0[med-NH] | 3.61 ± 2.20 | Corrugated spheres | Flaky agglomerated surface |
| 4-0[med-NH2] | 1.93 ± 1.84 | Wrinkled spheres | Smooth surface |
| 7-0[med-NH] | 2.34 ± 1.81 | Corrugated spheres | Finely agglomerated surface |
| 5-1[med-NH] | 2.18 ± 1.74 | Corrugated spheres | Agglomerated and crystalline |
| 5-7[med-NH] | - | Film formulation | - |
| 5-1[med-OH] | 2.12 ± 1.89 | Corrugated spheres | Grainy agglomerated structure |
| 5-0[med-Octyl] | 2.21 ± 1.75 | Corrugated spheres | Smooth surface |
| 4-1[med-NH] | 2.91 ± 2.18 | Partially fused corrugated spheres | Cracked surface |
| 7-1[med-NH] | - | Partially fused spheres and lamellae | Agglomerated and crystalline |
| 4-1[med-OH] | - | Fused agglomerated spheres | Bulky agglomerated structure |
| 7-1[med-OH] | - | Partially fused spheres and lamellae | Agglomerated and crystalline |
| 5-0[med-OH] | 2.99 ± 2.73 | Corrugated spheres | Smooth surface |
| 5-0[-] | - | Film formulation | - |
| 4-0[-] | 1.91 ± 1.44 | Corrugated spheres | Smooth surface |
| 7-0[-] | 2.59 ± 2.35 | Corrugated spheres | Grainy agglomerated structure |
| 5-1[-] | - | Fused crystalline spheres | Flaky crystalline surface |
| 4-1[-] | - | Fused agglomerated spheres | Bulky agglomerated structure |
| 7-1[-] | - | Fused agglomerated spheres | Bulky agglomerated structure |
| 5-0[med-OH] | 2.46 ± 2.23 | Corrugated spheres | Smooth surface |
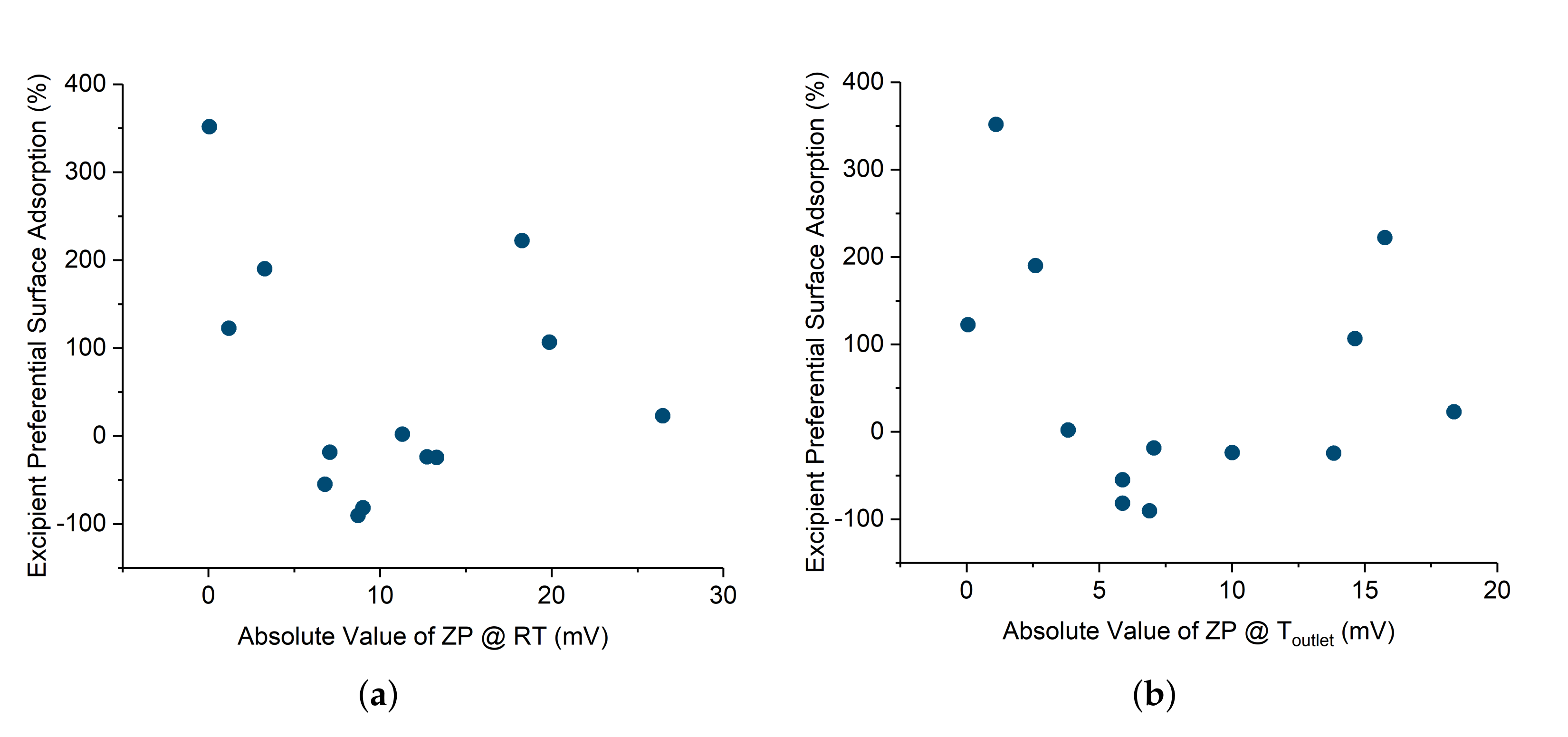
Appendix A.3. Zeta Potential/Preferential Adsorption Correlations

References
- Sou, T.; Kaminskas, L.M.; Nguyen, T.H.; Carlberg, R.; McIntosh, M.P.; Morton, D.A.V. The effect of amino acid excipients on morphology and solid-state properties of multi-component spray-dried formulations for pulmonary delivery of biomacromolecules. Eur. J. Pharm. Biopharm. 2013, 83, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Suihko, E.J.; Forbes, R.T.; Apperley, D.C. A solid-state NMR study of molecular mobility and phase separation in co-spray-dried protein—Sugar particles. Eur. J. Pharm. Sci. 2005, 25, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.D.; Morr, C.V. Microencapsulation Properties of Gum Arabic and Several Food Proteins: Spray-Dried Orange Oil Emulsion Particles. J. Agric. Food Chem. 1996, 44, 1314–1320. [Google Scholar] [CrossRef]
- Tan, S.; Zhong, C.; Langrish, T. Microencapsulation of pepsin in the spray-dried WPI (whey protein isolates) matrices for controlled release. J. Food Eng. 2019, 263, 147–154. [Google Scholar] [CrossRef]
- Sinsuebpol, C.; Chatchawalsaisin, J.; Kulvanich, P. Preparation and in vivo absorption evaluation of spray dried powders containing salmon calcitonin loaded chitosan nanoparticles for pulmonary delivery. Drug Des. Dev. Ther. 2013, 7, 861–873. [Google Scholar] [CrossRef] [Green Version]
- Angkawinitwong, U.; Sharma, G.; Khaw, P.T.; Brocchini, S.; Williams, G.R. Solid-state protein formulations. Ther. Deliv. 2015, 6, 59–82. [Google Scholar] [CrossRef]
- Zamani, M.; Prabhakaran, M.P.; Thian, E.S.; Ramakrishna, S. Protein encapsulated core–shell structured particles prepared by coaxial electrospraying: Investigation on material and processing variables. Int. J. Pharm. 2014, 473, 134–143. [Google Scholar] [CrossRef]
- Both, E.M. Powder Morphology Development during Spray Drying. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Liu, W.; Wu, W.D.; Selomulya, C.; Chen, X.D. Facile spray-drying assembly of uniform microencapsulates with tunable core-shell structures and controlled release properties. Langmuir 2011, 27, 12910–12915. [Google Scholar] [CrossRef]
- Miller, C.C. The Stokes-Einstein Law for Diffusion in Solution. Proc. R. Soc. Lond. Ser. A Contain. Pap. Math. Phys. Character 1924, 106, 724–749. [Google Scholar]
- Sen, D.; Mazumder, S.; Melo, J.S.; Khan, A.; Bhattyacharya, S.; D’Souza, S.F. Evaporation Driven Self-Assembly of a Colloidal Dispersion during Spray Drying: Volume Fraction Dependent Morphological Transition. Langmuir 2009, 25, 6690–6695. [Google Scholar] [CrossRef]
- Sen, D.; Melo, J.S.; Bahadur, J.; Mazumder, S.; Bhattacharya, S.; Ghosh, G.; Dutta, D.; D’Souza, S.F. Buckling-driven morphological transformation of droplets of a mixed colloidal suspension during evaporation-induced self-assembly by spray drying. Eur. Phys. J. E Soft Matter 2010, 31, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.D.; Liu, W.; Selomulya, C.; Chen, X.D. On spray drying of uniform silica-based microencapsulates for controlled release. Soft Matter 2011, 7, 11416. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Mei, F.; Bai, M.Y.; Zhao, S.; Chen, D.R. Release profile characteristics of biodegradable-polymer-coated drug particles fabricated by dual-capillary electrospray. J. Control. Release 2010, 145, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Sen, D.; Mazumder, S.; Ghosh, A.K.; Basak, C.B.; Dasgupta, K. Formation of nano-structured core–shell micro-granules by evaporation induced assembly. RSC Adv. 2015, 5, 85052–85060. [Google Scholar] [CrossRef]
- Tian, Y.; Fu, N.; Wu, W.D.; Zhu, D.; Huang, J.; Yun, S.; Chen, X.D. Effects of Co-spray Drying of Surfactants with High Solids Milk on Milk Powder Wettability. Food Bioprocess Technol. 2014, 7, 3121–3135. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Zhou, X.; Yu, J.; Wang, F.; Wang, J. Effects of glutaminase deamidation on the structure and solubility of rice glutelin. LWT Food Sci. Technol. 2011, 44, 2205–2210. [Google Scholar] [CrossRef]
- Akbarbaglu, Z.; Peighambardoust, S.H.; Sarabandi, K.; Jafari, S.M. Spray drying encapsulation of bioactive compounds within protein-based carriers; different options and applications. Food Chem. 2021, 359, 129965. [Google Scholar] [CrossRef]
- Pinto, J.T.; Faulhammer, E.; Dieplinger, J.; Dekner, M.; Makert, C.; Nieder, M.; Paudel, A. Progress in spray-drying of protein pharmaceuticals: Literature analysis of trends in formulation and process attributes. Dry. Technol. 2021, 39, 1415–1446. [Google Scholar] [CrossRef]
- Yoshii, H.; Buche, F.; Takeuchi, N.; Terrol, C.; Ohgawara, M.; Furuta, T. Effects of protein on retention of ADH enzyme activity encapsulated in trehalose matrices by spray drying. J. Food Eng. 2008, 87, 34–39. [Google Scholar] [CrossRef]
- Landström, K.; Alsins, J.; Bergenståhl, B. Competitive protein adsorption between bovine serum albumin and β-lactoglobulin during spray-drying. Food Hydrocoll. 2000, 14, 75–82. [Google Scholar] [CrossRef]
- Codrons, V.; Vanderbist, F.; Verbeeck, R.K.; Arras, M.; Lison, D.; Préat, V.; Vanbever, R. Systemic delivery of parathyroid hormone (1-34) using inhalation dry powders in rats. J. Pharm. Sci. 2003, 92, 938–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grubbs, F.E. Sample Criteria for Testing Outlying Observations. Ann. Math. Stat. 1950, 21, 27–58. [Google Scholar] [CrossRef]
- Rosen, J.E.; Gu, F.X. Surface Functionalization of Silica Nanoparticles with Cysteine: A Low-Fouling Zwitterionic Surface. Langmuir 2011, 27, 10507–10513. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Formulation Name | pH | [CaCl] (% w/v) | Excipient Inclusion (±) | Excipient Size | Surface Functional Group | [Total Dissolved Solids] (%w/w) | [Excipeint]/ ([Protein] + [Excipient]) (%w/w) |
|---|---|---|---|---|---|---|---|
| 5-0[small-OH] | 5.50 | 0.00 | + | small | OH | 3 | 20 |
| 5-0[med-OH] | 5.50 | 0.00 | + | med | OH | 3 | 20 |
| 5-0[large-OH] | 5.50 | 0.00 | + | large | OH | 3 | 20 |
| 5-0[med-NH] | 5.50 | 0.00 | + | med | (CH)NH | 3 | 20 |
| 4-0[med-NH] | 4.00 | 0.00 | + | med | (CH)NH | 3 | 20 |
| 7-0[med-NH] | 7.00 | 0.00 | + | med | (CH)NH | 3 | 20 |
| 5-1[med-NH] | 5.50 | 1.50 | + | med | (CH)NH | 3 | 20 |
| 5-1[med-NH] | 5.50 | 7.40 | + | med | (CH)NH | 3 | 20 |
| 5-1[med-OH] | 5.50 | 1.50 | + | med | OH | 3 | 20 |
| 5-0[med-Octyl] | 5.50 | 0.00 | + | med | CH(CH)CH | 3 | 20 |
| 4-1[med-NH] | 4.00 | 1.50 | + | med | (CH)NH | 3 | 20 |
| 7-1[med-NH] | 7.00 | 1.50 | + | med | (CH)NH | 3 | 20 |
| 4-1[med-OH] | 4.00 | 1.50 | + | med | OH | 3 | 20 |
| 7-1[med-OH] | 7.00 | 1.50 | + | med | OH | 3 | 20 |
| 5-0[med-OH] | 5.50 | 0.00 | + | med | OH | 15 | 20 |
| 5-0[-] | 5.50 | 0.00 | - | - | - | 3 | 0 |
| 4-0[-] | 4.00 | 0.00 | - | - | - | 3 | 0 |
| 7-0[-] | 7.00 | 0.00 | - | - | - | 3 | 0 |
| 5-1[-] | 5.50 | 1.50 | - | - | - | 3 | 0 |
| 4-1[-] | 4.00 | 1.50 | - | - | - | 3 | 0 |
| 7-1[-] | 7.00 | 1.50 | - | - | - | 3 | 0 |
| 5-0[med-OH] | 5.50 | 0.00 | + | med | OH | 3 | 50 |
| Formulation | D @ RT (nm) | D @ Toutlet (nm) | @ RT (mV) | @ Toutlet (mV) |
|---|---|---|---|---|
| 5-0[small-OH] | 52 | 98 | −9 | −6 |
| 5-0[med-OH] | 40 | 73 | −8 | −7 |
| 5-0[large-OH] | 128 | 225 | −13 | −10 |
| 5-0[med-NH] | 2312 | 1316 | 20 | 15 |
| 4-0[med-NH] | 1492 | 902 | 11 | 4 |
| 7-0[med-NH] | 1816 | 1026 | 3 | 3 |
| 5-1[med-NH] | 1958 | 949 | 26 | 18 |
| 5-7[med-NH] | 1988 | 936 | 18 | 16 |
| 5-1[med-OH] | 39 | 71 | −7 | −6 |
| 5-0[med-Octyl] | 111 | 178 | −7 | −7 |
| 4-1[med-NH] | 1101 | 906 | 13 | 14 |
| 7-1[med-NH] | ppt | ppt | 0 | 1 |
| 4-1[med-OH] | ppt | ppt | −13 | 0 |
| 7-1[med-OH] | ppt | ppt | 1 | 0 |
| Formulation | Overall % Si | Overall % S | Surface % Si | Surface % S | % Preferential Surface Adsorption (Si) | % Preferential Surface Adsorption (S) |
|---|---|---|---|---|---|---|
| 5-0[small-OH] | 3.60 | 0.24 | 0.66 | 0.00 | −82 | −100 |
| 5-0[med-OH] | 9.55 | 0.23 | 0.91 | 0.39 | −90 | 67 |
| 5-0[large-OH] | 5.80 | 0.37 | 4.42 | 0.47 | −24 | 26 |
| 5-0[med-NH] | 0.77 | 0.30 | 1.60 | 0.21 | 107 | −31 |
| 4-0[med-NH] | 2.04 | 0.28 | 2.08 | 0.24 | 2 | −14 |
| 7-0[med-NH] | 0.85 | 0.27 | 2.47 | 1.84 | 190 | 580 |
| 5-1[med-NH] | 0.96 | 0.43 | 1.18 | 0.32 | 23 | −26 |
| 5-7[med-NH] | 0.40 | 0.55 | 1.28 | 0.20 | 222 | −63 |
| 5-1[med-OH] | 2.36 | 0.43 | 1.06 | 0.31 | −55 | −28 |
| 5-0[med-Octyl] | 5.32 | 0.45 | 4.33 | 1.78 | −19 | 298 |
| 4-1[med-NH] | 0.94 | 0.46 | 0.71 | 0.21 | −25 | −55 |
| 7-1[med-NH] | 0.67 | 0.38 | 3.04 | 0.00 | 352 | −100 |
| 4-1[med-OH] | 0.06 | 0.43 | 0.68 | 0.20 | 944 | −53 |
| 7-1[med-OH] | 2.25 | 0.38 | 5.00 | 0.18 | 123 | −53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warner, N.; Osojnik Črnivec, I.G.; Rana, V.K.; Cruz, M.; Scherman, O.A. A Platform Approach to Protein Encapsulates with Controllable Surface Chemistry. Molecules 2022, 27, 2197. https://doi.org/10.3390/molecules27072197
Warner N, Osojnik Črnivec IG, Rana VK, Cruz M, Scherman OA. A Platform Approach to Protein Encapsulates with Controllable Surface Chemistry. Molecules. 2022; 27(7):2197. https://doi.org/10.3390/molecules27072197
Chicago/Turabian StyleWarner, Nina, Ilja Gasan Osojnik Črnivec, Vijay Kumar Rana, Menandro Cruz, and Oren A. Scherman. 2022. "A Platform Approach to Protein Encapsulates with Controllable Surface Chemistry" Molecules 27, no. 7: 2197. https://doi.org/10.3390/molecules27072197
APA StyleWarner, N., Osojnik Črnivec, I. G., Rana, V. K., Cruz, M., & Scherman, O. A. (2022). A Platform Approach to Protein Encapsulates with Controllable Surface Chemistry. Molecules, 27(7), 2197. https://doi.org/10.3390/molecules27072197






