Can the SARS-CoV-2 Omicron Variant Confer Natural Immunity against COVID-19?
Abstract
:1. Introduction
2. SARS-CoV-2 Mutation Results in the Emergence of Various Variants
3. Concepts of Immunity
3.1. Innate and Adaptive Immunity
3.2. Herd Immunity
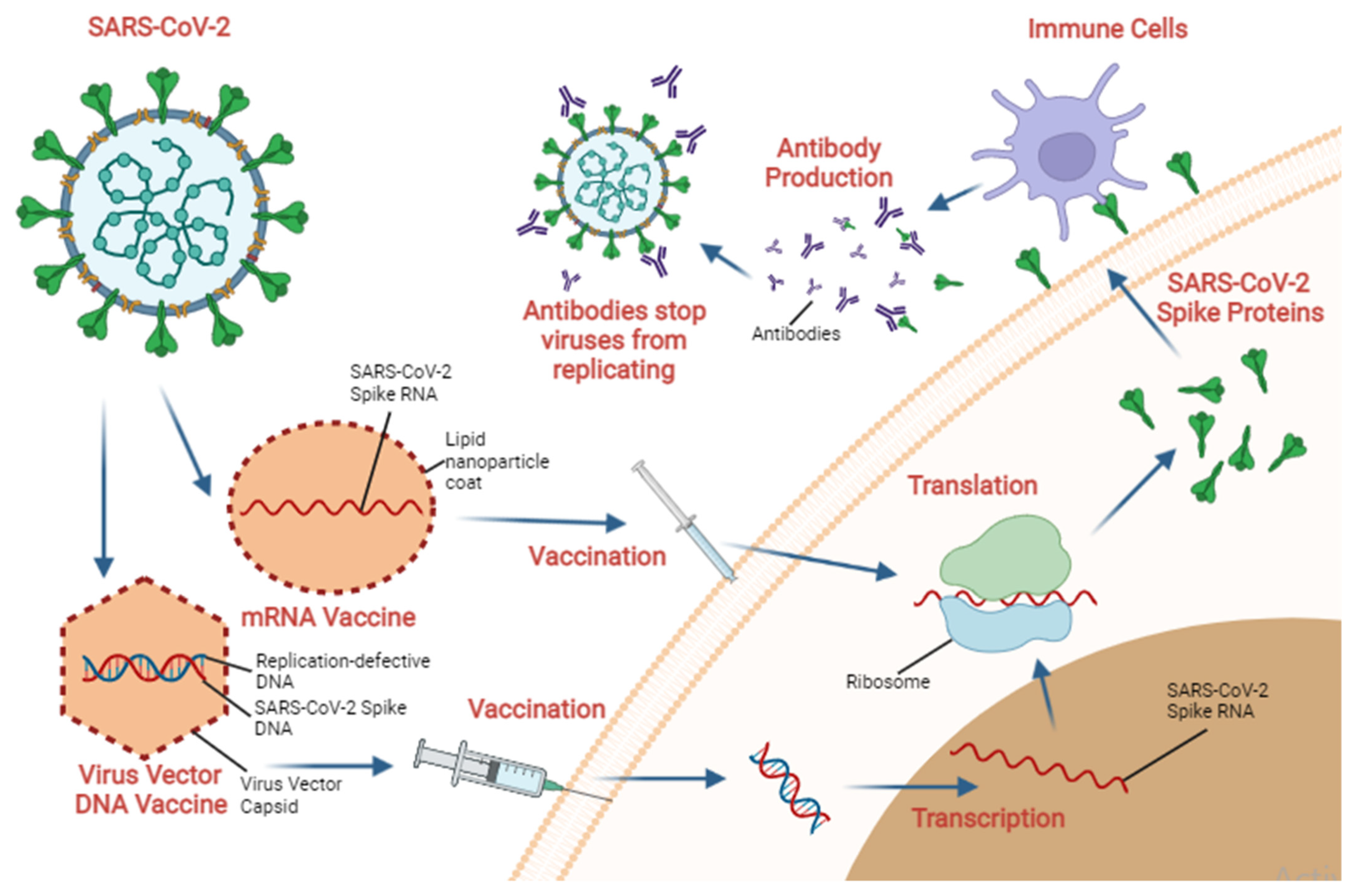
4. Vaccination
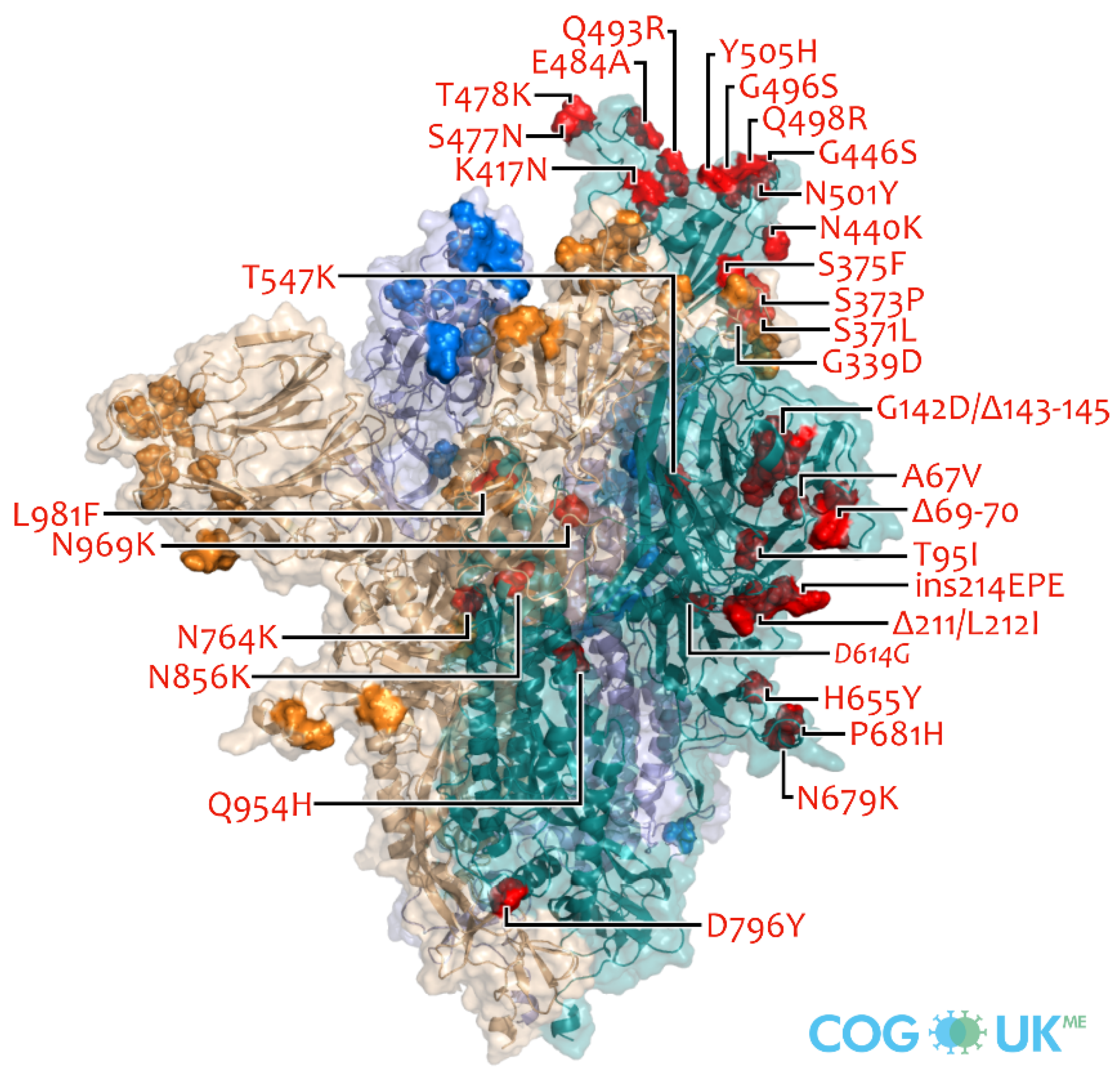
5. Omicron Variant and Its Characteristics
5.1. Omicron Variant and Its Characteristics
5.2. The Rationale for Presuming That Omicron Confers Natural Immunity
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pal, M.; Berhanu, G.; Desalegn, C.; Kandi, V. Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): An Update. Cureus 2020, 12, e7423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 infection: Emergence, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020, 24, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Sundar, V.; Bhaskar, E. Low secondary transmission rates of SARS-CoV-2 infection among contacts of construction laborers at open air environment. Germs 2021, 11, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- V’Kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Duffy, S. Why are RNA virus mutation rates so damn high? PLoS Biol. 2018, 16, e3000003. [Google Scholar] [CrossRef] [Green Version]
- Lauring, A.S.; Hodcroft, E.B. Genetic Variants of SARS-CoV-2—What Do They Mean? JAMA 2021, 325, 529–531. [Google Scholar] [CrossRef]
- Tasakis, R.N.; Samaras, G.; Jamison, A.; Lee, M.; Paulus, A.; Whitehouse, G.; Verkoczy, L.; Papavasiliou, F.N.; Diaz, M. SARS-CoV-2 variant evolution in the United States: High accumulation of viral mutations over time likely through serial Founder Events and mutational bursts. PLoS ONE 2021, 16, e0255169. [Google Scholar] [CrossRef]
- Peck, K.M.; Lauring, A.S. Complexities of Viral Mutation Rates. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [Green Version]
- Jamil, S.; Shafazand, S.; Pasnick, S.; Carlos, W.G.; Maves, R.; Cruz, C.D. Genetic Variants of SARS-CoV-2: What Do We Know So Far? Am. J. Respir. Crit. Care Med. 2021, 203, P30–P32. [Google Scholar] [CrossRef]
- Karim, S.S.A.; Karim, Q.A. Omicron SARS-CoV-2 variant: A new chapter in the COVID-19 pandemic. Lancet 2021, 398, 2126–2128. [Google Scholar] [CrossRef]
- Cameroni, E.; Bowen, J.E.; Rosen, L.E.; Saliba, C.; Zepeda, S.K.; Culap, K.; Pinto, D.; VanBlargan, L.A.; De Marco, A.; di Iulio, J.; et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature 2021, 602, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, R.K.; Chen, I.P.; Ma, T.; Syed, A.M.; Brazer, N.; Saldhi, P.; Simoneau, C.R.; Ciling, A.; Khalid, M.M.; Sreekumar, B.; et al. Limited cross-variant immunity after infection with the SARS-CoV-2 Omicron variant without vaccination. medRxiv 2022. [Google Scholar] [CrossRef]
- Madhi, A.S.; Ihekweazu, C.; Rees, H.; Pollard, A.J. Decoupling of omicron variant infections and severe COVID-19. Lancet 2022, 399, 1047–1048. [Google Scholar] [CrossRef]
- Celik, I.; Yadav, R.; Duzgun, Z.; Albogami, S.; El-Shehawi, A.M.; Fatimawali, F.; Idroes, R.; Tallei, T.E.; Bin Emran, T. Interactions of the Receptor Binding Domain of SARS-CoV-2 Variants with hACE2: Insights from Molecular Docking Analysis and Molecular Dynamic Simulation. Biology 2021, 10, 880. [Google Scholar] [CrossRef] [PubMed]
- Tallei, T.E.; Fatimawali; Adam, A.A.; Elseehy, M.M.; El-Shehawi, A.M.; Mahmoud, E.A.; Tania, A.D.; Niode, N.J.; Kusumawaty, D.; Rahimah, S.; et al. Fruit Bromelain-Derived Peptide Potentially Restrains the Attachment of SARS-CoV-2 Variants to hACE2: A Pharmacoinformatics Approach. Molecules 2022, 27, 260. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Govindarajulu, M.; Parise, R.S.; Neel, L.; Shankar, T.; Patel, S.; Lowery, P.; Smith, F.; Dhanasekaran, M.; Moore, T. Emerging SARS-CoV-2 Variants: A Review of Its Mutations, Its Implications and Vaccine Efficacy. Vaccines 2021, 9, 1195. [Google Scholar] [CrossRef]
- Rathinasamy, M.; Kandhasamy, S. An exploratory study on the propagation of SARS-CoV-2 variants: Omicron is the most predominant variant. J. Med Virol. 2022, 2022, 1–8. [Google Scholar] [CrossRef]
- Dubey, A.; Choudhary, S.; Kumar, P.; Tomar, S. Emerging SARS-CoV-2 Variants: Genetic Variability and Clinical Implications. Curr. Microbiol. 2021, 79, 20. [Google Scholar] [CrossRef]
- Viana, R.; Moyo, S.; Amoako, D.G.; Tegally, H.; Scheepers, C.; Althaus, C.L.; Anyaneji, U.J.; Bester, P.A.; Boni, M.F.; Chand, M.; et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 2022, 1–10. [Google Scholar] [CrossRef]
- Desingu, P.A.; Nagarajan, K.; Dhama, K. Emergence of Omicron third lineage BA.3 and its importance. J. Med Virol. 2022, 94, 1808–1810. [Google Scholar] [CrossRef] [PubMed]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.G.; et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2021, 602, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.; Muecksch, F.; Weisblum, Y.; Da Silva, J.; Bednarski, E.; Cho, A.; Wang, Z.; Gaebler, C.; Caskey, M.; Nussenzweig, M.C.; et al. Plasma neutralization properties of the SARS-CoV-2 Omicron variant. medRxiv 2021. [Google Scholar] [CrossRef]
- Liu, L.; Iketani, S.; Guo, Y.; Chan, J.F.-W.; Wang, M.; Liu, L.; Luo, Y.; Chu, H.; Huang, Y.; Nair, M.S.; et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature 2022, 602, 676–681. [Google Scholar] [CrossRef]
- Takashita, E.; Kinoshita, N.; Yamayoshi, S.; Sakai-Tagawa, Y.; Fujisaki, S.; Ito, M.; Iwatsuki-Horimoto, K.; Halfmann, P.; Watanabe, S.; Maeda, K.; et al. Efficacy of Antiviral Agents against the SARS-CoV-2 Omicron Subvariant BA.2. N. Engl. J. Med. 2022, 2022, NEJMc2201933. [Google Scholar] [CrossRef]
- Chen, J.; Wei, G.-W. Omicron BA.2 (B.1.1.529.2): High potential to becoming the next dominating variant. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Gad, S.C. Diesel Fuel, 2nd ed.; Wexler, P., Ed.; Elsevier: New York, NY, USA, 2005; pp. 665–698. ISBN 978-0-12-369400-3. [Google Scholar]
- Chowdhury, M.A.; Hossain, N.; Kashem, M.A.; Shahid, M.A.; Alam, A. Immune response in COVID-19: A review. J. Infect. Public Health 2020, 13, 1619–1629. [Google Scholar] [CrossRef]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef] [Green Version]
- Randolph, H.E.; Barreiro, L.B. Herd Immunity: Understanding COVID-19. Immunity 2020, 52, 737–741. [Google Scholar] [CrossRef]
- Aristizábal, B.; González, Á. Innate Immune System. In Autoimmunity: From Bench to Bedside; Anaya, J.M., Shoenfeld, Y., Rojas-Villarraga, A., Levy, R.A., Cervera, R., Eds.; El Rosario University Press: Bogota, Columbia, 2013. [Google Scholar]
- Devnath, P.; Hossain, M.J.; Emran, T.B.; Mitra, S. Massive third wave of COVID-19 outbreak in Bangladesh: A co-epidemic of dengue might worsen the situation. Future Virol. 2022, 2022, 1–4. [Google Scholar] [CrossRef]
- Turvey, S.E.; Broide, D.H. Innate immunity. J. Allergy Clin. Immunol. 2010, 125, S24–S32. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Titanium Alloys. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; Volume 5, pp. 213–224. ISBN 9780128012383. [Google Scholar]
- Clem, A.S. Fundamentals of vaccine immunology. J. Glob. Infect. Dis. 2011, 3, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Baxter, D. Active and passive immunity, vaccine types, excipients and licensing. Occup. Med. 2007, 57, 552–556. [Google Scholar] [CrossRef] [Green Version]
- Roth, K.D.R.; Wenzel, E.V.; Ruschig, M.; Steinke, S.; Langreder, N.; Heine, P.A.; Schneider, K.-T.; Ballmann, R.; Fühner, V.; Kuhn, P.; et al. Developing Recombinant Antibodies by Phage Display Against Infectious Diseases and Toxins for Diagnostics and Therapy. Front. Cell. Infect. Microbiol. 2021, 11, 697876. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Pulendran, B.; Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 2011, 12, 509–517. [Google Scholar] [CrossRef]
- Huber, S.E.; Beek, J.E.; de Jonge, J.; Eluytjes, W.; Baarle, D.E. T Cell Responses to Viral Infections–Opportunities for Peptide Vaccination. Front. Immunol. 2014, 5, 171. [Google Scholar] [CrossRef]
- Schmidt, M.E.; Varga, S.M. The CD8 T Cell Response to Respiratory Virus Infections. Front. Immunol. 2018, 9, 678. [Google Scholar] [CrossRef] [Green Version]
- Chandran, S.S.; Klebanoff, C.A. T cell receptor-based cancer immunotherapy: Emerging efficacy and pathways of resistance. Immunol. Rev. 2019, 290, 127–147. [Google Scholar] [CrossRef]
- Kim, C.; Williams, M.A. Nature and nurture: T-cell receptor-dependent and T-cell receptor-independent differentiation cues in the selection of the memory T-cell pool. Immunology 2010, 131, 310–317. [Google Scholar] [CrossRef]
- Whitmire, J.; Eam, B.; Whitton, J.L. Tentative T Cells: Memory Cells Are Quick to Respond, but Slow to Divide. PLoS Pathog. 2008, 4, e1000041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swadling, L.; Diniz, M.O.; Schmidt, N.M.; Amin, O.E.; Chandran, A.; Shaw, E.; Pade, C.; Gibbons, J.M.; Le Bert, N.; Tan, A.T.; et al. Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature 2022, 601, 110–117. [Google Scholar] [CrossRef]
- Louten, J. (Ed.) Chapter 8–Vaccines, Antivirals, and the Beneficial Uses of Viruses; Academic Press: Boston, MA, USA, 2016; pp. 133–154. ISBN 978-0-12-800947-5. [Google Scholar]
- Narayanaswamy, V.; Pentecost, B.T.; Schoen, C.N.; Alfandari, D.; Schneider, S.S.; Baker, R.; Arcaro, K.F. Neutralizing Antibodies and Cytokines in Breast Milk After Coronavirus Disease 2019 (COVID-19) mRNA Vaccination. Obstet. Gynecol. 2022, 139, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Lu, L.; Wu, H.; Shaman, J.; Cao, Y.; Fang, F.; Yang, Q.; He, Q.; Yang, Z.; Wang, M. Placental antibody transfer efficiency and maternal levels: Specific for measles, coxsackievirus A16, enterovirus 71, poliomyelitis I-III and HIV-1 antibodies. Sci. Rep. 2016, 6, 38874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tharmalingam, T.; Han, X.; Wozniak, A.; Saward, L. Polyclonal hyper immunoglobulin: A proven treatment and prophylaxis platform for passive immunization to address existing and emerging diseases. Hum. Vaccines Immunother. 2021, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.N.; Majumder, M.S. What Is Herd Immunity? JAMA J. Am. Med. Assoc. 2020, 324, 2113. [Google Scholar] [CrossRef] [PubMed]
- Delamater, P.L.; Street, E.J.; Leslie, T.F.; Yang, Y.T.; Jacobsen, K.H. Complexity of the Basic Reproduction Number (R0). Emerg. Infect. Dis. 2019, 25, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Mousazadeh, M.; Naghdali, Z.; Rahimian, N.; Hashemi, M.; Paital, B.; Al-Qodah, Z.; Mukhtar, A.; Karri, R.R.; Mahmoud, A.E.D.; Sillanpää, M.; et al. Chapter 9–Management of Environmental Health to Prevent an Outbreak of COVID-19: A Review; Hadi, D.M., Karri, R.R., Roy, S.B.T.-E., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 235–267. ISBN 978-0-323-85780-2. [Google Scholar]
- Plans-Rubió, P. Evaluation of the establishment of herd immunity in the population by means of serological surveys and vaccination coverage. Hum. Vaccines Immunother. 2012, 8, 184–188. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.; Plotkin, S.A. Impact of Vaccines; Health, Economic and Social Perspectives. Front. Microbiol. 2020, 11, 1526. [Google Scholar] [CrossRef]
- Vetter, V.; Denizer, G.; Friedland, L.R.; Krishnan, J.; Shapiro, M. Understanding modern-day vaccines: What you need to know. Ann. Med. 2018, 50, 110–120. [Google Scholar] [CrossRef]
- Iwasaki, A.; Omer, S.B. Why and How Vaccines Work. Cell 2020, 183, 290–295. [Google Scholar] [CrossRef]
- Otto, S.P.; Day, T.; Arino, J.; Colijn, C.; Dushoff, J.; Li, M.; Mechai, S.; Van Domselaar, G.; Wu, J.; Earn, D.J.; et al. The origins and potential future of SARS-CoV-2 variants of concern in the evolving COVID-19 pandemic. Curr. Biol. 2021, 31, R918–R929. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.; Archer, B.; Laurenson-Schafer, H.; Jinnai, Y.; Konings, F.; Batra, N.; Pavlin, B.; Vandemaele, K.; Van Kerkhove, M.D.; Jombart, T.; et al. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Eurosurveillance 2021, 26, 2100509. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, T.; Twohig, A.K.; Harris, R.J.; Seaman, S.R.; Flannagan, J.; Allen, H.; Charlett, A.; De Angelis, D.; Dabrera, G.; Presanis, A.M. Risk of hospital admission for patients with SARS-CoV-2 variant B.1.1.7: Cohort analysis. BMJ 2021, 373, n1412. [Google Scholar] [CrossRef] [PubMed]
- Paredes, M.I.; Lunn, S.M.; Famulare, M.; Frisbie, L.A.; Painter, I.; Burstein, R.; Roychoudhury, P.; Xie, H.; Mohamed Bakhash, S.A.; Perez, R.; et al. Associations between SARS-CoV-2 variants and risk of COVID-19 hospitalization among confirmed cases in Washington State: A retrospective cohort study. medRxiv 2021. [Google Scholar] [CrossRef]
- Sheikh, A.; McMenamin, J.; Taylor, B.; Robertson, C. SARS-CoV-2 Delta VOC in Scotland: Demographics, risk of hospital admission, and vaccine effectiveness. Lancet 2021, 397, 2461–2462. [Google Scholar] [CrossRef]
- Tareq, A.M.; Emran, T.B.; Dhama, K.; Dhawan, M.; Tallei, T.E. Impact of SARS-CoV-2 delta variant (B. 1.617. 2) in surging second wave of COVID-19 and efficacy of vaccines in tackling the ongoing pandemic. Hum. Vaccin. Immunother. 2021, 17, 4126–4127. [Google Scholar] [CrossRef]
- Tallei, T.E.; Fatimawali; Yelnetty, A.; Idroes, R.; Kusumawaty, D.; Emran, T.B.; Yesiloglu, T.Z.; Sippl, W.; Mahmud, S.; Alqahtani, T.; et al. An Analysis Based on Molecular Docking and Molecular Dynamics Simulation Study of Bromelain as Anti-SARS-CoV-2 Variants. Front. Pharmacol. 2021, 12, 2192. [Google Scholar] [CrossRef]
- Kupferschmidt, K. Where did ‘weird’ Omicron come from? Science 2021, 374, 1179. [Google Scholar] [CrossRef]
- Kumar, S.; Thambiraja, T.S.; Karuppanan, K.; Subramaniam, G. Omicron and Delta variant of SARS-CoV-2: A comparative computational study of spike protein. J. Med. Virol. 2021, 94, 1641–1649. [Google Scholar] [CrossRef]
- Chowdhury, S.; Bappy, M.H.; Chowdhury, S.; Chowdhury, S.; Chowdhury, S. Omicron variant (B.1.1.529) of SARS-CoV-2, a worldwide public health emergency! Eur. J. Clin. Med. 2022, 3, 8–12. [Google Scholar] [CrossRef]
- Meng, B.; Ferreira, I.; Abdullahi, A.; Kemp, S.A.; Goonawardane, N.; Papa, G.; Fatihi, S.; Charles, O.; Collier, D.; Choi, J. SARS-CoV-2 Omicron spike mediated immune escape, infectivity and cell-cell fusion. BioRxiv 2021. [Google Scholar] [CrossRef]
- Yang, Y.; Du, L. SARS-CoV-2 spike protein: A key target for eliciting persistent neutralizing antibodies. Signal Transduct. Target. Ther. 2021, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.; Muecksch, F.; Weisblum, Y.; Da Silva, J.; Bednarski, E.; Cho, A.; Wang, Z.; Gaebler, C.; Caskey, M.; Nussenzweig, M.C.; et al. Plasma Neutralization of the SARS-CoV-2 Omicron Variant. N. Engl. J. Med. 2022, 386, 599–601. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2021, 602, 657–663. [Google Scholar] [CrossRef]
- Nemet, I.; Kliker, L.; Lustig, Y.; Zuckerman, N.; Erster, O.; Cohen, C.; Kreiss, Y.; Alroy-Preis, S.; Regev-Yochay, G.; Mendelson, E.; et al. Third BNT162b2 Vaccination Neutralization of SARS-CoV-2 Omicron Infection. N. Engl. J. Med. 2021, 386, 492–494. [Google Scholar] [CrossRef]
- Lewnard, J.A.; Hong, V.X.; Patel, M.M.; Kahn, R.; Lipsitch, M.; Tartof, S.Y. Clinical Outcomes among Patients Infected with Omicron (B.1.1.529) SARS-CoV-2 Variant in Southern California. medRxiv 2022. [Google Scholar] [CrossRef]
- Maslo, C.; Friedland, R.; Toubkin, M.; Laubscher, A.; Akaloo, T.; Kama, B. Characteristics and Outcomes of Hospitalized Patients in South Africa During the COVID-19 Omicron Wave Compared with Previous Waves. J. Am. Med Assoc. 2021, 327, 583. [Google Scholar] [CrossRef]
- Callaway, E.; Ledford, H. How bad is Omicron? What scientists know so far. Nature 2021, 600, 197–199. [Google Scholar] [CrossRef]
- McMahan, K.; Giffin, V.; Tostanoski, L.H.; Chung, B.; Siamatu, M.; Suthar, M.S.; Halfmann, P.; Kawaoka, Y.; Piedra-Mora, C.; Martinot, A.J.; et al. Reduced Pathogenicity of the SARS-CoV-2 Omicron Variant in Hamsters. bioRxiv 2022. [Google Scholar] [CrossRef]
- Puhach, O.; Adea, K.; Hulo, N.; Sattonnet, P.; Genecand, C.; Iten, A.; Bausch, F.J.; Kaiser, L.; Vetter, P.; Eckerle, I.; et al. Infectious viral load in unvaccinated and vaccinated patients infected with SARS-CoV-2 WT, Delta and Omicron. medRxiv 2022. [Google Scholar] [CrossRef]
- Garrett, N.; Tapley, A.; Andriesen, J.; Seocharan, I.; Fisher, L.H.; Bunts, L.; Espy, N.; Wallis, C.L.; Randhawa, A.K.; Ketter, N.; et al. High Rate of Asymptomatic Carriage Associated with Variant Strain Omicron. medRxiv 2022. [Google Scholar] [CrossRef]
- Faustini, S.; Shields, A.; Banham, G.; Wall, N.; Al-Taei, S.; Tanner, C.; Ahmed, Z.; Efstathiou, E.; Townsend, N.; Goodall, M.; et al. Cross reactivity of spike glycoprotein induced antibody against Delta and Omicron variants before and after third SARS-CoV-2 vaccine dose in healthy and immunocompromised individuals. J. Infect. 2022, 2022, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Glatter, K.A.; Finkelman, P. History of the Plague: An Ancient Pandemic for the Age of COVID-19. Am. J. Med. 2021, 134, 176–181. [Google Scholar] [CrossRef]
- Hays, J.N. Plague and the end of Antiquity: The Pandemic of 541–750; Cambridge University Press: Cambridge, UK, 2007; pp. 1–382. ISBN 0521846390. [Google Scholar]
- Azizi, M.H.; Jalali, G.A.R.; Azizi, F. A history of the 1918 Spanish influenza pandemic and its impact on Iran. Arch. Iran. Med. 2010, 13, 262–265. Available online: https://pubmed.ncbi.nlm.nih.gov/20433236/ (accessed on 31 January 2022).
- Berkes, E.; Deschenes, O.; Gaetani, R.; Lin, J.; Severen, C. Lockdowns and Innovation: Evidence from the 1918 Flu Pandemic; National Bureau of Economic Research: Cambridge, MA, USA, 2020. [Google Scholar]
- Arora, S.; Grover, V.; Saluja, P.; Algarni, Y.A.; Saquib, S.A.; Asif, S.M.; Batra, K.; Alshahrani, M.Y.; Das, G.; Jain, R.; et al. Literature Review of Omicron: A Grim Reality Amidst COVID-19. Microorganisms 2022, 10, 451. [Google Scholar] [CrossRef]
- Hoffmann, M.; Krüger, N.; Schulz, S.; Cossmann, A.; Rocha, C.; Kempf, A.; Nehlmeier, I.; Graichen, L.; Moldenhauer, A.-S.; Winkler, M.S.; et al. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell 2022, 185, 447–456.e11. [Google Scholar] [CrossRef]
- Roth, C. Omicron: Is ′Natural Immunity′ Better than a Vaccine? Available online: https://www.dw.com/en/omicron-is-natural-immunity-better-than-a-vaccine/a-60425426 (accessed on 31 January 2022).
- Gazit, S.; Shlezinger, R.; Perez, G.; Lotan, R.; Peretz, A.; Ben-Tov, A.; Cohen, D.; Muhsen, K.; Chodick, G.; Patalon, T. Comparing SARS-CoV-2 natural immunity to vaccine-induced immunity: Reinfections versus breakthrough infections. medRxiv 2021. [Google Scholar] [CrossRef]
- Brehm, T.T.; Thompson, M.; Ullrich, F.; Schwinge, D.; Addo, M.M.; Spier, A.; Knobloch, J.K.; Aepfelbacher, M.; Lohse, A.W.; Lütgehetmann, M.; et al. Low SARS-CoV-2 infection rates and high vaccine-induced immunity among German healthcare workers at the end of the third wave of the COVID-19 pandemic. Int. J. Hyg. Environ. Health 2021, 238, 113851. [Google Scholar] [CrossRef]
- Crotty, S. Hybrid immunity. Science 2021, 372, 1392–1393. [Google Scholar] [CrossRef]
- Thiagarajan, K. Covid-19: The significance of India’s emerging “hybrid immunity”. BMJ 2021, 375, n3047. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Karim, F.; Cele, S.; San, J.E.; Lustig, G.; Tegally, H.; Bernstein, M.; Ganga, Y.; Jule, Z.; Reedoy, K. Omicron infection enhances neutralizing immunity against the Delta variant. medRxiv 2021. [Google Scholar] [CrossRef]
- Sharun, K.; Tiwari, R.; Dhama, K.; Emran, T.B.; Rabaan, A.A.; Al Mutair, A. Emerging SARS-CoV-2 variants: Impact on vaccine efficacy and neutralizing antibodies. Hum. Vaccin. Immunother. 2021, 17, 3491–3494. [Google Scholar] [CrossRef] [PubMed]
- Röltgen, K.; Powell, A.E.; Wirz, O.F.; Stevens, B.A.; Hogan, C.A.; Najeeb, J.; Hunter, M.; Wang, H.; Sahoo, M.K.; Huang, C.; et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci. Immunol. 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Lumley, S.F.; Wei, J.; O’Donnell, D.; Stoesser, E.N.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Cox, S.; James, T.; et al. The Duration, Dynamics, and Determinants of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibody Responses in Individual Healthcare Workers. Clin. Infect. Dis. 2021, 73, e699–e709. [Google Scholar] [CrossRef]
- Tarke, A.; Sidney, J.; Kidd, C.K.; Dan, J.M.; Ramirez, S.I.; Yu, E.D.; Mateus, J.; da Silva Antunes, R.; Moore, E.; Rubiro, P.; et al. Comprehensive analysis of T cell immunodominance and immunoprevalence of SARS-CoV-2 epitopes in COVID-19 cases. Cell Rep. Med. 2021, 2, 100204. [Google Scholar] [CrossRef] [PubMed]
- Geers, D.; Shamier, M.C.; Bogers, S.; den Hartog, G.D.; Gommers, L.; Nieuwkoop, N.N.; Schmitz, K.S.; Rijsbergen, L.C.; van Osch, J.A.T.; Dijkhuizen, E.; et al. SARS-CoV-2 variants of concern partially escape humoral but not T cell responses in COVID-19 convalescent donors and vaccine recipients. Sci. Immunol. 2021, 6, eabj1750. [Google Scholar] [CrossRef]
- Tarke, A.; Sidney, J.; Methot, N.; Yu, E.D.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; Wang, E.; et al. Impact of SARS-CoV-2 variants on the total CD4+ and CD8+ T cell reactivity in infected or vaccinated individuals. Cell Rep. Med. 2021, 2, 100355. [Google Scholar] [CrossRef]
- Redd, A.D.; Nardin, A.; Kared, H.; Bloch, E.M.; Pekosz, A.; Laeyendecker, O.; Abel, B.; Fehlings, M.; Quinn, T.C.; Tobian, A.A.R. CD8+ T-Cell Responses in COVID-19 Convalescent Individuals Target Conserved Epitopes From Multiple Prominent SARS-CoV-2 Circulating Variants. Open Forum Infect. Dis. 2021, 8, ofab143. [Google Scholar] [CrossRef]
- Bangaru, S.; Ozorowski, G.; Turner, H.L.; Antanasijevic, A.; Huang, D.; Wang, X.; Torres, J.L.; Diedrich, J.K.; Tian, J.-H.; Portnoff, A.D. Structural analysis of full-length SARS-CoV-2 spike protein from an advanced vaccine candidate. Science 2020, 370, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, Y.C.; Fischinger, S.; Siddiqui, S.M.; Chen, Z.; Yu, J.; Gebre, M.; Atyeo, C.; Gorman, M.J.; Zhu, A.L.; Kang, J. Discrete SARS-CoV-2 antibody titers track with functional humoral stability. Nat. Commun. 2021, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Amanna, I.J.; Slifka, M.K. Contributions of humoral and cellular immunity to vaccine-induced protection in humans. Virology 2011, 411, 206–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371. [Google Scholar] [CrossRef] [PubMed]
- Rakib, A.; Nain, Z.; Islam, M.A.; Sami, S.A.; Mahmud, S.; Islam, A.; Ahmed, S.; Siddiqui, A.B.F.; Babu, S.M.O.F.; Hossain, P.; et al. A molecular modelling approach for identifying antiviral selenium-containing heterocyclic compounds that inhibit the main protease of SARS-CoV-2: An in silico investigation. Brief. Bioinform. 2021, 22, 1476–1498. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.S.; Karami, A.; Haghighi, T.M.; Tumilaar, S.G.; Fatimawali; Idroes, R.; Mahmud, S.; Celik, I.; Ağagündüz, D.; Tallei, T.E.; et al. In Silico Evaluation of Iranian Medicinal Plant Phytoconstituents as Inhibitors against Main Protease and the Receptor-Binding Domain of SARS-CoV-2. Molecules 2021, 26, 5724. [Google Scholar] [CrossRef]
- Rakib, A.; Sami, S.A.; Islam, M.A.; Ahmed, S.; Faiz, F.B.; Khanam, B.H.; Marma, K.K.S.; Rahman, M.; Uddin, M.M.N.; Nainu, F.; et al. Epitope-Based Immunoinformatics Approach on Nucleocapsid Protein of Severe Acute Respiratory Syndrome-Coronavirus-2. Molecules 2020, 25, 5088. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Tirupathi, R.; Sule, A.A.; Aldali, J.; Mutair, A.A.; Alhumaid, S.; Muzaheed, G.; Nitin, K.; Thoyaja, A.; Ramesh, B.; et al. Viral Dynamics and Real-Time RT-PCR Ct Values Correlation with Disease Severity in COVID-19. Diagnostics 2021, 11, 1091. [Google Scholar] [CrossRef]
- Lavine, J.S.; Bjornstad, O.N.; Antia, R. Immunological characteristics govern the transition of COVID-19 to endemicity. Science 2021, 371, 741–745. [Google Scholar] [CrossRef]
- Wang, L.; Berger, N.A.; Kaelber, D.C.; Davis, P.B.; Volkow, N.D.; Xu, R. COVID infection severity in children under 5 years old before and after Omicron emergence in the US. medRxiv 2022. [Google Scholar] [CrossRef]
- Méthot, P.-O. Why do parasites harm their host? On the origin and legacy of Theobald Smith’s “law of declining virulence”—1900–1980. Hist. Philos. Life Sci. 2012, 34, 561–601. [Google Scholar]
- Geoghegan, J.; Holmes, E.C. The phylogenomics of evolving virus virulence. Nat. Rev. Genet. 2018, 19, 756–769. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.C. Current and future approaches to the therapy of human rabies. Antivir. Res. 2013, 99, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Sulis, G.; Roggi, A.; Matteelli, A.; Raviglione, M.C. Tuberculosis: Epidemiology and Control. Mediterr. J. Hematol. Infect. Dis. 2014, 6, e2014070. [Google Scholar] [CrossRef] [PubMed]
- Salata, C.; Calistri, A.; Parolin, C.; Palù, G. Coronaviruses: A paradigm of new emerging zoonotic diseases. Pathog. Dis. 2019, 77, ftaa006. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, S.; Biswas, S.; Kumar Paul, G.; Mita, M.A.; Afrose, S.; Robiul Hasan, M.; Sharmin Sultana Shimu, M.; Uddin, M.A.R.; Salah Uddin, M.; Zaman, S.; et al. Antiviral peptides against the main protease of SARS-CoV-2: A molecular docking and dynamics study. Arab. J. Chem. 2021, 14, 103315. [Google Scholar] [CrossRef]
- Sanjuán, R.; Domingo-Calap, P. Mechanisms of viral mutation. Cell. Mol. Life Sci. 2016, 73, 4433–4448. [Google Scholar] [CrossRef] [Green Version]
- Monroe, J.G.; Srikant, T.; Carbonell-Bejerano, P.; Becker, C.; Lensink, M.; Exposito-Alonso, M.; Klein, M.; Hildebrandt, J.; Neumann, M.; Kliebenstein, D.; et al. Mutation bias reflects natural selection in Arabidopsis thaliana. Nature 2022, 602, 101–105. [Google Scholar] [CrossRef]
- Mahmud, S.; Uddin, M.A.R.; Paul, G.K.; Shimu, M.S.S.; Islam, S.; Rahman, E.; Islam, A.; Islam, M.S.; Promi, M.M.; Emran, T.B.; et al. Virtual screening and molecular dynamics simulation study of plant derived compounds to identify potential inhibitor of main protease from SARS-CoV-2. Brief. Bioinform. 2021, 22, 1402–1414. [Google Scholar] [CrossRef]
- Mahmud, S.; Paul, G.K.; Afroze, M.; Islam, S.; Gupt, S.B.R.; Razu, M.H.; Biswas, S.; Zaman, S.; Uddin, M.S.; Khan, M.; et al. Efficacy of Phytochemicals Derived from Avicennia officinalis for the Management of COVID-19: A Combined In Silico and Biochemical Study. Molecules 2021, 26, 2210. [Google Scholar] [CrossRef]
- Mahmud, S.; Biswas, S.; Paul, G.K.; Mita, M.A.; Promi, M.M.; Afrose, S.; Hasan, M.; Zaman, S.; Uddin, M.; Dhama, K.; et al. Plant-based phytochemical screening by targeting main protease of SARS-CoV-2 to design effective potent inhibitors. Biology 2021, 10, 589. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Bibi, S.; Meem, A.F.K.; Islam, M.; Rahaman, M.; Bepary, S.; Rahman, M.; Elzaki, A.; Kajoak, S.; Osman, H.; et al. Natural Bioactive Molecules: An Alternative Approach to the Treatment and Control of COVID-19. Int. J. Mol. Sci. 2021, 22, 12638. [Google Scholar] [CrossRef] [PubMed]
- Arruda, E.F.; Das, S.S.; Dias, C.M.; Pastore, D.H. Modelling and optimal control of multi strain epidemics, with application to COVID-19. PLoS ONE 2021, 16, e0257512. [Google Scholar] [CrossRef] [PubMed]
- Mwai, P. Covid-19 Vaccinations: African Nations Miss WHO Target—BBC News. Available online: https://www.bbc.com/news/56100076 (accessed on 2 February 2022).
- Williams, D. Israel Mulls Offering 4th COVID Vaccine Dose to All Adults|Reuters. Available online: https://www.reuters.com/business/healthcare-pharmaceuticals/israel-mulls-offering-4th-covid-vaccine-dose-all-adults-2022-01-25/ (accessed on 2 February 2022).





| Greek Name | First Identified | Noteworthy Mutations | Transmission | Hospitalisation |
|---|---|---|---|---|
| Alpha | 20 September 2020, England | N501Y, P681H | +29% [58] | +52% [59] |
| Beta | May 2020, South Africa | K417N, E484K, N501Y | +25% [58] | Under research |
| Gamma | November 2020, Brazil | K417T, E484K, N501Y | +38% [58] | Increased [60] |
| Delta | October 2020, India | L452R, T478K, P681R | +97% [58] | +85% [61] |
| Omicron | 9 November 2021, South Africa | P681H, N440K, N501Y, S477N, and numerous others | Increased [62] | −41% [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abas, A.H.; Marfuah, S.; Idroes, R.; Kusumawaty, D.; Fatimawali; Park, M.N.; Siyadatpanah, A.; Alhumaydhi, F.A.; Mahmud, S.; Tallei, T.E.; et al. Can the SARS-CoV-2 Omicron Variant Confer Natural Immunity against COVID-19? Molecules 2022, 27, 2221. https://doi.org/10.3390/molecules27072221
Abas AH, Marfuah S, Idroes R, Kusumawaty D, Fatimawali, Park MN, Siyadatpanah A, Alhumaydhi FA, Mahmud S, Tallei TE, et al. Can the SARS-CoV-2 Omicron Variant Confer Natural Immunity against COVID-19? Molecules. 2022; 27(7):2221. https://doi.org/10.3390/molecules27072221
Chicago/Turabian StyleAbas, Abdul Hawil, Siti Marfuah, Rinaldi Idroes, Diah Kusumawaty, Fatimawali, Moon Nyeo Park, Abolghasem Siyadatpanah, Fahad A. Alhumaydhi, Shafi Mahmud, Trina Ekawati Tallei, and et al. 2022. "Can the SARS-CoV-2 Omicron Variant Confer Natural Immunity against COVID-19?" Molecules 27, no. 7: 2221. https://doi.org/10.3390/molecules27072221
APA StyleAbas, A. H., Marfuah, S., Idroes, R., Kusumawaty, D., Fatimawali, Park, M. N., Siyadatpanah, A., Alhumaydhi, F. A., Mahmud, S., Tallei, T. E., Emran, T. B., & Kim, B. (2022). Can the SARS-CoV-2 Omicron Variant Confer Natural Immunity against COVID-19? Molecules, 27(7), 2221. https://doi.org/10.3390/molecules27072221













