Hydrophobic AEROSIL®R972 Fumed Silica Nanoparticles Incorporated Monolithic Nano-Columns for Small Molecule and Protein Separation by Nano-Liquid Chromatography
Abstract
:1. Introduction
2. Results and Discussion
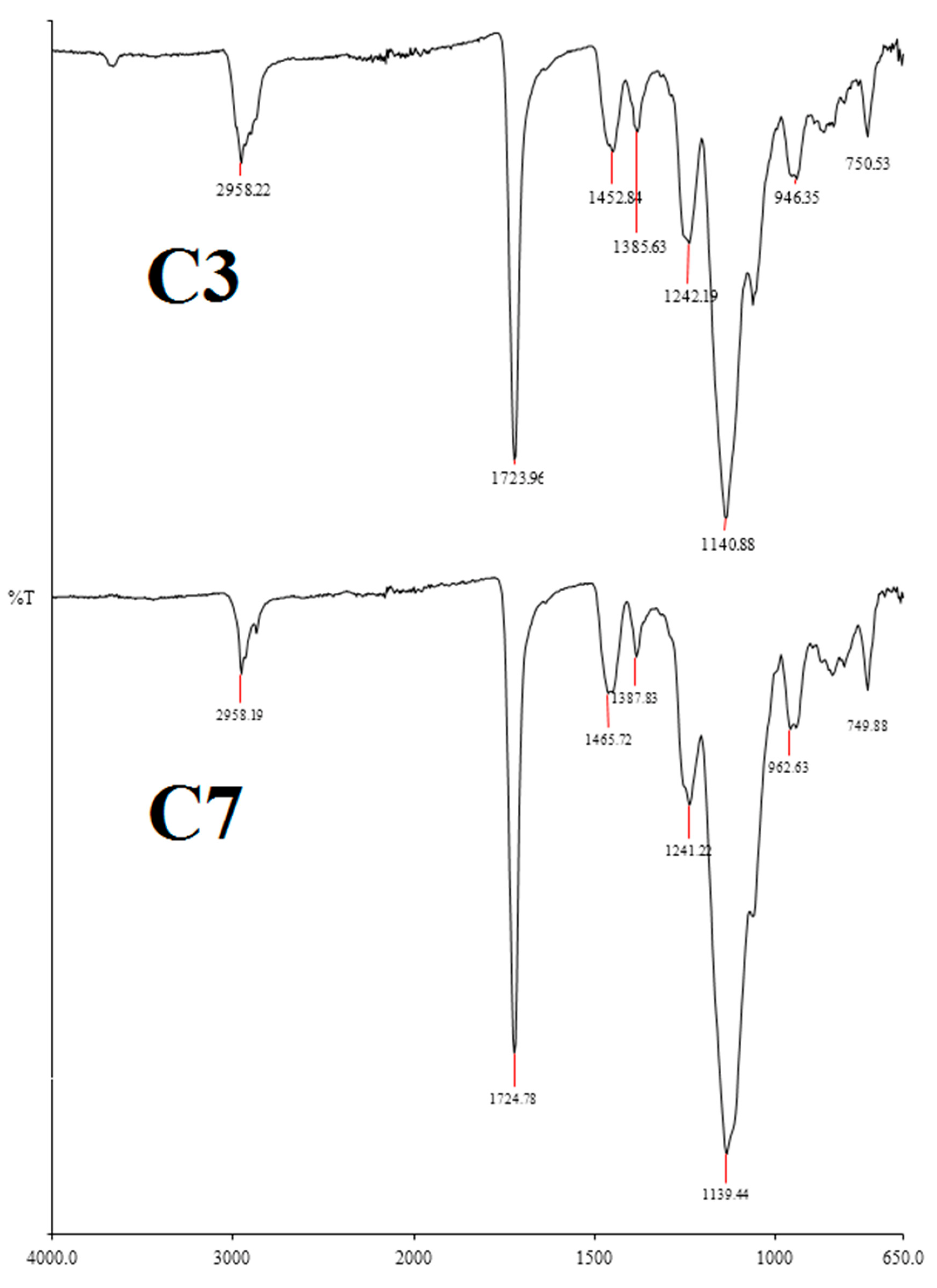
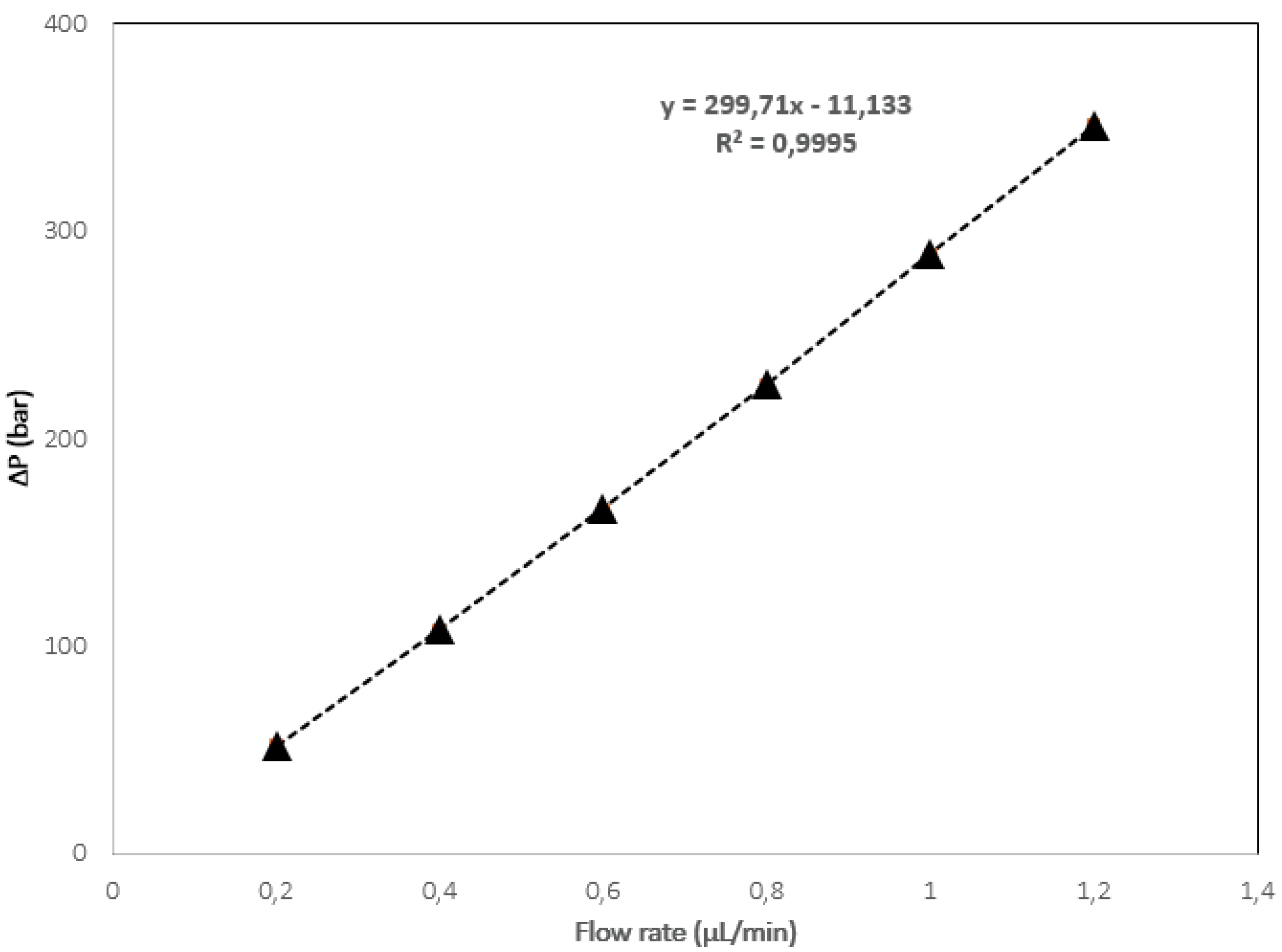
2.1. The Monolithic Column Preparation and Characterization with 50 µm ID
2.2. Chromatographic Evaluation and Application of HFSNPs-Based Monolithic Nanocolumn
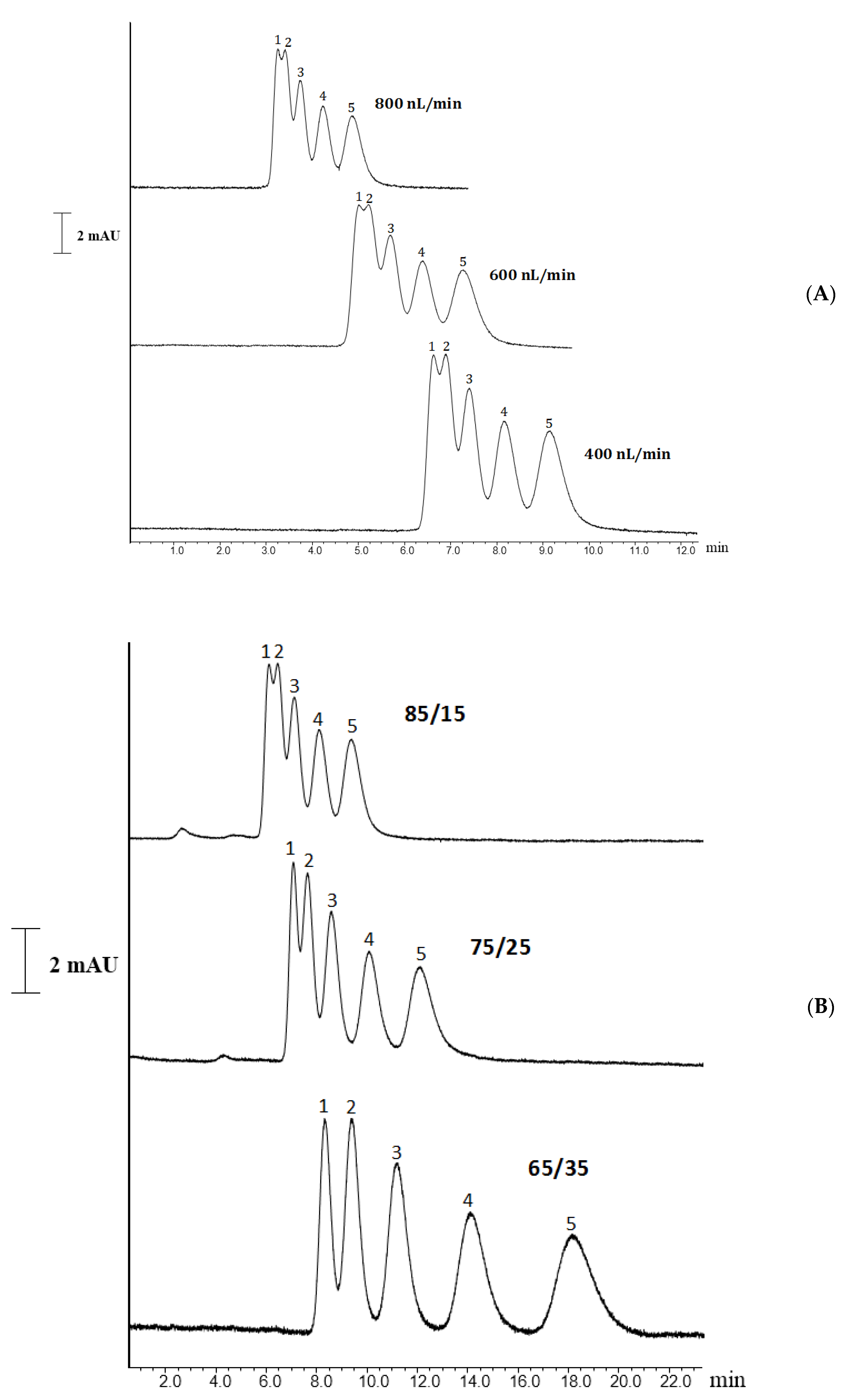
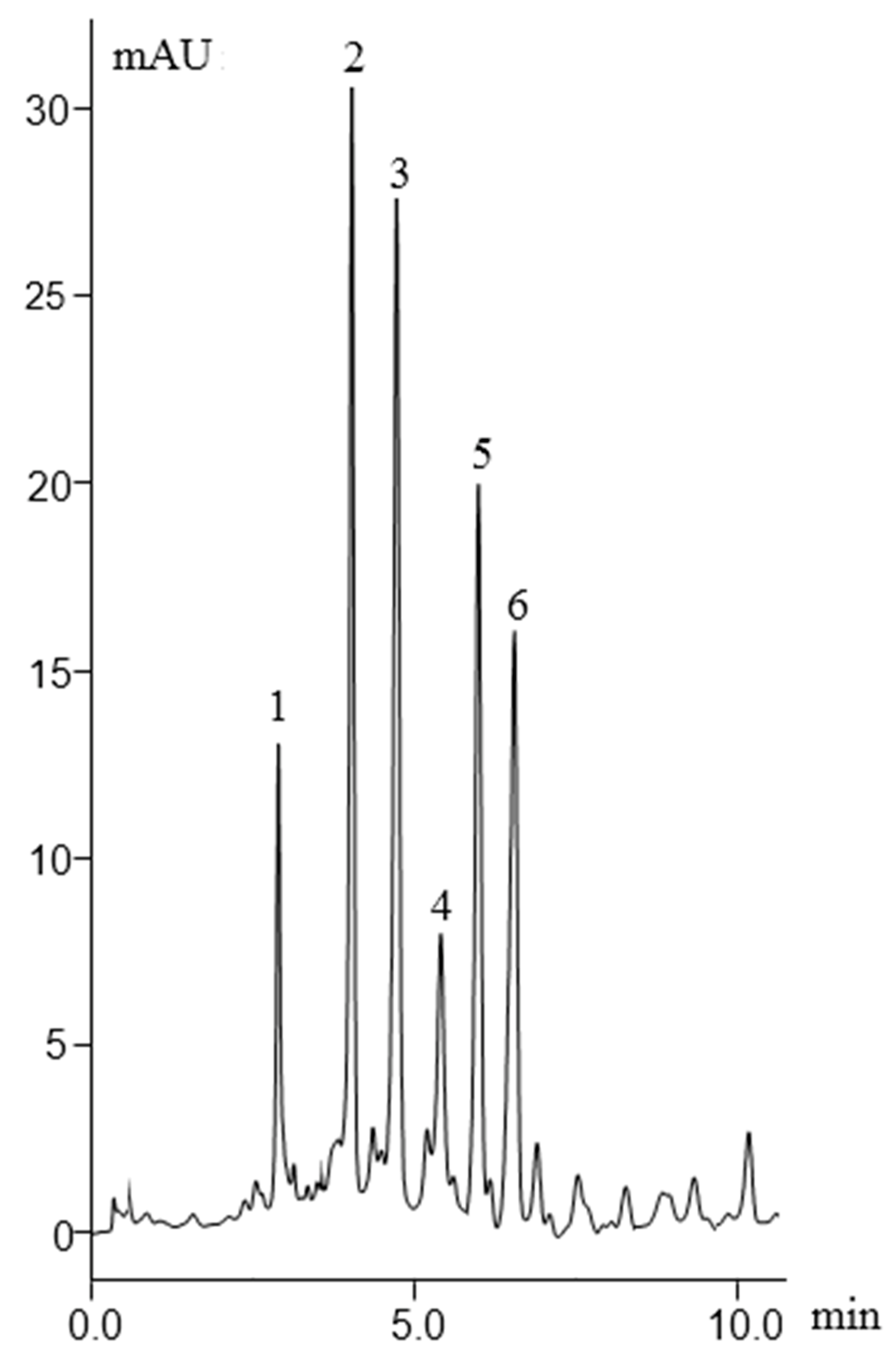
2.2.1. ABs Separation
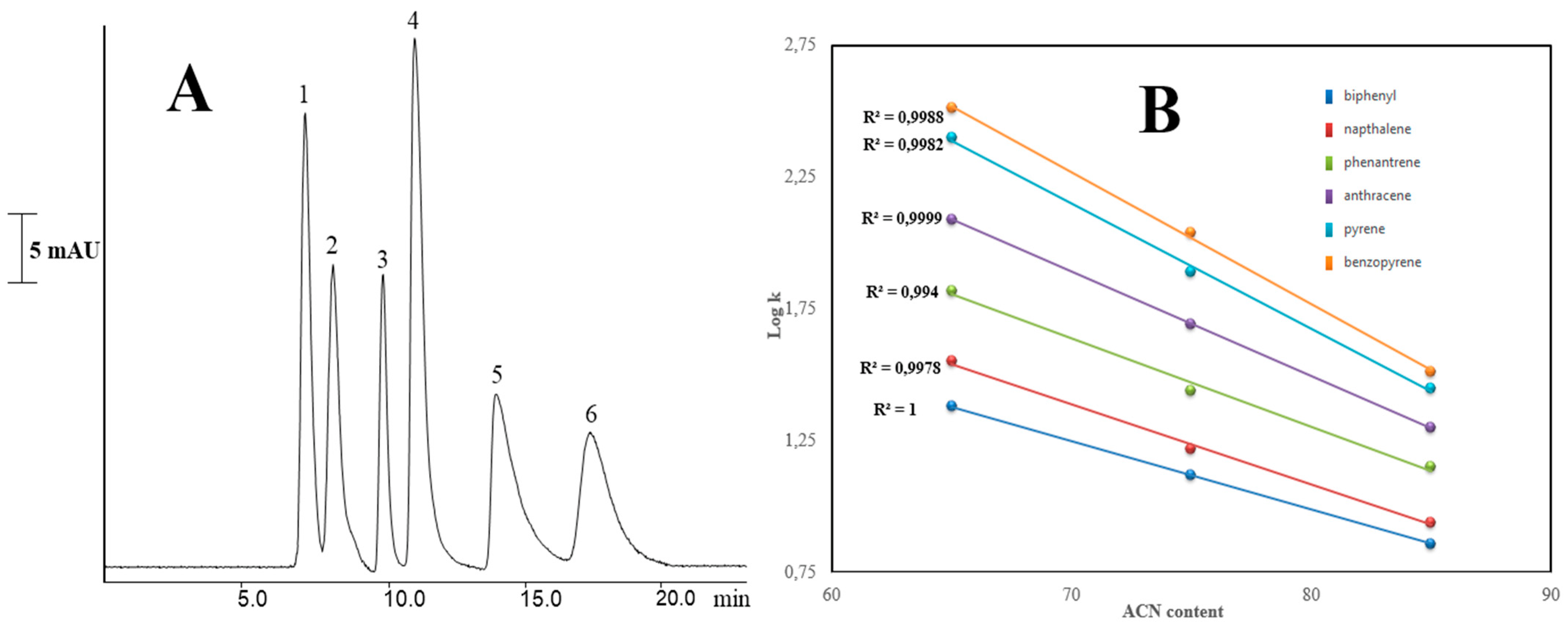
2.2.2. PAHs Separation
2.3. Protein Separation
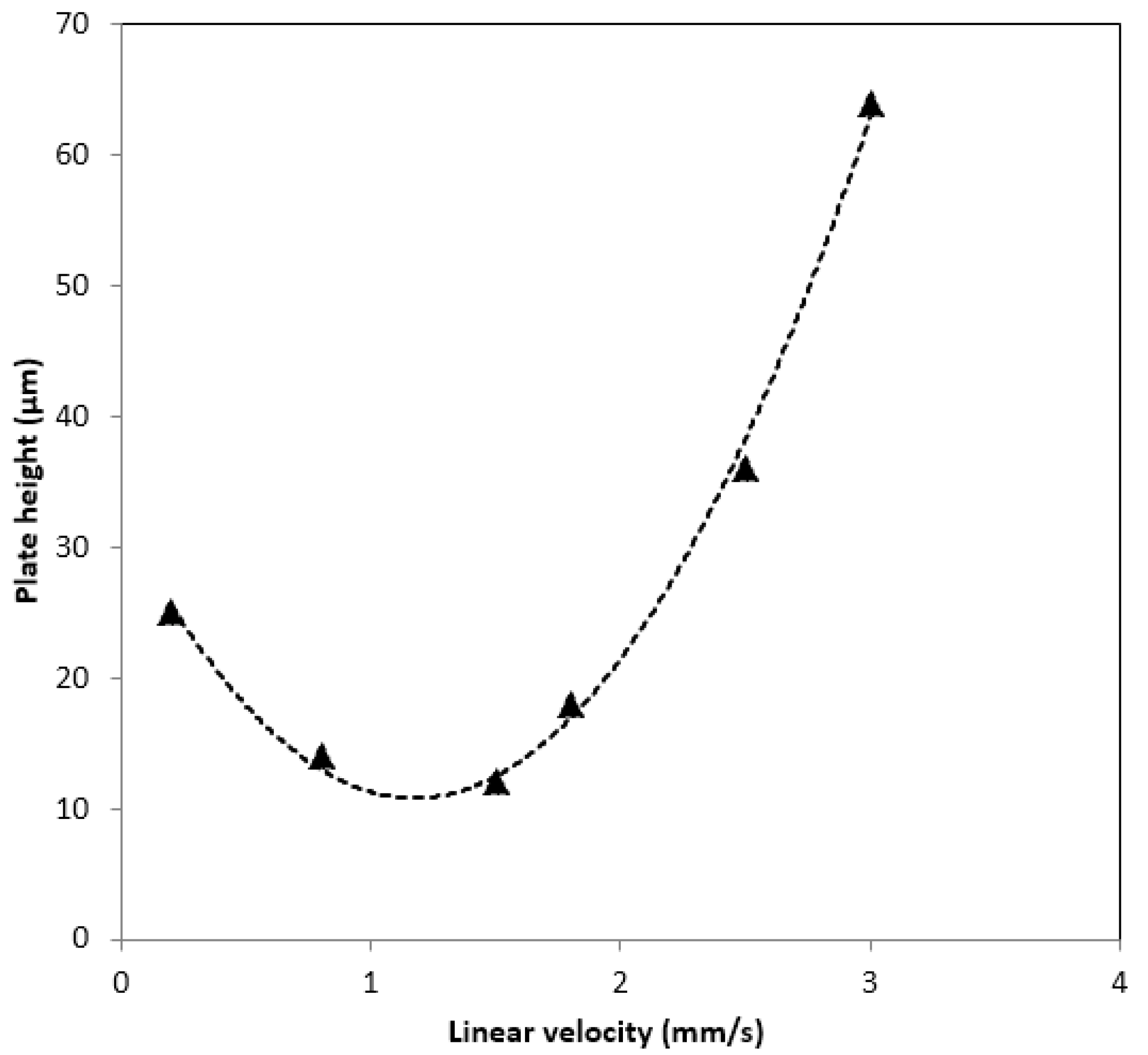
2.4. Stability and Repeatability
3. Materials and Methods
3.1. Instrumentation
3.2. Monolithic Nano-Column Preparation
3.3. Chromatographic Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saito, Y.; Jinno, K.; Greibrokk, T. Capillary columns in liquid chromatography: Between conventional columns and microchips. J. Sep. Sci. 2004, 27, 1379–1390. [Google Scholar] [CrossRef] [PubMed]
- Xiang, P.; Yang, Y.; Zhao, Z.; Chen, M.-L.; Liu, S. Ultrafast Gradient Separation with Narrow Open Tubular Liquid Chromatography. Anal. Chem. 2019, 91, 10738–10743. [Google Scholar] [CrossRef] [PubMed]
- Desmet, G.; Eeltink, S. Fundamentals for LC Miniaturization. Anal. Chem. 2012, 85, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Maciel, E.V.S.; de Toffoli, A.L.; Sobieski, E.; Nazário, C.E.D.; Lanças, F.M. Miniaturized liquid chromatography focusing on analytical columns and mass spectrometry: A review. Anal. Chim. Acta 2020, 1103, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Aydoğan, C.; Rigano, F.; Krčmová, L.K.; Chung, D.S.; Macka, M.; Mondello, L. Miniaturized LC in Molecular Omics. Anal. Chem. 2020, 92, 11485–11497. [Google Scholar] [CrossRef]
- Şeker, S.; Alharthi, S.; Aydoğan, C. Open tubular nano-liquid chromatography with a new polylysine grafted on graphene oxide stationary phase for the separation and determination of casein protein variants in milk. J. Chromatogr. A 2022, 1667, 462885. [Google Scholar] [CrossRef]
- Wilson, S.R.; Olsen, C.; Lundanes, E. Nano Liquid Chromatography Columns. Analyst 2019, 144, 7090–7104. [Google Scholar] [CrossRef]
- Wilson, S.R.; Vehus, T.; Berg, H.S.; Lundanes, E. Nano-LC in proteomics: Recent advances and approaches. Bioanalysis 2015, 7, 1799–1815. [Google Scholar] [CrossRef]
- Aydoğan, C. Nanoscale separations based on LC and CE for food analysis: A review. TrAC Trends Anal. Chem. 2019, 121, 115693. [Google Scholar] [CrossRef]
- Svec, F.; Lv, Y. Advances and Recent Trends in the Field of Monolithic Columns for Chromatography. Anal. Chem. 2014, 87, 250–273. [Google Scholar] [CrossRef]
- Lubomirsky, E.; Khodabandeh, A.; Preis, J.; Susewind, M.; Hofe, T.; Hilder, E.F.; Arrua, R.D. Polymeric stationary phases for size exclusion chromatography: A review. Anal. Chim. Acta 2021, 1151, 338244. [Google Scholar] [CrossRef] [PubMed]
- Vitek, R.; do Nascimento, F.H.; Masini, J.C. Polymer monoliths for the concentration of viruses from environmental waters: A review. J. Sep. Sci. 2021, 45, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Roberg-Larsen, H.; Wilson, S.R.; Lundanes, E. Recent advances in on-line upfront devices for sensitive bioanalytical nano LC methods. TrAC Trends Anal. Chem. 2021, 136, 116190. [Google Scholar] [CrossRef]
- Connolly, D.; Currivan, S.; Paull, B. Polymeric monolithic materials modified with nanoparticles for separation and detection of biomolecules: A review. Proteomics 2012, 12, 2904–2917. [Google Scholar] [CrossRef]
- Gama, M.R.; Rocha, F.R.P.; Bottoli, C.B.G. Monoliths: Synthetic routes, functionalization and innovative analytical applications. TrAC Trends Anal. Chem. 2019, 115, 39–51. [Google Scholar] [CrossRef]
- Beeram, S.R.; Rodriguez, E.; Doddavenkatanna, S.; Li, Z.; Pekarek, A.; Peev, D.; Goerl, K.; Trovato, G.; Hofmann, T.; Hage, D.S. Nanomaterials as stationary phases and supports in liquid chromatography. Electrophoresis 2017, 38, 2498–2512. [Google Scholar] [CrossRef]
- Reichelt, S.; Elsner, C.; Prager, A.; Naumov, S.; Kuballa, J.; Buchmeiser, M.R. Amino-functionalized monolithic spin-type columns for high-throughput lectin affinity chromatography of glycoproteins. Analyst 2012, 137, 2600–2607. [Google Scholar] [CrossRef]
- Aydoğan, C.; El Rassi, Z. Monolithic stationary phases with incorporated fumed silica nanoparticles. Part I. Polymethacrylate-based monolithic column with incorporated bare fumed silica nanoparticles for hydrophilic interaction liquid chromatography. J. Chromatogr. A 2016, 1445, 55–61. [Google Scholar] [CrossRef]
- Aydoğan, C.; El Rassi, Z. Monolithic stationary phases with incorporated fumed silica nanoparticles. Part II. Polymethacrylate-based monolithic column with “covalently” incorporated modified octadecyl fumed silica nanoparticles for reversed-phase chromatography. J. Chromatogr. A 2016, 1445, 62–67. [Google Scholar] [CrossRef]
- Aydoğan, C. Boronic acid-fumed silica nanoparticles incorporated large surface area monoliths for protein separation by nano-liquid chromatography. Anal. Bioanal. Chem. 2016, 408, 8457–8466. [Google Scholar] [CrossRef]
- Ganewatta, N.; El Rassi, Z. Poly(glyceryl monomethacrylate-co-ethylene glycol dimethacrylate) monolithic columns with incorporated bare and surface modified gluconamide fumed silica nanoparticles for hydrophilic interaction capillary electrochromatography. Talanta 2018, 179, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Greguš, M.; Kostas, J.C.; Ray, S.; Abbatiello, S.E.; Ivanov, A.R. Improved Sensitivity of Ultralow Flow LC–MS-Based Proteomic Profiling of Limited Samples Using Monolithic Capillary Columns and FAIMS Technology. Anal. Chem. 2020, 92, 14702–14712. [Google Scholar] [CrossRef] [PubMed]
- Eeltink, S.; Wouters, S.; Dores-Sousa, J.L.; Svec, F. Advances in organic polymer-based monolithic column technology for high-resolution liquid chromatography-mass spectrometry profiling of antibodies, intact proteins, oligonucleotides, and peptides. J. Chromatogr. A 2017, 1498, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Günyel, Z.; Aslan, H.; Demir, N.; Aydoğan, C. Nano-liquid chromatography with a new nano-structured monolithic nanocolumn for proteomics analysis. J. Sep. Sci. 2021, 44, 3996–4004. [Google Scholar] [CrossRef] [PubMed]
- Snyder, L.R.; Kirkland, J.J.; Dolan, J.W. Introduction to Modern Liquid Chromatography, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Fresco-Cala, B.; Carrasco-Correa, E.J.; Cárdenas, S.; Herrero-Martínez, J.M. Carbon nanostructures incorporated on methacrylate monoliths for separation of small molecules by nano-liquid chromatography. Microchem. J. 2018, 139, 222–229. [Google Scholar] [CrossRef]
- Aydogan, C.; El-Rassi, Z. Nano-scale separation techniques and their applications in proteomics: An overview. In Affinity Chromatography and Related Techniques; Denizli, A., El-Rassi, Z., Eds.; Kukla Kırtasiye Bilgisayar Malzeme Tic. Ltd.: Ankara, Turkey, 2018; Chapter 2; pp. 19–40. [Google Scholar]
- Lynch, K.B.; Ren, J.; Beckner, M.A.; He, C.; Liu, S. Monolith columns for liquid chromatographic separations of intact proteins: A review of recent advances and applications. Anal. Chim. Acta 2019, 1046, 48–68. [Google Scholar] [CrossRef]
- Ma, J.; Dai, Q.; Li, X.; Zhu, X.; Ma, T.; Qiao, X.; Shen, S.; Liu, X. Dipentaerythritol penta-/hexa-acrylate based-highly cross-linked hybrid monolithic column: Preparation and its applications for ultrahigh efficiency separation of proteins. Anal. Chim. Acta 2017, 963, 143–152. [Google Scholar] [CrossRef]

| Column | Monomers (µL) | Porogen (µL) | R2 b | Specific Surface Area (m2/g) | Permeability (m2) (×10−14) | |
|---|---|---|---|---|---|---|
| BMA:EDMA | HFSNPs a | DMF:H2O c | ||||
| C1 | 104:66 | - | 352:48 | No back pressure | - | - |
| C2 | 104:76 | - | 352:48 | No back pressure | - | - |
| C3 | 104:86 | - | 352:48 | 0.9990 | 71.3 | 9.14 |
| C4 | 104:86 | 1.63 | 352:48 | 0.9995 | - | - |
| C5 | 104:86 | 4.94 | 352:48 | 0.9997 | - | - |
| C6 | 104:86 | 8.29 | 352:48 | 0.9995 | - | - |
| C7 | 104:86 | 13.4 | 352:48 | 0.9999 | 321.4 | 1.48 |
| C8 | 104:86 | 15.2 | 352:48 | - | - | - |
| C9 | 104:86 | 20.2 | 352:48 | - | - |
| Type and Number (n) of Experimental Repetitions | Average Retention Time (min) | RSD% of Retention Time |
|---|---|---|
| Run-to-run (n = 3) | 2.15 | 2.30 |
| Day-to-day (n = 3) | 2.21 | 2.48 |
| Column-to-column (n = 3) | 2.39 | 2.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydoğan, C.; Erdoğan, İ.Y.; El-Rassi, Z. Hydrophobic AEROSIL®R972 Fumed Silica Nanoparticles Incorporated Monolithic Nano-Columns for Small Molecule and Protein Separation by Nano-Liquid Chromatography. Molecules 2022, 27, 2306. https://doi.org/10.3390/molecules27072306
Aydoğan C, Erdoğan İY, El-Rassi Z. Hydrophobic AEROSIL®R972 Fumed Silica Nanoparticles Incorporated Monolithic Nano-Columns for Small Molecule and Protein Separation by Nano-Liquid Chromatography. Molecules. 2022; 27(7):2306. https://doi.org/10.3390/molecules27072306
Chicago/Turabian StyleAydoğan, Cemil, İbrahim Y. Erdoğan, and Ziad El-Rassi. 2022. "Hydrophobic AEROSIL®R972 Fumed Silica Nanoparticles Incorporated Monolithic Nano-Columns for Small Molecule and Protein Separation by Nano-Liquid Chromatography" Molecules 27, no. 7: 2306. https://doi.org/10.3390/molecules27072306






