Photocatalytic Degradation of Orange G Dye by Using Bismuth Molybdate: Photocatalysis Optimization and Modeling via Definitive Screening Designs
Abstract
:1. Introduction
- (1)
- To evaluate the application of the bismuth molybdate materials towards the photocatalytic degradation of ORG dye;
- (2)
- To estimate the significant outcome of six process parameters, such as photocatalyst loading, dye concentration, irradiation time, temperature, pH, and the role of H2O2, as added for electron (e−) scavenger;
- (3)
- The modeling and optimization of process variables for Photocatalytic degradation by statistical method; Definitive Screening design, and a response surface designs method.
2. Materials and Methods
2.1. Materials for Synthesis
2.2. Synthesis of Bismuth Molybdate
2.3. Instrumentation
2.4. Photocatalytic Measurements
2.5. Experimental Design and Data Analysis
3. Results and Discussion
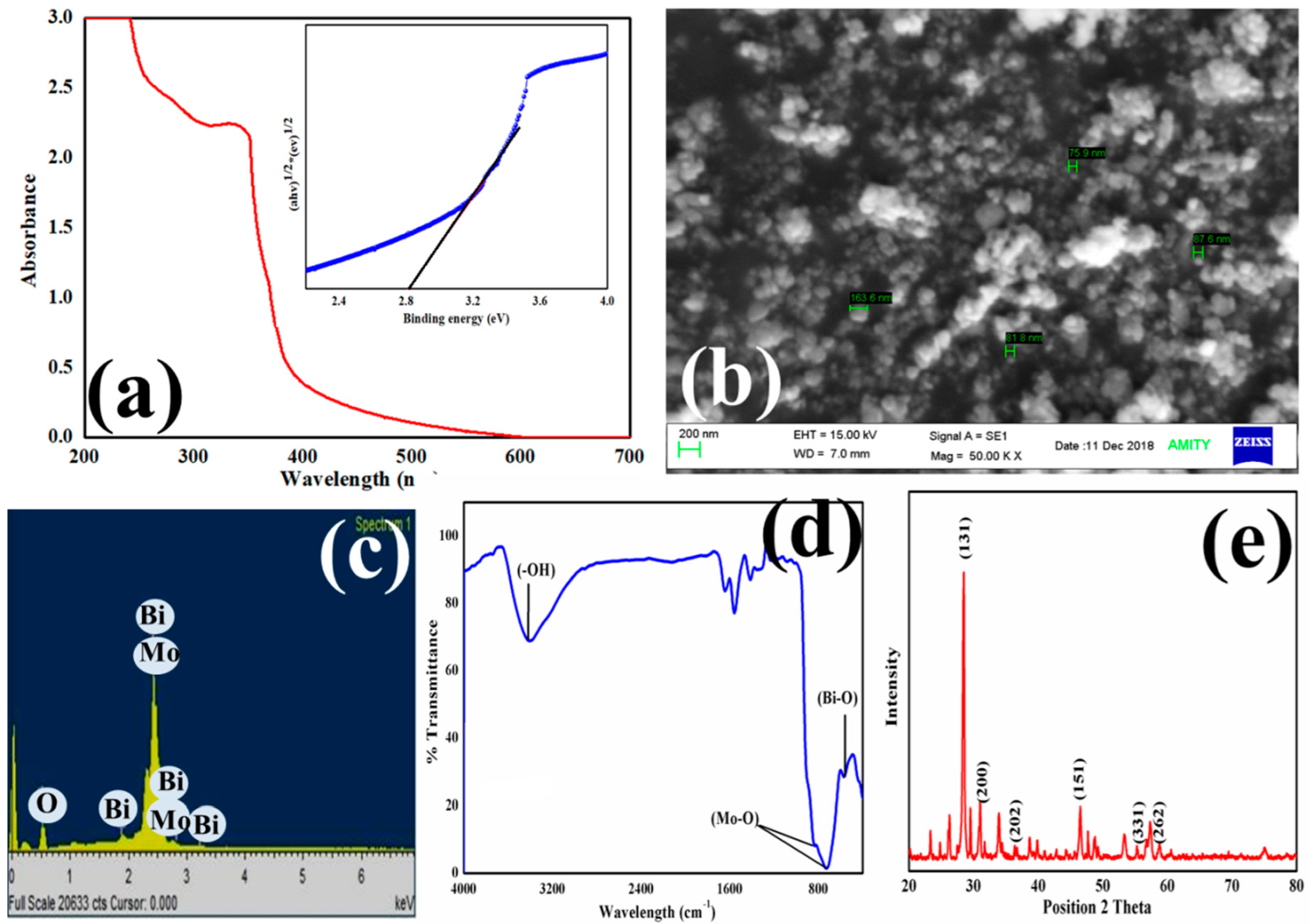
3.1. UV-Visible, SEM, FT-IR, and XRD Characterization of Bi2MoO6
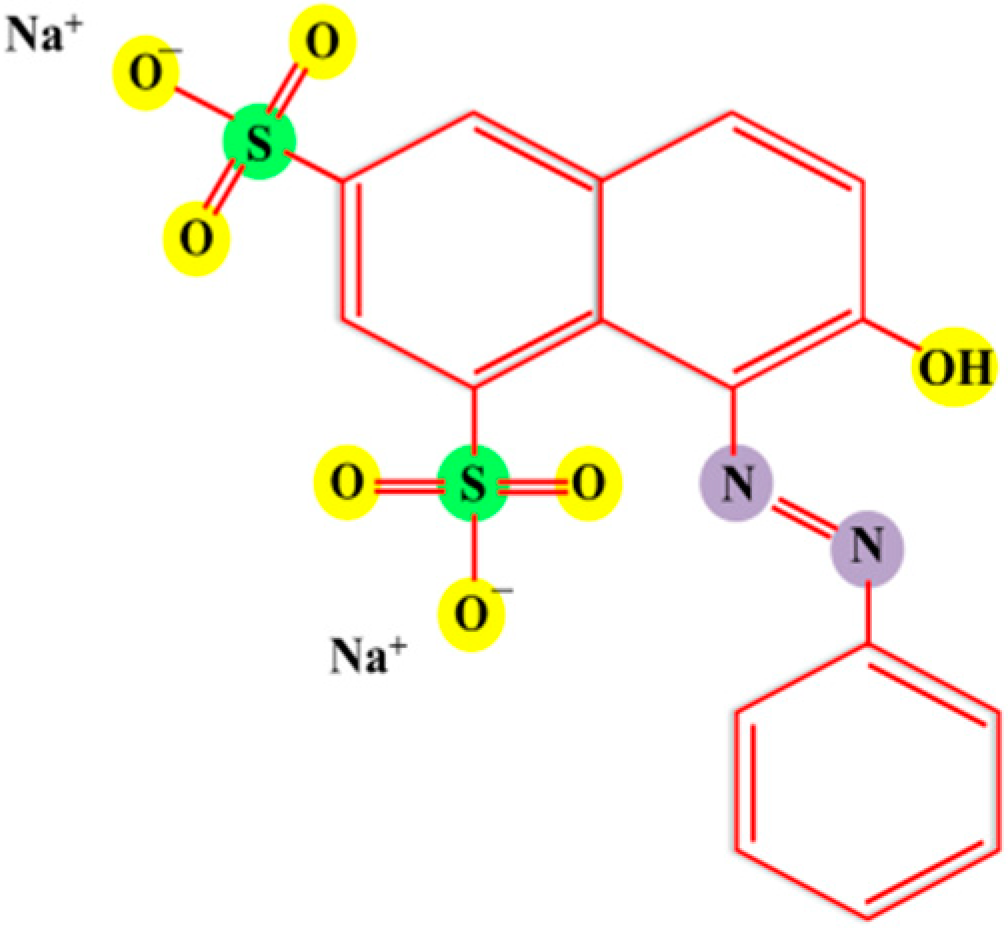
3.2. Photocatalytic Degradation of ORG
3.2.1. Preliminary Experiments
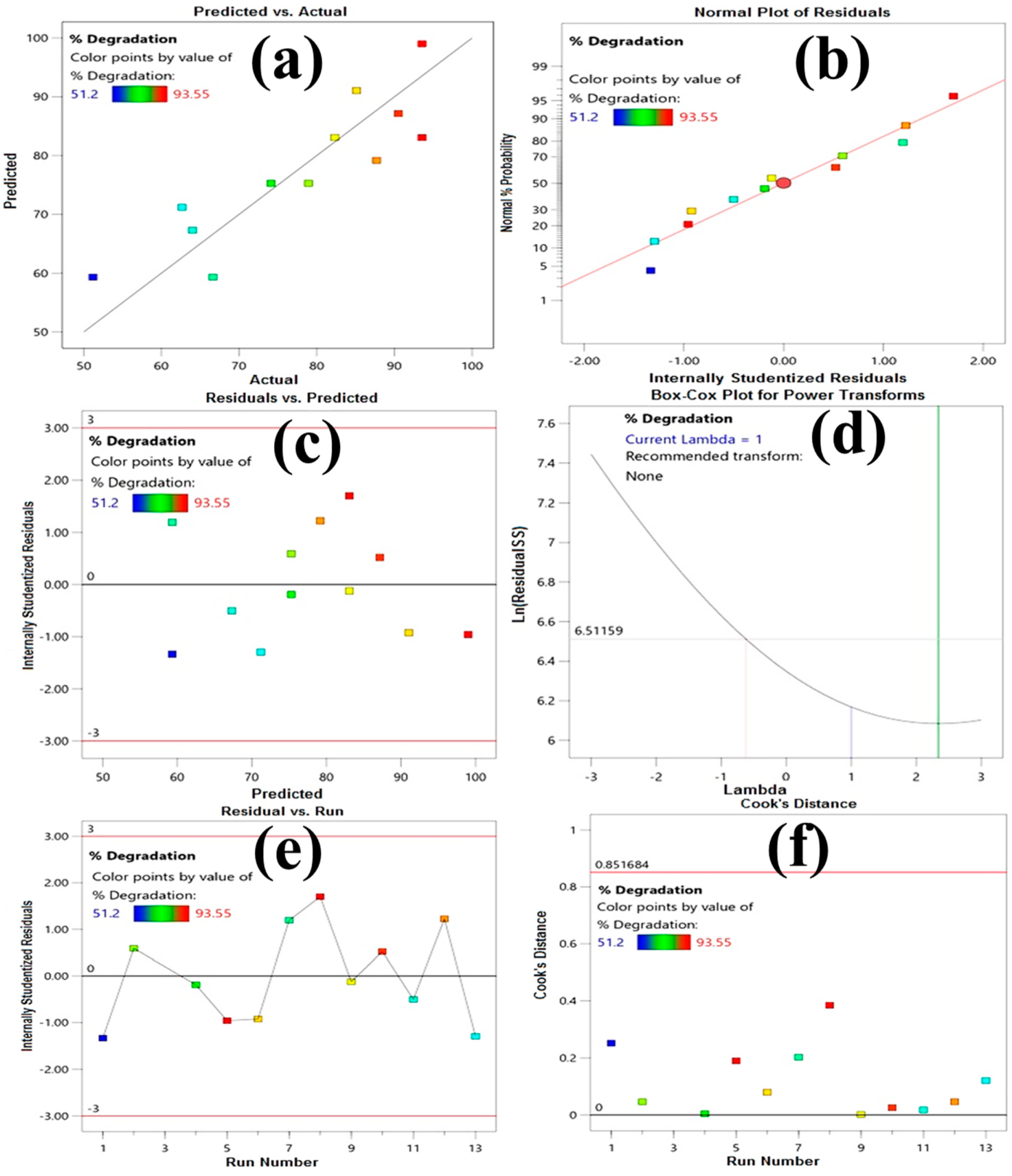
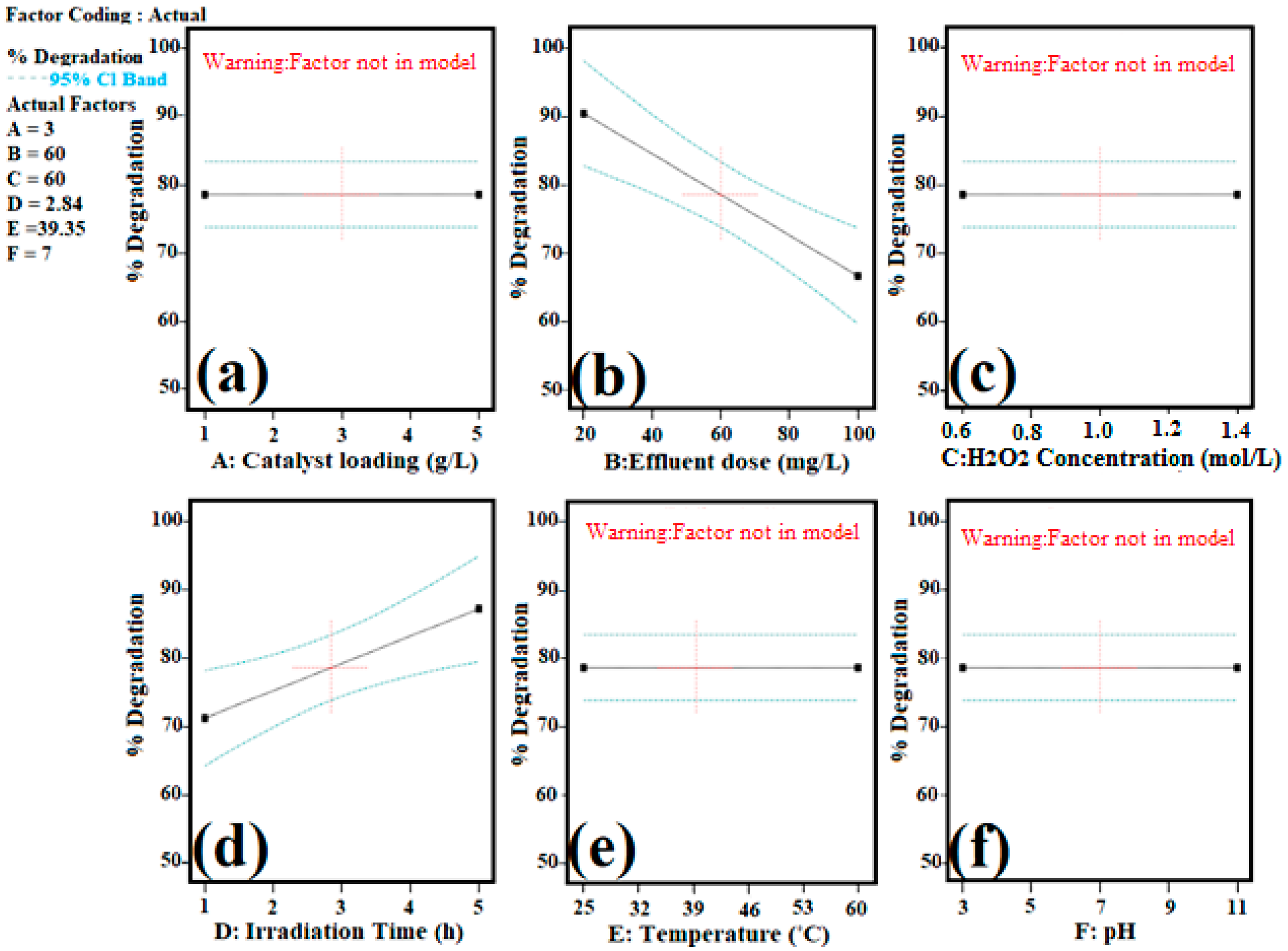
3.2.2. Process Parameters Optimization with Statistical Modeling for ORG Photocatalytic Degradation
| Catalyst | Synthesis Method | ORG Dye (mg/L) | Light Sources | Degradation | References |
|---|---|---|---|---|---|
| N-doped TiO2 | hydrolysis and calcination of tetrabutyl titanate | 25 | Visible | 96.29% | [68] |
| ZnO/HS | precipitation and calcination | 50 | Solar UV | 49.78% 94.36% | [69] |
| Sn(IV)/TiO2/activated carbon | Dip-coating technique with TiO2 sol–gel | 50 | UV | 99.1% | [70] |
| ZnO/activated carbon | Carbonization and Precipitation | 20 mg | Visible UV | 95% 90% | [20] |
| Bi2MoO6 | Co-precipitation | 20–100 mg | Visible | 98% | This work |
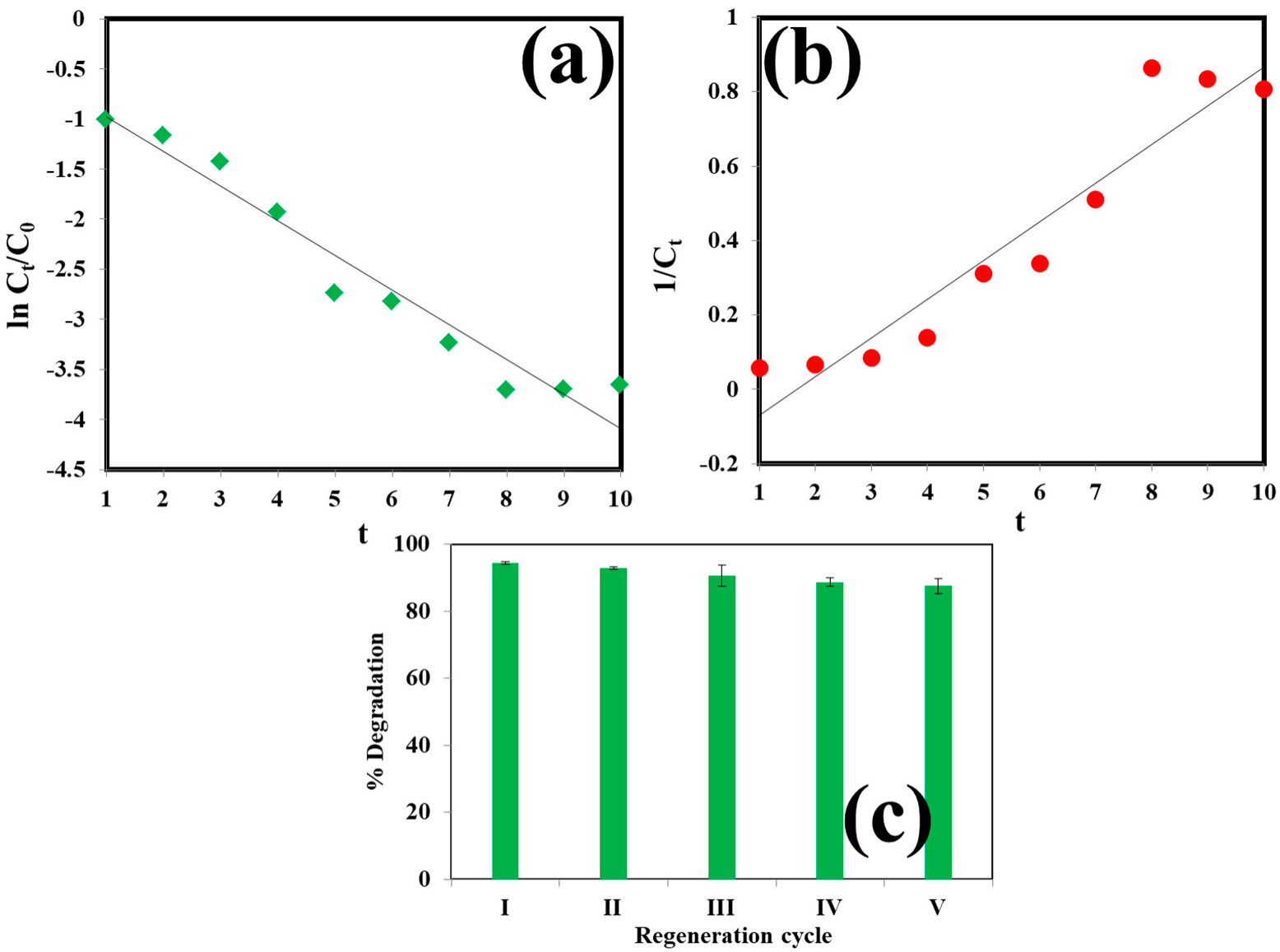
3.2.3. Model Validation, Kinetic and Isotherm Studies
3.3. Reusability of the Catalyst
3.4. Mechanism of Orange G Dye Degradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jianping, L.; Minrong, L.; Jinnan, W.; Jianjian, L.; Hongwen, S.; Maoxing, H. Global Environmental Issues and Human Wellbeing. In Report on Global Environmental Competitiveness; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–21. [Google Scholar] [CrossRef] [Green Version]
- Larsson, D.G.; de Pedro, C.; Paxeus, N. Effluent from drug manufactures contains extremely high levels of pharmaceuticals. J. Hazard. Mater. 2007, 148, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Alinsafi, A.; Evenou, F.; Abdulkarim, E.M.; Pons, M.N.; Zahraa, O.; Benhammou, A.; Yaacoubi, A.; Nejmeddine, A. Treatment of textile industry wastewater by supported photocatalysis. Dye. Pigment. 2007, 74, 439–445. [Google Scholar] [CrossRef]
- Shanker, U.; Rani, M.; Jassal, V. Degradation of hazardous organic dyes in water by nanomaterials. Environ. Chem. Lett. 2017, 15, 623–642. [Google Scholar] [CrossRef]
- Eren, Z. Ultrasound as a basic and auxiliary process for dye remediation: A review. J. Environ. Manag. 2012, 104, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Samsami, S.; Mohamadizaniani, M.; Sarrafzadeh, M.-H.; Rene, E.R.; Firoozbahr, M. Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives. Process Saf. Environ. Prot. 2020, 143, 138–163. [Google Scholar] [CrossRef]
- Verma, P.; Samanta, S.K. Microwave-enhanced advanced oxidation processes for the degradation of dyes in water. Environ. Chem. Lett. 2018, 16, 969–1007. [Google Scholar] [CrossRef]
- Obama, B. The irreversible momentum of clean energy. Science 2017, 355, 126–129. [Google Scholar] [CrossRef] [Green Version]
- Gautam, S.; Shandilya, P.; Priya, B.; Singh, V.P.; Raizada, P.; Rai, R.; Valente, M.A.; Singh, P. Superparamagnetic MnFe2O4 dispersed over graphitic carbon sand composite and bentonite as magnetically recoverable photocatalyst for antibiotic mineralization. Sep. Purif. Technol. 2017, 172, 498–511. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, Z.; Khan, A.U.; Mastoi, N.R.; Aslam, M.; Kim, J. Photocatalytic systems as an advanced environmental remediation: Recent developments, limitations and new avenues for applications. J. Environ. Chem. Eng. 2016, 4, 4143–4164. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Dwivedi, A.D.; Lee, W.; Zhao, X.; Liu, W.; Sillanpää, M.; Zhao, D.; Huang, C.H.; Fu, J. Application of nanotechnologies for removing pharmaceutically active compounds from water: Development and future trends. Environ. Sci. Nano 2018, 5, 27–47. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Somaudon, V.; Watcharakitti, J.; Nanan, S. Solar light-driven photocatalyst based on bismuth molybdate (Bi4MoO9) for detoxification of anionic azo dyes in wastewater. J. Mater. Sci. Mater. Electron. 2021, 32, 1977–1991. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, L.; Wang, D.; Yang, F.; Ye, J. Synthesis of bismuth molybdate photocatalysts for CO2 photo-reduction. J. CO2 Util. 2019, 29, 196–204. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, Y.; Park, S.-J. Recent Advances in Carbonaceous Photocatalysts with Enhanced Photocatalytic Performances: A Mini Review. Materials 2019, 12, 1916. [Google Scholar] [CrossRef] [Green Version]
- Imam, S.S.; Muhammad, A.I.; Babamale, H.F.; Zango, Z.U. Removal of Orange G Dye from Aqueous Solution by Adsorption: A Short Review. J. Environ. Treat. Tech. 2021, 9, 318–327. [Google Scholar]
- Madhavan, J.; Grieser, F.; Ashokkumar, M. Degradation of orange-G by advanced oxidation processes. Ultrason. Sonochem. 2010, 17, 338–343. [Google Scholar] [CrossRef]
- Tarkwa, J.-B.; Oturan, N.; Acayanka, E.; Laminsi, S.; Oturan, M.A. Photo-Fenton oxidation of Orange G azo dye: Process optimization and mineralization mechanism. Environ. Chem. Lett. 2018, 17, 473–479. [Google Scholar] [CrossRef]
- Vinayagam, M.; Saranya, R.; Ramya, V.; Sivasamy, A. Photocatalytic degradation of orange G dye using ZnO/biomass activated carbon nanocomposite. J. Environ. Chem. Eng. 2017, 6, 3726–3734. [Google Scholar] [CrossRef]
- Asma, R.; Muhammad, I.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar]
- Abbasi_Asl, H.; Moradi, Z.; Ghaedi, M.; Sabzehmeidani, M.M. Degradation of Orange G and Trypan blue using Ag2C2O4/Ag/g-C3N4 composites as efficient photocatalyst under solar irradiation. J. Photochem. Photobiol. A Chem. 2020, 401, 112755. [Google Scholar] [CrossRef]
- Tekin, D.; Tekin, T.; Kiziltas, H. Photocatalytic degradation kinetics of Orange G dye over ZnO and Ag/ZnO thin film catalysts. Sci. Rep. 2019, 9, 17544. [Google Scholar] [CrossRef] [PubMed]
- Pedneka, P.P.K.; Mulani, I.; Mandake, M. Photocatalytic Degradation of Orange-G Dye Using Cu/Al2O3 Photocatalyst. 2017. Available online: https://www.semanticscholar.org/paper/Photocatalytic-Degradation-of-Orange-G-dye-using-Pawar-Pednekar/a421179faebdeff5dfb7d7304f023fd32beb94a1 (accessed on 3 March 2022).
- Thennarasu, G.; Kavithaa, S.; Sivasamy, A. Photocatalytic degradation of Orange G dye under solar light using nanocrystalline semiconductor metal oxide. Environ. Sci. Pollut. Res. Int. 2011, 19, 2755–2765. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, Z. Bismuth-based photocatalytic semiconductors: Introduction, challenges and possible approaches. J. Mol. Catal. A Chem. 2016, 423, 533–549. [Google Scholar] [CrossRef]
- Li, M.; Yu, S.; Huang, H.; Li, X.; Feng, Y.; Wang, C.; Wang, Y.; Ma, T.; Guo, L.; Zhang, Y. Unprecedented Eighteen-Faceted BiOCl with a Ternary Facet Junction Boosting Cascade Charge Flow and Photo-redox. Angew. Chem. Int. Ed. 2019, 58, 9517–9521. [Google Scholar] [CrossRef]
- Chen, P.; Liu, H.; Sun, Y.; Li, J.; Cui, W.; Wang, L.; Zhang, W.; Yuan, X.; Wang, Z.; Zhang, Y.; et al. Bi metal prevents the deactivation of oxygen vacancies in Bi2O2CO3 for stable and efficient photocatalytic NO abatement. Appl. Catal. B Environ. 2020, 264, 118545. [Google Scholar] [CrossRef]
- Priya, B.; Raizada, P.; Singh, N.; Thakur, P.; Singh, P. Adsorptional photocatalytic mineralization of oxytetracycline and ampicillin antibiotics using Bi2O3/BiOCl supported on graphene sand composite and chitosan. J. Colloid Interface Sci. 2016, 479, 271–283. [Google Scholar] [CrossRef]
- Garg, S.; Yadav, M.; Chandra, A.; Hernadi, K. A Review on BiOX (X = Cl, Br and I) Nano-/Microstructures for Their Photocatalytic Applications. J. Nanosci. Nanotechnol. 2019, 19, 280–294. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Gu, S.; Zhao, Y.; Zhou, G.; Li, W. BiVO4, Bi2WO6 and Bi2MoO6 photocatalysis: A brief review. J. Mater. Sci. Technol. 2020, 56, 45–68. [Google Scholar] [CrossRef]
- Lopes, O.F.; Carvalho, K.T.G.; Avansi, W.; Miloric, D.M.B.; Ribeiro, C. Insights into the photocatalytic performance of Bi2O2CO3/BiVO4 heterostructures prepared by one-step hydrothermal method. RSC Adv. 2018, 8, 10889–10897. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Sudhaik, A.; Raizada, P.; Hosseini-Bandegharaei, A.; Thakur, V.K.; Saini, A.; Saini, V.; Singh, P. An overview on bismuth molybdate based photocatalytic systems: Controlled morphology and enhancement strategies for photocatalytic water purification. J. Environ. Chem. Eng. 2020, 8, 104291. [Google Scholar] [CrossRef]
- Bhatia, V.; Dhir, A.; Ray, A. Photocatalytic Degradation of Atenolol with Graphene Oxide/Zinc Oxide Composite: Optimization of Process Parameters using Statistical Method. J. Photochem. Photobiol. A Chem. 2021, 409, 113136. [Google Scholar] [CrossRef]
- Ray, S.; Lalman, J.A.; Biswas, N. Using the Box-Benkhen technique to statistically model phenol photocatalytic degradation by titanium dioxide nanoparticles. Chem. Eng. J. 2009, 150, 15–24. [Google Scholar] [CrossRef]
- Czitrom, V. One-Factor-at-a-Time versus Designed Experiments. Am. Stat. 1999, 53, 126–131. [Google Scholar]
- Voelkel, J.G. The Efficiencies of Fractional Factorial Designs. Technometrics 2005, 47, 488–494. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, T.; Zhao, X. Controllable synthesis of Bi2MoO6 and effect of morphology and variation in local structure on photocatalytic activities. Appl. Catal. B Environ. 2010, 98, 138–146. [Google Scholar] [CrossRef]
- Bi, J.; Wu, L.; Li, J.; Li, Z.; Wang, X.; Fu, X. Simple solvothermal routes to synthesize nanocrystalline Bi2MoO6 photocatalysts with different morphologies. Acta. Mater. 2007, 55, 4699–4705. [Google Scholar] [CrossRef]
- Kandi, D.; Behera, A.; Sahoo, S.; Parida, K. CdS QDs modified BiOI/Bi2MoO6 nanocomposite for degradation of quinolone and tetracycline types of antibiotics towards environmental remediation. Sep. Purif. Technol. 2020, 253, 117523. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, H.; Shen, Y.; Qu, J. Photocatalytic reduction of bromate at C60 modified Bi2MoO6 under visible light irradiation. Appl. Catal. B Environ. 2011, 106, 63–68. [Google Scholar] [CrossRef]
- Tanji, K.; Navio, J.A.; Chaqroune, A.; Naja, J.; Puga, F.; Hidalgo, M.C.; Kherbeche, A. Fast photodegradation of rhodamine B and caffeine using ZnO-hydroxyapatite composites under UV-light illumination. Catal. Today 2020, 388–389, 176–186. [Google Scholar] [CrossRef]
- Rajesh, C. Solid-phase photodegradation of polystyrene by nano TiO2 under ultraviolet radiation. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100229. [Google Scholar]
- Antonopoulou, M.; Konstantinou, I. Photocatalytic degradation of pentachlorophenol by visible light Ν-F-TiO₂ in the presence of oxalate ions: Optimization, modeling, and scavenging studies. Environ. Sci. Pollut. Res. Int. 2015, 22, 9438–9448. [Google Scholar] [CrossRef] [PubMed]
- Elhosiny, A.H.; Khairy, Y. Facile low temperature synthesis and characterization of bismuth molybdate (Bi2MoO6) nanostructures: An effect surfactant concentration. Optik 2019, 178, 90–96. [Google Scholar] [CrossRef]
- Abid, N.; Al-keisy, A.; Ahmed, D.; Salih, A.; Khammas, A. pH dependent synthesis and characterization of bismuth molybdate nanostructure for photocatalysis degradation of organic pollutants. Environ. Sci. Pollut. Res. 2022, 1–11. [Google Scholar] [CrossRef]
- Krishnapandi, A.; Muthukutty, B.; Chen, S.M.; Arul, K.T.; Shiuan, H.J.; Selvaganapathy, M. Bismuth molybdate incorporated functionalized carbon nanofiber as an electrocatalytic tool for the pinpoint detection of organic pollutant in life samples. Ecotoxicol. Environ. Saf. 2021, 209, 111828. [Google Scholar] [CrossRef]
- Shameem, A.; Devendran, P.; Siva, V.; Murugan, A.; Sasikumar, S.; Nallamuthu, N.; Hussain, S.; Asath Bahadur, S. Robust one-step synthesis of bismuth molybdate nanocomposites: A promising negative electrode for high end ultracapacitors. Solid State Sci. 2020, 106, 106303. [Google Scholar] [CrossRef]
- Ding, J.; Yang, Z.; He, C.; Tong, X.; Li, Y.; Niu, X.; Zhang, H. UiO-66(Zr) coupled with Bi(2)MoO(6) as photocatalyst for visible-light promoted dye degradation. J. Colloid. Interface Sci. 2017, 497, 126–133. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, F.; Zhan, S.; Zhu, Z.; He, Q. Mechanisms on the enhanced sterilization performance of fluorocarbon resin composite coatings modified by g-C3N4/Bi2MoO6 under the visible-light. J. Photochem. Photobiol. A Chem. 2018, 350, 10–16. [Google Scholar] [CrossRef]
- Vaez, M.; Moghaddam, A.Z.; Alijani, S. Optimization and Modeling of Photocatalytic Degradation of Azo Dye Using a Response Surface Methodology (RSM) Based on the Central Composite Design with Immobilized Titania Nanoparticles. Ind. Eng. Chem. Res. 2012, 51, 4199–4207. [Google Scholar] [CrossRef]
- Maqbool, Z.; Hussain, S.; Mahmood, F.; Shahid, M.; Shahzad, T.; Ahmad, T.; Sahar, A.; Imran, M.; Hafeez, F. Metal-tolerant Pseudomonas aeruginosa Strain ZM130 has the Potential for Concurrent Dye Decolorization and Plant Growth Promotion. Int. J. Agric. Biol. 2018, 20, 2621–2631. [Google Scholar]
- Antonopoulou, M.; Konstantinou, I. Optimization and Modeling of the Photocatalytic Degradation of the Insect Repellent DEET in Aqueous TiO2 Suspensions. CLEAN-Soil Air Water 2013, 41, 593–600. [Google Scholar] [CrossRef]
- Majdi, H.; Esfahani, J.; Mohebbi, M. Optimization of convective drying by response surface methodology. Comput. Electron. Agric. 2019, 156, 574–584. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Zhou, Z.; Hong, Y. Symmetry Analysis of the Uncertain Alternative Box-Cox Regression Model. Symmetry 2022, 14, 22. [Google Scholar] [CrossRef]
- Chaker, H.; Attar, A.E.; Djennas, M.; Fourmentin, S. A statistical modeling-optimization approach for efficiency photocatalytic degradation of textile azo dye using cerium-doped mesoporous ZnO: A central composite design in response surface methodology. Chem. Eng. Res. Des. 2021, 171, 198–212. [Google Scholar] [CrossRef]
- Rawat, S.; Singh, J.; Koduru, J.R. Effect of ultrasonic waves on degradation of phenol and para-nitrophenol by iron nanoparticles synthesized from Jatropha leaf extract. Environ. Technol. Innov. 2021, 24, 101857. [Google Scholar] [CrossRef]
- Li, S.; Lin, Q.; Liu, X.; Yang, L.; Ding, J.; Dong, F.; Li, Y.; Irfana, M.; Zhang, P. Fast photocatalytic degradation of dyes using low-power laser-fabricated Cu 2 O–Cu nanocomposites. RSC Adv. 2018, 8, 20277–20286. [Google Scholar] [CrossRef] [Green Version]
- Sboui, M.; Nsib, M.F.; Rayes, A.; Ochiai, T.; Houas, A. Application of solar light for photocatalytic degradation of Congo red by a floating salicylic acid-modified TiO2/palm trunk photocatalyst. C. R. Chim. 2017, 20, 181–189. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Hossaini, H.; Nasseri, S.; Azizi, N.; Shahmoradi, B.; Khosravi, T. Optimization of photocatalytic degradation of methyl orange using immobilized scoria-Ni/TiO2 nanoparticles. J. Nanostruct. Chem. 2020, 10, 143–159. [Google Scholar] [CrossRef]
- Meetani, M.A.; Rauf, M.A.; Hisaindee, S.; Khaleel, A.; AlZamly, A.; Ahmad, A. Mechanistic studies of photoinduced degradation of Orange G using LC/MS. RSC Adv. 2011, 1, 490–497. [Google Scholar] [CrossRef]
- Zhou, F.-S.; Yan, C.; Wang, H. Photocatalytic degradation of Orange G using sepiolite-TiO2 nanocomposites: Optimization of physicochemical parameters and kinetics studies. Chem. Eng. Sci. 2018, 183, 231–239. [Google Scholar] [CrossRef]
- Ou, X.; Yan, J.; Zhang, F.; Zhang, C. Accelerated degradation of orange G over a wide pH range in the presence of FeVO4. Front. Environ. Sci. Eng. 2018, 12, 7. [Google Scholar] [CrossRef]
- Martínez-de la Cruz, A.; Alfaro, S.O. Synthesis and characterization of γ-Bi2MoO6 prepared by co-precipitation: Photoassisted degradation of organic dyes under vis-irradiation. J. Mol. Catal. A Chem. 2010, 320, 85–91. [Google Scholar] [CrossRef]
- Gonçalves, N.P.F.; Minella, M.; Mailhot, G.; Brigante, M.; Prevot, A.B. Photo-activation of persulfate and hydrogen peroxide by humic acid coated magnetic particles for Bisphenol A degradation. Catal. Today 2021, 361, 43–49. [Google Scholar] [CrossRef]
- Ökte, A.N.; Resat, M.S.; Inel, Y. Influence of hydrogen peroxide and methanol on the photocatalytic degradation of 1,3-dihydroxybenzene. Toxicol. Environ. Chem. 2001, 79, 171–178. [Google Scholar] [CrossRef]
- Sun, J.; Qiao, L.; Sun, S.; Wang, G. Photocatalytic degradation of Orange G on nitrogen-doped TiO2 catalysts under visible light and sunlight irradiation. J. Hazard. Mater. 2008, 155, 312–319. [Google Scholar] [CrossRef]
- Al-Beladi, A.A.; Kosaa, S.A.; Wahab, R.A.; Salam, M.A. Removal of Orange G dye from water using halloysite nanoclay-supported ZnO nanoparticles. Desalination Water Treat. 2020, 196, 287–298. [Google Scholar] [CrossRef]
- Sun, J.; Wang, X.; Sun, J.; Sun, R.; Sun, S.-P.; Qiao, L. Photocatalytic degradation and kinetics of Orange G using nano-sized Sn (IV)/TiO2/AC photocatalyst. J. Mol. Catal. A Chem. 2006, 260, 241–246. [Google Scholar] [CrossRef]
- Mapukata, S.; Kobayashi, N.; Kimura, M.; Nyokong, T. Asymmetrical and symmetrical zinc phthalocyanine-cobalt ferrite conjugates embedded in electrospun fibers for dual photocatalytic degradation of azo dyes: Methyl Orange and Orange G. J. Photochem. Photobiol. A Chem. 2019, 379, 112–122. [Google Scholar] [CrossRef]
- Liu, R.; Hou, Y.; Jiang, H.; Nie, B. Ag(I)-Hived Fullerene Microcube as an Enhanced Catalytic Substrate for the Reduction of 4-Nitrophenol and the Photodegradation of Orange G Dye. Langmuir 2020, 36, 5236–5242. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Y.; Yin, B.; Liu, Y.; Nguyen, T.T.; Zhang, S.; Wei, Y.; Du, C.; Li, L.; Guo, M. Table tennis bat poplar baseplate-derived bismuth molybdate with efficient visible-light photocatalytic activity. Colloid Interface Sci. Commun. 2021, 44, 100478. [Google Scholar] [CrossRef]
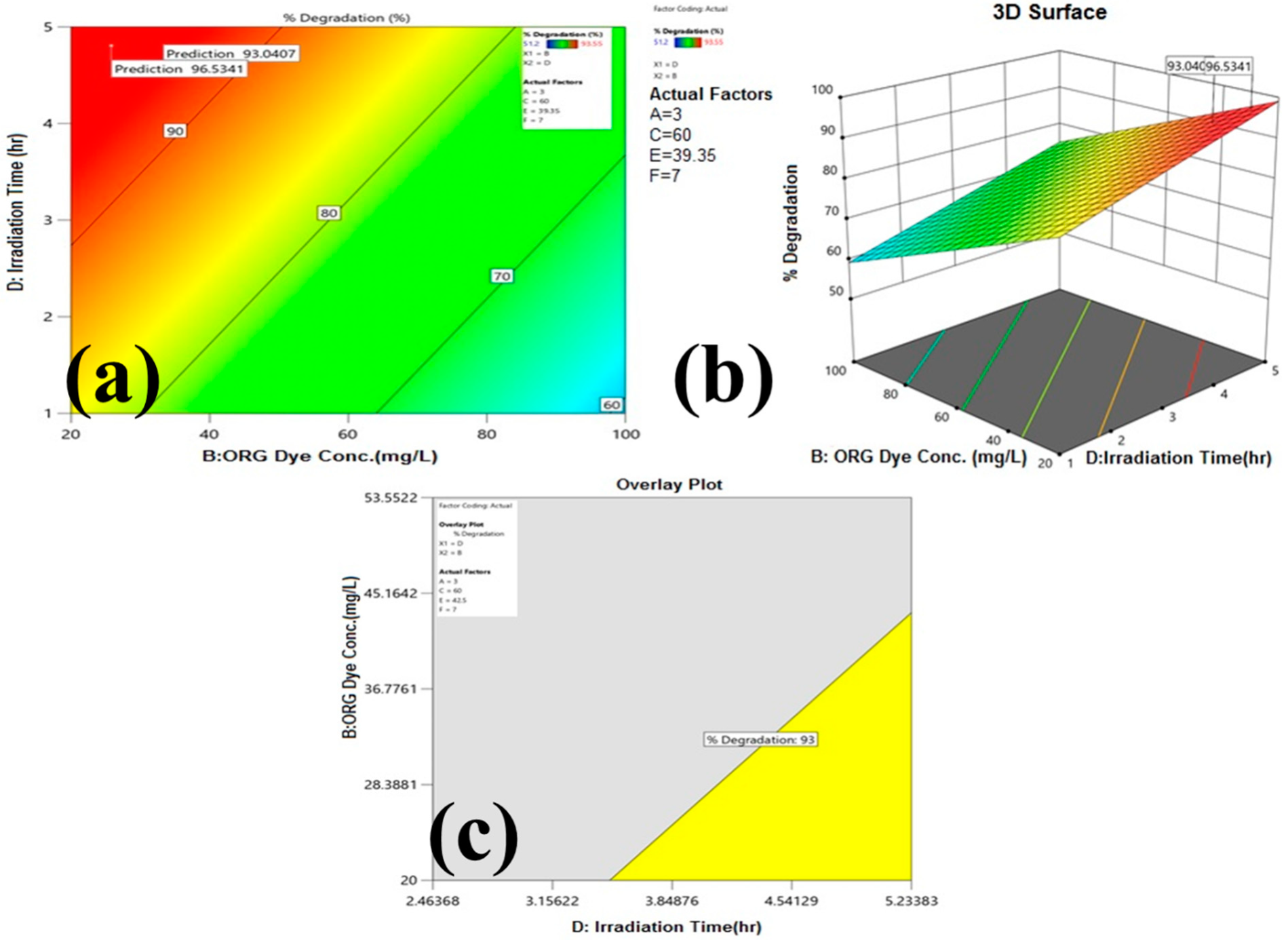
| Design Summary | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| File version | 13.0.2.0 | ||||||||
| Study type | Response Surface | Subtype | Randomized | ||||||
| Design Type | Definitive Screening | Runs | 13 | ||||||
| Design model | Reduced Quadratic | Blocks | No Blocks | ||||||
| Factors and Response | |||||||||
| Factors | Name | Units | Type | Min | Max | Coded low | Coded high | ||
| A | Catalyst Loading | g/L | Numeric | 1 | 5 | −1 | +1 | ||
| B | Effluent Dose | mg/L | Numeric | 20 | 100 | −1 | +1 | ||
| C | H2O2 | Mole | Numeric | 0.6 | 1.4 | −1 | +1 | ||
| D | Irradiation Time | H | Numeric | 1 | 5 | −1 | +1 | ||
| E | Temperature | °C | Numeric | 25 | 60 | −1 | +1 | ||
| F | pH | Numeric | 3 | 11 | −1 | +1 | |||
| Response | Name | Units | Observations | Min | Max | Mean | SD | ||
| R1 | % Degradation | % | 13 | 51.2 | 93.55 | 77.65 | 13.22 | ||
| Design Sheet, Response and model prediction | |||||||||
| F 1 | F 2 | F 3 | F 4 | F 5 | F 6 | Degradation | |||
| Std | Run | A: Catalyst Loading | B: Effluent Dose | C: H2O2 | D: Irradiation Time | E: Temperature | F: pH | Experimental | Predicted |
| g L−1 | mg L−1 | mol L−1 | H | °C | % | % | |||
| 9 | 1 | 5 | 100 | 1.4 | 1 | 42.5 | 3 | 51.2 | 59.33 |
| 1 | 2 | 3 | 100 | 1.4 | 5 | 60 | 11 | 78.93 | 75.29 |
| 10 | 3 | 1 | 20 | 0.6 | 5 | 42.5 | 11 | 79.06 | NA |
| 6 | 4 | 1 | 100 | 1 | 5 | 25 | 3 | 74.12 | 75.29 |
| 11 | 5 | 5 | 20 | 1.4 | 5 | 25 | 7 | 93.55 | 99.03 |
| 8 | 6 | 1 | 20 | 1.4 | 3 | 60 | 3 | 85.12 | 91.05 |
| 12 | 7 | 1 | 100 | 0.6 | 1 | 60 | 7 | 66.63 | 59.33 |
| 5 | 8 | 5 | 20 | 1 | 1 | 60 | 11 | 93.55 | 83.08 |
| 2 | 9 | 3 | 20 | 0.6 | 1 | 25 | 3 | 82.34 | 83.08 |
| 3 | 10 | 5 | 60 | 0.6 | 5 | 60 | 3 | 90.52 | 87.16 |
| 7 | 11 | 5 | 100 | 0.6 | 3 | 25 | 11 | 64 | 67.31 |
| 13 | 12 | 3 | 60 | 1 | 3 | 42.5 | 7 | 87.71 | 79.18 |
| 4 | 13 | 1 | 60 | 1.4 | 1 | 25 | 11 | 62.66 | 71.2 |
| ANOVA for Reduced Linear Model | ||||||
|---|---|---|---|---|---|---|
| Response 1: % Degradation of ORG dye | ||||||
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value | |
| Model | 1618.74 | 2 | 809.37 | 15.26 | 0.0013 | Significant |
| B- Effluent Dose | 1237.98 | 1 | 1237.98 | 23.33 | 0.0009 | |
| D- Irradiation Time | 559.01 | 1 | 559.01 | 10.54 | 0.0101 | |
| Residual | 477.5 | 9 | 53.06 | |||
| Cor Total | 2096.24 | 11 | ||||
| Fit Statistics | ||||||
| SD | 7.28 | R2 | 0.7722 | |||
| Mean | 77.53 | Adjusted R2 | 0.7216 | |||
| C.V. % | 9.4 | Predicted R2 | 0.5931 | |||
| Adeq Precision | 10.8995 | |||||
| Coefficients in Terms of Coded Factors | ||||||
| Factor | Coefficient Estimate | df | Standard Error | 95% CI Low | 95% CI High | VIF |
| Intercept | 79.18 | 1 | 2.12 | 74.37 | 83.99 | |
| B-Effluent Dose | −11.87 | 1 | 2.46 | −17.43 | −6.31 | 1.01 |
| D-Time for Irradiation | 7.98 | 1 | 2.46 | 2.42 | 13.54 | 1.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shukla, B.K.; Rawat, S.; Gautam, M.K.; Bhandari, H.; Garg, S.; Singh, J. Photocatalytic Degradation of Orange G Dye by Using Bismuth Molybdate: Photocatalysis Optimization and Modeling via Definitive Screening Designs. Molecules 2022, 27, 2309. https://doi.org/10.3390/molecules27072309
Shukla BK, Rawat S, Gautam MK, Bhandari H, Garg S, Singh J. Photocatalytic Degradation of Orange G Dye by Using Bismuth Molybdate: Photocatalysis Optimization and Modeling via Definitive Screening Designs. Molecules. 2022; 27(7):2309. https://doi.org/10.3390/molecules27072309
Chicago/Turabian StyleShukla, Brijesh Kumar, Shalu Rawat, Mayank Kumar Gautam, Hema Bhandari, Seema Garg, and Jiwan Singh. 2022. "Photocatalytic Degradation of Orange G Dye by Using Bismuth Molybdate: Photocatalysis Optimization and Modeling via Definitive Screening Designs" Molecules 27, no. 7: 2309. https://doi.org/10.3390/molecules27072309
APA StyleShukla, B. K., Rawat, S., Gautam, M. K., Bhandari, H., Garg, S., & Singh, J. (2022). Photocatalytic Degradation of Orange G Dye by Using Bismuth Molybdate: Photocatalysis Optimization and Modeling via Definitive Screening Designs. Molecules, 27(7), 2309. https://doi.org/10.3390/molecules27072309






