Gas Chromatography Combustion Isotope Ratio Mass Spectrometry to Detect Differences in Four Compartments of Simmental Cows Fed on C3 and C4 Diets
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fatty Acids Quantification and Carbon Isotope Ratio Analysis: Diet Effects
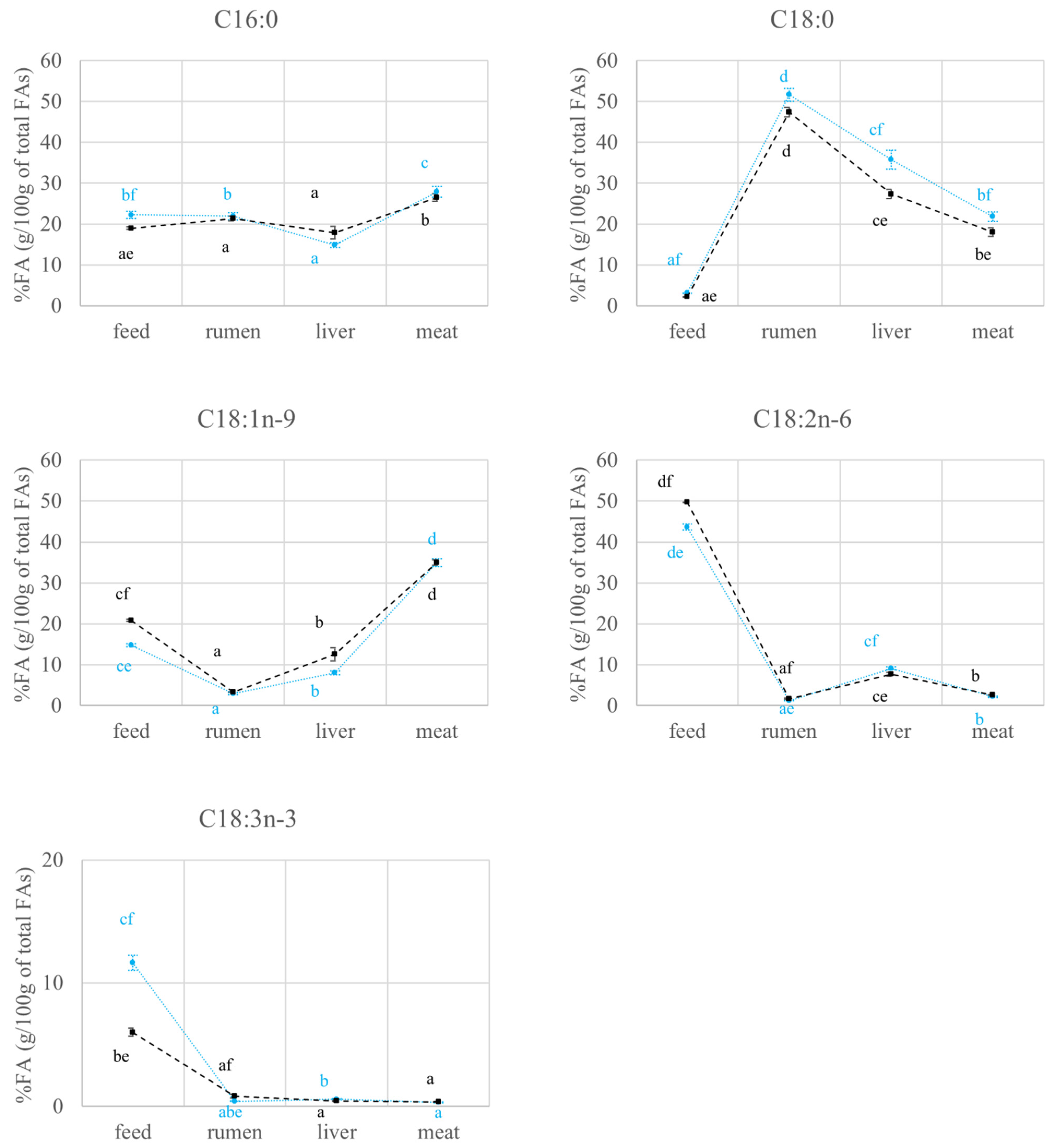
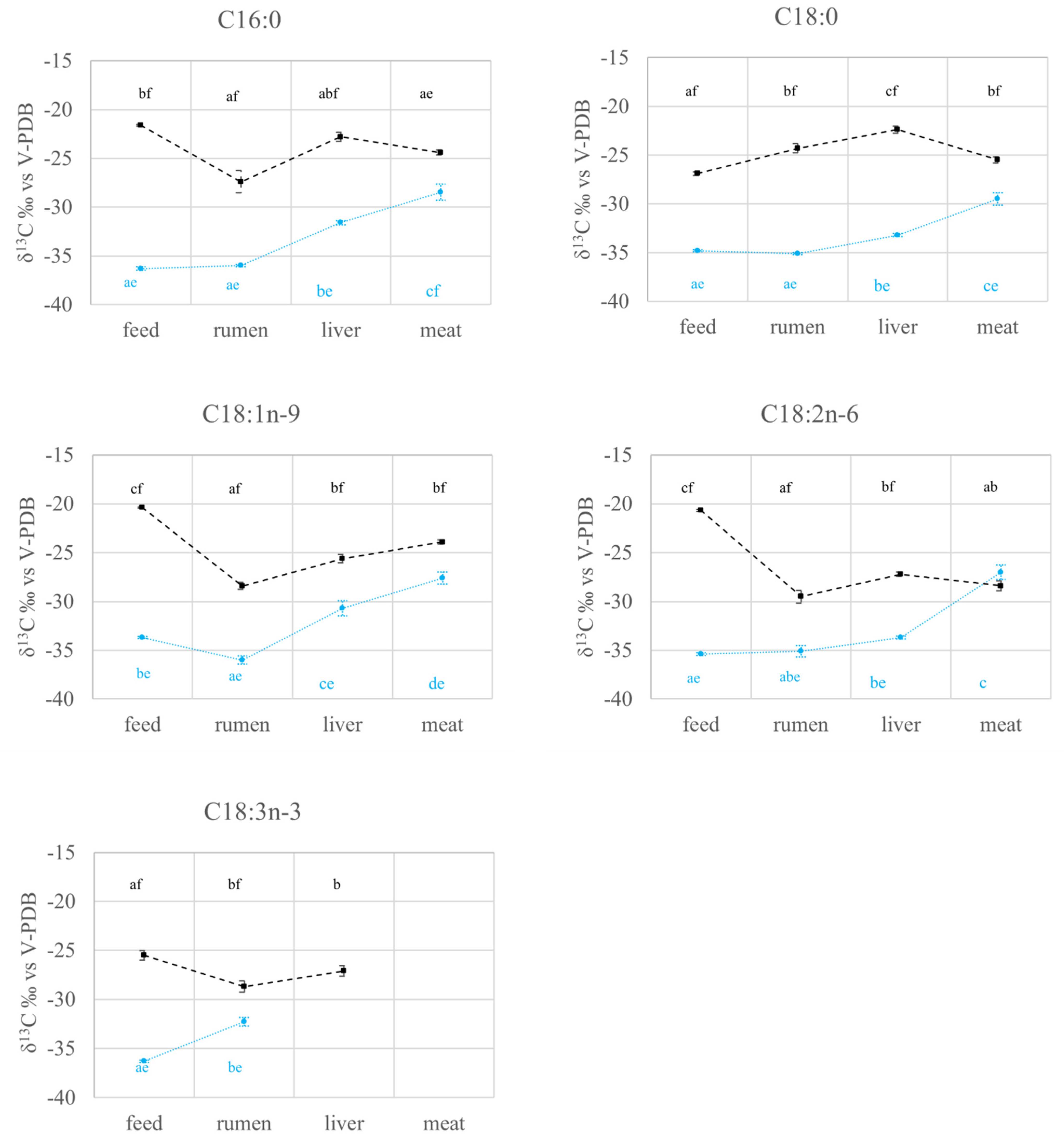
2.2. FAs Quantification and Carbon Isotope Ratio Analysis: Compartment Effects
2.2.1. FAs Quantification and Carbon Isotope Ratio Analysis in Feed and Rumen
2.2.2. FAs Quantification and Carbon Isotope Ratio Analysis in the Liver
2.2.3. FAs Quantification and Carbon Isotope Ratio Analysis in the Meat
3. Materials and Methods
3.1. Description of the Samples
3.1.1. Diet
3.1.2. Slaughter
3.2. Extraction, Derivatization and Quantification of the FAs
3.2.1. Lipid Extraction
3.2.2. Derivatization
3.2.3. FAs Quantification by GC-MS
3.3. Isotopic Analysis of FAs by GC-IRMS
Results Elaboration
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Sample Availability
References
- Galli, C.; Risé, P. Origin of Fatty Acids in the Body: Endogenous Synthesis versus Dietary Intakes. Eur. J. Lipid Sci. Technol. 2006, 108, 521–525. [Google Scholar] [CrossRef]
- Manca, G.; Camin, F.; Coloru, G.C.; Del Caro, A.; Depentori, D.; Franco, M.A.; Versini, G. Characterization of the Geographical Origin of Pecorino Sardo Cheese by Casein Stable Isotope ((13)c/(12)c and (15)n/(14)n) Ratios and Free Amino Acid Ratios. J. Agric. Food Chem. 2001, 49, 1404–1409. [Google Scholar] [CrossRef] [PubMed]
- Bontempo, L.; Barbero, A.; Bertoldi, D.; Camin, F.; Larcher, R.; Perini, M.; Sepulcri, A.; Zicarelli, L.; Piasentier, E. Isotopic and Elemental Profiles of Mediterranean Buffalo Milk and Cheese and Authentication of Mozzarella di Bufala Campana PDO: An Initial Exploratory Study. Food Chem. 2019, 285, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Rossmann, A.; Haberhauer, G.; Hölzl, S.; Horn, P.; Pichlmayer, F.; Voerkelius, S. The Potential of Multielement Stable Isotope Analysis for Regional Origin Assignment of Butter. Eur. Food Res. Technol. 2000, 211, 32–40. [Google Scholar] [CrossRef]
- Pianezze, S.; Camin, F.; Perini, M.; Corazzin, M.; Piasentier, E. Tracing Lamb Meat with Stable Isotope Ratio Analysis: A Review. Small Rumin. Res. 2021, 203, 106482. [Google Scholar] [CrossRef]
- Osorio, M.T.; Moloney, A.P.; Schmidt, O.; Monahan, F.J. Beef Authentication and Retrospective Dietary Verification Using Stable Isotope Ratio Analysis of Bovine Muscle and Tail Hair. J. Agric. Food Chem. 2011, 59, 3295–3305. [Google Scholar] [CrossRef]
- Bahar, B.; Monahan, F.J.; Moloney, A.P.; O’Kiely, P.; Scrimgeour, C.M.; Schmidt, O. Alteration of the Carbon and Nitrogen Stable Isotope Composition of Beef by Substitution of Grass Silage with Maize Silage. Rapid Commun. Mass Spectrom. 2005, 19, 1937–1942. [Google Scholar] [CrossRef]
- De Smet, S.; Balcaen, A.; Claeys, E.; Boeckx, P.; Van Cleemput, O. Stable Carbon Isotope Analysis of Different Tissues of Beef Animals in Relation to Their Diet. Rapid Commun. Mass Spectrom. 2004, 18, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Rojas, J.M.; Vasta, V.; Lanza, A.; Luciano, G.; Ladroue, V.; Guillou, C.; Priolo, A. Stable Isotopes to Discriminate Lambs Fed Herbage or Concentrate Both Obtained from C(3) Plants. Rapid Commun. Mass Spectrom. 2008, 22, 3701–3705. [Google Scholar] [CrossRef]
- Prache, S. Diet Authentication in Sheep from the Composition of Animal Tissues and Products. Rev. Bras. Zootec. 2009, 38, 362–370. [Google Scholar] [CrossRef] [Green Version]
- Galimov, E.M. Causes of Fractionation of Isotopes. Biol. Fractionat. Isot. 1985, 1, 1–15. [Google Scholar]
- Molkentin, J.; Giesemann, A. Differentiation of Organically and Conventionally Produced Milk by Stable Isotope and Fatty Acid Analysis. Anal. Bioanal. Chem. 2007, 388, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Molkentin, J.; Giesemann, A. Follow-up of Stable Isotope Analysis of Organic versus Conventional Milk. Anal. Bioanal. Chem. 2010, 398, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Knobbe, N.; Vogl, J.; Pritzkow, W.; Panne, U.; Fry, H.; Lochotzke, H.M.; Preiss-Weigert, A. C and N Stable Isotope Variation in Urine and Milk of Cattle Depending on the Diet. Anal. Bioanal. Chem. 2006, 386, 104–108. [Google Scholar] [CrossRef]
- Twining, C.W.; Taipale, S.J.; Ruess, L.; Bec, A.; Martin-Creuzburg, D.; Kainz, M.J. Stable Isotopes of Fatty Acids: Current and Future Perspectives for Advancing Trophic Ecology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190641. [Google Scholar] [CrossRef]
- van Leeuwen, K.A.; Prenzler, P.D.; Ryan, D.; Camin, F. Gas Chromatography-Combustion-Isotope Ratio Mass Spectrometry for Traceability and Authenticity in Foods and Beverages. Compr. Rev. Food Sci. Food Saf. 2014, 13, 814–837. [Google Scholar] [CrossRef] [Green Version]
- van Leeuwen, K.A.; Camin, F.; Jerónimo, E.; Vasta, V.; Prenzler, P.D.; Ryan, D.; Bessa, R.J.B. Dietary Effects on Stable Carbon Isotope Composition of Fatty Acids in Polar and Neutral Fractions of Intramuscular Fat of Lambs. J. Agric. Food Chem. 2017, 65, 9404–9411. [Google Scholar] [CrossRef] [PubMed]
- Pianezze, S.; Corazzin, M.; Perini, M.; Camin, F.; Paolini, M.; Sepulcri, A.; Saccà, E.; Fabro, C.; Piasentier, E. Fatty Acids Stable Carbon Isotope Fractionation in the Bovine Organism. A Compound-Specific Isotope Analysis through Gas Chromatography Combustion Isotope Ratio Mass Spectrometry. J. Chromatogr. A 2021, 1641, 461966. [Google Scholar] [CrossRef]
- Richter, E.K.; Spangenberg, J.E.; Willems, H.; Kreuzer, M.; Leiber, F. Stable Carbon Isotope Composition of Perirenal Adipose Tissue Fatty Acids from Engadine Sheep Grazing Either Mountain or Lowland Pasture. J. Anim. Sci. 2012, 90, 905–913. [Google Scholar] [CrossRef] [Green Version]
- Constable, I.J.; Pierce, C.M.; Lai, C.-M.; Magno, A.L.; Degli-Esposti, M.A.; French, M.A.; McAllister, I.L.; Butler, S.; Barone, S.B.; Schwartz, S.D.; et al. Phase 2a Randomized Clinical Trial: Safety and Post Hoc Analysis of Subretinal rAAV.sFLT-1 for Wet Age-Related Macular Degeneration. eBioMedicine 2016, 14, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Chilliard, Y.; Glasser, F.; Ferlay, A.; Bernard, L.; Rouel, J.; Doreau, M. Diet, Rumen Biohydrogenation and Nutritional Quality of Cow and Goat Milk Fat. Eur. J. Lipid Sci. Technol. 2007, 109, 828–855. [Google Scholar] [CrossRef]
- Glasser, F.; Doreau, M.; Maxin, G.; Baumont, R. Fat and Fatty Acid Content and Composition of Forages: A Meta-Analysis. Anim. Feed Sci. Technol. 2013, 185, 19–34. [Google Scholar] [CrossRef]
- Boerman, J.P.; Firkins, J.L.; St-Pierre, N.R.; Lock, A.L. Intestinal Digestibility of Long-Chain Fatty Acids in Lactating Dairy Cows: A Meta-Analysis and Meta-Regression. J. Dairy Sci. 2015, 98, 8889–8903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enjalbert, F.; Combes, S.; Zened, A.; Meynadier, A. Rumen Microbiota and Dietary Fat: A Mutual Shaping. J. Appl. Microbiol. 2017, 123, 782–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craninx, M.; Fievez, V.; Vlaeminck, B.; De Baets, B. Artificial Neural Network Models of the Rumen Fermentation Pattern in Dairy Cattle. Comput. Electron. Agric. 2008, 60, 226–238. [Google Scholar] [CrossRef]
- Doreau, M.; Ferlay, A. Digestion and Utilisation of Fatty Acids by Ruminants. Anim. Feed Sci. Technol. 1994, 45, 379–396. [Google Scholar] [CrossRef]
- Jenkins, T.C. Lipid Metabolism in the Rumen. J. Dairy Sci. 1993, 76, 3851–3863. [Google Scholar] [CrossRef]
- Boschker, H.T.S.; Middelburg, J.J. Stable Isotopes and Biomarkers in Microbial Ecology. FEMS Microbiol. Ecol. 2002, 40, 85–95. [Google Scholar] [CrossRef]
- Leite, R.F.; de Carvalho, V.B.; de Almeida Teixeira, I.A.M. Main driving factors influencing passage kinetics in ruminants. Ciênc. Anim. 2018, 28, 98–113. [Google Scholar]
- Bickhart, D.M.; Weimer, P.J. Symposium Review: Host-Rumen Microbe Interactions May Be Leveraged to Improve the Productivity of Dairy Cows. J. Dairy Sci. 2018, 101, 7680–7689. [Google Scholar] [CrossRef]
- Bruss, M.L. Metabolic Fatty Liver of Ruminants. Adv. Vet. Sci. Comp. Med. 1993, 37, 417–449. [Google Scholar] [PubMed]
- Hocquette, J.F.; Bauchart, D. Intestinal Absorption, Blood Transport and Hepatic and Muscle Metabolism of Fatty Acids in Preruminant and Ruminant Animals. Reprod. Nutr. Dev. 1999, 39, 27–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bionaz, M.; Vargas-Bello-Pérez, E.; Busato, S. Advances in fatty acids nutrition in dairy cows: From gut to cells and effects on performance. J. Anim. Sci. Biotech. 2020, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Wurie, H.R.; Buckett, L.; Zammit, V.A. Diacylglycerol acyltransferase 2 acts upstream of diacylglycerol acyltransferase 1 and utilizes nascent diglycerides and de novo synthesized fatty acids in HepG2 cells. FEBS J. 2012, 279, 3033–3047. [Google Scholar] [CrossRef]
- Cadorniga-Valino, C.; Grummer, R.R.; Armentano, L.E.; Donkin, S.S.; Bertics, S.J. Effects of fatty acids and hormones on fatty acid metabolism and gluconeogenesis in bovine hepatocytes. J. Dairy Sci. 1997, 80, 646–656. [Google Scholar] [CrossRef]
- Zammit, V.A. Role of insulin in hepatic fatty acid partitioning: Emerging concepts. Biochem. J. 1996, 314, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Van der Walt, J. Nitrogen metabolism of the ruminant liver. Aust. J. Agric. Sci. 1993, 44, 381–403. [Google Scholar] [CrossRef]
- Pearce, J. Fatty acid synthesis in liver and adipose tissue. Proc. Nutr. Soc. 1983, 42, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Pethick, D.W.; Dunshea, F.R. Fat metabolism and turnover. In Quantitative Aspects of Ruminant Digestion and Metabolism; Forbes, J.M., France, J., Eds.; CAB International: Wallingford, UK, 1993; pp. 291–311. [Google Scholar]
- Smith, S.B.; Lunt, D.K.; Chung, K.Y.; Choi, C.B.; Tume, R.K.; Zembayashi, M. Adiposity, Fatty Acid Composition, and Delta-9 Desaturase Activity during Growth in Beef Cattle. Anim. Sci. J. 2006, 77, 478–486. [Google Scholar] [CrossRef]
- Sampath, H.; Ntambi, J.M. The Fate and Intermediary Metabolism of Stearic Acid. Lipids 2005, 40, 1187–1191. [Google Scholar] [CrossRef]
- Richter, E.K.; Spangenberg, J.E.; Klevenhusen, F.; Soliva, C.R.; Kreuzer, M.; Leiber, F. Stable Carbon Isotope Composition of c9,t11-Conjugated Linoleic Acid in Cow’s Milk as Related to Dietary Fatty Acids. Lipids 2012, 47, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Edmonson, J.M. Gastric Freezing: The View a Quarter Century Later. J. Lab. Clin. Med. 1989, 114, 613–614. [Google Scholar]
- Agabriel, J.; Institut National de la Recherche Agronomique (France). Alimentation des Bovins, Ovins et Caprins: Besoins des Animaux, Valeurs des Aliments: Tables Inra 2007; Editions Quae: Versailles Cedex, France, 2007; ISBN 9782759200207. [Google Scholar]
- Romanzin, A.; Corazzin, M.; Piasentier, E.; Bovolenta, S. Concentrate Supplement Modifies the Feeding Behavior of Simmental Cows Grazing in Two High Mountain Pastures. Animals 2018, 8, 76. [Google Scholar] [CrossRef] [Green Version]
- Corazzin, M.; Berlese, M.; Sturaro, E.; Ramanzin, M.; Gallo, L.; Aprea, E.; Gasperi, F.; Gianelle, D.; Bovolenta, S. Effect of Feeding Adaptation of Italian Simmental Cows before Summer Grazing on Animal Behavior and Milk Characteristics. Animals 2020, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Pahl, C.; Hartung, E.; Grothmann, A.; Mahlkow-Nerge, K.; Haeussermann, A. Suitability of Feeding and Chewing Time for Estimation of Feed Intake in Dairy Cows. Animal 2016, 10, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 279, 3033–3047. [Google Scholar] [CrossRef]
- Perini, M.; Paolini, M.; Pace, R.; Camin, F. The Use of Stable Isotope Ratio Analysis to Characterise Saw Palmetto (Serenoa Repens) Extract. Food Chem. 2019, 274, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Wang and LA Goonewardene, Z. The Use of MIXED Models in the Analysis of Animal Experiments with Repeated Measures Data. Can. J. Anim. Sci. 2004, 84, 1–11. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pianezze, S.; Corazzin, M.; Bontempo, L.; Sepulcri, A.; Saccà, E.; Perini, M.; Piasentier, E. Gas Chromatography Combustion Isotope Ratio Mass Spectrometry to Detect Differences in Four Compartments of Simmental Cows Fed on C3 and C4 Diets. Molecules 2022, 27, 2310. https://doi.org/10.3390/molecules27072310
Pianezze S, Corazzin M, Bontempo L, Sepulcri A, Saccà E, Perini M, Piasentier E. Gas Chromatography Combustion Isotope Ratio Mass Spectrometry to Detect Differences in Four Compartments of Simmental Cows Fed on C3 and C4 Diets. Molecules. 2022; 27(7):2310. https://doi.org/10.3390/molecules27072310
Chicago/Turabian StylePianezze, Silvia, Mirco Corazzin, Luana Bontempo, Angela Sepulcri, Elena Saccà, Matteo Perini, and Edi Piasentier. 2022. "Gas Chromatography Combustion Isotope Ratio Mass Spectrometry to Detect Differences in Four Compartments of Simmental Cows Fed on C3 and C4 Diets" Molecules 27, no. 7: 2310. https://doi.org/10.3390/molecules27072310