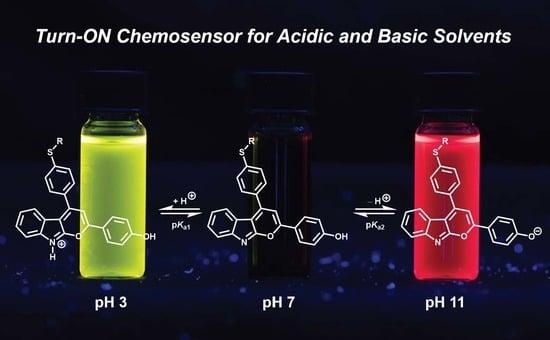
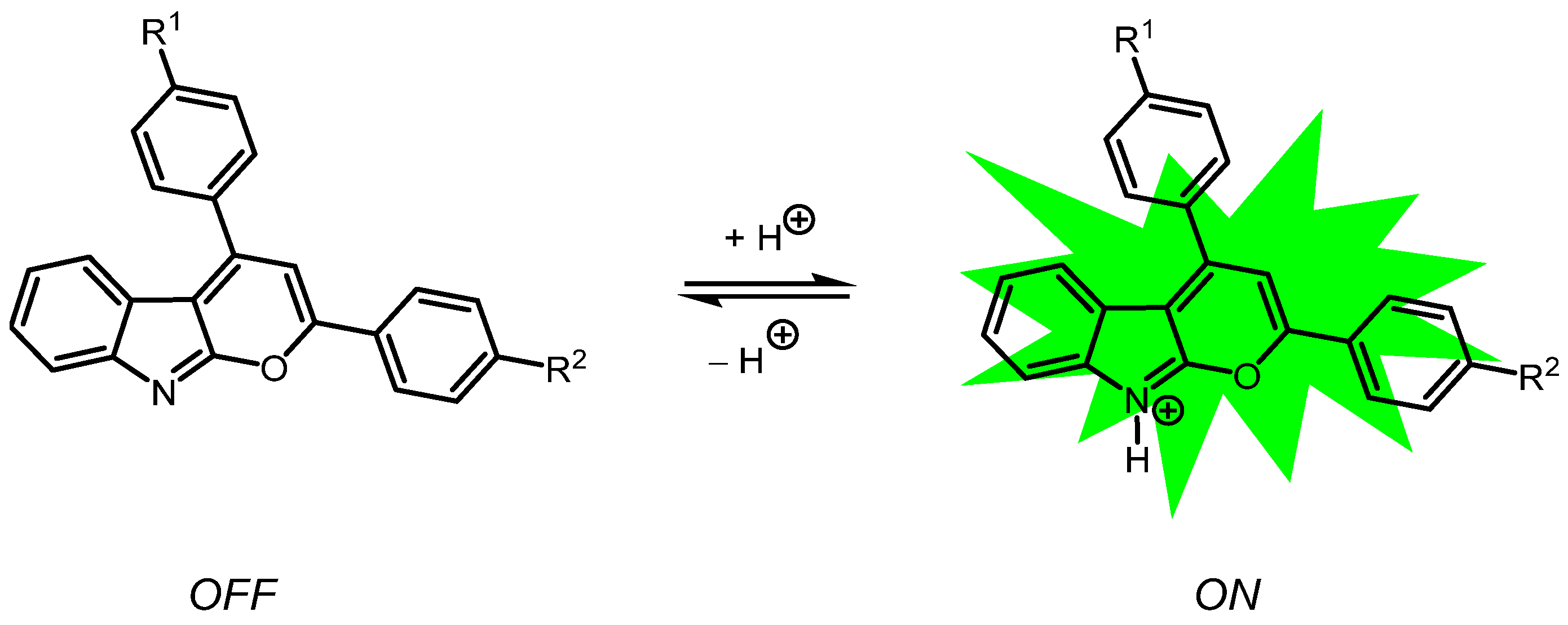
Amphipolar, Amphiphilic 2,4-diarylpyrano[2,3-b]indoles as Turn-ON Luminophores in Acidic and Basic Media
Abstract
:1. Introduction
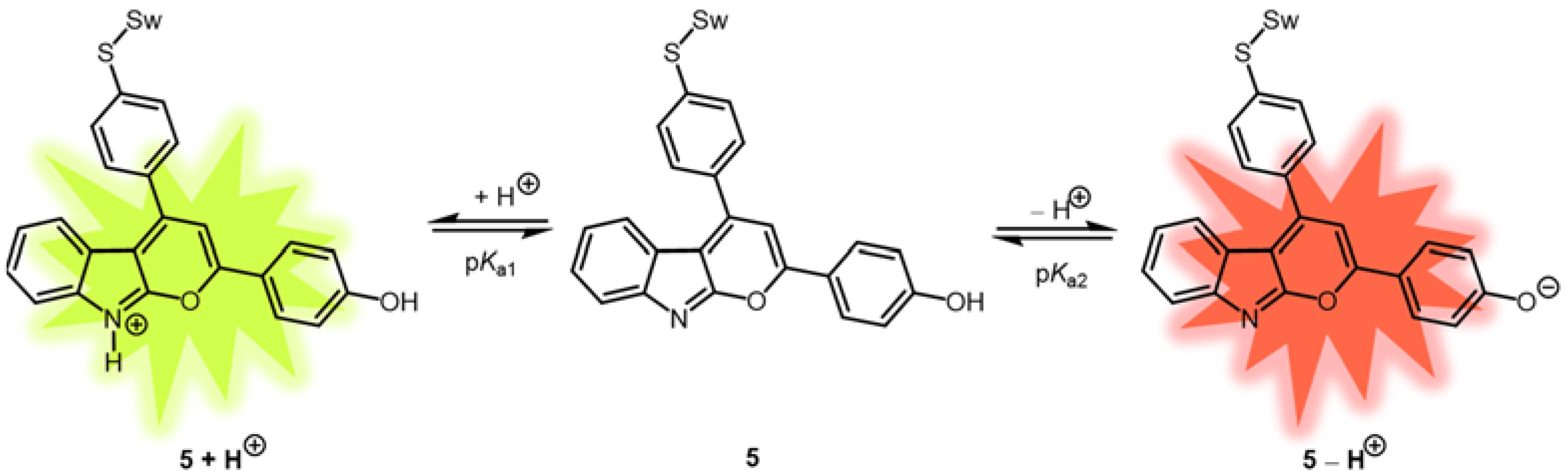
2. Results and Discussion
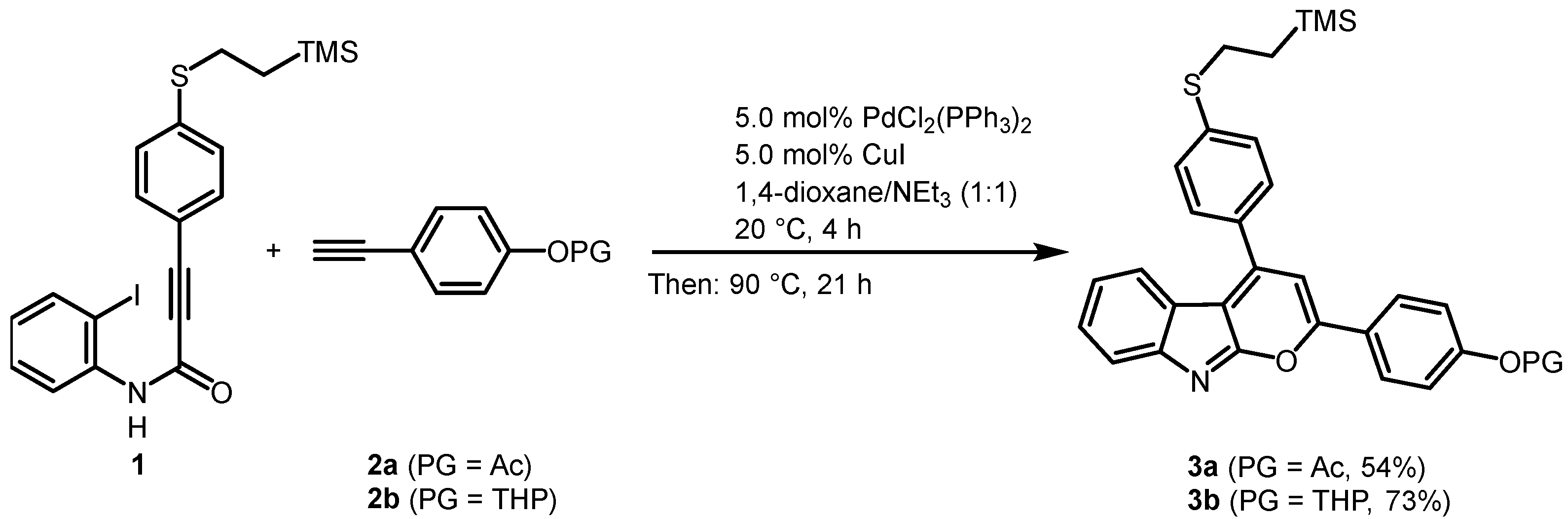
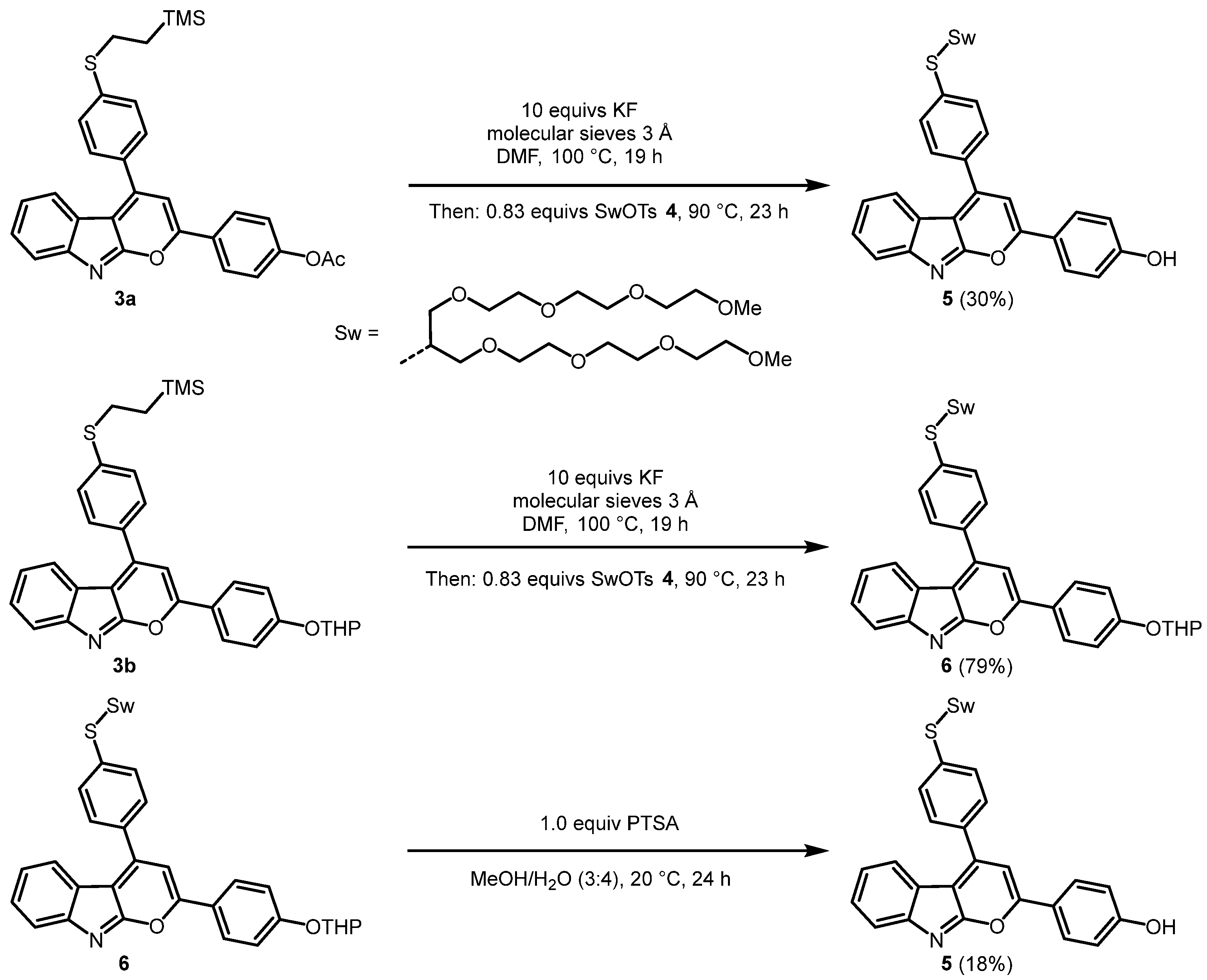
2.1. Synthesis of the 2,4-diarylpyrano[2,3-b]indole Chromophores
2.2. Solubility of 2,4-diarylpyrano[2,3-b]indole 5
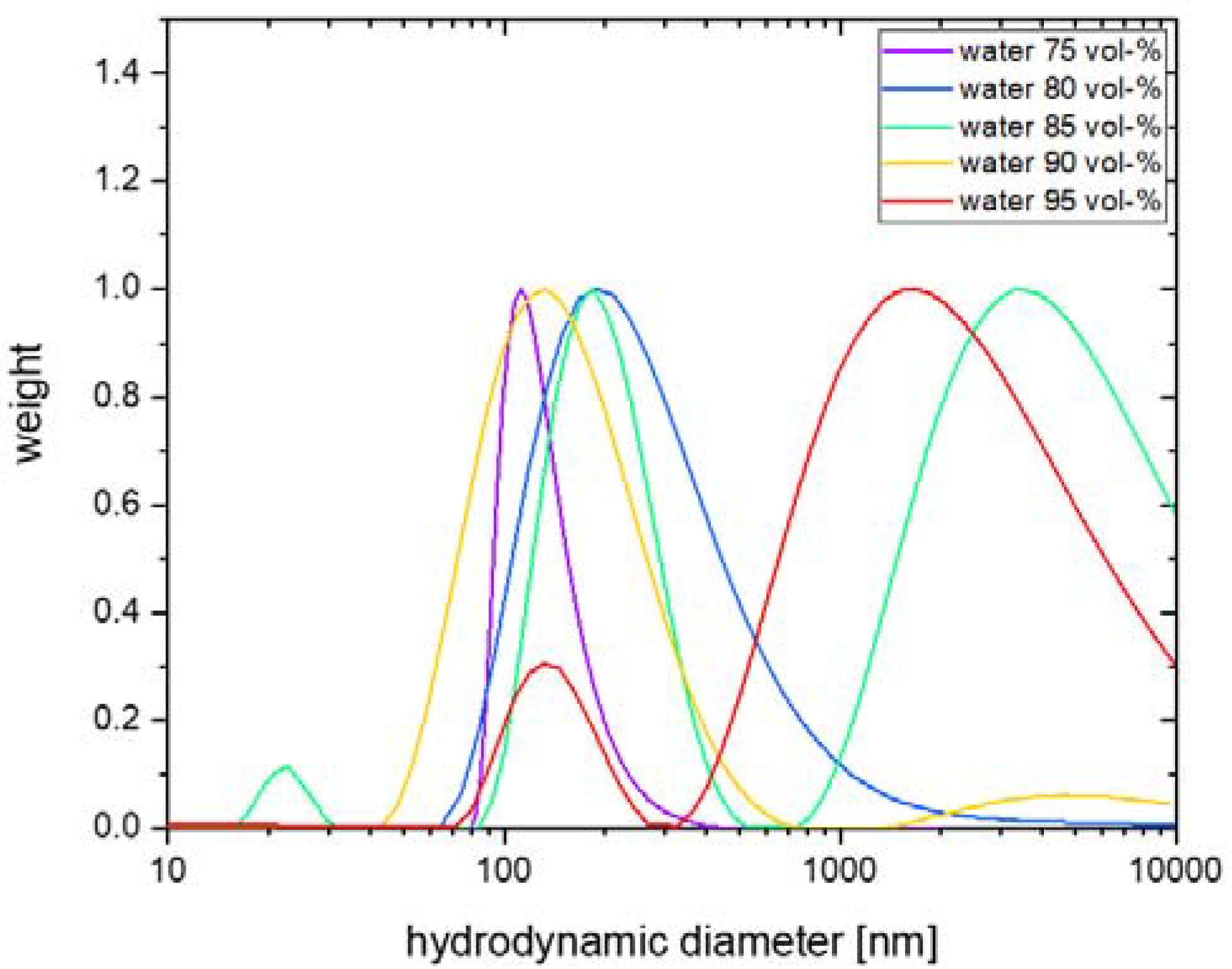
2.3. Particle size of 2,4-diarylpyrano[2,3-b]indole 5 in Aqueous Solutions
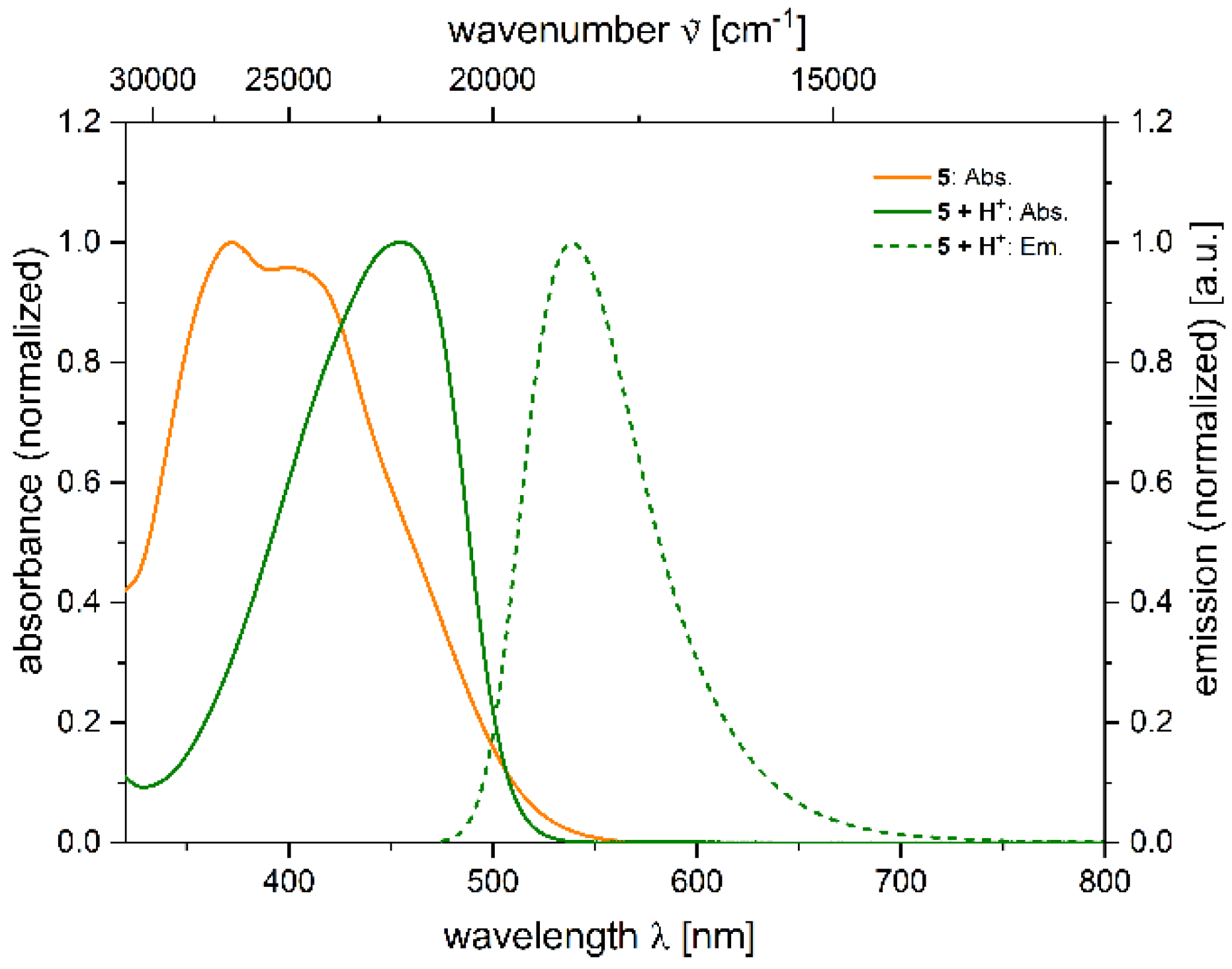
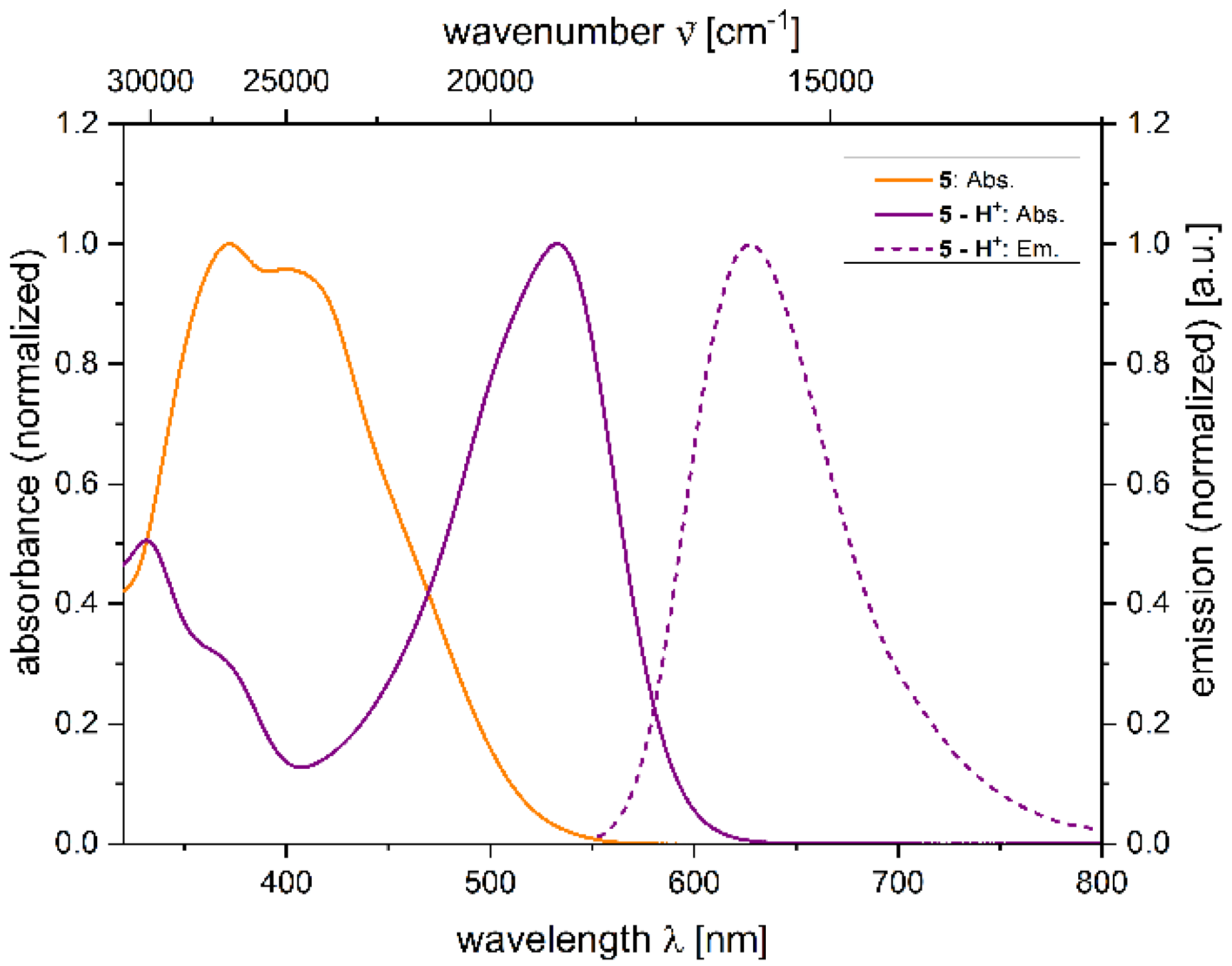
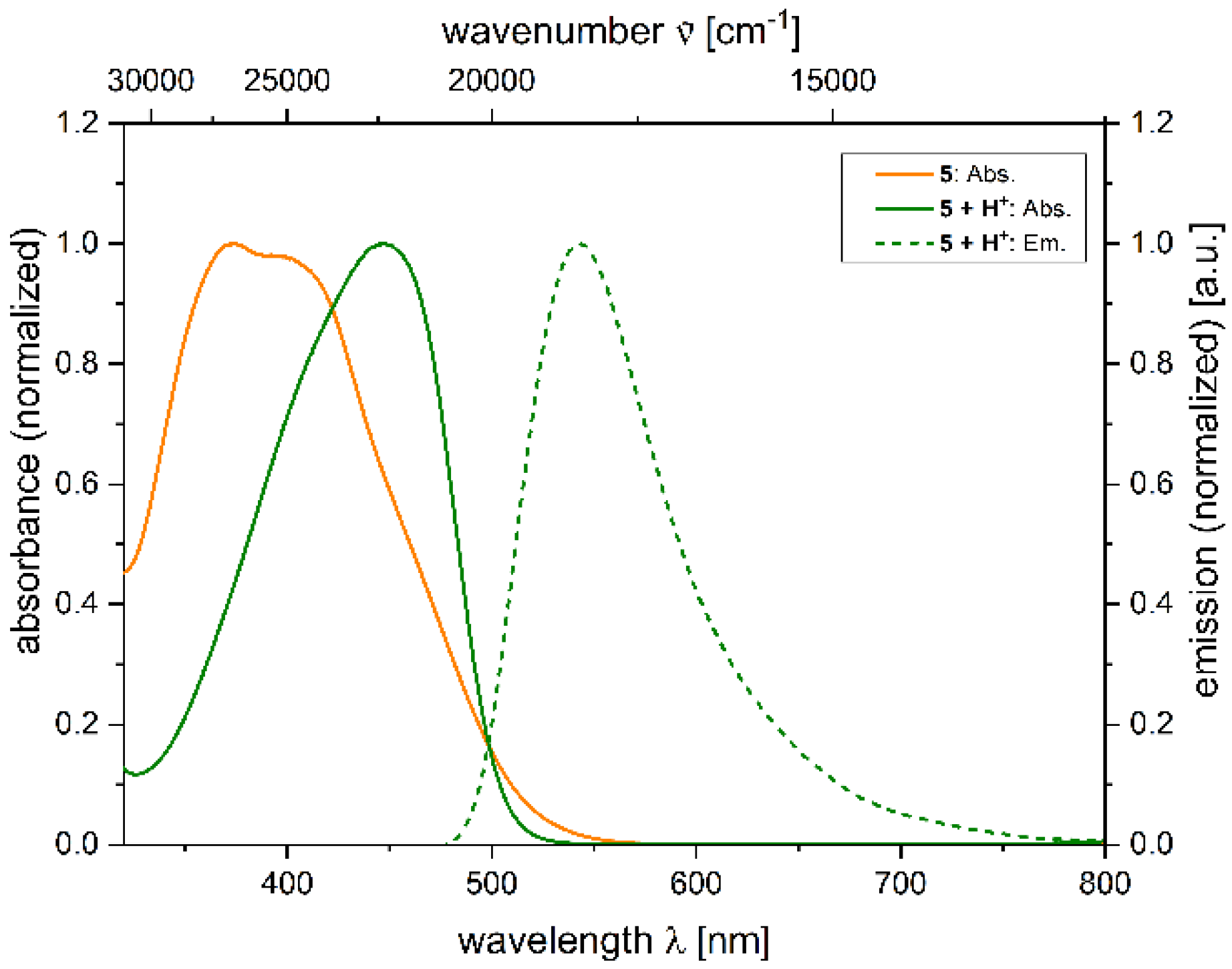
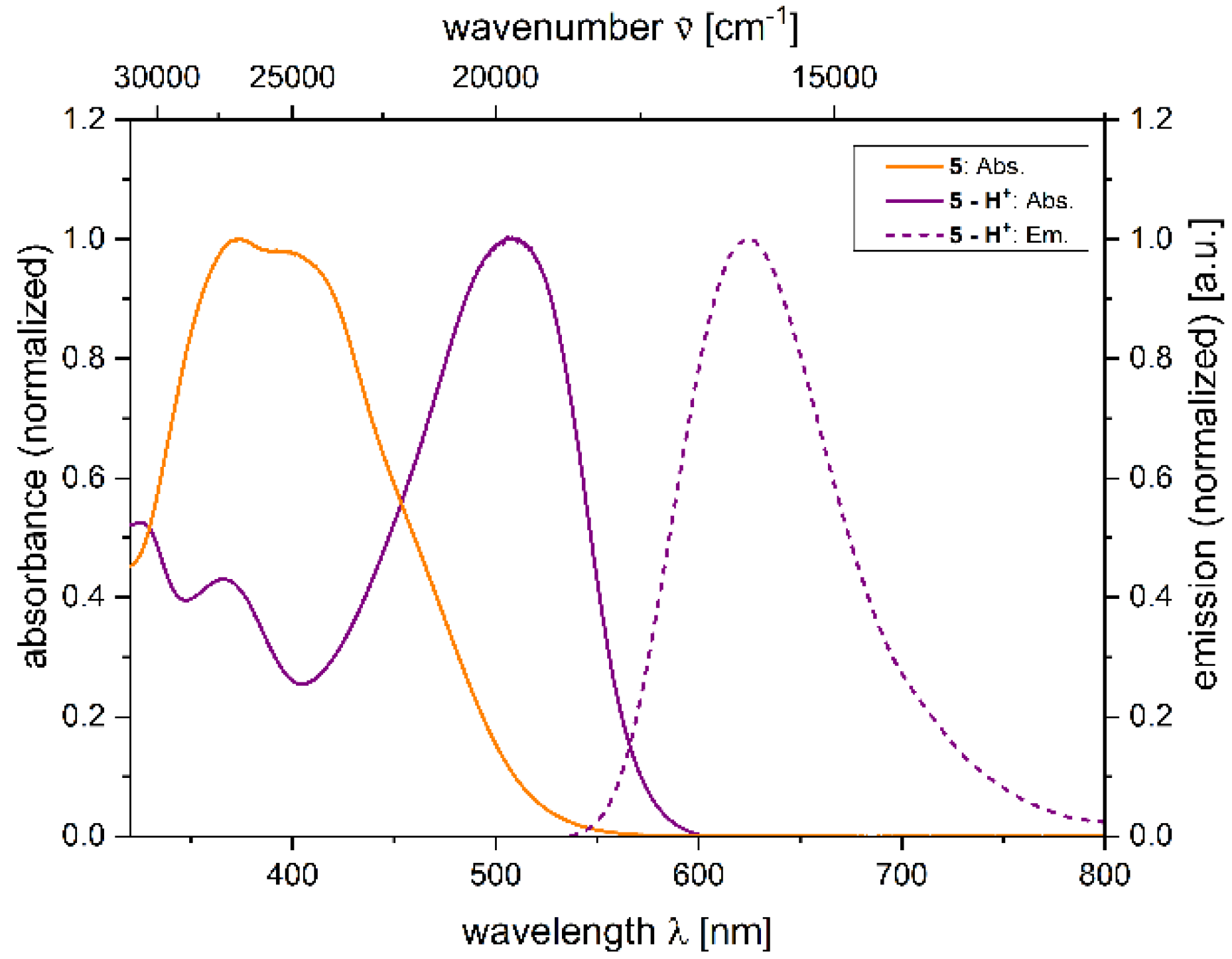
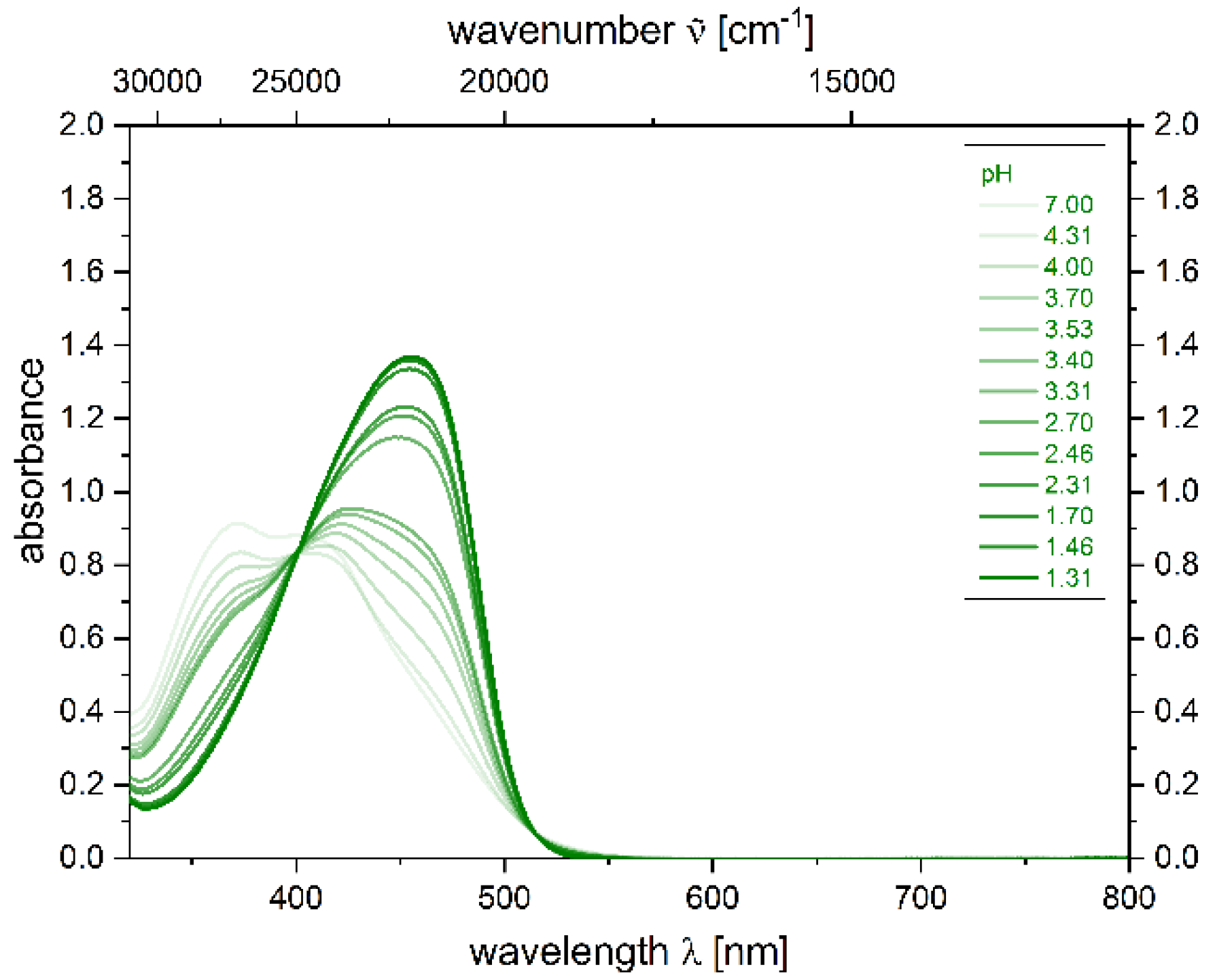
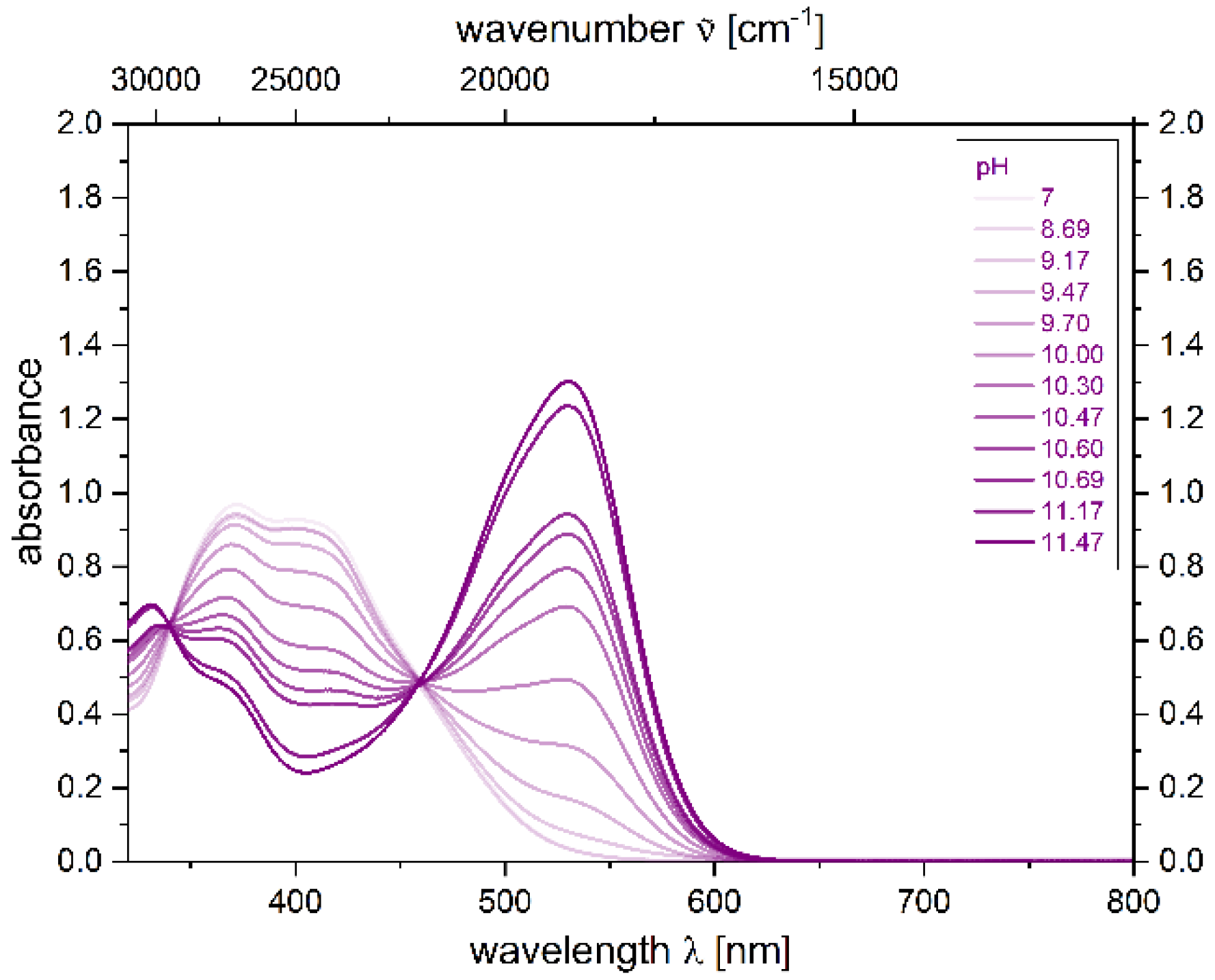
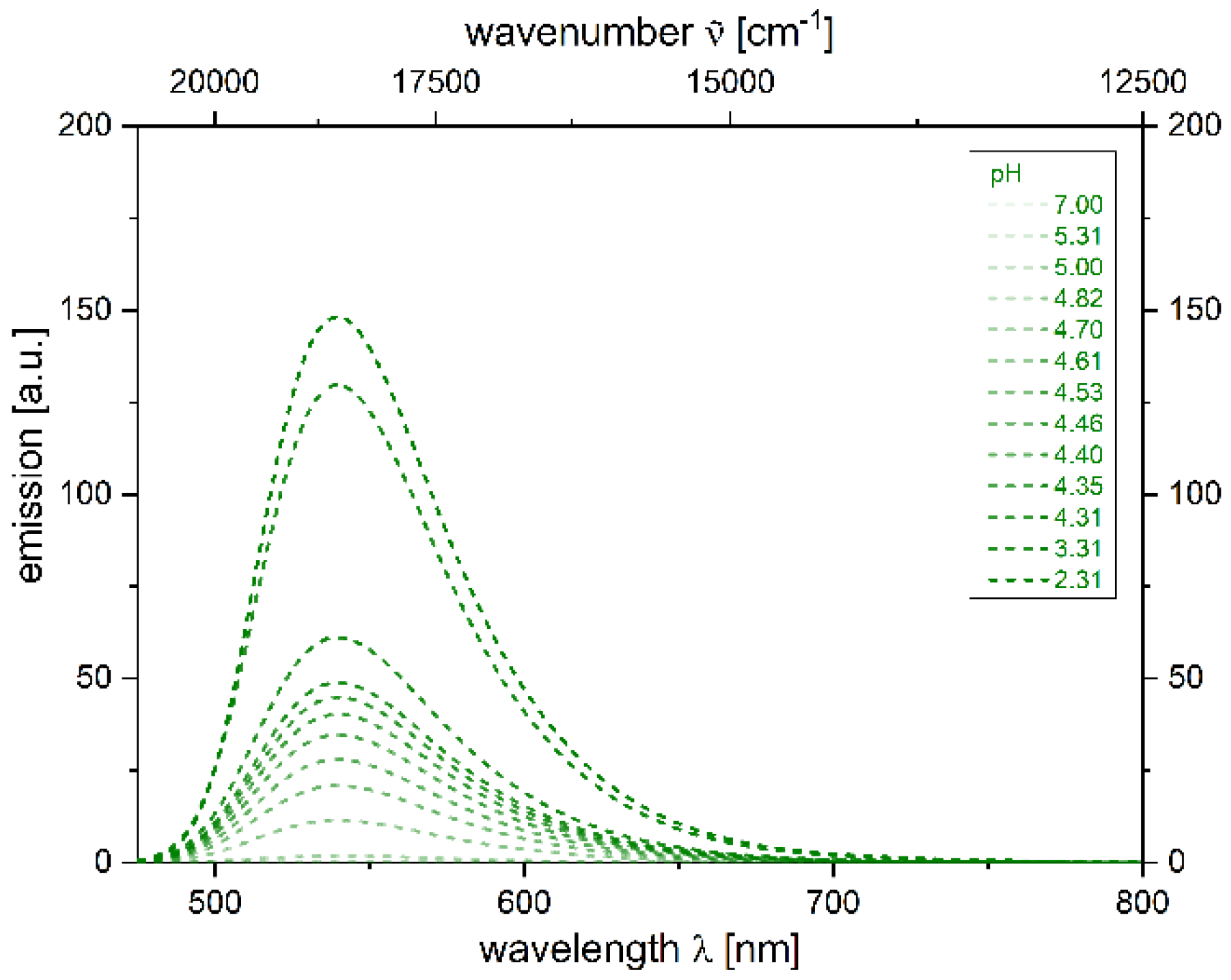
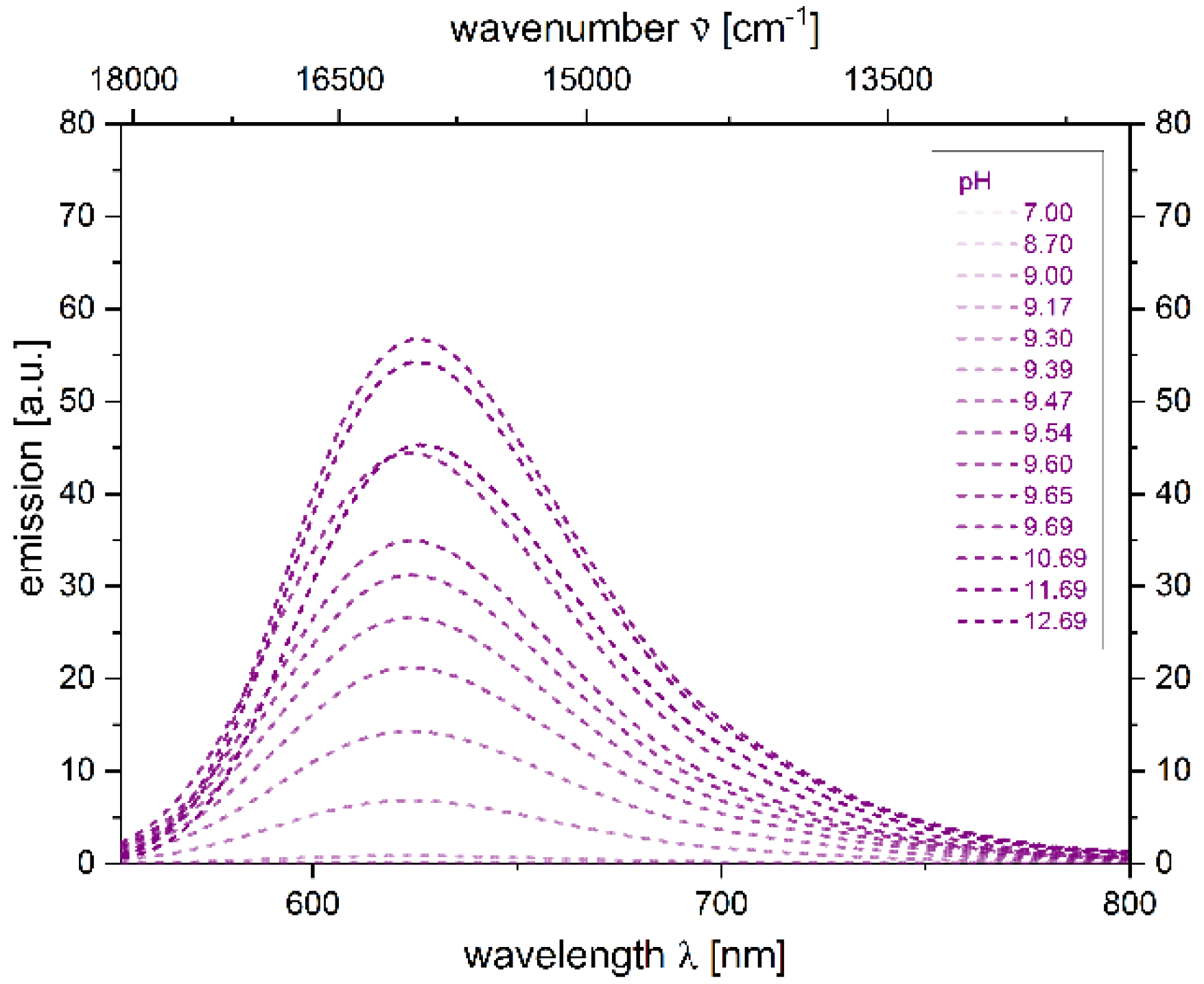
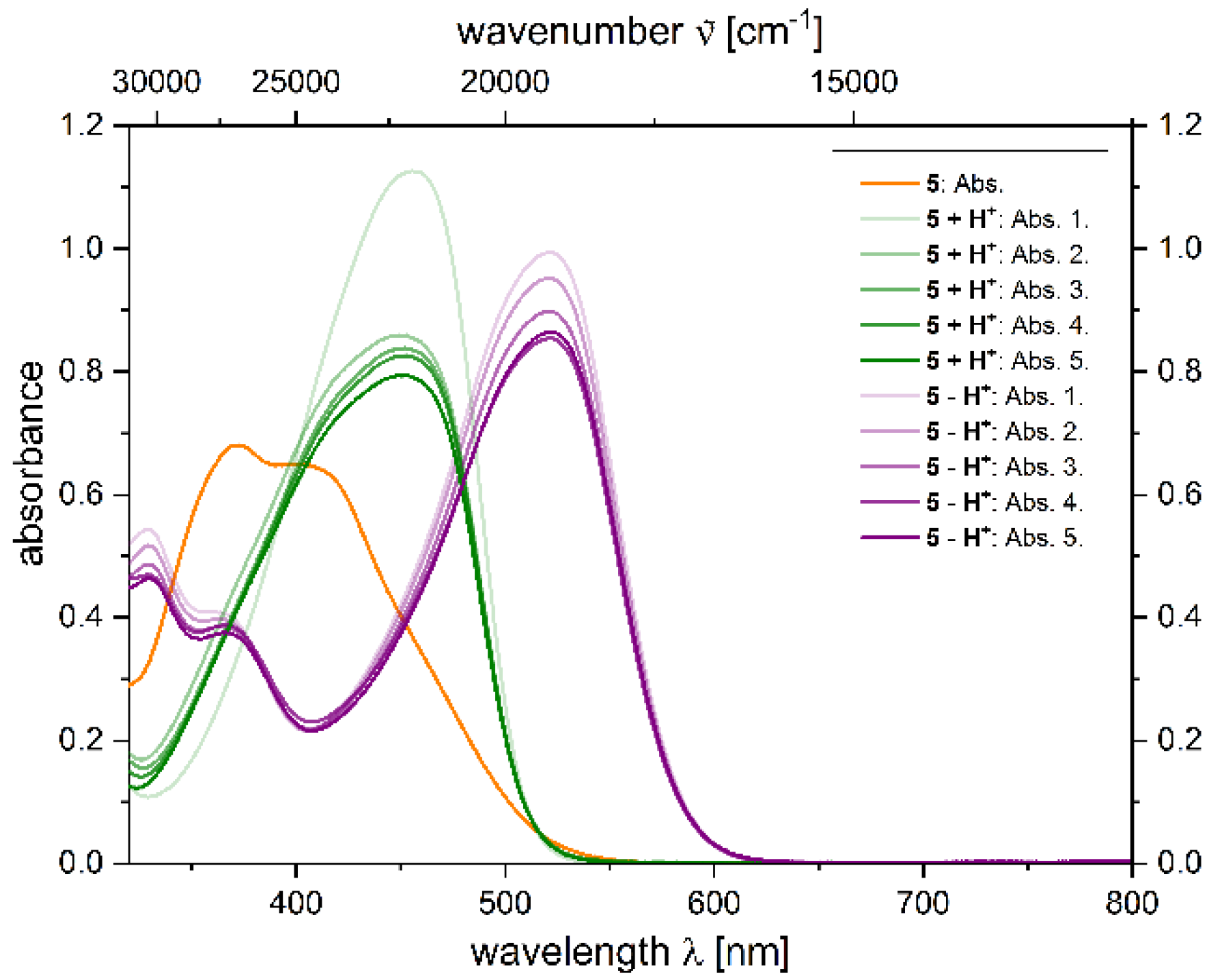
2.4. Photophysical Properties of 2,4-diarylpyrano[2,3-b]indole 5
3. Materials and Methods
3.1. General Considerations
3.2. Synthesis of 4-(4-(4-((2-(trimethylsilyl)ethyl)thio)phenyl)pyrano[2,3-b]indol-2-yl)phenyl acetate (3a)
3.3. Synthesis of 2-(4-((tetrahydro-2H-pyran-2-yl)oxy)phenyl)-4-(4-((2-(trimethylsilyl)ethyl)-thio)phenyl)pyrano[2,3-b]indole (3b)
3.4. Synthesis of 4-(4-(4-((2,5,8,11,15,18,21,24-octaoxapentacosan-13-yl)thio)phenyl)-pyrano[2,3-b]indol-2-yl)phenol (5)
3.5. Synthesis of 4-(4-((2,5,8,11,15,18,21,24-octaoxapentacosan-13-yl)thio)phenyl)-2-(4-((tetrahydro-2H-pyran-2-yl)oxy)phenyl)pyrano[2,3-b]indole (6)
3.6. Alternative Synthesis of 4-(4-(4-((2,5,8,11,15,18,21,24-octaoxapentacosan-13-yl)thio)phenyl)-pyrano[2,3-b]indol-2-yl)phenol (5)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Müller, T.J.J.; Bunz, U.H.F. (Eds.) Functional Organic Materials; Wiley-VHC: Weinheim, Germany, 2007. [Google Scholar] [CrossRef]
- Xie, L.; Tang, W.; Liu, Z.; Tang, W.; Yuan, Z.; Qin, Y.; Yan, L.; Zhu, X.; Zhu, W.; Wang, X. Effects of side-chain engineering with the S Atom in Thieno3,2-bthiophene-porphyrin to obtain small-molecule donor materials for organic solar cells. Molecules 2021, 26, 6134. [Google Scholar] [CrossRef] [PubMed]
- Braveenth, R.; Bae, I.-J.; Han, J.-H.; Qiong, W.; Seon, G.; Raagulan, K.; Yang, K.; Park, Y.H.; Kim, M.; Chai, K.Y. Utilizing a spiro core with acridine-and phenothiazine-based new hole transporting materials for highly efficient green phosphorescent organic light-emitting diodes. Molecules 2018, 23, 713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Zhang, Y.; Wang, H.; Zhang, S. Novel conjugated polymers prepared by direct (Hetero) arylation: An eco-friendly tool for organic electronics. Molecules 2018, 23, 408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, B.; Khalid, M.; Asim, S.; Khan, M.U.; Iqbal, Z.; Hussain, A.; Hussain, R.; Ahmed, S.; Ali, A.; Hussain, A.; et al. Key electronic, linear and nonlinear optical properties of designed disubstituted quinoline with carbazole compounds. Molecules 2021, 26, 2760. [Google Scholar] [CrossRef]
- Bracciale, M.P.; Kwon, G.; Ho, D.; Kim, C.; Santarelli, M.L.; Marrocchi, A. Synthesis, characterization, and thin-film transistor response of Benzoipentahelicene-3,6-dione. Molecules 2022, 27, 863. [Google Scholar] [CrossRef]
- Guo, R.; Leng, P.; Zhang, Q.; Wang, Y.; Lv, X.; Sun, S.; Ye, S.; Duan, Y.; Wang, L. Donor engineering for diphenylsulfone derivatives with both thermally activated delayed fluorescence and aggregation-induced emission properties. Dyes Pigm. 2021, 184, 108781. [Google Scholar] [CrossRef]
- Chen, G.; Qiu, Z.; Tan, J.-H.; Chen, W.-C.; Zhou, P.; Xing, L.; Ji, S.; Qin, Y.; Zhao, Z.; Huo, Y. Deep-blue organic light-emitting diodes based on push-pull π-extended imidazole-fluorene hybrids. Dyes Pigm. 2021, 184, 108754. [Google Scholar] [CrossRef]
- Zheng, B.; Huo, L. Recent advances of dithienobenzodithiophene-based organic semiconductors for organic electronics. Sci. China Chem. 2021, 64, 358–384. [Google Scholar] [CrossRef]
- Mokhtar, S.M.A.; Alvarez de Eulate, E.; Yamada, M.; Prow, T.W.; Evans, D.R. Conducting polymers in wearable devices. Med. Devices Sens. 2021, 4, e10160. [Google Scholar] [CrossRef]
- Che, Y.; Zhang, Y.; Yang, Y.; Liu, C.-H.; Izquierdo, R.; Xiao, S.S.; Perepichka, D.F. Understanding the photovoltaic behavior of A-D-A molecular semiconductors through a permutation of end groups. J. Org. Chem. 2020, 85, 52–61. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, X.; Tsui, G.C.; Miao, Q. Trifluoromethylation of anthraquinones for n-Type organic semiconductors in field effect transistors. J. Org. Chem. 2020, 85, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Shankar, S.; Philips, D.S.; Ajayaghosh, A. Diketopyrrolopyrrole-based functional supramolecular polymers: Next-generation materials for optoelectronic applications. Mater. Today Chem. 2020, 16, 100242. [Google Scholar] [CrossRef]
- Arias-Gómez, A.; Godoy, A.; Portilla, J. Functional Pyrazolo1,5-apyrimidines: Current approaches in synthetic transformations and uses as an antitumor scaffold. Molecules 2021, 26, 2708. [Google Scholar] [CrossRef] [PubMed]
- Ravichandiran, P.; Boguszewska-Czubara, A.; Masłyk, M.; Bella, A.P.; Johnson, P.M.; Subramaniyan, S.A.; Shim, K.S.; Yoo, D.J. A phenoxazine-based fluorescent chemosensor for dual channel detection of Cd2+ and CN-ions and its application to bio-imaging in live cells and zebrafish. Dyes Pigm. 2020, 172, 107828. [Google Scholar] [CrossRef]
- Municoy, S.; Álvarez Echazú, M.I.; Antezana, P.E.; Galdopórpora, J.M.; Olivetti, C.; Mebert, A.M.; Foglia, M.L.; Tuttolomondo, M.V.; Alvarez, G.S.; Hardy, J.G.; et al. Stimuli-Responsive materials for tissue engineering and drug delivery. Int. J. Mol. Sci. 2020, 21, 4724. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, F.; Linhardt, R.J. Porphyrin-based compounds and their applications in materials and medicine. Dyes Pigm. 2021, 188, 109136. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Xu, Y.; Li, L.; Sun, Y.; Huang, W. Recent advances in the development of NIR-II organic emitters for biomedicine. Coord. Chem. Rev. 2020, 415, 213318. [Google Scholar] [CrossRef]
- Wu, D.; Zhou, J.; Creyer, M.N.; Yim, W.; Chen, Z.; Messersmith, P.B.; Jokerst, J.V. Phenolic-enabled nanotechnology: Versatile particle engineering for biomedicine. Chem. Soc. Rev. 2021, 50, 4432–4483. [Google Scholar] [CrossRef]
- Khan, S.A.; Kumar, P. Photophysical and Physicochemical investigation of newly synthesized polycyclic pyrazoline-benzothiazole as fluorescence Chemosensor for the detection of Cu2+ metal ion. Polycycl. Aromat. Compd. 2021, 41, 576–592. [Google Scholar] [CrossRef]
- Tavallali, H.; Deilamy-Rad, G.; Parhami, A.; Asghari, K.; Ahmadi, A. Bismuth triggered selective colorimetric naked-eye detection for oxalate ions based on bromopyrogallol red that works as a molecular keypad lock. Int. J. Environ. Anal. Chem. 2021, 101, 648–667. [Google Scholar] [CrossRef]
- Diana, R.; Caruso, U.; Di Costanzo, L.; Gentile, F.S.; Panunzi, B. Colorimetric recognition of multiple first-row transition metals: A single water-soluble chemosensor in acidic and basic conditions. Dyes Pigm. 2021, 184, 108832. [Google Scholar] [CrossRef]
- Sánchez-Portillo, P.; Hernández-Sirio, A.; Godoy-Alcántar, C.; Lacroix, P.G.; Agarwal, V.; Santillán, R.; Barba, V. Colorimetric metal ion (II) Sensors Based on imine boronic esters functionalized with pyridine. Dyes Pigm. 2021, 186, 108991. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, B.; Wang, B.; Fan, P.; Jiu, Y.; Zhang, M.; Jiang, L.; Hou, J.-T. A highly selective phenothiazine-based fluorescent chemosensor for phosgene. Dyes Pigm. 2020, 173, 107933. [Google Scholar] [CrossRef]
- Samanta, S.K.; Maiti, K.; Ali, S.S.; Guria, U.N.; Ghosh, A.; Datta, P.; Mahapatra, A.K. A solvent directed D-π-A fluorescent chemodosimeter for selective detection of hazardous hydrazine in real water sample and living cell. Dyes Pigm. 2020, 173, 107997. [Google Scholar] [CrossRef]
- Malkondu, S.; Erdemir, S.; Karakurt, S. Red and blue emitting fluorescent probe for cyanide and hypochlorite ions: Biological sensing and environmental analysis. Dyes Pigm. 2020, 174, 108019. [Google Scholar] [CrossRef]
- Bathula, C.; Henry, O.; Ashok Kumar, K.; Subalakshmi, K.; Jana, A.; Rabani, I.; Choi, J.-H.; Jeon, J.-H.; Kim, H.-S. Facile synthesis of an indacenodithiophene-based conjugated polymer for acid vapor sensing. Dyes Pigm. 2021, 184, 108847. [Google Scholar] [CrossRef]
- Nagarajan, R.; Varadaraju, C.; Lee, K.H. Recent advancements in the role of N-heterocyclic receptors on heavy metal ion sensing. Dyes Pigm. 2021, 191, 109331. [Google Scholar] [CrossRef]
- Rashdan, H.R.M.; Farag, M.M.; El-Gendey, M.S.; Mounier, M.M. Toward rational design of novel anti-cancer drugs based on targeting, solubility, and bioavailability exemplified by 1,3,4-thiadiazole derivatives synthesized under solvent-free conditions. Molecules 2019, 24, 2371. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Lei, Y.; Zhang, J.; Li, N.; Zhang, F.; Wang, H.; He, F. Rational design for multicolor flavone-based fluorophores with aggregation-induced emission enhancement characteristics and applications in mitochondria-imaging. Molecules 2018, 23, 2290. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.-B.; Zhang, S.; Qi, J.; Liang, X.-J.; Yoon, J. Advances in application of azobenzene as a trigger in biomedicine: Molecular design and spontaneous assembly. Adv. Mater. 2021, 33, e2007290. [Google Scholar] [CrossRef]
- Li, B.; Zhao, M.; Zhang, F. Rational design of Near-Infrared-II organic molecular dyes for bioimaging and biosensing. ACS Mater. Lett. 2020, 2, 905–917. [Google Scholar] [CrossRef]
- Amabili, P.; Biavasco, F.; Brenciani, A.; Citterio, B.; Corbisiero, D.; Ferrazzano, L.; Fioriti, S.; Guerra, G.; Orena, M.; Rinaldi, S. Simple amphiphilic α-hydrazido acids: Rational design, synthesis, and in vitro bioactivity profile of a novel class of potential antimicrobial compounds. Eur. J. Med. Chem. 2020, 189, 112072. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.V.; Chenoweth, D.M.; Petersson, E.J. Rational design of small molecule fluorescent probes for biological applications. Org. Biomol. Chem. 2020, 18, 5747–5763. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, N.N.; Saha, S.; Pramanik, A.; Sarkar, P.; Pal, S. Molecular design of porphyrin dyes using different electron-withdrawing moieties for high performance dye-sensitized solar cells. Comput. Theor. Chem. 2020, 1182, 112846. [Google Scholar] [CrossRef]
- Dai, J.; Wu, X.; Ding, S.; Lou, X.; Xia, F.; Wang, S.; Hong, Y. Aggregation-Induced emission photosensitizers: From molecular design to photodynamic therapy. J. Med. Chem. 2020, 63, 1996–2012. [Google Scholar] [CrossRef]
- Bronstein, H.; Nielsen, C.B.; Schroeder, B.C.; McCulloch, I. The role of chemical design in the performance of organic semiconductors. Nat. Rev. Chem. 2020, 4, 66–77. [Google Scholar] [CrossRef]
- Poriel, C.; Rault-Berthelot, J. Blue Single-Layer organic light-emitting diodes using fluorescent materials: A molecular design view point. Adv. Funct. Mater. 2020, 30, 1910040. [Google Scholar] [CrossRef]
- Gerasimova, T.P.; Burganov, T.I.; Katsyuba, S.A.; Kalinin, A.A.; Islamova, L.N.; Fazleeva, G.M.; Ahmadeev, B.S.; Mustafina, A.R.; Monari, A.; Assfeld, X.; et al. Halochromic luminescent quinoxalinones as a basis for pH-sensing in organic and aqueous solutions. Dyes Pigm. 2021, 186, 108958. [Google Scholar] [CrossRef]
- Kurishita, Y.; Kohira, T.; Ojida, A.; Hamachi, I. Rational design of FRET-based ratiometric chemosensors for in vitro and in cell fluorescence analyses of nucleoside polyphosphates. J. Am. Chem. Soc. 2010, 132, 13290–13299. [Google Scholar] [CrossRef]
- Bamesberger, A.; Schwartz, C.; Song, Q.; Han, W.; Wang, Z.; Cao, H. Rational design of a rapid fluorescent approach for detection of inorganic fluoride in MeCN–H2O: A new fluorescence switch based on N-aryl-1,8-naphthalimide. New J. Chem. 2014, 38, 884–888. [Google Scholar] [CrossRef]
- Wang, B.; Han, J.; Zhang, H.; Bender, M.; Biella, A.; Seehafer, K.; Bunz, U.H.F. Detecting counterfeit brandies. Chem. Eur. J. 2018, 24, 17361–17366. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.-H.; Qin, J.-C.; Wang, Y.-W.; Peng, Y.; Zhang, Y.-M.; Zhao, D.-M.; Zhang, Z.-H. A quinoline-based chromogenic and ratiometric fluorescent probe for selective detection of Mg2+ ion: Design, synthesis and its application in salt lake brines and bioimaging. Dyes Pigm. 2021, 185, 108896. [Google Scholar] [CrossRef]
- Jia, Y.; Li, P.; Song, W.; Zhao, G.; Zheng, D.; Li, D.; Wang, Y.; Wang, J.; Li, C.; Han, K. Rational design of a profluorescent substrate for S-Adenosylhomocysteine hydrolase and its applications in bioimaging and inhibitor screening. ACS Appl. Mater. Interfaces 2016, 8, 25818. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Hu, Z.; Peng, C.; Liu, B.; Li, W.; Gao, C. Rational design of a colorimetric and fluorescence turn-on chemosensor with benzothiazolium moiety for cyanide detection in aqueous solution. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 224, 117409. [Google Scholar] [CrossRef]
- Han, Q.; Liu, X.; Wang, X.; Yin, R.; Jiang, H.; Ru, J.; Liu, W. Rational design of a lysosomal-targeted ratiometric two-photon fluorescent probe for imaging hydrogen polysulfides in live cells. Dyes Pigm. 2020, 173, 107877. [Google Scholar] [CrossRef]
- Lu, Y.; Ruan, G.; Du, W.; Li, J.; Yang, N.; Wu, Q.; Lu, L.; Zhang, C.; Li, L. Recent progress in rational design of fluorescent probes for Fe2+ and bioapplication. Dyes Pigm. 2021, 190, 109337. [Google Scholar] [CrossRef]
- Peng, Z.; Shi, L.; Zeng, X.; Yang, S.; Xiao, J.; Gong, S.; Xiang, H.; Shao, G. Rational design of the ratiometric fluorescent probes for sulfur dioxide derivatives and the study on the sensing performance of cyano-containing groups. Dyes Pigm. 2021, 186, 108972. [Google Scholar] [CrossRef]
- Wang, B.; Anslyn, E.V. Chemosensors: Principles, Strategies and Applications; Wiley-Blackwell: Oxford, UK, 2011. [Google Scholar]
- Chua, M.H.; Shah, K.W.; Zhou, H.; Xu, J. Recent advances in aggregation-induced emission chemosensors for anion sensing. Molecules 2019, 24, 2711. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhong, H.; Huang, Y.; Zhao, R. Recent advances in Aiegens for metal ion biosensing and bioimaging. Molecules 2019, 24, 4593. [Google Scholar] [CrossRef] [Green Version]
- Kwon, N.; Hu, Y.; Yoon, J. Fluorescent Chemosensors for various analytes including reactive oxygen species, biothiol, metal ions, and toxic gases. ACS Omega 2018, 3, 13731–13751. [Google Scholar] [CrossRef]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present and future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borelli, M.; Iasilli, G.; Minei, P.; Pucci, A. Fluorescent polystyrene films for the detection of volatile organic compounds using the twisted intramolecular charge transfer mechanism. Molecules 2017, 22, 1306. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.U.; Qin, Y.; Nambiar, S.; Yeow, J.T.; Howlader, M.M.; Hu, N.-X.; Deen, M.J. Polymers and organic materials-based pH sensors for healthcare applications. Prog. Mater. Sci. 2018, 96, 174–216. [Google Scholar] [CrossRef]
- Bindhu, M.R.; Umadevi, M. Silver and gold nanoparticles for sensor and antibacterial applications. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 128, 37–45. [Google Scholar] [CrossRef]
- Chah, S.; Hammond, M.R.; Zare, R.N. Gold nanoparticles as a colorimetric sensor for protein conformational changes. Chem. Biol. 2005, 12, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Horak, E.; Kassal, P.; Murković Steinberg, I. Benzimidazole as a structural unit in fluorescent chemical sensors: The hidden properties of a multifunctional heterocyclic scaffold. Supramol. Chem. 2018, 30, 838–857. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, N.; Bai, W.; Bao, Y. Fluorescent chemosensors based on conjugated polymers with N-heterocyclic moieties: Two decades of progress. Polym. Chem. 2020, 11, 3095–3114. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006. [Google Scholar]
- Schönhaber, J.; Frank, W.; Müller, T.J.J. Insertion-coupling-cycloisomerization domino synthesis and cation-induced halochromic fluorescence of 2,4-diarylpyrano[2,3-b]indoles. Org. Lett. 2010, 12, 4122–4125. [Google Scholar] [CrossRef]
- Wilcke, T.; Glißmann, T.; Lerch, A.; Karg, M.; Müller, T.J.J. Acidochromic Turn-on 2,4-Diarylpyrano[2,3-b]indole Luminophores with Solubilizing Groups for A Broad Range of Polarity. ChemistrySelect 2018, 3, 10345–10351. [Google Scholar] [CrossRef]
- Mitsumori, S.; Tsuri, T.; Honma, T.; Hiramatsu, Y.; Okada, T.; Hashizume, H.; Inagaki, M.; Arimura, A.; Yasui, K.; Asanuma, F.; et al. Synthesis and biological activity of various derivatives of a novel class of potent, selective, and orally active prostaglandin D2 receptor antagonists. 1. Bicyclo2.2.1heptane derivatives. J. Med. Chem. 2003, 46, 2436–2445. [Google Scholar] [CrossRef]
- Chimenti, F.; Fioravanti, R.; Bolasco, A.; Chimenti, P.; Secci, D.; Rossi, F.; Yáñez, M.; Orallo, F.; Ortuso, F.; Alcaro, S. Chalcones: A valid scaffold for monoamine oxidases inhibitors. J. Med. Chem. 2009, 52, 2818–2824. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C. Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Bourguignon, L.Y.; Chu, A.; Jin, H.; Brandt, N.R. Ryanodine receptor-ankyrin interaction regulates internal Ca2+ release in mouse T-lymphoma cells. J. Biol. Chem. 1995, 270, 17917–17922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altura, B.M.; Carella, A.; Altura, B.T. Adverse effects of tris, hepes and mops buffers on contractile responses of arterial and venous smooth muscle induced by prostaglandins. Prostaglandins Med. 1980, 5, 123–130. [Google Scholar] [CrossRef]
- Provencher, S.W. A constrained regularization method for inverting data represented by linear algebraic or integral equations. Comput. Phys. Commun. 1982, 27, 213–227. [Google Scholar] [CrossRef]
- Jones, G.; Jackson, W.R.; Choi, C.Y.; Bergmark, W.R. Solvent effects on emission yield and lifetime for coumarin laser dyes. Requirements for a rotatory decay mechanism. J. Phys. Chem. 1985, 89, 294–300. [Google Scholar] [CrossRef]
- Moore, J.W.; Pearson, R.G.; Frost, A.A. Kinetics and Mechanism; Wiley: New York, NY, USA, 1981. [Google Scholar]
- Shirahase, H.; Takahashi, K.; Shoji, Y. Novel Aromatic Compound and Use Thereof. Patent WO2015030189, 5 March 2015. [Google Scholar]
- Pisula, W.; Kastler, M.; Wasserfallen, D.; Pakula, T.; Müllen, K. Exceptionally long-range self-assembly of hexa-peri-hexabenzocoronene with dove-tailed alkyl substituents. J. Am. Chem. Soc. 2004, 126, 8074–8075. [Google Scholar] [CrossRef]
- Lauter, U.; Meyer, W.H.; Enkelmann, V.; Wegner, G. Supramolecular structures of poly(p-phenylenes) with oxyethylene side chains and their mixtures with lithium salts. Macromol. Chem. Phys. 1998, 199, 2129–2140. [Google Scholar] [CrossRef]
- Li, Y.; Urbas, A.; Li, Q. Synthesis and characterization of light-driven dithienylcyclopentene switches with axial chirality. J. Org. Chem. 2011, 76, 7148–7156. [Google Scholar] [CrossRef]
- Tsai, C.; Jamison, J.M.; Summers, J.L. Ascorbate, Vitamin K3, and Hydroxytolans in the Treatment of Cancer. Patent WO2008157745, 24 December 2008. [Google Scholar]


| Solvent | Solubility of Compound 5 |
|---|---|
| cyclohexane | – |
| n-pentane | – |
| n-hexane | – |
| toluene | + |
| benzene | + |
| diethyl ether | – |
| methyl tert-butyl ether | – |
| 1,4-dioxane | + |
| tetrahydrofuran | + |
| ethyl acetate | + |
| chloroform | + |
| dichloromethane | + |
| acetone | + |
| DMF | + |
| dimethyl sulfoxide | + |
| acetonitrile | + |
| propan-2-ol | + |
| ethanol | + |
| methanol | + |
| water | – |
| Solvent | Solubility of Compound 5 |
|---|---|
| water | – |
| buffer, HEPES, 0.1 mol·L−1, pH 8 | – |
| buffer, MOPS, 0.1 mol·L−1, pH 7 | – |
| water, HCl(aq), pH 1 | + |
| water, HCl(aq), pH 3 | – |
| water, pH 7 | – |
| water, NaOH(aq), pH 11 | – |
| water, NaOH(aq), pH 13 | + |
| tetrahydrofuran/water | 25:75 |
| dimethyl sulfoxide/water | 70:30 |
| propan-2-ol/water | 35:65 |
| Compound | λmax,abs [nm] | λmax,em [nm] | Stokes Shift Δῦ | Quantum Yield |
|---|---|---|---|---|
| (ε) [L·cm−1·mol−1] | [cm−1] | Φf | ||
| 5 | 372 (14,200) | - | - | <0.010 |
| 5 + H+[a] | 455 (22,400) | 539 | 3400 | 0.039 [b] |
| 0.058 [c] | ||||
| 5 − H+[a] | 533 (24,500) | 628 | 2800 | 0.059 [c] |
| Compound | λmax,abs [nm] | λmax,em[nm] | Stokes Shift Δῦ | Quantum Yield |
|---|---|---|---|---|
| (ε) [L·cm−1·mol−1] | [cm−1] | Φf | ||
| 5 | 373 (24200) | - | - | <0.010 |
| 5 + H+[a] | 447 (37900) | 543 | 4000 | 0.014 [b] |
| 5 − H+[a] | 509 (34500) | 624 | 3600 | 0.018 [b] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilcke, T.; Postole, A.; Krüsmann, M.; Karg, M.; Müller, T.J.J. Amphipolar, Amphiphilic 2,4-diarylpyrano[2,3-b]indoles as Turn-ON Luminophores in Acidic and Basic Media. Molecules 2022, 27, 2354. https://doi.org/10.3390/molecules27072354
Wilcke T, Postole A, Krüsmann M, Karg M, Müller TJJ. Amphipolar, Amphiphilic 2,4-diarylpyrano[2,3-b]indoles as Turn-ON Luminophores in Acidic and Basic Media. Molecules. 2022; 27(7):2354. https://doi.org/10.3390/molecules27072354
Chicago/Turabian StyleWilcke, Tobias, Alexandru Postole, Marcel Krüsmann, Matthias Karg, and Thomas J. J. Müller. 2022. "Amphipolar, Amphiphilic 2,4-diarylpyrano[2,3-b]indoles as Turn-ON Luminophores in Acidic and Basic Media" Molecules 27, no. 7: 2354. https://doi.org/10.3390/molecules27072354
APA StyleWilcke, T., Postole, A., Krüsmann, M., Karg, M., & Müller, T. J. J. (2022). Amphipolar, Amphiphilic 2,4-diarylpyrano[2,3-b]indoles as Turn-ON Luminophores in Acidic and Basic Media. Molecules, 27(7), 2354. https://doi.org/10.3390/molecules27072354