Development of New Thiophene-Containing Triaryl Pyrazoline Derivatives as PI3Kγ Inhibitors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Acitivity
2.2.1. PI3K Inhibition Assay
2.2.2. The Anti-Proliferation Assay
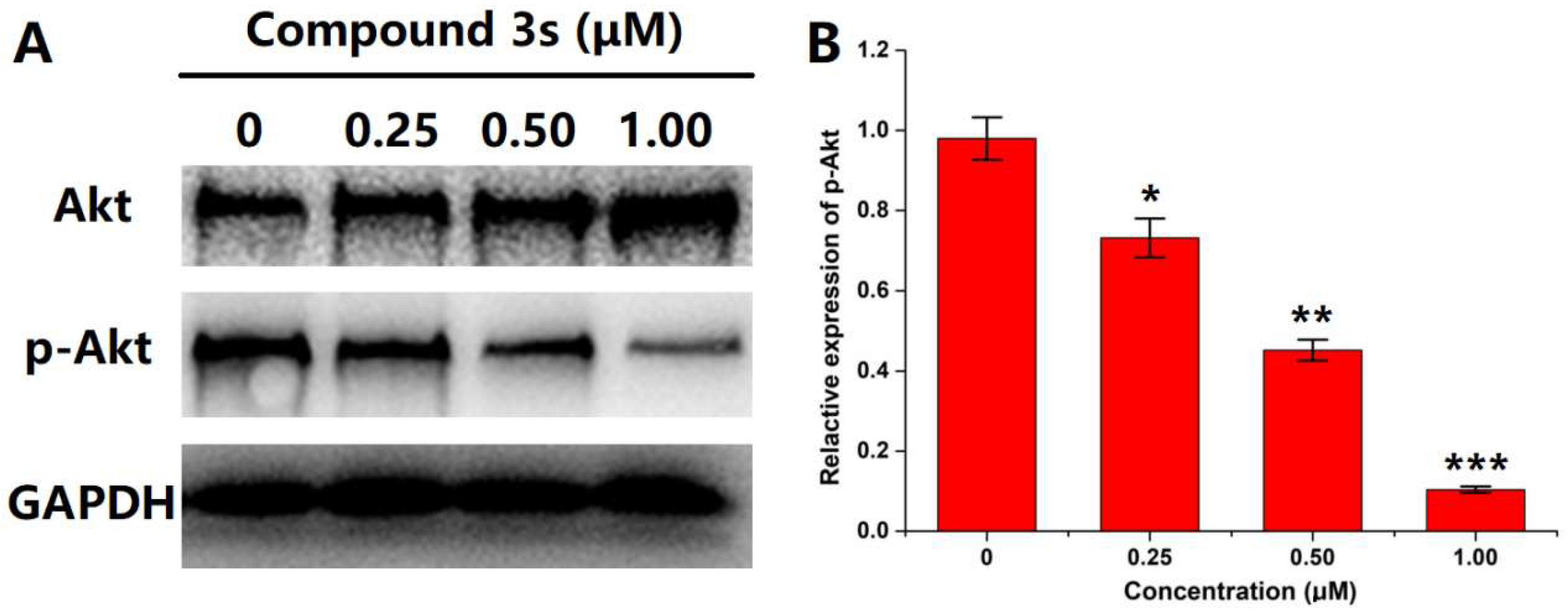
2.2.3. Western Blot
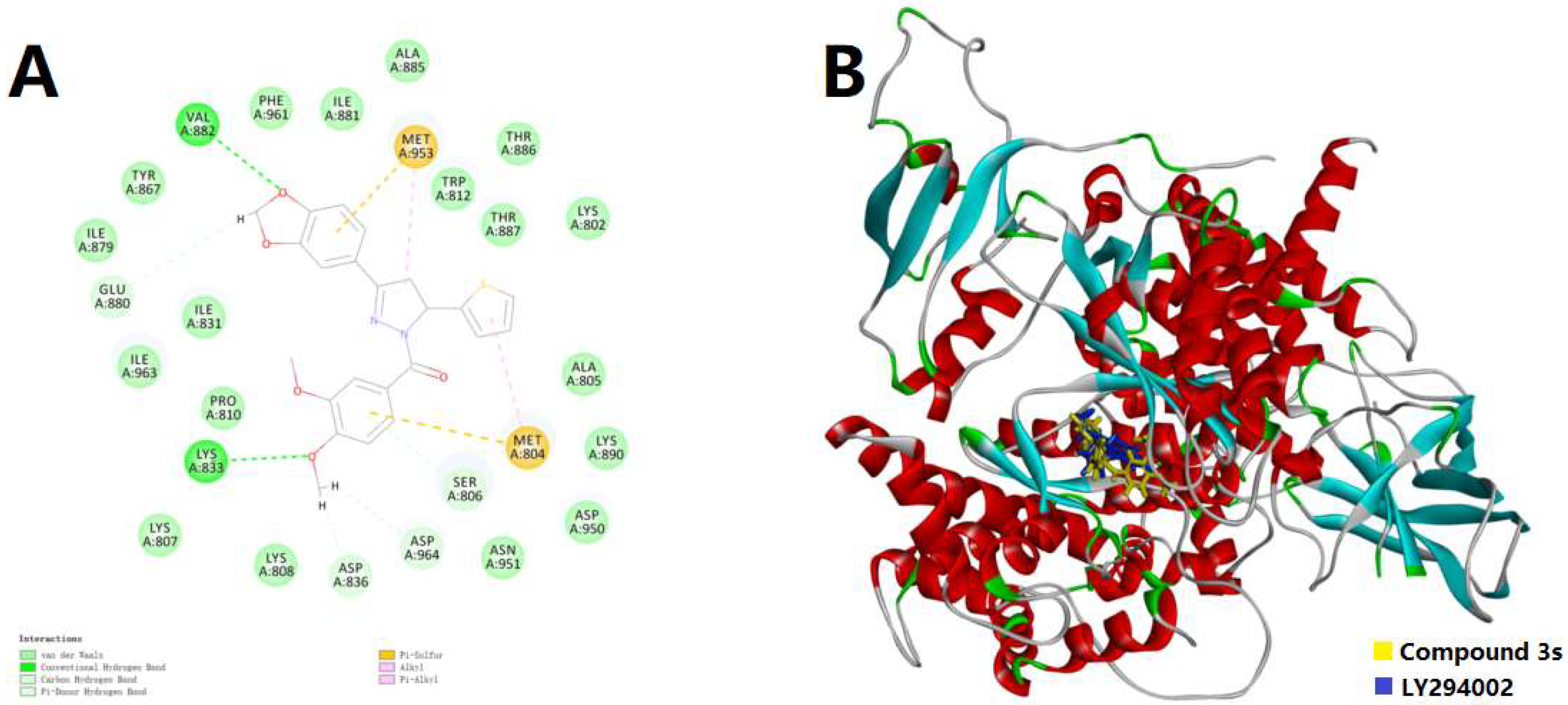
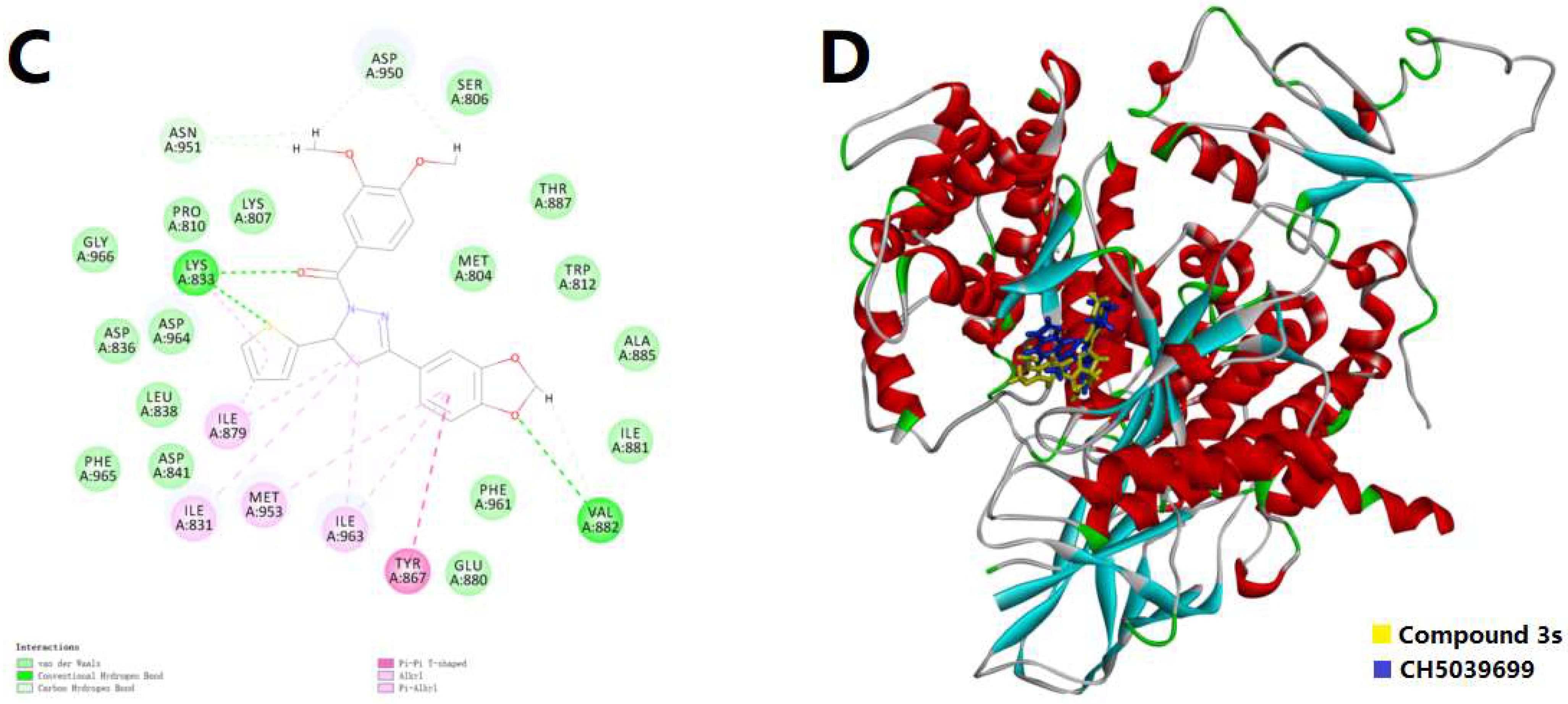
2.3. Molecular Docking Simulation
3. Materials and Methods
3.1. Materials and Apparatus
3.2. Chemical Syntheses
3.2.1. Synthesis of Chalcones (1a–1t)
3.2.2. Synthesis of 3-Aryl-5-(thiophen-2-yl)-4,5-dihydro-1H-pyrazole Derivatives (2a–2t)
3.2.3. Synthesis of (3,4-Dimethoxyphenyl)(3-aryl-5-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)methanone Derivatives(3a–3t)
3.2.4. (3,4-Dimethoxyphenyl)(3-phenyl-5-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)methanone (3a)
3.2.5. (3,4-Dimethoxyphenyl)(3-(2-fluorophenyl)-5-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)methanone (3b)
3.2.6. (3,4-Dimethoxyphenyl)(3-(3-fluorophenyl)-5-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)methanone (3c)
3.2.7. (3-(3-Bromophenyl)-5-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)(3,4-dimethoxyphenyl)methanone (3d)
3.2.8. (3,4-Dimethoxyphenyl)(3-(3-nitrophenyl)-5-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)methanone (3e)
3.2.9. (3,4-Dimethoxyphenyl)(3-(4-methoxyphenyl)-5-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)methanone (3f)
3.2.10. (3-(4-(Benzyloxy)phenyl)-5-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)(3,4-dimethoxyphenyl)methanone (3g)
3.2.11. (3-(4-((λ1-Sulfanyl)-λ5-methyl) phenyl)-5-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl) (3,4-dimethoxyphenyl)methanone (3h)
3.2.12. (3,4-Dimethoxyphenyl)(5-(thiophen-2-yl)-3-(p-tolyl)-4,5-dihydro-1H-pyrazol-1-yl)methanone (3i)
3.2.13. (3-([1,1’-Biphenyl]-4-yl)-5-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)(3,4-dimethoxyphenyl)methanone (3j)
3.2.14. (3-(4’-Bromo-[1,1’-biphenyl]-4-yl)-5-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)(3,4-dimethoxyphenyl)methanone (3k)
3.2.15. (3-(4-Bromophenyl)-5-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)(3,4-dimethoxyphenyl)methanone (3l)
3.2.16. (3,4-Dimethoxyphenyl)(3-(4-iodophenyl)-5-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)methanone (3m)
3.2.17. (3,4-Dimethoxyphenyl)(3-(naphthalen-2-yl)-5-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)methanone (3n)
3.2.18. (3,4-Dimethoxyphenyl)(3-(4-methoxynaphthalen-1-yl)-5-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)methanone (3o)
3.2.19. (3-(6-((λ1-Oxidanyl)-λ5-methyl) naphthalen-2-yl)-5-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)(3,4-dimethoxyphenyl)methanone (3p)
3.2.20. (3,4-Dimethoxyphenyl)(3-(3,4-dimethoxyphenyl)-5-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)methanone (3q)
3.2.21. (3,4-Dimethoxyphenyl)(3-(3,4-dimethylphenyl)-5-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)methanone (3r)
3.2.22. (3-(Benzo[d][1,3]dioxol-5-yl)-5-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)(3,4-dimethoxyphenyl)methanone (3s)
3.2.23. (3-(5-Bromopyridin-2-yl)-5-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)(3,4-dimethoxyphenyl)methanone (3t)
3.3. Biological Assay
3.3.1. The PI3K Inhibition Assay
3.3.2. Anti-Proliferation Assay
3.3.3. Western Blot
3.4. Protocol of Docking Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Singh, V.J.; Sharma, B.; Chawla, P.A. Recent developments in mitogen activated protein kinase inhibitors as potential anticancer agents. Bioorg. Chem. 2021, 114, 105161. [Google Scholar] [CrossRef] [PubMed]
- Rascio, F.; Spadaccino, F.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Netti, G.S.; Ranieri, E. The pathogenic role of PI3K/AKT pathway in cancer onset and drug resistance: An updated review. Cancers 2021, 13, 3949. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Vashishta, M.; Kong, L.; Wu, X.; Lu, J.J.; Guha, C.; Dwarakanath, B.S. The role of Notch, Hedgehog, and Wnt signaling pathways in the resistance of tumors to anticancer therapies. Front. Cell Dev. Biol. 2021, 9, 650772. [Google Scholar] [CrossRef]
- Meng, D.; He, W.; Zhang, Y.; Liang, Z.; Zheng, J.; Zhang, X.; Zheng, X.; Zhan, P.; Chen, H.; Li, W.; et al. Development of PI3K inhibitors: Advances in clinical trials and new strategies (Review). Pharmacol. Res. 2021, 173, 105900. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, D.A.; Hajjo, R.; Bardaweel, S.K.; Zhong, H.A. Phosphatidylinositol 3-kinase (PI3K) inhibitors: A recent update on inhibitor design and clinical trials (2016–2020). Expert Opin. Ther. Pat. 2021, 31, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Rathinaswamy, M.K.; Gaieb, Z.; Fleming, K.D.; Borsari, C.; Harris, N.J.; Moeller, B.E.; Wymann, M.P.; Amaro, R.E.; Burke, J.E. Disease-related mutations in PI3Ky disrupt regulatory C-terminal dynamics and reveal a path to selective inhibitors. eLife 2021, 10, e64691. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Abak, A.; Shoorei, H.; Mohaqiq, M.; Majidpoor, J.; Sayad, A.; Taheri, M. Regulatory role of microRNAs on PTEN signaling. Biomed. Pharmacother. 2021, 133, 110986. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, H.; Gao, X.; Ryu, J.H.; Yang, Y.; Kwon, I.C.; Roberts, T.M.; Kim, S.H. Multi-targeting siRNA nanoparticles for simultaneous inhibition of PI3K and Rac1 in PTEN-deficient prostate cancer. J. Ind. Eng. Chem. 2021, 99, 196–203. [Google Scholar] [CrossRef]
- Calderón, L.; Schindler, K.; Malin, S.G.; Schebesta, A.; Sun, Q.; Schwickert, T.; Alberti, C.; Fischer, M.; Jaritz, M.; Tagoh, H.; et al. Pax5 regulates B cell immunity by promoting PI3K signaling via PTEN down-regulation. Sci. Immunol. 2021, 6, eabg5003. [Google Scholar] [CrossRef]
- Rathinaswamy, M.K.; Dalwadi, U.; Fleming, K.D.; Adams, C.; Stariha, J.T.B.; Pardon, E.; Baek, M.; Vadas, O.; Dimaio, F.; Steyaert, J.; et al. Structure of the phosphoinositide 3-kinase (PI3K) p110γ-p101 complex reveals molecular mechanism of GPCR activation. Sci. Adv. 2021, 7, eabj4282. [Google Scholar] [CrossRef]
- Li, X.; He, S.; Ma, B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer 2020, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Meng, L.-H. Emerging roles of class I PI3K inhibitors in modulating tumor microenvironment and immunity. Acta Pharmacol. Sin. 2020, 41, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Bilanges, B.; Posor, Y.; Vanhaesebroeck, B. PI3K isoforms in cell signalling and vesicle traffickin. Nat. Rev. Mol. Cell Biol. 2019, 20, 515–534. [Google Scholar] [CrossRef] [PubMed]
- Endicott, S.J.; Ziemba, Z.J.; Beckmann, L.J.; Boynton, D.N.; Miller, R.A. Inhibition of class I PI3K enhances chaperone-mediated autophagy. J. Cell Biol. 2020, 219, e202001031. [Google Scholar] [CrossRef]
- Aksoy, E.; Saveanu, L.; Manoury, B. The isoform selective roles of PI3Ks in dendritic cell biology and function. Front. Immunol. 2018, 9, 2574. [Google Scholar] [CrossRef]
- Sadasivan, C.; Zhabyeyev, P.; Labib, D.; White, J.A.; Paterson, D.I.; Oudit, G.Y. Cardiovascular toxicity of PI3Kα inhibitors. Clin. Sci. 2020, 134, 2595–2622. [Google Scholar] [CrossRef]
- Pennino, F.P.; Murakami, M.; Zollo, M.; Robertson, E.S. The metastasis suppressor protein NM23-H1 modulates the PI3K-AKT axis through interaction with the p110α catalytic subunit. Oncogenesis 2021, 10, 34. [Google Scholar] [CrossRef]
- Marshall, J.D.S.; Whitecross, D.E.; Mellor, P.; Anderson, D.H. Impact of p85α alterations in cancer. Biomolecules 2019, 9, 29. [Google Scholar] [CrossRef]
- Luff, D.H.; Wojdyla, K.; Oxley, D.; Chessa, T.; Hudson, K.; Hawkins, P.T.; Stephens, L.R.; Barry, S.T.; Okkenhaug, K. PI3Kδ forms distinct multiprotein complexes at the TCR signalosome in naïve and differentiated CD4+ T Cells. Front. Immunol. 2021, 12, 631271. [Google Scholar] [CrossRef]
- Fujita, A.; Kan-o, K.; Tonai, K.; Yamamoto, N.; Ogawa, T.; Fukuyama, S.; Nakanishi, Y.; Matsumoto, K. Inhibition of PI3Kδ enhances poly I: C-induced antiviral responses and inhibits replication of human metapneumovirus in murine lungs and human bronchial epithelial cells. Front. Immunol. 2020, 11, 432. [Google Scholar] [CrossRef] [Green Version]
- de Assis Lima, I.V.; Bellozi, P.M.Q.; Batista, E.M.; Vilela, L.R.; Brandão, I.L.; Ribeiro, F.M.; Moraes, M.F.D.; Moreira, F.A.; de Oliveira, A.C.P. Cannabidiol anticonvulsant effect is mediated by the PI3Kγ pathway. Neuropharmacology 2020, 176, 108156. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.W.D.; Abdulai, R.; Mogemark, M.; Petersen, J.; Thomas, M.J.; Valastro, B.; Eriksson, A.W. Evolution of PI3Kγ and δ inhibitors for inflammatory and qutoimmune diseases. J. Med. Chem. 2019, 62, 4783–4814. [Google Scholar] [CrossRef] [PubMed]
- Vanhaesebroeck, B.; Perry, M.W.D.; Brown, J.R.; André, F.; Okkenhaug, K. PI3K inhibitors are finally coming of age. Nat. Rev. Drug Discov. 2021, 20, 741–769. [Google Scholar] [CrossRef] [PubMed]
- Rodon, J.; Dienstmann, R.; Serra, V.; Tabernero, J. Development of PI3K inhibitors: Lessons learned from early clinical trials. Nat. Rev. Clin. Oncol. 2013, 10, 143–153. [Google Scholar] [CrossRef]
- Ghareghomi, S.; Ahmadian, S.; Zarghami, N.; Hemmati, S. hTERT-molecular targeted therapy of ovarian cancer cells via folate-functionalized PLGA nanoparticles co-loaded with MNPs/siRNA/wortmannin. Life Sci. 2021, 277, 119621. [Google Scholar] [CrossRef]
- Wang, J.; Lv, X.; Guo, X.; Dong, Y.; Peng, P.; Huang, F.; Wang, P.; Zhang, H.; Zhou, J.; Wang, Y.; et al. Feedback activation of STAT3 limits the response to PI3K/AKT/mTOR inhibitors in PTEN-deficient cancer cells. Oncogenesis 2021, 10, 8. [Google Scholar] [CrossRef]
- Down, K.; Amour, A.; Baldwin, I.R.; Cooper, A.W.J.; Deakin, A.M.; Felton, L.M.; Guntrip, S.B.; Hardy, C.; Harrison, Z.A.; Jones, K.L.; et al. Optimization of novel indazoles as highly potent and selective inhibitors of phosphoinositide 3-kinase δ for the treatment of respiratory disease. J. Med. Chem. 2015, 58, 7381–7399. [Google Scholar] [CrossRef]
- Wallin, J.J.; Guan, J.; Prior, W.W.; Lee, L.B.; Berry, L.; Belmont, L.D.; Koeppen, H.; Belvin, M.; Friedman, L.S.; Sampath, D. GDC-0941, a novel class I selective PI3K inhibitor, enhances the efficacy of docetaxel in human breast cancer models by increasing cell death in vitro and in vivo. Clin. Cancer Res. 2012, 18, 3901–3911. [Google Scholar] [CrossRef] [Green Version]
- Omeljaniuk, W.J.; Krętowski, R.; Ratajczak-Wrona, W.; Jablońska, E.; Cechowska-Pasko, M. Novel dual PI3K/mTOR inhibitor, Apitolisib (GDC-0980), inhibits growth and induces apoptosis in human glioblastoma cells. Int. J. Mol. Sci. 2021, 22, 11511. [Google Scholar] [CrossRef]
- Lauder, S.N.; Smart, K.; Kersemans, V.; Allen, D.; Scott, J.; Pires, A.; Milutinovic, S.; Somerville, M.; Smart, S.; Kinchesh, P.; et al. Enhanced antitumor immunity through sequential targeting of PI3Kδ and LAG3. J. Immunother. Cancer 2020, 8, e000693. [Google Scholar] [CrossRef]
- Yin, Y.; Sha, S.; Wu, X.; Wang, S.-F.; Qiao, F.; Song, Z.-C.; Zhu, H.-L. Development of novel chromeno[4,3-c]pyrazol-4(2H)-one derivates bearing sulfonylpiperazine as antitumor inhibitors targeting PI3Kα. Eur. J. Med. Chem. 2019, 182, 111630. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zhou, Y.; Sha, S.; Wu, X.; Wang, S.-F.; Qiao, F.; Song, Z.-C.; Zhu, H.-L. Development of novel chromeno[4,3-c]pyrazol-4(2H)-one derivates containing piperazine as inhibitors of PI3Kα. Bioorg. Chem. 2019, 92, 103238. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.-F.; Lv, X.-H.; Wang, X.-L.; Sun, J.; Zhang, Y.-B.; Yang, Y.-S.; Gong, H.-B.; Zhu, H.-L. Design, synthesis, biological evaluation and molecular modeling of novel 1,3,4-oxadiazole derivatives based on Vanillic acid as potential immunosuppressive agents. Bioorg. Med. Chem. 2012, 20, 4226–4236. [Google Scholar] [CrossRef]
- Elewa, S.I.; Mansour, E.; Nassar, I.F.; Mekawey, A.A.I. Synthesis of some new pyrazoline-based thiazole derivatives and evaluation of their antimicrobial, antifungal, and anticancer activities. Russ. J. Bioorg. Chem. 2020, 46, 382–392. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, J.; Wang, F.; Hu, X.-W.; Shi, Y. Pyrazoline derivatives as tubulin polymerization inhibitors with one hit for Vascular Endothelial Growth Factor Receptor 2 inhibition. Bioorg. Chem. 2021, 114, 105134. [Google Scholar] [CrossRef] [PubMed]
- Alex, J.M.; Singh, S.; Kumar, R. 1-Acetyl-3,5-diaryl-4,5-dihydro(1H)pyrazoles: Exhibiting anticancer activity through intracellular ROS scavenging and the Mitochondria-dependent death pathway. Arch. Pharm. 2014, 347, 717–727. [Google Scholar] [CrossRef]
- Qin, Y.-J.; Li, Y.-J.; Jiang, A.-Q.; Yang, M.-R.; Zhu, Q.-Z.; Dong, H.; Zhu, H.-L. Design, synthesis and biological evaluation of novel pyrazoline-containing derivatives as potential tubulin assembling inhibitor. Eur. J. Med. Chem. 2015, 94, 447–457. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, Y.-L.; Fan, J.; Ma, X.; Qin, Y.-J.; Zhu, H.-L. Novel nicotinoyl pyrazoline derivates bearing N-methyl indole moiety as antitumor agents: Design, synthesis and evaluation. Eur. J. Med. Chem. 2018, 156, 722–737. [Google Scholar] [CrossRef]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blazquez-Castro, A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.-H.; Liu, X.; Shen, M.-Y.; Nie, S.-P.; Zhang, H.; Li, C.; Gong, D.-M.; Xie, M.-Y. Purification, physicochemical characterisation and anticancer activity of a polysaccharide from Cyclocarya paliurus leaves. Food Chem. 2013, 136, 1453–1460. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Su, M.-M.; Li, H.-L.; Liu, Q.-X.; Xu, C.; Yang, Y.-S.; Zhu, H.-L. A fluorescent sensor for discrimination of HSA from BSA through selectivity evolution. Anal. Chim. Acta 2018, 1043, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Han, H.-W.; Sun, W.-X.; Yang, Y.-S.; Tang, C.-Y.; Lu, G.-H.; Qi, J.-L.; Wang, X.-M.; Yang, Y.-H. Design and characterization of α-lipoic acyl shikonin ester twin drugs as tubulin and PDK1 dual inhibitors. Eur. J. Med. Chem. 2018, 144, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Code | IC50 (μM) | CC50 (μM) | GI50 (μM) | ||
|---|---|---|---|---|---|
| PI3Kα | PI3Kγ | L02 | HeLa | HepG2γ | |
| 3a | >100.000 | 7.480 ± 0.220 | 174.000 ± 10.000 | 109.000 ± 5.800 | 61.100 ± 3.200 |
| 3b | >100.000 | 4.430 ± 0.200 | 152.000 ± 9.500 | 77.900 ± 3.900 | 50.200 ± 1.600 |
| 3c | 30.900 ± 1.300 | 4.210 ± 0.072 | 94.500 ± 4.300 | 26.300 ± 1.700 | 20.400 ± 2.000 |
| 3d | 78.700 ± 4.000 | 2.220 ± 0.072 | 120.000 ± 4.700 | 57.100 ± 2.600 | 42.600 ± 1.900 |
| 3e | 2.080 ± 0.099 | 1.120 ± 0.081 | 58.400 ± 2.600 | 5.900 ± 0.200 | 5.510 ± 0.200 |
| 3f | 26.600 ± 1.010 | 0.570 ± 0.044 | 112.000 ± 8.300 | 23.700 ± 1.200 | 15.100 ± 0.420 |
| 3g | 0.920 ± 0.056 | 26.900 ± 1.400 | 103.500 ± 5.400 | 31.600 ± 0.900 | 31.600 ± 1.400 |
| 3h | 64.400 ± 3.600 | 18.100 ± 1.700 | 180.000 ± 4.600 | 61.300 ± 2.300 | 53.300 ± 1.900 |
| 3i | 89.500 ± 3.400 | 3.030 ± 0.085 | 151.000 ± 8.900 | 66.600 ± 1.200 | 48.700 ± 1.800 |
| 3j | 3.920 ± 0.064 | >100.000 | 196.000 ± 10.000 | 75.700 ± 2.900 | 50.600 ± 1.000 |
| 3k | 1.100 ± 0.061 | 42.700 ± 2.600 | 178.000 ± 8.700 | 44.600 ± 2.100 | 46.600 ± 1.300 |
| 3l | 17.100 ± 0.580 | 5.110 ± 0.083 | 86.600 ± 3.500 | 20.100 ± 1.700 | 15.500 ± 0.870 |
| 3m | 75.600 ± 2.400 | 2.520 ± 0.055 | 141.000 ± 5.600 | 57.900 ± 3.600 | 40.100 ± 1.400 |
| 3n | 12.000 ± 1.100 | 1.790 ± 0.049 | 62.600 ± 2.600 | 11.100 ± 1.200 | 8.850 ± 0.310 |
| 3o | 8.810 ± 0.170 | 9.910 ± 0.260 | 137.000 ± 4.700 | 18.100 ± 0.850 | 16.900 ± 0.360 |
| 3p | 0.560 ± 0.045 | 3.760 ± 0.190 | 67.100 ± 3.900 | 6.810 ± 0.240 | 6.310 ± 0.540 |
| 3q | 5.240 ± 0.130 | 0.430 ± 0.036 | 41.300 ± 2.600 | 4.720 ± 0.120 | 4.220 ± 0.130 |
| 3r | 4.130 ± 0.062 | 1.370 ± 0.055 | 52.500 ± 3.200 | 6.200 ± 0.220 | 5.550 ± 0.410 |
| 3s | 42.600 ± 2.900 | 0.066 ± 0.005 | 181.000 ± 9.000 | 13.700 ± 1.100 | 12.900 ± 1.300 |
| 3t | >100.000 | 9.150 ± 0.140 | >300.000 | 124.000 ± 9.700 | 109.700 ± 8.100 |
| LY294002 | 0.447 ± 0.038 | 0.777 ± 0.071 | 51.300 ± 1.400 | 21.100 ± 2.000 | 26.500 ± 1.900 |
| Code | Structure | Code | Structure |
|---|---|---|---|
| 3a | 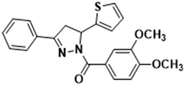 | 3k |  |
| 3b |  | 3l | 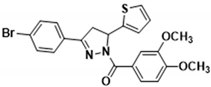 |
| 3c |  | 3m | 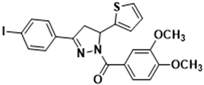 |
| 3d | 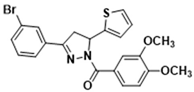 | 3n | 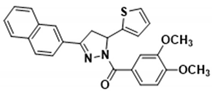 |
| 3e | 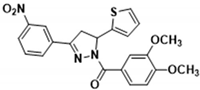 | 3o |  |
| 3f | 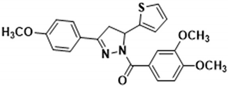 | 3p | 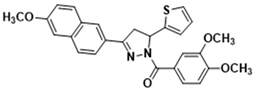 |
| 3g | 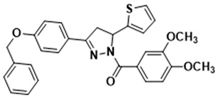 | 3q | 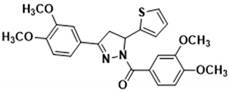 |
| 3h | 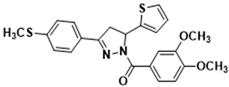 | 3r | 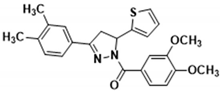 |
| 3i | 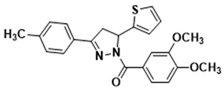 | 3s |  |
| 3j | 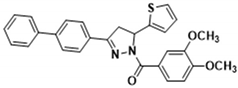 | 3t | 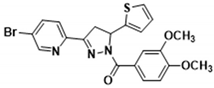 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Zhang, B.; Zhao, Q.; Li, J.; Shi, Y. Development of New Thiophene-Containing Triaryl Pyrazoline Derivatives as PI3Kγ Inhibitors. Molecules 2022, 27, 2404. https://doi.org/10.3390/molecules27082404
Yang B, Zhang B, Zhao Q, Li J, Shi Y. Development of New Thiophene-Containing Triaryl Pyrazoline Derivatives as PI3Kγ Inhibitors. Molecules. 2022; 27(8):2404. https://doi.org/10.3390/molecules27082404
Chicago/Turabian StyleYang, Bing, Bo Zhang, Qun Zhao, Jin Li, and Yujun Shi. 2022. "Development of New Thiophene-Containing Triaryl Pyrazoline Derivatives as PI3Kγ Inhibitors" Molecules 27, no. 8: 2404. https://doi.org/10.3390/molecules27082404
APA StyleYang, B., Zhang, B., Zhao, Q., Li, J., & Shi, Y. (2022). Development of New Thiophene-Containing Triaryl Pyrazoline Derivatives as PI3Kγ Inhibitors. Molecules, 27(8), 2404. https://doi.org/10.3390/molecules27082404





