Optimization of Headspace Solid-Phase Micro-Extraction Conditions (HS-SPME) and Identification of Major Volatile Aroma-Active Compounds in Chinese Chive (Allium tuberosum Rottler)
Abstract
:1. Introduction
2. Results and Discussion
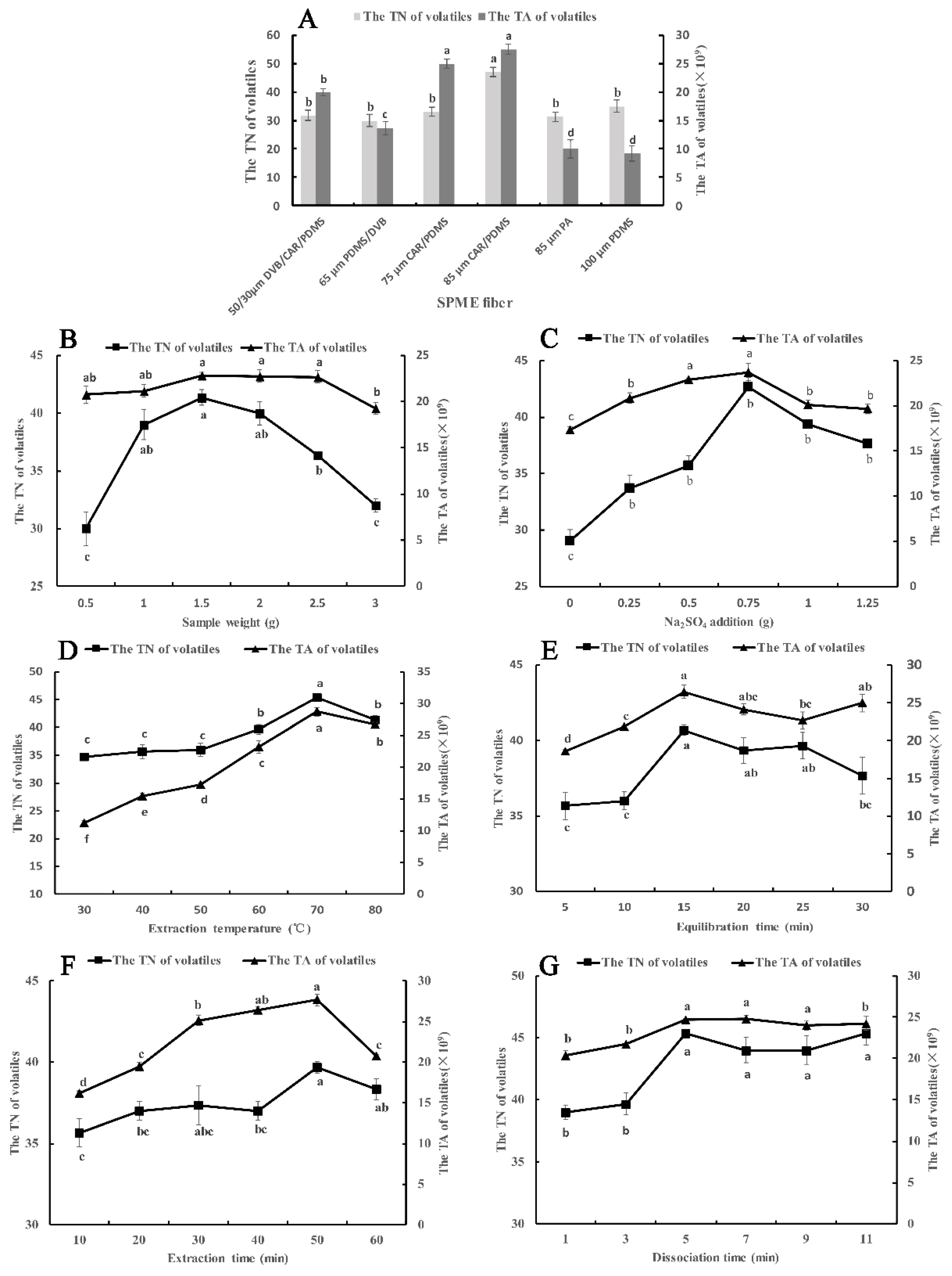
2.1. The Optimization of HS-SPME
2.1.1. Selection of SPME Fiber
2.1.2. Effect of Sample Weight
2.1.3. Effect of Na2SO4 Amount
2.1.4. Effect of Extraction Temperature
2.1.5. Effect of Equilibration Time
2.1.6. Effect of Extraction Time
2.1.7. Effect of Desorption Time
2.2. Validation of the Analytical Reproducibility
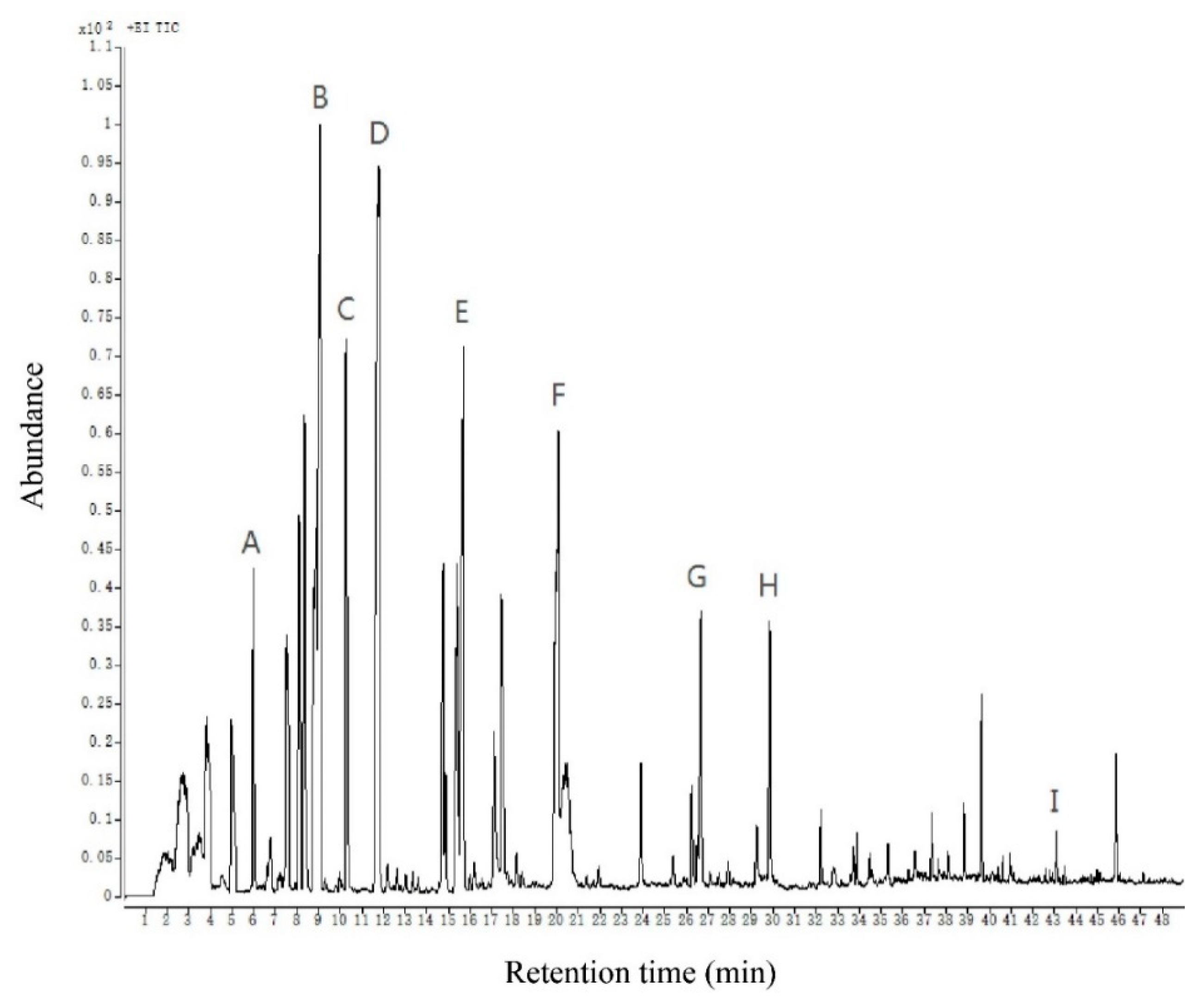
2.3. Analysis of Volatile Compounds of Chinese Chive
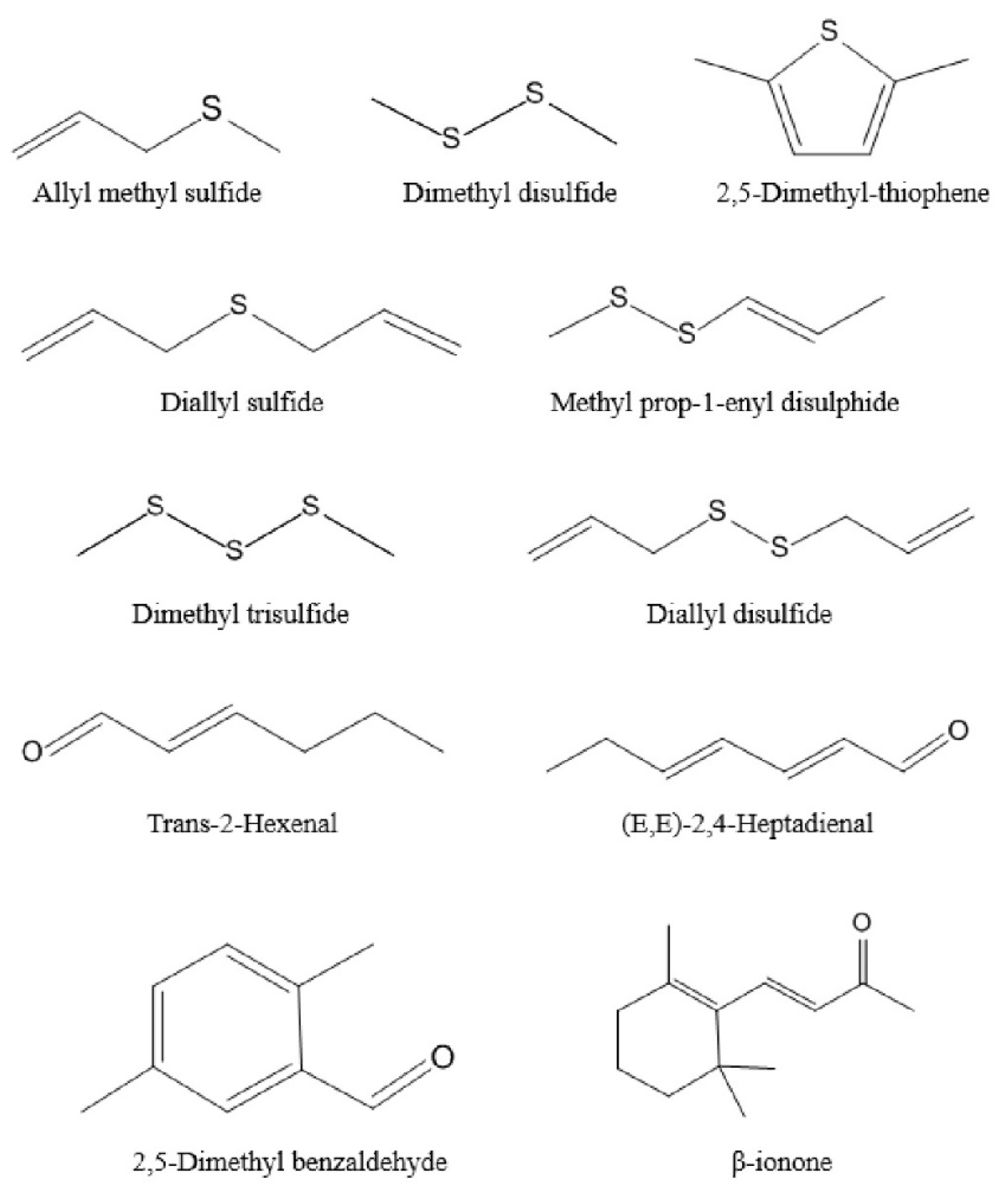
2.3.1. Ethers
2.3.2. Aldehydes
2.3.3. Alcohols and Ketones
2.3.4. Hydrocarbons, Esters and Phenols
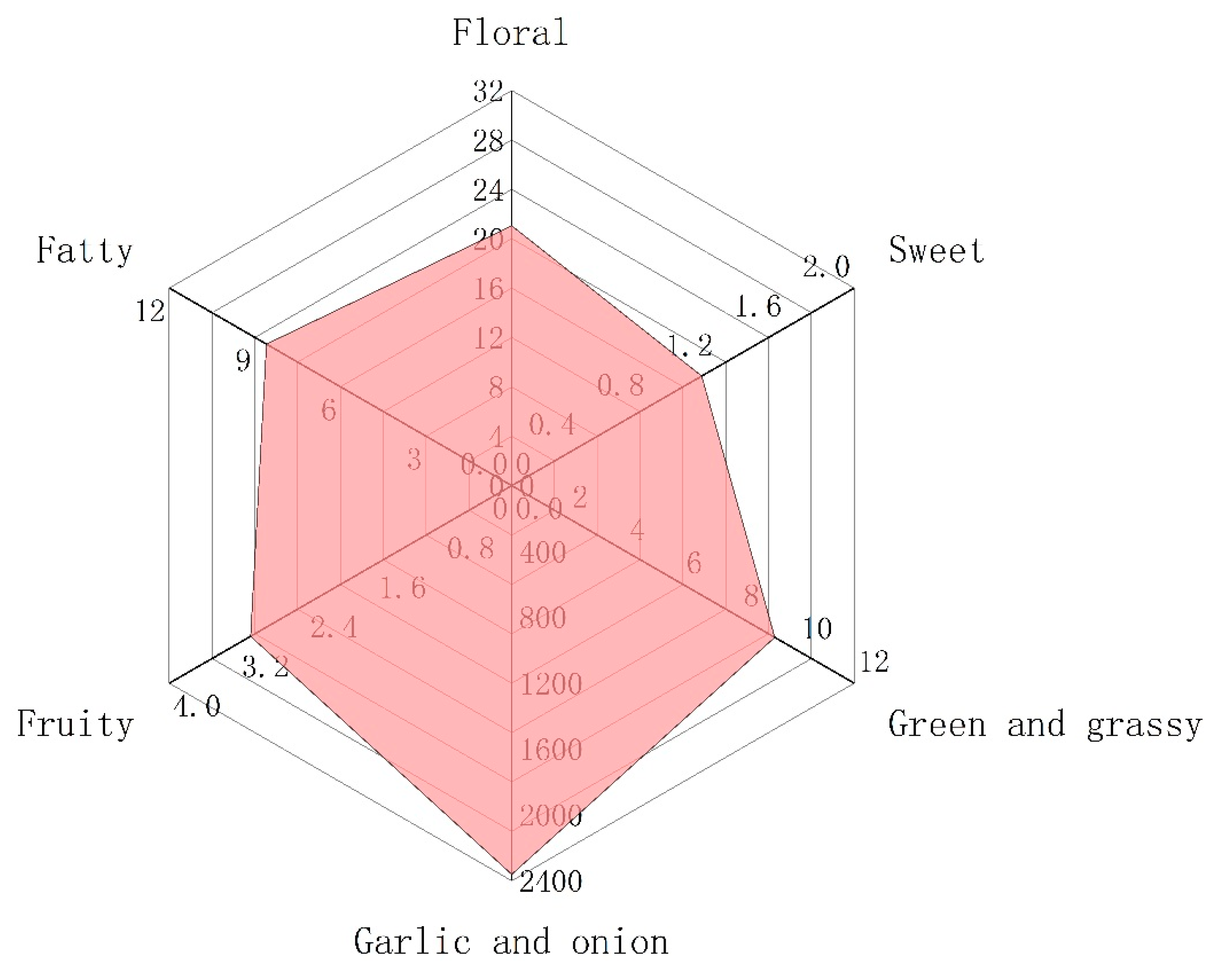
2.4. Odour Activity Values (OAVs) Analysis of Volatile Compounds
3. Materials and Methods
3.1. Plant Material
3.2. Reagents and Instruments
3.3. Optimization of HS-SPME
3.4. GC-MS Analysis
3.5. Qualitative and Quantitative Analysis of Volatile Compounds
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cecchi, L.; Ieri, F.; Vignolini, P.; Mulinacci, N.; Romani, A. Characterization of Volatile and Flavonoid Composition of Different Cuts of Dried Onion (Allium cepa L.) by HS-SPME-GC-MS, HS-SPME-GCxGC-TOF and HPLC-DAD. Molecules 2020, 25, 408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asemani, Y.; Zamani, N.; Bayat, M.; Amirghofran, Z. Allium vegetables for possible future of cancer treatment. Phytother. Res. PTR 2019, 33, 3019–3039. [Google Scholar] [CrossRef] [PubMed]
- Hiyasat, B.; Sabha, D.; Grotzinger, K.; Kempfert, J.; Rauwald, J.W.; Mohr, F.W.; Dhein, S. Antiplatelet activity of Allium ursinum and Allium sativum. Pharmacology 2009, 83, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Shibamoto, T. Chemical Compositions and Antioxidant/Anti-inflammatory Activities of Steam Distillate from Freeze-Dried Onion (Allium cepa L.) Sprout. J. Agric. Food Chem. 2008, 56, 10462–10467. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, D.-B.; Lee, S.; Park, J.; Shin, D.; Yoon, M. Profiling of organosulphur compounds using HPLC-PDA and GC/MS system and antioxidant activities in hooker chive (Allium hookeri). Nat. Prod. Res. 2016, 30, 2798–2804. [Google Scholar] [CrossRef]
- Sobolewska, D.; Michalska, K.; Podolak, I.; Grabowska, K. Steroidal saponins from the genus Allium. Phytochem. Rev. 2016, 15, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Han, S.H.; Suh, W.S.; Park, K.J.; Kim, K.H.; Lee, K.R. Two new phenylpropane glycosides from Allium tuberosum Rottler. Arch. Pharm. Res. 2015, 38, 1312–1316. [Google Scholar] [CrossRef]
- Zhang, W.N.; Zhang, H.L.; Lu, C.Q.; Luo, J.P.; Zha, X.Q. A new kinetic model of ultrasound-assisted extraction of polysaccharides from Chinese chive. Food Chem. 2016, 212, 274–281. [Google Scholar] [CrossRef]
- Pino, J.A.; Fuentes, V.; Correa, M.T. Volatile Constituents of Chinese Chive (Allium tuberosum Rottl. ex Sprengel) and Rakkyo (Allium chinense G. Don). J. Agric. Food Chem. 2001, 49, 1328–1330. [Google Scholar] [CrossRef]
- Rose, P.; Whiteman, M.; Moore, P.K.; Zhu, Y.Z. Bioactive S-alk(en)yl cysteine sulfoxide metabolites in the genus Allium: The chemistry of potential therapeutic agents. Nat. Prod. Rep. 2005, 22, 351–368. [Google Scholar] [CrossRef]
- Yoshimoto, N.; Saito, K. S-Alk(en)ylcysteine sulfoxides in the genus Allium: Proposed biosynthesis, chemical conversion, and bioactivities. J. Exp. Bot. 2019, 70, 4123–4137. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Tong, J.; Hu, M.; Ji, Y.; Wang, B.; Liang, H.; Liu, M.; Wu, Z. Transcriptome landscapes of multiple tissues highlight the genes involved in the flavor metabolic pathway in Chinese chive (Allium tuberosum). Genomics 2021, 113, 2145–2157. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, Y.; Mukaida, Y.; Saito, Y.; Oshima, K.; Takahashi, T.; Muroi, E.; Hashimoto, K.; Uda, Y. Characterisation of volatile sulphur-containing compounds generated in crushed leaves of Chinese chive (Allium tuberosum Rottler). Food Chem. 2010, 120, 343–348. [Google Scholar] [CrossRef]
- Pawliszyn, J. Solid Phase Microextraction: Theory and Practice; VCH: New York, NY, USA, 1997. [Google Scholar]
- Mnayer, D.; Fabiano-Tixier, A.S.; Petitcolas, E.; Hamieh, T.; Nehme, N.; Ferrant, C.; Fernandez, X.; Chemat, F. Chemical composition, antibacterial and antioxidant activities of six essentials oils from the Alliaceae family. Molecules 2014, 19, 20034–20053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beltran Sanahuja, A.; Ponce Landete, M.; Domingo Martinez, M.I.; Prats Moya, M.S.; Valdes Garcia, A. Optimization of Volatile Compounds Extraction from Industrial Celery (Apium graveolens) By-Products by Using Response Surface Methodology and Study of Their Potential as Antioxidant Sources. Foods 2021, 10, 2664. [Google Scholar] [CrossRef]
- Risticevic, S.; Lord, H.; Gorecki, T.; Arthur, C.L.; Pawliszyn, J. Protocol for solid-phase microextraction method development. Nat. Protoc. 2010, 5, 122–139. [Google Scholar] [CrossRef]
- de Fatima Alpendurada, M. Solid-phase microextraction: A promising technique for sample preparation in environmental analysis. J. Chromatogr. A 2000, 889, 3–14. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Panigrahi, S. Solid-Phase Microextraction (SPME) Techniques for Quality Characterization of Food Products: A Review. Food Bioprocess Technol. 2010, 4, 1–26. [Google Scholar] [CrossRef]
- Ho, C.W.; Wan Aida, W.M.; Maskat, M.Y.; Osman, H. Optimization of headspace solid phase microextraction (HS-SPME) for gas chromatography mass spectrometry (GC-MS) analysis of aroma compound in palm sugar (Arenga pinnata). J. Food Compos. Anal. 2006, 19, 822–830. [Google Scholar] [CrossRef]
- Câmara, J.S.; Alves, M.A.; Marques, J.C. Development of headspace solid-phase microextraction-gas chromatography–mass spectrometry methodology for analysis of terpenoids in Madeira wines. Anal. Chim. Acta 2006, 555, 191–200. [Google Scholar] [CrossRef]
- Manousi, N.; Zachariadis, G.A. Determination of Volatile Compounds in Nut-Based Milk Alternative Beverages by HS-SPME Prior to GC-MS Analysis. Molecules 2019, 24, 3091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, M.; Carvalho, M.; Henrique, R.; Jeronimo, C.; Moreira, N.; de Lourdes Bastos, M.; de Pinho, P.G. Analysis of volatile human urinary metabolome by solid-phase microextraction in combination with gas chromatography-mass spectrometry for biomarker discovery: Application in a pilot study to discriminate patients with renal cell carcinoma. Eur. J. Cancer 2014, 50, 1993–2002. [Google Scholar] [CrossRef]
- Wei, S.; Xiao, X.; Wei, L.; Li, L.; Li, G.; Liu, F.; Xie, J.; Yu, J.; Zhong, Y. Development and comprehensive HS-SPME/GC-MS analysis optimization, comparison, and evaluation of different cabbage cultivars (Brassica oleracea L. var. capitata L.) volatile components. Food Chem. 2021, 340, 128166. [Google Scholar] [PubMed]
- Robbat, A., Jr.; Liu, T.-Y.; Abraham, B.M. On-Site Detection of Polycyclic Aromatic Hydrocarbons in Contaminated Soils by Thermal Desorption Gas Chromatography/Mass Spectrometry. Anal. Chem. 1992, 64, 1477–1483. [Google Scholar] [CrossRef]
- Ma, Q.L.; Hamid, N.; Bekhit, A.E.D.; Robertson, J.; Law, T.F. Optimization of headspace solid phase microextraction (HS-SPME) for gas chromatography mass spectrometry (GC–MS) analysis of aroma compounds in cooked beef using response surface methodology. Microchem. J. 2013, 111, 16–24. [Google Scholar] [CrossRef]
- Souza Silva, E.A.; Saboia, G.; Jorge, N.C.; Hoffmann, C.; Dos Santos Isaias, R.M.; Soares, G.L.G.; Zini, C.A. Development of a HS-SPME-GC/MS protocol assisted by chemometric tools to study herbivore-induced volatiles in Myrcia splendens. Talanta 2017, 175, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Machiels, D.; Istasse, L. Evaluation of two commercial solid-phase microextraction fibres for the analysis of target aroma compounds in cooked beef meat. Talanta 2003, 61, 529–537. [Google Scholar] [CrossRef]
- Zhang, S.; Du, Z.; Li, G. Graphene-supported zinc oxide solid-phase microextraction coating with enhanced selectivity and sensitivity for the determination of sulfur volatiles in Allium species. J. Chromatogr. A 2012, 1260, 1–8. [Google Scholar] [CrossRef]
- Iida, H.; Hashimoto, S.; Miyazawa, M.; Kameoka, H. Volatile Flavor Components of Nira (Allium tuberosum Rottl.). J. Food Sci. 1983, 48, 660–661. [Google Scholar] [CrossRef]
- Molina-Calle, M.; Priego-Capote, F.; Luque de Castro, M.D. Headspace−GC–MS volatile profile of black garlic vs fresh garlic: Evolution along fermentation and behavior under heating. Lwt 2017, 80, 98–105. [Google Scholar] [CrossRef]
- Choi, S.M.; Lee, D.J.; Kim, J.Y.; Lim, S.T. Volatile composition and sensory characteristics of onion powders prepared by convective drying. Food Chem. 2017, 231, 386–392. [Google Scholar] [CrossRef]
- Noordermeer, M.A.; Goot, W.V.D.; Kooij, A.J.V.; Veldsink, J.W.; Veldink, G.A.; Vliegenthart, J.F.G. Development of a Biocatalytic Process for the Production of C6-Aldehydes from Vegetable Oils by Soybean Lipoxygenase and Recombinant Hydroperoxide Lyase. J. Agric. Food Chem. 2002, 50, 4270–4274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigo, D.; Jolie, R.; Loey, A.V.; Hendrickx, M. Thermal and high pressure stability of tomato lipoxygenase and hydroperoxide lyase. J. Food Eng. 2007, 79, 423–429. [Google Scholar] [CrossRef]
- Zhao, C.; Quan, P.; Liu, C.; Li, Q.; Fang, L. Effect of isopropyl myristate on the viscoelasticity and drug release of a drug-in-adhesive transdermal patch containing blonanserin. Acta Pharm. Sin. B 2016, 6, 623–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Kannan, K. Quantitative identification of and exposure to synthetic phenolic antioxidants, including butylated hydroxytoluene, in urine. Environ. Int. 2019, 128, 24–29. [Google Scholar] [CrossRef]
- Czerny, M.; Christlbauer, M.; Christlbauer, M.; Fischer, A.; Granvogl, M.; Hammer, M.; Hartl, C.; Hernandez, N.M.; Schieberle, P. Re-investigation on odour thresholds of key food aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. Eur. Food Res. Technol. 2008, 228, 265–273. [Google Scholar] [CrossRef]
- Tian, P.; Zhan, P.; Tian, H.; Wang, P.; Lu, C.; Zhao, Y.; Ni, R.; Zhang, Y. Analysis of volatile compound changes in fried shallot (Allium cepa L. var. aggregatum) oil at different frying temperatures by GC-MS, OAV, and multivariate analysis. Food Chem. 2021, 345, 128748. [Google Scholar] [CrossRef]
- Chen, S.; Tang, J.; Fan, S.; Zhang, J.; Chen, S.; Liu, Y.; Yang, Q.; Xu, Y. Comparison of Potent Odorants in Traditional and Modern Types of Chinese Xiaoqu Liquor (Baijiu) Based on Odor Activity Values and Multivariate Analyses. Foods 2021, 10, 2392. [Google Scholar] [CrossRef]
- Anon, A.; Lopez, J.F.; Hernando, D.; Orriols, I.; Revilla, E.; Losada, M.M. Effect of five enological practices and of the general phenolic composition on fermentation-related aroma compounds in Mencia young red wines. Food Chem. 2014, 148, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Zhang, W.; Lao, F.; Mi, R.; Liao, X.; Luo, D.; Wu, J. Isolation and identification of putative precursors of the volatile sulfur compounds and their inhibition methods in heat-sterilized melon juices. Food Chem. 2021, 343, 128459. [Google Scholar] [CrossRef]
- Van Gemert, L.J. Odour Thresholds: Compilations of Odour Threshold Values in Air, Water and Other Medhia; Oliemans Punter & Partners BV: Utrecht, The Netherlands, 2011. [Google Scholar]
- Alenyorege, E.A.; Ma, H.; Aheto, J.H.; Agyekum, A.A.; Zhou, C. Effect of sequential multi-frequency ultrasound washing processes on quality attributes and volatile compounds profiling of fresh-cut Chinese cabbage. Lwt 2020, 117, 108666. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients; CRC Press LLC: Boca Raton, FL, USA, 2010. [Google Scholar]
- Wang, M.Q.; Ma, W.J.; Shi, J.; Zhu, Y.; Lin, Z.; Lv, H.P. Characterization of the key aroma compounds in Longjing tea using stir bar sorptive extraction (SBSE) combined with gas chromatography-mass spectrometry (GC-MS), gas chromatography-olfactometry (GC-O), odor activity value (OAV), and aroma recombination. Food Res. Int. 2020, 130, 108908. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Wu, Q.; Niu, Y.; Wu, M.; Zhu, J.; Zhou, X.; Chen, X.; Wang, H.; Li, J.; Kong, J. Characterization of the Key Aroma Compounds in Five Varieties of Mandarins by Gas Chromatography-Olfactometry, Odor Activity Values, Aroma Recombination, and Omission Analysis. J. Agric. Food Chem. 2017, 65, 8392–8401. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, M.; Siebert, T.E.; Varela, C.; Pretorius, I.S.; Henschke, P.A. Effect of ammonium nitrogen supplementation of grape juice on wine volatiles and non-volatiles composition of the aromatic grape variety Albariño. Food Chem. 2012, 133, 124–131. [Google Scholar] [CrossRef]
| NO. | RT a (min) | Compound | CAS | Molecule Formula | Content (μg/kg) | RT b | RI c | Identification Method d |
|---|---|---|---|---|---|---|---|---|
| Ethers | ||||||||
| 1 | 3.2107 | Propylene sulfide | 1072-43-1 | C3H6S | 153.53 | 944 | 915 | MS/RI |
| 2 | 3.5648 | Allyl methyl sulfide | 10152-76-8 | C4H8S | 480.72 | 971 | 956 | MS/RI |
| 3 | 4.9748 | Dimethyl disulfide | 624-92-0 | C2H6S2 | 1880.64 | 1077 | 1077 | MS/RI |
| 4 | 6.0674 | Diallyl sulfide | 592-88-1 | C6H10S | 333.89 | 1142 | 1148 | MS/RI |
| 5 | 6.391 | (1E)-1-[(1E)-1-propenylsulfanyl]-1-propene | 33922-80-4 | C6H10S | 108.09 | 1159 | 1158 | MS/RI |
| 6 | 6.5802 | 2,5-dimethyl-thiophene | 638-02-8 | C6H8S | 27.56 | 1169 | 1168 | MS/RI |
| 7 | 6.7878 | (Z)-allyl(prop-1-en-1-yl)sulfane | 104324-69-8 | C6H10S | 39.98 | 1180 | - | MS |
| 8 | 6.8244 | (E)-allyl 1-propenyl sulfide | 104324-36-9 | C6H10S | 391.04 | 1182 | - | MS |
| 9 | 7.1677 | 2,4-dimethylthiophene | 638-00-6 | C6H8S | 199.85 | 1201 | 1197 | MS/RI |
| 10 | 7.9414 | Methyl propyl disulfide | 2179-60-4 | C4H10S2 | 3.20 | 1233 | 1239 | MS/RI |
| 11 | 8.3443 | Methyl prop-1-enyl disulphide | 5905-47-5 | C4H8S2 | 1913.80 | 1250 | 1269 | MS/RI |
| 12 | 8.4175 | 3,4-dimethyl-thiophene | 632-15-5 | C6H8S | 2206.77 | 1253 | 1252 | MS/RI |
| 13 | 9.1317 | Allyl methyl disulfide | 2179-58-0 | C4H8S2 | 8146.24 | 1282 | 1281 | MS/RI |
| 14 | 9.5041 | 2-vinyl-thiophene | 1918-82-7 | C6H6S | 49.78 | 1298 | 1312 | MS/RI |
| 15 | 9.7665 | (Z)-1-methyl-2-(prop-1-en-1-yl)disulfane | 23838-18-8 | C4H8S2 | 5.22 | 1307 | 1303 | MS/RI |
| 16 | 9.9497 | (E)-1-methyl-2-(prop-1-en-1-yl)disulfane | 23838-19-9 | C4H8S2 | 8865.14 | 1313 | 1327 | MS/RI |
| 17 | 11.9946 | Dimethyl trisulfide | 3658-80-8 | C2H6S3 | 10,623.30 | 1377 | 1377 | MS/RI |
| 18 | 13.5938 | 1-[[(Z)-prop-1-enyl]disulfanyl]propane | 23838-20-2 | C6H12S2 | 159.20 | 1422 | 1421 | MS/RI |
| 19 | 15.6021 | Diallyl disulfide | 2179-57-9 | C6H10S2 | 2924.25 | 1474 | 1475 | MS/RI |
| 20 | 15.8645 | (E)-1-allyl-2-(prop-1-en-1-yl)disulfane | 122156-02-9 | C6H10S2 | 3047.31 | 1480 | - | MS |
| 21 | 17.0908 | 3H-1,2-dithiole | 288-26-6 | C3H4S2 | 1197.11 | 1510 | 1510 | MS/RI |
| 22 | 20.5892 | (E)-1-methyl-3-(prop-1-en-1-yl)trisulfane | 23838-25-7 | C4H8S3 | 2223.95 | 1586 | 1586 | MS/RI |
| 23 | 20.8639 | Methyl allyl trisulfide | 34135-85-8 | C4H8S3 | 4927.59 | 1592 | 1593 | MS/RI |
| 24 | 26.6202 | 3-ethenyl-3,6-dihydrodithiine | 62488-52-2 | C6H8S2 | 197.57 | 1711 | 1750 | MS/RI |
| 25 | 27.9874 | 2-ethenyl-1,3-dithiane | 61685-40-3 | C6H10S2 | 43.36 | 1739 | 1723 | MS/RI |
| 26 | 28.0119 | 2-ethylidene-1,3-dithiane | 51102-62-6 | C6H10S2 | 51.49 | 1740 | 1778 | MS/RI |
| 27 | 29.3366 | Diallyl trisulfide | 2050-87-5 | C6H10S3 | 361.58 | 1766 | 1805 | MS/RI |
| 28 | 31.7294 | 2-ethenyl-4H-1,3-dithiine | 80028-57-5 | C6H8S2 | 37.82 | 1819 | 1857 | MS/RI |
| Aldehydes | ||||||||
| 29 | 4.5109 | 2-butenal | 4170-30-3 | C4H6O | 164.26 | 1043 | 1047 | MS/RI |
| 30 | 5.9454 | 2-methylpent-4-enal | 5187-71-3 | C6H10O | 47.41 | 1135 | 1141 | MS/RI |
| 31 | 6.2872 | 2-methyl-2-pentenal | 623-36-9 | C6H10O | 3.01 | 1153 | 1155 | MS/RI |
| 32 | 7.1967 | 2-hexenal | 505-57-7 | C6H10O | 166.93 | 1202 | 1213 | MS/RI |
| 33 | 7.5752 | (E)-2-hexenal | 6728-26-3 | C6H10O | 2996.96 | 1218 | 1216 | MS/RI |
| 34 | 10.5235 | 2-ethyl-2-hexanal | 645-62-5 | C8H14O | 12.49 | 1331 | 1333 | MS/RI |
| 35 | 12.373 | Nonanal | 124-19-6 | C9H18O | 53.79 | 1389 | 1391 | MS/RI |
| 36 | 12.6294 | 2,4-hexadienal | 142-83-6 | C6H8O | 55.48 | 1397 | 1400 | MS/RI |
| 37 | 13.0994 | 5-ethylcyclopentene-1-carbaldehyde | 36431-60-4 | C8H12O | 94.34 | 1410 | 1410 | MS/RI |
| 38 | 16.0477 | (E,E)-2,4-heptadienal | 881395 | C7H10O | 83.96 | 1485 | 1495 | MS/RI |
| 39 | 16.3651 | Decanal | 112-31-2 | C10H20O | 86.80 | 1493 | 1498 | MS/RI |
| 40 | 16.9999 | 1,3,4-trimethylcyclohex-3-enecarbaldehyde | 40702-26-9 | C10H16O | 15.88 | 1508 | 1525 | MS/RI |
| 41 | 17.8301 | (E)-2-nonenal | 18829-56-6 | C9H16O | 8.38 | 1526 | 1534 | MS/RI |
| 42 | 25.4542 | 2,5-dimethylbenzaldehyde | 5779-94-2 | C9H10O | 296.75 | 1687 | 1683 | MS/RI |
| 43 | 27.6628 | 2-undecenal | 2463-77-6 | C11H20O | 25.78 | 1732 | 1751 | MS/RI |
| Alcohols | ||||||||
| 44 | 5.8781 | Allyl alcohol | 107-18-6 | C3H6O | 28.69 | 1131 | 1123 | MS/RI |
| 45 | 7.3127 | 2-hexyn-1-ol | 764-60-3 | C6H10O | 86.04 | 1207 | 1207 | MS/RI |
| 46 | 16.23 | 2-ethylhexanol | 104-76-7 | C8H18O | 10.30 | 1490 | 1491 | MS/RI |
| 47 | 18.6782 | Linalool | 78-70-6 | C10H18O | 15.53 | 1545 | 1547 | MS/RI |
| 48 | 34.897 | α-ionol | 25312-34-9 | C13H22O | 17.09 | 1905 | 1895 | MS/RI |
| 49 | 47.7179 | Phytol | 150-86-7 | C20H40O | 12.11 | 2609 | 2622 | MS/RI |
| Ketones | ||||||||
| 50 | 10.1694 | 2,5-octanedione | 3214-41-3 | C8H14O2 | 212.64 | 1319 | 1319 | MS/RI |
| 51 | 12.1469 | 2,2-dimethylcyclohexanone | 1193-47-1 | C8H14O | 39.50 | 1382 | 1382 | MS/RI |
| 52 | 32.7916 | 6,10-dimethyl-5,9-undecadien-2-one | 689-67-8 | C13H22O | 111.26 | 1848 | 1841 | MS/RI |
| 53 | 35.331 | β-ionone | 79-77-6 | C13H20O | 167.56 | 1921 | 1940 | MS/RI |
| 54 | 41.1062 | o-acetyl-p-cresol | 1450-72-2 | C9H10O2 | 42.73 | 2185 | 2185 | MS/RI |
| Hydrocarbons | ||||||||
| 55 | 12.7233 | 3-hexylcyclohexene | 15232-78-7 | C12H22 | 81.14 | 1400 | 1392 | MS/RI |
| 56 | 25.7543 | 4-methoxystyrene | 637-69-4 | C9H10O | 647.64 | 1694 | 1684 | MS/RI |
| Esters | ||||||||
| 57 | 38.0841 | Isopropyl myristate | 110-27-0 | C17H34O2 | 44.63 | 2032 | 2027 | MS/RI |
| Phenols | ||||||||
| 58 | 34.7746 | Butylated hydroxytoluene | 128-37-0 | C15H24O | 10.02 | 1900 | 1909 | MS/RI |
| 59 | 43.1876 | 2,4-di-tert-butylphenol | 96-76-4 | C14H22O | 11.42 | 2309 | 2318 | MS/RI |
| NO a. | Compound | CAS | Molecule Formula | Content (μg/kg) | Odor Threshold b (μg/kg) | Odor Activity Values (OAVs) | Odor Description c |
|---|---|---|---|---|---|---|---|
| Ethers | |||||||
| 2 | Allyl methyl sulfide | 10152-76-8 | C4H8S | 480.72 | 22 | 21.85 | Alliaceous, garlic, onion |
| 3 | Dimethyl disulfide | 624-92-0 | C2H6S2 | 1880.64 | 12 | 156.72 | Siffuse, intense onion odor |
| 4 | Diallyl sulfide | 592-88-1 | C6H10S | 333.89 | 32.5 | 10.27 | Characteristic garlic odor |
| 6 | 2,5-dimethyl-thiophene | 638-02-8 | C6H8S | 27.56 | 0.7 | 39.37 | Nutty sulfury |
| 9 | 2,4-dimethylthiophene | 638-00-6 | C6H8S | 199.85 | 3000 | 0.07 | Not clear |
| 11 | Methyl prop-1-enyl disulphide | 5905-47-5 | C4H8S2 | 1913.80 | 6.3 | 303.78 | A strong odor in garlic and onion |
| 12 | 3,4-dimethyl-thiophene | 632-15-5 | C6H8S | 2206.77 | 5000 | 0.44 | Savory roasted onion |
| 17 | Dimethyl trisulfide | 3658-80-8 | C2H6S3 | 10,623.30 | 6 | 1770.55 | Powerful, diffusive, fresh onion. |
| 19 | Diallyl disulfide | 2179-57-9 | C6H10S2 | 2924.25 | 30 | 97.47 | Characteristic garlic odor |
| Aldehydes | |||||||
| 29 | 2-butenal | 4170-30-3 | C4H6O | 164.26 | 1400 | 0.12 | Flower |
| 31 | 2-methyl-2-pentenal | 623-36-9 | C6H10O | 3.01 | 290 | 0.01 | Powerful, grassy-green, slightly fruity odor |
| 32 | 2-hexenal | 505-57-7 | C6H10O | 166.93 | 850 | 0.20 | Fragrant, apple, vegetable odor |
| 33 | (E)-2-hexenal | 6728-26-3 | C6H10O | 2996.96 | 1125 | 2.66 | Green, banana, fatty |
| 35 | Nonanal | 124-19-6 | C9H18O | 53.79 | 300 | 0.18 | Fatty, orange, rose odor |
| 36 | 2,4-hexadienal | 142-83-6 | C6H8O | 55.48 | 60 | 0.92 | Sweet, green aroma |
| 38 | (E,E)-2,4-heptadienal | 4313-03-5 | C7H10O | 83.96 | 15.4 | 5.45 | Fatty, green odor |
| 39 | Decanal | 112-31-2 | C10H20O | 86.80 | 650 | 0.13 | Penetrating, sweet, floral, fatty odor |
| 41 | (E)-2-nonenal | 18829-56-6 | C9H16O | 8.38 | 50 | 0.17 | Fatty green cucumber aldehydic citrus |
| 42 | 2,5-dimethyl benzaldehyde | 5779-94-2 | C9H10O | 296.75 | 200 | 1.48 | Not clear |
| Alcohols | |||||||
| 46 | 2-ethyl-1-hexanol | 104-76-7 | C8H18O | 10.30 | 198 | 0.05 | Mild, sweet, slightly floral odor |
| 47 | Linalool | 78-70-6 | C10H18O | 15.53 | 37 | 0.42 | A typical pleasant floral odor |
| Ketones | |||||||
| 53 | β-ionone | 79-77-6 | C13H20O | 167.56 | 8.4 | 19.95 | Flowery, violet-like |
| Phenol | |||||||
| 58 | Butylated hydroxytoluene | 128-37-0 | C15H24O | 10.02 | 1000 | 0.01 | Faint, musty odor |
| 59 | 2,4-di-t-butylphenol | 96-76-4 | C14H22O | 11.42 | 500 | 0.02 | Phenolic |
| Optimized Parameters | Levels of Optimized Parameters | |||||
|---|---|---|---|---|---|---|
| SPME fiber | 50/30 μm DVB/CAR/PDMS | 65 μm PDMS/DVB | 75 μm CAR/PDMS | 85 μm PA | 85 μm CAR/PDMS | 100 μm PDMS |
| Sample weight (g) | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 |
| Na2SO4 weight (g) | 0 | 0.25 | 0.5 | 0.75 | 1 | 1.25 |
| Extraction temperature (°C) | 30 | 40 | 50 | 60 | 70 | 80 |
| Equilibration time (min) | 5 | 10 | 15 | 20 | 25 | 30 |
| Extraction time (min) | 10 | 20 | 30 | 40 | 50 | 60 |
| Desorption time (min) | 1 | 3 | 5 | 7 | 9 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, B.; Wu, Q.; Wei, S.; Li, H.; Wei, J.; Hanif, M.; Li, J.; Liu, Z.; Xiao, X.; Yu, J. Optimization of Headspace Solid-Phase Micro-Extraction Conditions (HS-SPME) and Identification of Major Volatile Aroma-Active Compounds in Chinese Chive (Allium tuberosum Rottler). Molecules 2022, 27, 2425. https://doi.org/10.3390/molecules27082425
Xie B, Wu Q, Wei S, Li H, Wei J, Hanif M, Li J, Liu Z, Xiao X, Yu J. Optimization of Headspace Solid-Phase Micro-Extraction Conditions (HS-SPME) and Identification of Major Volatile Aroma-Active Compounds in Chinese Chive (Allium tuberosum Rottler). Molecules. 2022; 27(8):2425. https://doi.org/10.3390/molecules27082425
Chicago/Turabian StyleXie, Bojie, Qian Wu, Shouhui Wei, Haiyan Li, Jinmei Wei, Medhia Hanif, Ju Li, Zeci Liu, Xuemei Xiao, and Jihua Yu. 2022. "Optimization of Headspace Solid-Phase Micro-Extraction Conditions (HS-SPME) and Identification of Major Volatile Aroma-Active Compounds in Chinese Chive (Allium tuberosum Rottler)" Molecules 27, no. 8: 2425. https://doi.org/10.3390/molecules27082425
APA StyleXie, B., Wu, Q., Wei, S., Li, H., Wei, J., Hanif, M., Li, J., Liu, Z., Xiao, X., & Yu, J. (2022). Optimization of Headspace Solid-Phase Micro-Extraction Conditions (HS-SPME) and Identification of Major Volatile Aroma-Active Compounds in Chinese Chive (Allium tuberosum Rottler). Molecules, 27(8), 2425. https://doi.org/10.3390/molecules27082425





