Sustainable Green Processing of Grape Pomace Using Micellar Extraction for the Production of Value-Added Hygiene Cosmetics
Abstract
:1. Introduction
2. Results and Discussion
2.1. Development of Loan Chemical Extraction for Obtaining Cosmetically Valuable Compounds from Waste Plant Material Using Components Borrowed from the Final Product
2.2. Determination of Selected Compounds by UPLC-MS/MS
2.3. Determination of Anthocyanins by UPLC-ESI-MS/MS
2.4. Total Phenolic, Flavonoid, and Anthocyanin Content
2.5. Antioxidant Activity (DPPH, ABTS)
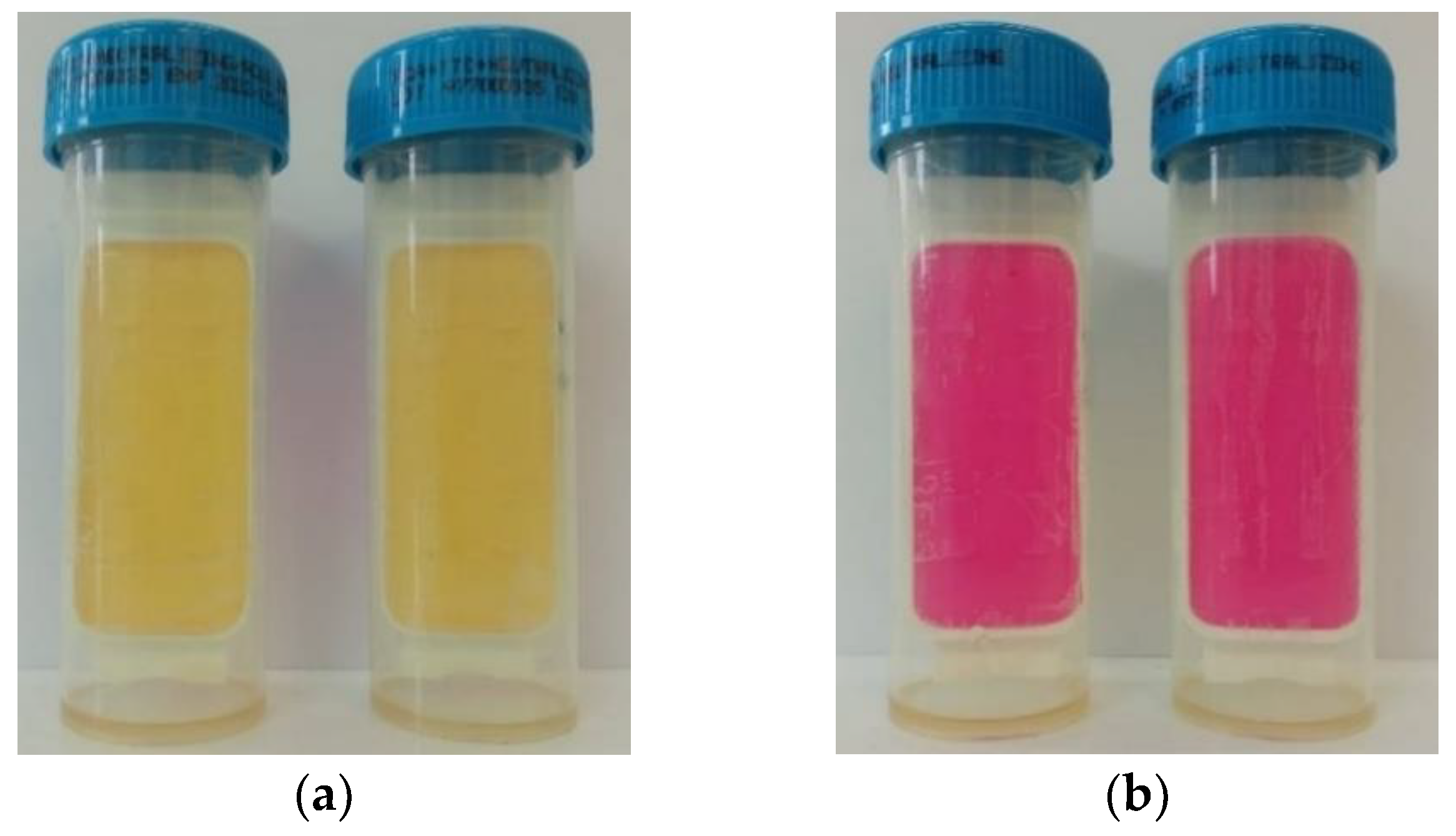
2.6. Determination of the Color Parameters of Grape Pomace Extracts
2.7. Application Analysis
2.7.1. Interactions of Model Washing Gels with the Skin
2.7.2. Determination of the Color Parameters of Cosmetics Containing Extracts
2.7.3. Rheological Behavior
2.7.4. Foam Ability
2.7.5. Microbiological Stability
2.7.6. Stability
3. Materials and Methods
3.1. Materials
3.2. Plant Material
3.3. Extraction Medium Characterisation
3.3.1. Surface Tension Measurements
3.3.2. Dynamic Light Scattering (DLS)
3.4. Preparation of Micellar Extracts from Grape Pomace Using LCE, as Cosmetic Components Borrowed from the Final Product (Shower Gel)
3.5. Determination of Bioactive Compounds by UPLC-ESI–MS/MS
3.5.1. Quantitative UPLC-ESI-MS/MS Analysis of Selected Compounds in Grape Pomace Extracts
3.5.2. Qualitative UPLC-ESI-MS/MS Determination of Selected Anthocyanins in Grape Pomace Extracts
3.6. Determination of Antioxidant Properties
3.6.1. Total Phenolics Content (TPC)
3.6.2. Total Flavonoid Content (TFC)
3.6.3. Total Anthocyanin Content (TAC)
3.6.4. Antioxidant Activity (DPPH Test)
3.6.5. Antioxidant Activity (ABTS Test)
3.7. Preparation of Model Cosmetics (Shower Gels)
3.8. Determination of the Color Parameters of Grape Pomace Extracts and Cosmetics (Shower Gels) Containing Extracts
3.9. Rheological Behavior
3.10. Foam Ability
3.11. Determination of Irritant Potential—Zein Value
3.12. Transepidermal Water Loss (TEWL) and Skin Hydration Measurements
3.13. Microbiological Stability
3.14. Stability
3.15. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statements
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bujak, T.; Wasilewski, T.; Nizioł-Łukaszewska, Z. Effect of molecular weight of polyvinylpyrrolidone on the skin irritation potential and properties of body wash cosmetics in the coacervate form. Pure Appl. Chem. 2019, 91, 1521–1532. [Google Scholar] [CrossRef]
- Lukic, M.; Pantelic, I.; Savic, S. An Overview of Novel Surfactants for Formulation of Cosmetics with Certain Emphasis on Acidic Active Substances. Tenside Surf. Det. 2016, 53, 7–19. [Google Scholar] [CrossRef]
- Bujak, T.; Nizioł-Łukaszewska, Z.; Wasilewski, T. Sodium Lauryl Sulfate vs. Sodium Coco Sulfate. Study of the safety of use anionic surfactants with respect to their interaction with the skin. Tenside Surf. Det. 2019, 56, 126–133. [Google Scholar] [CrossRef]
- González-Minero, F.J.; Bravo-Díaz, L. The Use of Plants in Skin-Care Products, Cosmetics and Fragrances: Past and Present. Cosmetics 2018, 5, 50. [Google Scholar] [CrossRef] [Green Version]
- Okereke, J.N.; Udebuani, A.C.; Ezeji, E.U.; Obasi, K.O.; Nnoli, M.C. Possible Health Implications Associated with Cosmetics: A Review. Sci. J. Public Health 2015, 3, 58–63. [Google Scholar]
- Nizioł-Łukaszewska, Z.; Furman-Toczek, D.; Bujak, T.; Wasilewski, T.; Hordyjewicz-Baran, Z. Moringa oleifera L. Extracts as Bioactive Ingredients That Increase Safety of Body Wash Cosmetics. Dermatol. Res. Pract. 2020, 2020, 8197902. [Google Scholar] [CrossRef] [PubMed]
- Bujak, T.; Zagórska-Dziok, M.; Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Lal, K.; Wasilewski, T.; Hordyjewicz-Baran, Z. Flower Extracts as Multifunctional Dyes in the Cosmetics Industry. Molecules 2022, 27, 922. [Google Scholar] [CrossRef] [PubMed]
- Seweryn, A.; Wasilewski, T. Detergents in the coacervate form with plant extracts obtained under supercritical carbon dioxide conditions as examples of sustainable products. J. Dispers. Sci. Technol. 2020, 41, 797–808. [Google Scholar] [CrossRef]
- Wasilewski, T.; Yong-Qiang, S.; Hreczuch, W.; Seweryn, A.; Bujak, T. Evaluation of Ethoxylated Rapeseed Oil Fatty Acids Methyl Esters as nonionic co-surfactants in Hand Dishwashing Liquids. Tenside Surf. Det. 2019, 56, 279–286. [Google Scholar] [CrossRef]
- Wasilewski, T.; Bujak, T. Effect of the Type of Nonionic Surfactant on the Manufacture and Properties of Hand Dishwashing Liquids in the Coacervate Form. Ind. Eng. Chem. Res. 2014, 53, 13356–13361. [Google Scholar] [CrossRef]
- Seweryn, A.; Wasilewski, T.; Bocho-Janiszewska, A. Correlation between Sequestrant Type and Properties of Mild Soap Based Hand Washing Products. Ind. Eng. Chem. Res. 2018, 57, 12683–12688. [Google Scholar] [CrossRef]
- Bujak, T.; Nizioł-Łukaszewska, Z.; Wasilewski, T. Effect of Molecular Weight of Polymers on the Properties of Delicate Facial Foams. Tenside Surf. Det. 2018, 55, 96–102. [Google Scholar] [CrossRef]
- Bujak, T.; Wasilewski, T.; Nizioł-Łukaszewska, Z. Role of macromolecules in the safety of use of body wash cosmetics. Colloids Surf. B 2015, 135, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, T.; Arct, J.; Pytkowska, K.; Bocho-Janiszewska, A.; Krajewski, M.; Bujak, T. Technologiczne i fizykochemiczne aspekty wytwarzania koncentratów kosmetyków myjących. Przemysl Chem. 2015, 94, 741–747. [Google Scholar]
- Wasilewski, T. Coacervates as a modern delivery system of hand dishwashing liquid. J. Surfactants Deterg. 2010, 13, 513–520. [Google Scholar] [CrossRef]
- Brito, A.G.; Peixoto, J.; Oliveria, J.M.; Oliveria, J.A.; Costa, C.; Nogueira, R.; Rodrigues, A. Brewery and winery wastewater treatment: Some focal points of design and operation. In Utilization of By-Products and Treatment of Waste in the Food Industry; Oreopoulou, V., Russ, W., Eds.; Springer Science & Business Media: New York, NY, USA, 2007; pp. 109–131. [Google Scholar]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J. Food Compos. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Xia, E.; Deng, G.; Guo, Y.; Li, H. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Murthy, K.N.C.; Singh, R.P.; Jayaprakasha, G.K. Antioxidant activities of grape Vitis vinifera pomace extracts. J. Agric. Food Chem. 2002, 50, 5909–5914. [Google Scholar] [CrossRef]
- Ilyas, T.; Chowdhary, P.; Chaurasia, D.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Sustainable green processing of grape pomace for the production of value-added products: An overview. Environ. Technol. Innov. 2021, 23, 101592. [Google Scholar] [CrossRef]
- Kalli, E.; Lappa, I.; Bouchagier, P.; Tarantilis, P.A.; Skotti, E. Novel application and industrial exploitation of winery by-products. Bioresour. Bioprocess. 2018, 5, 46. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Pellegrini, N.; Colombi, B.; Salvatore, S.; Brenna, O.V.; Galaverna, G.; Del Rio, D.; Bianchi, M.; Bennett, R.N.; Brighenti, F. Evaluation of antioxidant capacity of some fruit and vegetable foods: Efficiency of extraction of a sequence of solvents. J. Sci. Food Agric. 2007, 87, 103–111. [Google Scholar] [CrossRef]
- Martinez Vidal, J.L.; Belmonte Vega, A.; Garrido Frenich, A.; Egea Gonzalez, F.J.; Arrebola Liebanas, F.J. Determination of fifteen priority phenolic compounds in environmental samples from Andalusia (Spain) by liquid chromatrography–mass spectrometry. Anal. Bioanal. Chem. 2004, 379, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Negro, N.; Tommasi, L.; Miceli, A. Phenolic compounds and antioxidant activity from red grape marc extracts. Bioresour. Technol. 2003, 87, 41–44. [Google Scholar] [CrossRef]
- Lapornik, B.; Prošek, M.; Wondra, A.G. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride: Urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.J.; Kumar, G.V.; Dash, A.; Scholz, P.; Banerjee, R. Sustainable green solvents and techniques for lipid extraction from microalgae: A review. Algal Res. 2017, 21, 138–147. [Google Scholar] [CrossRef]
- Marr, R.; Gamse, T. Use of supercritical fluids for different processes including new developments—A review. Chem. Eng. Process. 2000, 39, 19–28. [Google Scholar] [CrossRef]
- Lang, Q.; Wai, C.M. Supercritical fluid extraction in herbal and natural product studies—A practical review. Talanta 2001, 53, 771–782. [Google Scholar] [CrossRef]
- Meireles, A.; Angela, M. Supercritical extraction from solid: Process design data (2001–2003). Curr. Opin. Solid State Mater. Sci. 2003, 7, 321–330. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, J.; Cheng, Y.; Li, D.; Hu, F.; Li, H. Determination of Prometryne in water and soil by HPLC–UV using cloud-point extraction. Talanta 2009, 79, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Vichapong, J.; Santaladchaiyakit, Y.; Burakham, R.; Supalax, S. Cloud-point extraction and reversed-phase high performance liquid chromatography for analysis of phenolic compounds and their antioxidant activity in Thai local wines. J. Food Sci. Technol. 2014, 51, 664–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arya, S.S.; Kaimal, A.M.; Chib, M.; Sonawane, S.K.; Show, P.L. Novel, energy efficient and green cloud point extraction: Technology and applications in food processing. J. Food Sci. Technol. 2019, 56, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Santalad, A.; Srijaranai, S.; Burakham, R.; Glennon, J.D.; Deming, R.L. Cloud-point extraction and reversed-phase high-performance liquid chromatography for the determination of carbamate insecticide residues in fruits. Anal. Bioanal. Chem. 2009, 394, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I.; Azhari, N.H. Vernonia cinerea leaves as the source of phenolic compounds, antioxidants, and anti-diabetic activity using microwave-assisted extraction technique. Ind. Crop. Prod. 2018, 122, 533–544. [Google Scholar] [CrossRef]
- Vinatour, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Howard, L.R. Effects of Solvent and Temperature on Pressurized Liquid Extraction of Anthocyanins and Total Phenolics from Dried Red Grape Skin. J. Agric. Food Chem. 2003, 51, 5207–5213. [Google Scholar] [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; Sannita, E.; Perego, P. High-pressure high-temperature extraction of phenolic compounds from grape skins. Int. J. Food Sci. Technol. 2012, 47, 399–405. [Google Scholar] [CrossRef]
- Boussetta, N.; Lebovka, N.; Vorobiev, E.N.; Adenier, H.; Bedel-Cloutour, C.; Lanoisellé, J.-L. Electrically Assisted Extraction of Soluble Matter from Chardonnay Grape Skins for Polyphenol Recovery. J. Agric. Food Chem. 2009, 57, 1491–1497. [Google Scholar] [CrossRef]
- Toepfl, S.; Mathys, A.; Heinz, V.; Knorr, D. Review: Potential of high hydrostatic pressure and pulsed electric fields for energy efficiency and environmentally friendly food processing. Food Rev. Int. 2006, 22, 405–423. [Google Scholar] [CrossRef]
- Gaur, R.; Sharma, A.; Khare, S.K.; Gupta, M.N. A novel process for extraction of edible oils: Enzyme assisted three phase partitioning (EATPP). Bioresour. Technol. 2007, 98, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Gomez-García, R.; Martínez-Avila, G.C.G.; Aguilar, C.N. Enzyme-assisted extraction of antioxidative phenolics from grape (Vitis vinifera L.) residues. 3 Biotech 2012, 3, 297–300. [Google Scholar] [CrossRef] [Green Version]
- de Camargo, A.C.; Regitano-d’Arce, M.A.; Biasoto, A.C.; Shahidi, F. Enzyme assisted extraction of phenolics from winemaking by-products: Antioxidant potential and inhibition of alpha-glucosidase and lipase activities. Food Chem. 2016, 212, 395–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [Green Version]
- Kusumawati, I.; Indrayanto, G. Natural Antioxidants in Cosmetics. Stud. Nat. Prod. Chem. 2013, 40, 485–505. [Google Scholar]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Fariña, L.O.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2019, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds; a Review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [Green Version]
- Duba, K.; Fiori, L. Supercritical CO2 extraction of grape seeds oil: Scale-up and economic analysis. Int. J. Food Sci. Technol. 2019, 54, 1306–1312. [Google Scholar] [CrossRef]
- Sapkale, G.N.; Patil, S.M.; Surwase, U.S.; Bhatbhage, P.K. Supercritical fluid extraction. Int. J. Chem. Sci. 2010, 8, 729–743. [Google Scholar]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Kupe, M.; Karatas, N.; Unal, M.S.; Ercisli, S.; Baron, M.; Sochor, J. Phenolic Composition and Antioxidant Activity of Peel, Pulp and Seed Extracts of Different Clones of the Turkish Grape Cultivar ‘Karaerik’. Plants 2021, 10, 2154. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.L.; Falqué, E.; Domínguez, H. Relevance of Natural Phenolics from Grape and Derivative Products in the Formulation of Cosmetics. Cosmetics 2015, 2, 259–276. [Google Scholar] [CrossRef] [Green Version]
- Maluf, D.F.; Gonçalves, M.M.; D’Angelo, R.W.O.; Girassol, A.B.; Tulio, A.P.; Pupo, Y.M.; Farago, P.V. Cytoprotection of Antioxidant Biocompounds from Grape Pomace: Further Exfoliant Phytoactive Ingredients for Cosmetic Products. Cosmetics 2018, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Tikhonova, A.; Ageeva, N.; Globa, E. Grape pomace as a promising source of biologically valuable components. In BIO Web of Conferences; EDP Sciences: Les Ulis, France, 2021; Volume 34, p. 06002. [Google Scholar]
- Chan, C.-F.; Lien, C.-Y.; Lai, Y.-C.; Huang, C.-L.; Liao, W.C. Influence of purple sweet potato extracts on the UV absorption properties of a cosmetic cream. J. Cosmet. Sci. 2010, 61, 333–341. [Google Scholar]
- Mathur, P.; George, R.; Mathur, A. Anthocyanin: A Revolutionary Pigment for Textile industry. Curr. Trends Biotechnol. Microbiol. 2020, 1, 75–77. [Google Scholar]
- Hoss, I.; Rajha, H.N.; El Khoury, R.; Youssef, S.; Manca, M.L.; Manconi, M.; Louka, N.; Maroun, R.G. Valorization of Wine-Making By-Products’ Extracts in Cosmetics. Cosmetics 2021, 8, 109. [Google Scholar] [CrossRef]
- Cádiz-Gurrea, M.; Silva, A.; Delerue-Matos, C.; Rodrigues, F. Cosmetics—Food waste recovery. In Food Waste Recovery; Academic Press: Cambridge, MA, USA, 2021; pp. 503–528. ISBN 978-0-12-820563-1. [Google Scholar]
- Matos, M.S.; Romero-Díez, R.; Álvarez, A.; Bronze, M.R.; Rodríguez-Rojo, S.; Mato, R.B.; Cocero, M.J.; Matias, A.A. Polyphenol-Rich Extracts Obtained from Winemaking Waste Streams as Natural Ingredients with Cosmeceutical Potential. Antioxidants 2019, 8, 355. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alibade, A.; Batra, G.; Bozinou, E.; Salakidou, C.; Lalas, S. Optimization of the extraction of antioxidants from winery wastes using cloud point extraction and a surfactant of natural origin (lecithin). Chem. Pap. 2020, 74, 4517–4524. [Google Scholar] [CrossRef]
- Kourilova, X.; Pernicova, I.; Vidlakova, M.; Krejcirik, R.; Mrazova, K.; Hrubanova, K.; Krzyzanek, V.; Nebesarova, J.; Obruca, S. Biotechnological Conversion of Grape Pomace to Poly(3-hydroxybutyrate) by Moderately Thermophilic Bacterium Tepidimonas taiwanensis. Bioengineering 2021, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Negro, C.; Aprile, A.; Luvisi, A.; De Bellis, L.; Miceli, A. Antioxidant Activity and Polyphenols Characterization of Four Monovarietal Grape Pomaces from Salento (Apulia, Italy). Antioxidants 2021, 10, 1406. [Google Scholar] [CrossRef]
- Ramirez-Lopez, L.M.; McGlynn, W.; Goad, C.L.; Mireles DeWitt, C.A. Simultaneous determination of phenolic compounds in Cynthiana grape (Vitis aestivalis) by high performance liquid chromatography–electrospray ionisation–mass spectrometry. Food Chem. 2014, 149, 15–24. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Hardeland, R.; Lopez-Burillo, S.; Mayo, J.C.; Sainz, R.M.; Reiter, R.J. Melatonin: A hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J. Pineal Res. 2003, 34, 75–78. [Google Scholar] [CrossRef]
- Valiente, C.; Arrigoni, E.; Esteban, R.M.; Amado, R. Grape Pomace as a Potential Food Fiber. J. Food Sci. 1995, 60, 818–820. [Google Scholar] [CrossRef]
- Iora, S.R.F.; Maciel, G.M.; Zielinski, A.A.F.; da Silva, M.V.; Pontes, P.V.D.A.; Haminiuk, C.W.I.; Granato, D. Evaluation of the bioactive compounds and the antioxidant capacity of grape pomace. Int. J. Food Sci. 2015, 50, 62–69. [Google Scholar] [CrossRef]
- Jin, Q.; O’Hair, J.; Stewart, A.C.; O’Keefe, S.F.; Neilson, A.P.; Kim, Y.-T.; McGuire, M.; Lee, A.; Wilder, G.; Huang, H. Compositional Characterization of Different Industrial White and Red Grape Pomaces in Virginia and the Potential Valorization of the Major Components. Foods 2019, 8, 667. [Google Scholar] [CrossRef] [Green Version]
- Baron, G.; Ferrario, G.; Marinello, C.; Carini, M.; Morazzoni, P.; Aldini, G. Effect of Extraction Solvent and Temperature on Polyphenol Profiles, Antioxidant and Anti-Inflammatory Effects of Red Grape Skin By-Product. Molecules 2021, 26, 5454. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Rodrigues, E.; Gonzaga, L.V.; Caliari, V.; Genovese, M.I.; Gonçalves, A.E.S.S.; Fett, R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011, 127, 174–179. [Google Scholar] [CrossRef]
- Eyiz, V.; Tontul, I.; Turker, S. Optimization of green extraction of phytochemicals from red grape pomace by homogenizer assisted extraction. J. Food Meas. Charact. 2020, 14, 39–47. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Silva, G.L.; Rodrigues, E.; Gonzaga, L.V.; Fett, R. Atividade antioxidante de extratos de bagaço de uva das variedades Regente e Pinot Noir (Vitis vinifera). Rev. Do Inst. Adolfo Lutz 2007, 66, 158–163. [Google Scholar]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz-Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Int. Food Res. J. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Jackson, C.T.; Paye, M.; Maibach, H. Mechanism of Skin Irritation by Surfactants and Anti-Irritants for Surfactants Base Products. In Handbook of Cosmetic Science and Technology, 4th ed.; Barel, A., Paye, M., Maibach, H., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 353–365. [Google Scholar]
- Farn, R.J. Chemistry and Technology of Surfactants; Blackwell Publishing: Hoboken, NY, USA, 2006; pp. 46–90. [Google Scholar]
- Rosen, M.J. Surfactants and Interfacial Phenomena, 3rd ed.; John Wiley & Sons Inc.: New York, NY, USA, 2006. [Google Scholar]
- Dominguez, J.G.; Balaguer, F.; Parra, J.L.; Pelejero, C.M. The inhibitory effect of some amphoteric surfactants on the irritation potential of alkylsulphates. Int. J. Cosmet. Sci. 1981, 3, 57–68. [Google Scholar] [CrossRef]
- Moore, P.N.; Puvvada, S.; Blankschtein, D. Challenging the surfactant monomer skin penetration model: Penetration of sodium dodecyl sulfate micelles into the epidermis. J. Cosmet. Sci. 2003, 54, 29–46. [Google Scholar]
- Dasilva, S.C.; Sahu, R.P.; Konger, R.L.; Perkins, S.M.; Kaplan, M.H.; Travers, J.B. Increased skin barrier disruption by sodium lauryl sulfate in mice expressing a constitutively active STAT6 in T cells. Arch. Dermatol. Res. 2012, 304, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Faucher, J.A.; Goddard, E.D. Interaction of keratinous substrates with sodium lauryl sulfate. I. Sorption. J. Soc. Cosmet. Chem. 1978, 29, 323–337. [Google Scholar]
- Hall-Manning, T.J.; Holland, G.H.; Rennie, G.; Revell, P.; Hines, J.; Barratt, M.D.; Basketter, D.A. Skin irritation potential of mixed surfactant systems. Food Chem. Toxicol. 1998, 36, 233–238. [Google Scholar] [CrossRef]
- Nielsen, G.D.; Nielsen, J.B.; Andersen, K.E.; Grandjean, P. Effects of industrial detergents on the barier function of human skin. Int. J. Occup. Environ. Health 2000, 6, 138–142. [Google Scholar] [CrossRef]
- Wasilewski, T.; Seweryn, A.; Bujak, T. Supercritical carbon dioxide blackcurrant seed extract as an anti-irritant additive for hand dishwashing liquids. Green Chem. Lett. Rev. 2016, 9, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Nizioł-Łukaszewska, Z.; Osika, P.; Wasilewski, T.; Bujak, T. Hydrophilic Dogwood Extracts as Materials for Reducing the Skin Irritation Potential of Body Wash Cosmetics. Molecules 2017, 22, 320. [Google Scholar] [CrossRef]
- Bujak, T.; Nizioł-Łukaszewska, Z.; Zan, A. Amphiphilic cationic polymers as effective substances improving the safety of use of body wash gels. Int. J. Biol. Macromol. 2020, 15, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Lips, A.; Ananthapadmanabhan, K.P.; Vethamuthu, M.; Hua, X.Y.; Yang, L.; Vincent, C.; Deo, N.; Somasundaran, P. Role of Surfactant Micelle Charge in Protein Denaturation and Surfactant-Induced Skin Irritation. In Surfactants in Personal Care Products and Decorative Cosmetics, 3rd ed.; Rhein, L., Schlossman, M., Lenick, A., Somasundaran, P., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2007; pp. 177–189. [Google Scholar]
- Aburjai, T.; Natsheh, F.M. Plants used as cosmetics. Phytother. Res. 2003, 17, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Visscher, M.O.; Said, D.; Wickett, R. Transepidermal water loss: Effect of hand hygiene. Skin Res. Technol. 2010, 16, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Kancheva, V.D.; Kasaikina, O.T. Bio-antioxidants—A chemical base of their antioxidant activity and beneficial effect on human health. Curr. Med. Chem. 2013, 20, 4784–4805. [Google Scholar] [CrossRef]
- Bordignon-Luiz, M.T.; Gauche, C.; Gris, E.F.; Falcão, L.D. Colour stability of anthocyanins from Isabel grapes (Vitis labrusca L.) in model systems. LWT—Food Sci. Technol. 2007, 40, 594–599. [Google Scholar] [CrossRef]
- Louli, V.; Ragoussis, N.; Magoulas, K. Recovery of phenolic antioxidants from wine industry by-products. Bioresour. Technol. 2004, 92, 201–208. [Google Scholar] [CrossRef]
- Compendium of International Methods of Wine and Must Analysis of the International Organisation of Vine and Wine; International Organisation of Vine and Wine: Paris, France, 2020; Volume 1, Included: Resolution adopted in Geneva (Switzerland) 19 July 2019.
- Kumar, B.; Tikariha, D.; Ghosh, K.K. Effects of Electrolytes on Micellar and Surface Properties of Some Monomeric Surfactants. J. Dispers. Sci. Technol. 2012, 33, 265–271. [Google Scholar] [CrossRef]
- Saxena, N.; Pal, N.; Ojha, K.; Dey, S.; Mandal, A. Synthesis, characterization, physical and thermodynamic properties of a novel anionic surfactant derived from Sapindus laurifolius. RSC Adv. 2018, 8, 24485–24499. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]









| Compound | Quantification/ Confirmation Transition | Family | GPE_10pDG2p [mg/L] | GPE_20pDG2p [mg/L] | |
|---|---|---|---|---|---|
| 1 | Tartaric acid | 148.9 > 87.0 148.9 > 73.0 | organic acids | 1104 ± 11 b | 2817 ± 17 a |
| 2 | Maleic acid | 114.9 > 70.9 114.9 > 45.0 | organic acids | 4.20 ± 0.80 a | 5.15 ± 0.48 a |
| 3 | DL-malic acid | 132.9 > 114.9 132.9 > 71.0 | organic acids | 848 ± 11 b | 1147 ± 17 a |
| Sum of organic acids | 1956 b | 3969 a | |||
| 4 | Gallic acid | 168.9 > 124.8 168.9 > 78.9 | phenolic acids | 1.87 ± 0.19 b | 4.91 ± 0.33 a |
| 5 | D-(−)-quinic acid | 190.9 > 84.9 190.9 > 93.0 | phenolic acids | 4.88 ± 0.26 b | 10.86 ± 0.17 a |
| 6 | Quercetin | 300.9 > 151.0 300.9 > 179.0 | flavonols | 0.26 ± 0.08 b | 0.27 ± 0.03 a |
| 7 | (+)-Catechin | 290.9 > 139.0 290.9 > 123.0 | flavanols | 5.76 ± 0.06 a | 5.36 ± 0.1 a |
| 8 | (−)-Epicatechin | 290.9 > 139.0 290.9 > 123.0 | flavanols | 4.40 ± 0.22 a | 4.64 ± 0.10 a |
| 9 | (−)-Catechin 3-gallate | 306.9 > 288.8 306.9 > 163.0 | flavanols | 2.67 ± 0.10 b | 4.23 ± 0.17 a |
| Sum of phenolic compounds | 19.9 b | 30.3 a | |||
| 10 | L-methionine | 150.0 > 103.9 150.0 > 132.9 | amino acids | 2.78 ± 0.08 b | 6.10 ± 0.77 a |
| 11 | L-tryptophan | 205.1 > 188.0 205.1 > 145.9 | amino acids | 3.05 ± 0.25 b | 6.96 ± 0.16 a |
| Sum of amino acids | 5.8 b | 13.1 a | |||
| 12 | D-(+)-xylose | 149.9 > 104.0 149.9 > 89.9 | sugars | 776 ± 12 b | 1417 ± 39 a |
| 13 | Sucrose | 340.9 > 179.0 340.9 > 88.9 | sugars | 2.77 ± 0.13 b | 3.50 ± 0.13 a |
| Sum of sugars | 779 b | 1420 a | |||
| TOTAL | 2760 b | 5432 a | |||
| Peak | Retention Time [min] | Identification | Molecular Formula | Molar Mass [Da] | Precursor Ion m/z | Main Product Ions MS2 [m/z] | CE [V] | GPE_20pDG2p/GPE_10pDG2p Ratio |
|---|---|---|---|---|---|---|---|---|
| 1 | 9.5 | Cyanidin 3-glucoside (Cy 3-glc) | C21H21O11+ | 449 | 449 [M + H]+ | 287 [M-C6H11O5]+ 315 [M-C5H10O4]+ | 24 | 1.3 ± 0.1 b |
| 2 | 10.3 | Petunidin 3-glucoside (Pet 3-glc) | C22H23O12+ | 478 | 479 [M + H]+ | 317 [M-C6H11O5]+ 302 [M-C6H11O4]+ | 10 | 1.9 ± 0.2 a |
| 3 | 11.6 | Peonidin 3-glucoside (Peo 3-glc) | C22H23O11+ | 462 | 463 [M + H]+ | 301 [M-C6H11O5]+ 201 [C9H5O4]+ | 20 | 1.1 ± 0.1 b |
| 4 | 11.7 | Malvidin-3-(6”-acetoyl)glucoside-5-glucoside | C31H37O18+ | 696 | 697 [M + H]+ | 535 [M-C6H10O5]+ 331 [M-C14H24O11]+ | 34 | 1.8 ± 0.1 a |
| 5 | 12.1 | Malvidin 3-glucoside (Mv 3-glc) | C23H25O12+ | 492 | 493 [M + H]+ | 331 [M-C6H11O5] +315 [M-C5H10O4]+ | 15 | 1.2 ± 0.2 b |
| 6 | 16.0 | Cyanidin 3-(acetylglucoside) (Cy 3-acglc) | C23H23O12+ | 490 | 491 [M + H]+ | 287 [M-C8H13O6]+ 163 [M-C17H13O7]+ | 18 | 1.2 ± 0.1 b |
| 7 | 17.6 | Malvidin 3-(6”acetyl) glucoside (Mv 3-(6-acglc)) | C25H27O13+ | 534 | 535 [M + H]+ | 331 [M-C8H13O6]+ 315 [M-C8H13O7]+ | 12 | 1.2 ± 0.1 b |
| 8 | 19.9 | Cyanidin 3-O-p-coumarylglucoside (Cy 3-cumglc) | C30H27O13+ | 594 | 595 [M + H]+ | 287 [M-C15H17O7]+ 415 [M-C9H8O4]+ | 19 | 1.0 ± 0.1 b |
| 9 | 20.1 | Petunidin 3-(6”-cumaroyl)-glucoside (Pet 3-(6-cum)glc) | C31H29O14+ | 624 | 625 [M + H]+ | 317 [M-C15H17O7]+ 301 [M-C15H18O8]+ | 23 | 1.4 ± 0.1 ab |
| 10 | 21.0 | Malvidin 3-(6”-cumaroyl)-glucoside (Mv 3-(6-cum)glc) | C32H31O14+ | 638 | 639 [M + H]+ | 331 [M-C15H17O7]+ 447 [M-C6H10O5]+ | 35 | 1.1 ± 0.1 b |
| 11 | 21.0 | Peonidin 3-(6”-cumaroyl)-glucoside (Peo 3-(6-cum)glc) | C31H29O13+ | 608 | 609 [M + H]+ | 301 [M-C15H17O7]+ 492 [M-C9H8O4]+ | 20 | 0.9 ± 0.1 b |
| TPC [mg GAE/L] | TFC [mg QE/L] | TAC [mg Cyd-3-glu/L] | DPPH [mg TE/L] | ABTS [mg TE/L] | |
|---|---|---|---|---|---|
| TPC ± SD | TFC ± SD | TAC ± SD | DPPH ± SD | ABTS ± SD | |
| GP_10pDG2p | 543.1 b ± 12.7 | 139.2 b ± 11.2 | 236.7 b ± 18.6 | 815.4 b ± 10.3 | 743.6 b ± 5.2 |
| GP_20pDG2p | 709.1 a ± 6.7 | 177.6 a ± 5.6 | 395.7 a ± 6.9 | 1126.6 a ± 24.7 | 954.3 a ± 10.2 |
| L* | a* | b* | C* | ho | ΔE | |
|---|---|---|---|---|---|---|
| DG2p | 27.16 ± 0.13 c | −0.55 ± 0.05 c | 0.18 ± 0.04 c | 0.6 ± 0.1 c | −18.1 ± 0.3 c | - |
| GPE_10pDG2p | 5.25 ± 0.05 a | 20.53 ± 0.07 a | 8.38 ± 0.07 a | 22.2 ± 0.2 a | 22.2 ± 0.3 a | 31.5 ± 0.4 a |
| GPE_20pDG2p | 2.55 ± 0.03 b | 15.62 ± 0.06 b | 4.44 ± 0.05 b | 16.2 ± 0.2 b | 15.7 ± 0.2 b | 29.7 ± 0.4 b |
| L* | a* | b* | C* | ho | ΔE | |
|---|---|---|---|---|---|---|
| SG | 30.72 ± 0.12 c | −0.06 ± 0.05 c | 0.55 ± 0.03 c | 0.6 ± 0.1 c | −83.8 ± 0.3 c | - |
| SG_GPE_10pDG2p | 7.75 ± 0.06 a | 30.45 ± 0.06 a | 11.87 ± 0.05 a | 32.7 ± 0.2 a | 21.3 ± 0.2 a | 39.8 ± 0.3 a |
| SG_GPE_20pDG2p | 4.16 ± 0.04 b | 23.17 ± 0.05 b | 7.16 ± 0.04 b | 24.3 ± 0.2 b | 17.2 ± 0.2 b | 35.9 ± 0.4 b |
| Acidity [g/L] | pH | Sugar [°Bx] | |||||
|---|---|---|---|---|---|---|---|
| Tartaric Acid | Malic Acid | Citric Acid | Acetic Acid | Sulphuric Acid | |||
| Regent | 6.75 | 6.03 | 5.76 | 5.40 | 4.41 | 3.46 | 20 |
| Léon Millot | 8.80 | 7.86 | 7.51 | 7.04 | 5.75 | 3.43 | 21 |
| Rondo | 9.15 | 8.17 | 7.81 | 7.32 | 5.98 | 3.23 | 20 |
| Ingredient (INCI Name) | GPE_10pDG2p | GPE_20pDG2p | |
|---|---|---|---|
| 1 | Decyl glucoside | 2 | 2 |
| 2 | Benzyl alcohol, benzoic acid, dehydroacetic acid, tocopherol | 0.5 | 0.5 |
| 3 | Aqua | 87.5 | 77.5 |
| 4 | Grape pomace | 10 | 20 |
| Ingredient (INCI Name) | SG_GPE_10pDG2p [%] | SG_GPE_20pDG2p [%] | |
|---|---|---|---|
| 1 | Sodium coco-sulfate | 4.5 | 4.5 |
| 2 | Aqua | to 100 | to 100 |
| 3 | GPE_10pDG2p | 65 | - |
| 4 | GPE_20pDG2p | - | 65 |
| 5 | Decyl glucoside | 3.8 | 3.8 |
| 6 | Benzyl alcohol, benzoic acid, dehydroacetic acid, tocopherol | 0.5 | 0.5 |
| 7 | Citric acid | to pH 5.5 | to pH 5.5 |
| 8 | Parfum | 0.5 | 0.5 |
| 9 | Cocamidopropyl betaine | 2 | 2 |
| 10 | Sodium chloride | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasilewski, T.; Hordyjewicz-Baran, Z.; Zarębska, M.; Stanek, N.; Zajszły-Turko, E.; Tomaka, M.; Bujak, T.; Nizioł-Łukaszewska, Z. Sustainable Green Processing of Grape Pomace Using Micellar Extraction for the Production of Value-Added Hygiene Cosmetics. Molecules 2022, 27, 2444. https://doi.org/10.3390/molecules27082444
Wasilewski T, Hordyjewicz-Baran Z, Zarębska M, Stanek N, Zajszły-Turko E, Tomaka M, Bujak T, Nizioł-Łukaszewska Z. Sustainable Green Processing of Grape Pomace Using Micellar Extraction for the Production of Value-Added Hygiene Cosmetics. Molecules. 2022; 27(8):2444. https://doi.org/10.3390/molecules27082444
Chicago/Turabian StyleWasilewski, Tomasz, Zofia Hordyjewicz-Baran, Magdalena Zarębska, Natalia Stanek, Ewa Zajszły-Turko, Magdalena Tomaka, Tomasz Bujak, and Zofia Nizioł-Łukaszewska. 2022. "Sustainable Green Processing of Grape Pomace Using Micellar Extraction for the Production of Value-Added Hygiene Cosmetics" Molecules 27, no. 8: 2444. https://doi.org/10.3390/molecules27082444






