Physicochemical, Antioxidant and Anticancer Characteristics of Seed Oil from Three Chenopodium quinoa Genotypes
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Profiles of Three QSOs
2.2. Oxidative Stability
2.3. Analysis of Fatty Acids in QSOs
2.4. Analysis of Tocopherols and Phytosterols
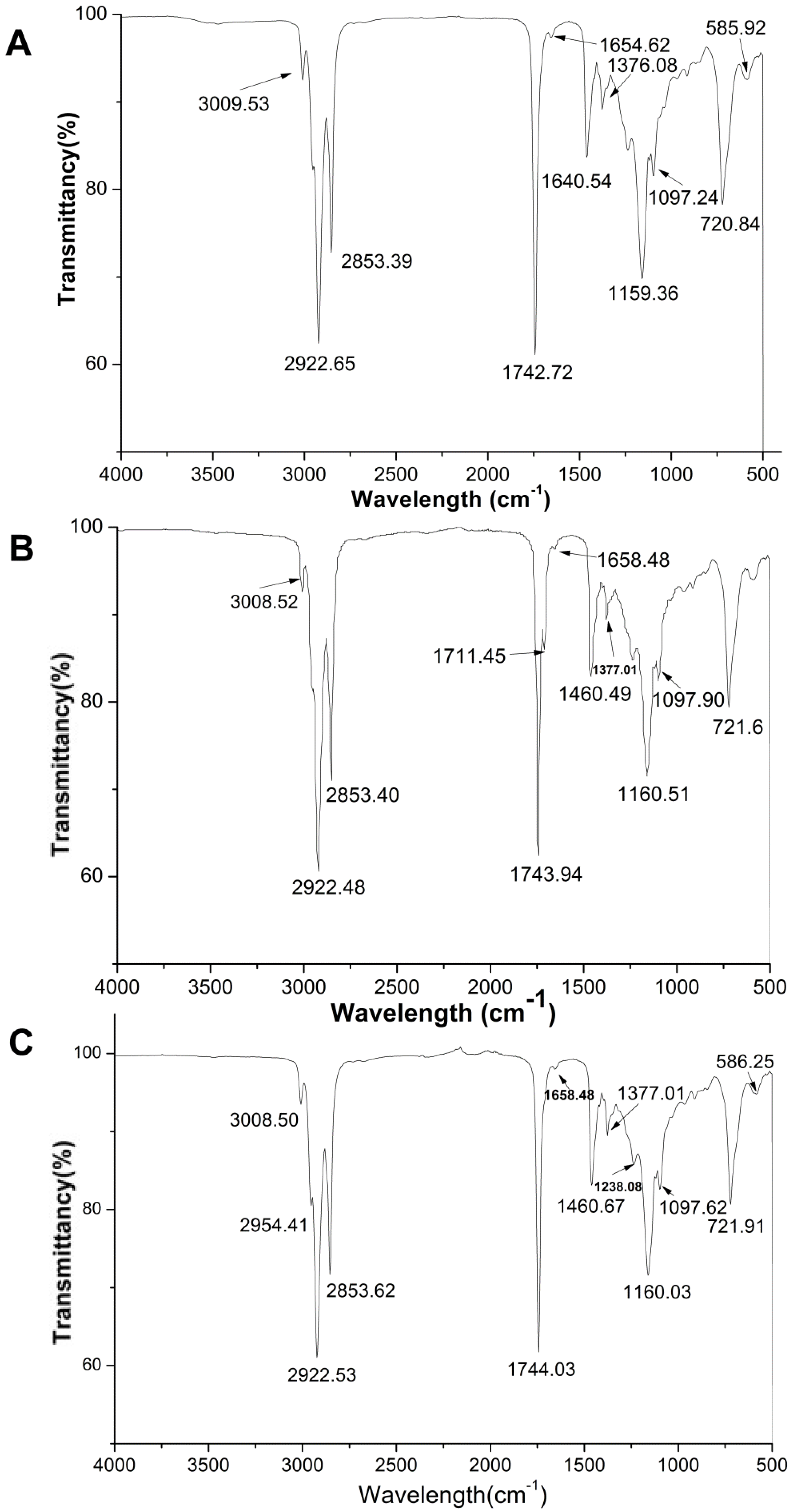
2.5. FTIR Analysis of QSOs
2.6. Antioxidant Activities of QSOs
2.7. Cell Viability and Cytotoxicity of QSOs
2.8. BSO-Induced Morphological Changes of HCT116 Cells
2.9. BSO-Induced Apoptosis of HCT116 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Extraction of C. quinoa Wild. Seed Oils
4.3. Content of Quinoa Seed Oils (QSOs)
4.4. Chemical Properties
4.4.1. Oxidative Stability of QSOs
4.4.2. Color Determination
4.4.3. Analysis of Fatty Acid Profiles
4.4.4. Analysis of sn-2 Fatty Acids
4.4.5. Triacylglycerol Composition
4.4.6. Fourier Transform Infrared (FTIR) Spectroscopy
4.5. Antioxidant Activity
4.6. Anticancer Activity In Vitro
4.7. BSO-Induced Apoptotic Morphological Changes of HCT116 Cells
4.8. Detection of Apoptotic Cells by Hoechst 33342 Staining
4.9. Flow Cytometric Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Repo-Carrasco, R.; Espinoza, C.; Jacobsen, S.E. Nutritional Value and Use of the Andean Crops Quinoa (Chenopodium quinoa) and Kañiwa (Chenopodium pallidicaule). Food Rev. Int. 2003, 19, 179–189. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Zhang, B.; Chen, P.X.; Liu, R.; Tsao, R. Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 166, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Vega-Galvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L.; Martinez, E.A. Nutrition facts and functional potential of quinoa (Chenopodium quinoa Willd.), an ancient Andean grain: A review. J. Sci. Food Agric. 2010, 90, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Regional Office for Latin America and the Caribbean. Quinoa: An Ancient Crop to Contribute to World Food Security; Regional Office for Latin America and the Caribbean: Santiago, Chile, 2011. [Google Scholar]
- Gawlik-Dziki, U.; Swieca, M.; Sulkowski, M.; Dziki, D.; Baraniak, B.; Czyz, J. Antioxidant and anticancer activities of Chenopodium quinoa leaves extracts—In vitro study. Food Chem. Toxicol. 2013, 57, 154–160. [Google Scholar] [CrossRef]
- Miranda, M.; Delatorre-Herrera, J.; Vega-Gálvez, A.; Jorquera, E.; Quispe-Fuentes, I.; Martínez, E.A. Antimicrobial Potential and Phytochemical Content of Six Diverse Sources of Quinoa Seeds (Chenopodium quinoa Willd.). Agric. Sci. 2014, 5, 1015. [Google Scholar]
- Graf, B.L.; Poulev, A.; Kuhn, P.; Grace, M.H.; Lila, M.A.; Raskin, I. Quinoa seeds leach phytoecdysteroids and other compounds with anti-diabetic properties. Food Chem. 2014, 163, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Lee, Y.J.; Kim, Y.H.; Yoon, K.S. Antioxidant and Antimicrobial Activities of Quinoa (Chenopodium quinoa Willd.) Seeds Cultivated in Korea. Prev. Nutr. Food Sci. 2017, 22, 195–202. [Google Scholar] [CrossRef]
- Sons, J.W. Bailey’s Industrial Oil and Fat Products, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Orsavova, J.; Misurcova, L.; Ambrozova, J.V.; Vicha, R.; Mlcek, J. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Torres, M.M.; Maestri, D.M. The effects of genotype and extraction methods on chemical composition of virgin olive oils from Traslasierra Valley (Córdoba, Argentina). Food Chem. 2006, 96, 507–511. [Google Scholar] [CrossRef]
- El Yamani, M.; Sakar, E.; Boussakouran, A.; Rharrabti, Y. Effect of storage time and conditions on the quality characteristics of ‘Moroccan Picholine’ olive oil. Biocatal. Agric. Biotechnol. 2022, 39, 102244. [Google Scholar] [CrossRef]
- Sakar, E.; El Yamani, M.; Boussakouran, A.; Ainane, A.; Ainane, T.; Gharby, S.; Rharrabti, Y. Variability of oil content and its physicochemical traits from the main almond [Prunus dulcis Mill. DA Webb] cultivars grown under contrasting environments in north-eastern Morocco. Biocatal. Agric. Biotech. 2021, 32, 101952. [Google Scholar] [CrossRef]
- Hou, G.; Ablett, G.; Pauls, K.; Rajcan, I. Environmental effects on fatty acid levels in soybean oil. J. Am. Oil Chem. Soc. 2006, 83, 759–763. [Google Scholar] [CrossRef]
- Graf, B.L.; Rojas-Silva, P.; Rojo, L.E.; Delatorre-Herrera, J.; Baldeon, M.E.; Raskin, I. Innovations in Health Value and Functional Food Development of Quinoa (Chenopodium quinoa Willd.). Compr. Rev. Food Sci. Food Saf. 2015, 14, 431–445. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.B.; Zheng, L.Y.; Jin, J.; Li, X.J.; Fu, J.N.; Wang, M.Z.; Guan, Y.F.; Song, X. Phytochemical and Biological Characteristics of Mexican Chia Seed Oil. Molecules 2018, 23, 3219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, L.Y.; Jin, J.; Shi, L.K.; Huang, J.H.; Chang, M.; Wang, X.G.; Zhang, H.; Jin, Q.Z. Gamma tocopherol, its dimmers, and quinones: Past and future trends. Crit. Rev. Food Sci. 2020, 60, 3916–3930. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.Y.; Zhang, T.; Xie, L.L.; Karrar, E.; Shi, L.K.; Jin, J.; Wang, X.G.; Jin, Q.Z. Physicochemical characteristics of Actinostemma lobatum Maxim. kernel oil by supercritical fluid extraction and conventional methods. Ind. Crops Prod. 2020, 152, 112516. [Google Scholar] [CrossRef]
- Deng, D.H.; Xu, L.; Ye, Z.H.; Cui, H.F.; Cai, C.B.; Yu, X.P. FTIR Spectroscopy and Chemometric Class Modeling Techniques for Authentication of Chinese Sesame Oil. J. Am. Oil Chem. Soc. 2012, 89, 1003–1009. [Google Scholar] [CrossRef]
- Che Man, Y.B.; Mirghani, M.E.S. Detection of lard mixed with body fats of chicken, lamb, and cow by fourier transform infrared spectroscopy. J. Am. Oil Chem. Soc. 2001, 78, 753–761. [Google Scholar] [CrossRef]
- van de Voort, F.R.; Ismail, A.A.; Sedman, J.; Emo, G. Monitoring the oxidation of edible oils by Fourier transform infrared spectroscopy. J. Am. Oil Chem. Soc. 1994, 71, 243–253. [Google Scholar] [CrossRef]
- Srivastava, Y.; Semwal, A.D. A study on monitoring of frying performance and oxidative stability of virgin coconut oil (VCO) during continuous/prolonged deep fat frying process using chemical and FTIR spectroscopy. J. Food Sci. Technol. 2015, 52, 984–991. [Google Scholar] [CrossRef] [Green Version]
- Sim, S.F.; Lee, T.Z.E.; Mohd Irwan Lu, N.A.L.; Samling, B. Synchronized Analysis of FTIR Spectra and GCMS Chromatograms for Evaluation of the Thermally Degraded Vegetable Oils. J. Anal. Methods Chem. 2014, 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Guillén, M.D.; Cabo, N. Infrared spectroscopy in the study of edible oils and fats. J. Sci. Food Agric. 1997, 75, 1–11. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Holse, M.; Hansen, Å.; Arendt, E.; Gallagher, E. Impact of Baking on Vitamin E Content of Pseudocereals Amaranth, Quinoa, and Buckwheat. Cereal Chem. 2009, 86, 0909041222–0909041515. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Chen, P.X.; Zhang, B.; Hernandez, M.; Zhang, H.; Marcone, M.F.; Liu, R.; Tsao, R. Characterisation of fatty acid, carotenoid, tocopherol/tocotrienol compositions and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 174, 502–508. [Google Scholar] [CrossRef]
- Maszewska, M.; Florowska, A.; Dluzewska, E.; Wroniak, M.; Marciniak-Lukasiak, K.; Zbikowska, A. Oxidative Stability of Selected Edible Oils. Molecules 2018, 23, 1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.C.; Kim, I.H.; Chang, J.; Rhee, Y.K.; Oh, H.I.; Park, H.K. Chemical composition and oxidative stability of safflower oil prepared with expeller from safflower seeds roasted at different temperatures. J. Food Sci. 2004, 69, C33–C38. [Google Scholar] [CrossRef]
- Rozanska, M.B.; Kowalczewski, P.L.; Tomaszewska-Gras, J.; Dwiecki, K.; Mildner-Szkudlarz, S. Seed-Roasting Process Affects Oxidative Stability of Cold-Pressed Oils. Antioxidants 2019, 8, 313. [Google Scholar] [CrossRef] [Green Version]
- Rekas, A.; Wroniak, M.; Siger, A.; Scibisz, I. Chemical composition and resistance to oxidation of high-oleic rapeseed oil pressed from microwave pre-treated intact and de-hulled seeds. Grasas Aceites 2017, 68, e225. [Google Scholar] [CrossRef] [Green Version]
- Syed, A. Chapter 4—Oxidative Stability and Shelf Life of Vegetable Oils. In Oxidative Stability and Shelf Life of Foods Containing Oils and Fats; Hu, M., Jacobsen, C., Eds.; AOCS Press: Urbana, IL, USA, 2016; pp. 187–207. [Google Scholar]
- Neff, W.E.; El-Agaimy, M. Effect of Linoleic Acid Position in Triacylglycerols on their Oxidative Stability. LWT-Food Sci. Technol. 1996, 29, 772–775. [Google Scholar] [CrossRef] [Green Version]
- Decker, E.A.; Elias, R.J.; McClements, D.J. Oxidation in Foods and Beverages and Antioxidant Applications. Volume 2: Management in Different Industry Sectors; Woodhead Publishing Ltd.: Cambridge, UK, 2010; pp. 203–205. [Google Scholar]
- Pereira, E.; Encina-Zelada, C.; Barros, L.; Gonzales-Barron, U.; Cadavez, V.; Ferreira, I.C.F.R. Chemical and nutritional characterization of Chenopodium quinoa Willd (quinoa) grains: A good alternative to nutritious food. Food Chem. 2019, 280, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Villa, D.Y.G.; Russo, L.; Kerbab, K.; Landi, M.; Rastrelli, L. Chemical and nutritional characterization of Chenopodium pallidicaule (canihua) and Chenopodium quinoa (quinoa) seeds. Emir. J. Food Agric. 2014, 26, 609–615. [Google Scholar] [CrossRef] [Green Version]
- Villa-Rodríguez, J.A.; Molina-Corral, F.J.; Ayala-Zavala, J.F.; Olivas, G.I.; González-Aguilar, G.A. Effect of maturity stage on the content of fatty acids and antioxidant activity of ‘Hass’ avocado. Food Res. Int. 2011, 44, 1231–1237. [Google Scholar] [CrossRef]
- Liu, H.J.; Li, X.Q.; Zhu, Y.L.; Huang, Y.; Zhang, Q.; Lin, S.; Fang, C.; Li, L.Y.; Lv, Y.L.; Mei, W.H.; et al. Effect of Plant-Derived n-3 Polyunsaturated Fatty Acids on Blood Lipids and Gut Microbiota: A Double-Blind Randomized Controlled Trial. Front. Nutr. 2022, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Zhao, Y.Y.; Huang, L.J.; Zhang, X.Y.; Wu, Y. The Effect of n-3 Polyunsaturated Fatty Acids on Plasma Lipids and Lipoproteins in Patients with Chronic Renal Failure—A Meta-Analysis of Randomized Controlled Trials. J. Renal Nutr. 2012, 22, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef] [Green Version]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Kim, R.; Emi, M.; Tanabe, K. The role of apoptosis in cancer cell survival and therapeutic outcome. Cancer Biol. Ther. 2006, 5, 1429–1442. [Google Scholar] [CrossRef] [Green Version]
- CNSMC, China National Standardization Management Committee. Determination of Oil Content Valid GB/T 14488.2-2008; China National Standardization Management Committee: Beijing, China, 2008. [Google Scholar]
- Azadmard-Damirchi, S.; Habibi-Nodeh, F.; Hesari, J.; Nemati, M.; Achachlouei, B.F. Effect of pretreatment with microwaves on oxidative stability and nutraceuticals content of oil from rapeseed. Food Chem. 2010, 121, 1211–1215. [Google Scholar] [CrossRef]
- Committee, A.C. Color of Fats and Oils, Lovibond (Wesson) Wesson Method Using Color Glasses Calibrated in Accordance with the Aocs-Tintometer Color Scale. In AOCS Official Method Cc 13b-45; AOCS: Urbana, IL, USA, 2008. [Google Scholar]
- Jin, J.; Wang, Y.; Su, H.; Warda, P.; Xie, D.; Liu, Y.; Wang, X.; Huang, J.; Jin, Q.; Wang, X. Oxidative stabilities of mango kernel fat fractions produced by three-stage fractionation. Int. J. Food Prop. 2017, 20, 2817–2829. [Google Scholar] [CrossRef] [Green Version]
- AOCS. Individual Triglycerides in Oils and Fats by HPLC; AOCS: Urbana, IL, USA, 2009. [Google Scholar]




| Chenopodium quinoa Willd. Seed | |||
|---|---|---|---|
| Oil Parameters | White | Red | Black |
| Oil content (g/100g) | 6.19 ± 0.08 a | 5.73 ± 0.02 b | 5.68 ± 0.04 b |
| Oil stability (induction period)/h | 1.57 ± 0.01 b | 7.56 ± 0.00 a | 7.50 ± 0.01 a |
| Oil color/(units) | |||
| Red | 4.8 | 5.0 | 4.0 |
| Yellow | 70.4 | 70.0 | 73.6 |
| Fatty acid profile (%) | |||
| C14:0 | 0.42 ± 0.00 a | 0.21 ± 0.03 b | 0.15 ± 0.00 b |
| C16:0 | 13.16 ± 0.14 a | 11.14 ± 0.02 b | 9.77 ± 0.04 c |
| C16:1 | 1.17 ± 0.02 a | 0.18 ± 0.01 b | - |
| C17:1 | - | 0.17 ± 0.00 b | 0.26 ± 0.00 a |
| C18:0 | 0.85 ± 0.04 b | 1.25 ± 0.03 a | 0.69 ± 0.03 c |
| C18:1 | 25.14 ± 0.07 a | 23.91 ± 0.07 b | 25.79 ± 0.01 a |
| C18:2 Trans | 0.90 ± 0.07 b | 1.13 ± 0.02 a | 0.91 ± 0.01 b |
| C18:2 | 43.74 ± 0.01 c | 49.17 ± 0.07 b | 51.75 ± 0.09 a |
| C18:3 | 8.24 ± 0.02 a | 7.09 ± 0.00 b | 4.59 ± 0.02 c |
| C20:0 | 0.56 ± 0.02 | - | - |
| C20:1 | 1.45 ± 0.03 b | 1.50 ± 0.00 b | 1.69 ± 0.00 a |
| C20:4 | 0.55 ± 0.04 a | 0.46 ± 0.00 b | 0.53 ± 0.00 a |
| C23:0 | 1.14 ± 0.02 b | 1.51 ± 0.02 a | 1.54 ± 0.01 a |
| C24:0 | 2.67 ± 0.11 a | 2.28 ± 0.13 a | 2.34 ± 0.03 a |
| SFA | 18.87 ± 0.10 a | 16.4 ± 0.13 ab | 14.48 ± 0.09 b |
| PUFA | 52.53 ± 0.07 b | 56.72 ± 0.07 ab | 56.87 ± 0.11 a |
| sn-2 Fatty acid composition (%) | |||
| C16:0 | 1.89 | 2.93 | 2.39 |
| C18:0 | 0.59 | 1.15 | 1.39 |
| C18:1 | 21.69 | 22.2 | 19.97 |
| C18:2 | 59.35 | 67.79 | 71.87 |
| C18:3 | 6.21 | 5.93 | 3.54 |
| C21:0 | - | - | 0.84 |
| C22:6 | 7.89 | - | - |
| C24:1 | 2.39 | - | - |
| Chenopodium quinoa Wild | |||
|---|---|---|---|
| White | Red | Black | |
| Fat compositions/% | |||
| TAG | 88.57 ± 1.51 ab | 81.98 ± 0.9 b | 91.42 ± 0.22 a |
| 1,3-DAG | 0.98 ± 0.12 c | 4.33 ± 0.54 a | 2.11 ± 0.17 b |
| 1,2(2,3)-DAG | 6.56 ± 0.19 a | 1.85 ± 0.34 c | 2.26 ± 0.10 b |
| FFA | 3.89 ± 1.58 b | 11.84 ± 0.02 a | 4.21 ± 0.28 b |
| TAG compositions/% | |||
| LLLn | 3.42 ± 0.10 | 2.81 ± 0.03 | 0.87 ± 0.01 |
| PLnLn | 1.26 ± 0.04 | 1.04 ± 0.17 | 0.33 ± 0.04 |
| MLLn | 0.22 ± 0.05 | - | - |
| PLnL | 7.01 ± 0.56 | 9.27 ± 0.36 | 8.50 ± 0.24 |
| LLL | 10.78 ± 0.96 | 10.13 ± 0.42 | 6.09 ± 0.35 |
| LnLO | 1.31 ± 0.08 | 1.35 ± 0.03 | 0.41 ± 0.06 |
| LLO | 33.45 ± 0.21 | 31.15 ± 0.09 | 40.08 ± 0.13 |
| LLP | 8.46 ± 0.10 | 12.38 ± 0.09 | 10.01 ± 0.05 |
| OOL | 18.63 ± 0.14 | 14.48 ± 0.05 | 17.48 ± 0.12 |
| PLO | 9.25 ± 0.22 | 11.64 ± 0.01 | 10.78 ± 0.02 |
| PPL | 0.61 ± 0.03 | 1.02 ± 0.05 | 0.69 ± 0.04 |
| OOP | 1.09 ± 0.00 | 1.44 ± 0.02 | 1.44 ± 0.16 |
| LOS | 2.73 ± 0.03 | 1.63 ± 0.19 | 1.74 ± 0.15 |
| OOO | 1.81 ± 0.10 | 1.66 ± 0.06 | 1.59 ± 0.00 |
| Tocopherols (mg/kg) | |||
| α-tocopherol | 38.05 ± 4.44 a | 33.52 ± 1.06 b | 34.09 ± 0.64 b |
| β-tocopherol | 2.40 ± 0.21 b | 1.93 ± 0.40 c | 3.34 ± 0.07 a |
| γ-tocopherol | 66.10 ± 1.96 c | 72.65 ± 0.88 b | 105.75 ± 0.85 a |
| δ-tocopherol | 16.53 ± 1.63 | 9.19 ± 1.42 | 13.49 ± 1.16 |
| Total tocopherols | 123.09 ± 4.32 ab | 117.29 ± 1.64 b | 156.67 ± 2.58 a |
| Phytosterols (mg/kg) | |||
| Campesterol | 160.82 ± 8.87 b | 221.39 ± 21.08 a | 236.08 ± 1.04 a |
| Stigmasterol | 3488.37 ± 500.12 b | 4291.21 ± 43.64 ab | 4914.16 ± 177.27 a |
| β-Sitosterol | 5797.71 ± 822.15 b | 7664.25 ± 141.76 a | 7095.52 ± 32.69 a |
| Total phytosterols | 9446.91 ± 1331.14 b | 12176.85 ± 206.49 a | 12245.77 ± 208.93 a |
| Samples | Antioxidant Activity (IC50, mg/mL) | Cytotoxicity (IC50, μg/mL) | |
|---|---|---|---|
| DPPH | ABTS | HCT116 | |
| White | 102.3 a | 72.1 a | 647.4 a |
| Red | 74.5 b | 63.2 b | 381.3 b |
| Black | 24.6 c | 40.4 c | 281.9 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Zheng, L.; Peng, Y.; Zhu, X.; Liu, F.; Yang, X.; Li, H. Physicochemical, Antioxidant and Anticancer Characteristics of Seed Oil from Three Chenopodium quinoa Genotypes. Molecules 2022, 27, 2453. https://doi.org/10.3390/molecules27082453
Shen Y, Zheng L, Peng Y, Zhu X, Liu F, Yang X, Li H. Physicochemical, Antioxidant and Anticancer Characteristics of Seed Oil from Three Chenopodium quinoa Genotypes. Molecules. 2022; 27(8):2453. https://doi.org/10.3390/molecules27082453
Chicago/Turabian StyleShen, Yingbin, Liyou Zheng, Yao Peng, Xucheng Zhu, Fu Liu, Xinquan Yang, and Haimei Li. 2022. "Physicochemical, Antioxidant and Anticancer Characteristics of Seed Oil from Three Chenopodium quinoa Genotypes" Molecules 27, no. 8: 2453. https://doi.org/10.3390/molecules27082453
APA StyleShen, Y., Zheng, L., Peng, Y., Zhu, X., Liu, F., Yang, X., & Li, H. (2022). Physicochemical, Antioxidant and Anticancer Characteristics of Seed Oil from Three Chenopodium quinoa Genotypes. Molecules, 27(8), 2453. https://doi.org/10.3390/molecules27082453






