The Potential Antipyretic Mechanism of Ellagic Acid with Brain Metabolomics Using Rats with Yeast-Induced Fever
Abstract
:1. Introduction
2. Results
2.1. Antipyretic Effects of Ellagic Acid
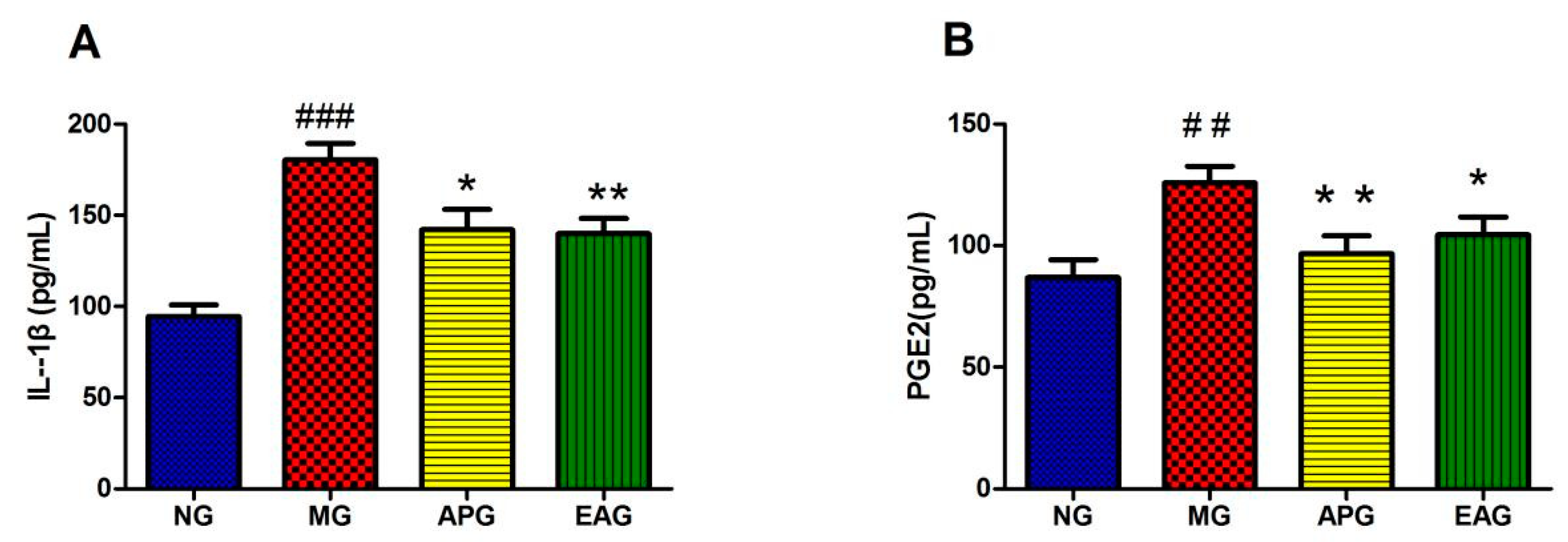
2.2. ELISA Results of Inflammatory Factor IL-1β and PGE2 in Serum
2.3. Brain Metabolomics Profile and Multivariate Data Analysis
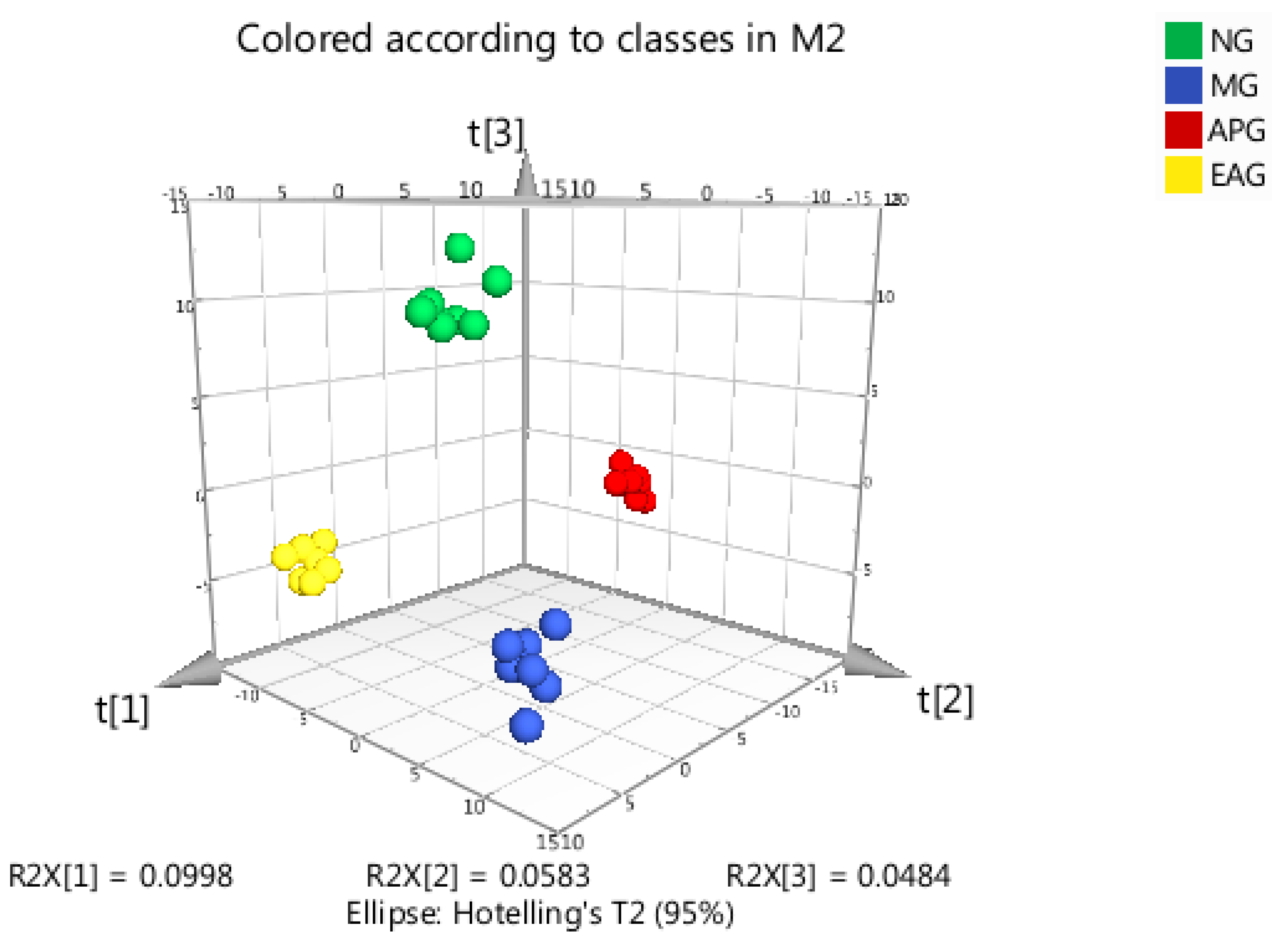
2.3.1. Principal Component Analysis
2.3.2. Orthogonal Partial Least Squares-Discriminant Analysis
2.3.3. Identification of Potential Biomarkers and the Variation Trends among Four Groups
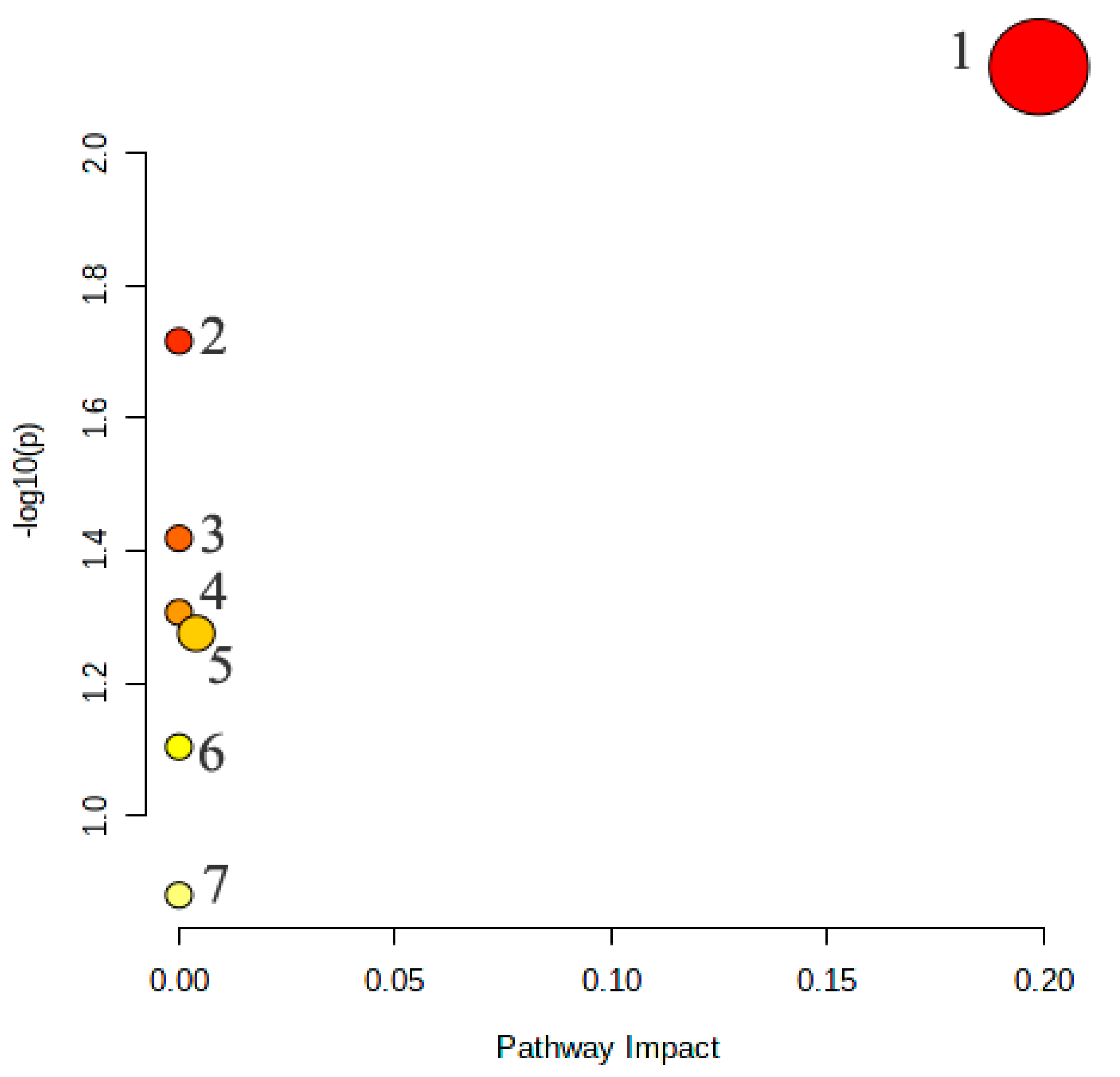
2.3.4. Metabolic Pathway Analysis of the Potential Biomarkers
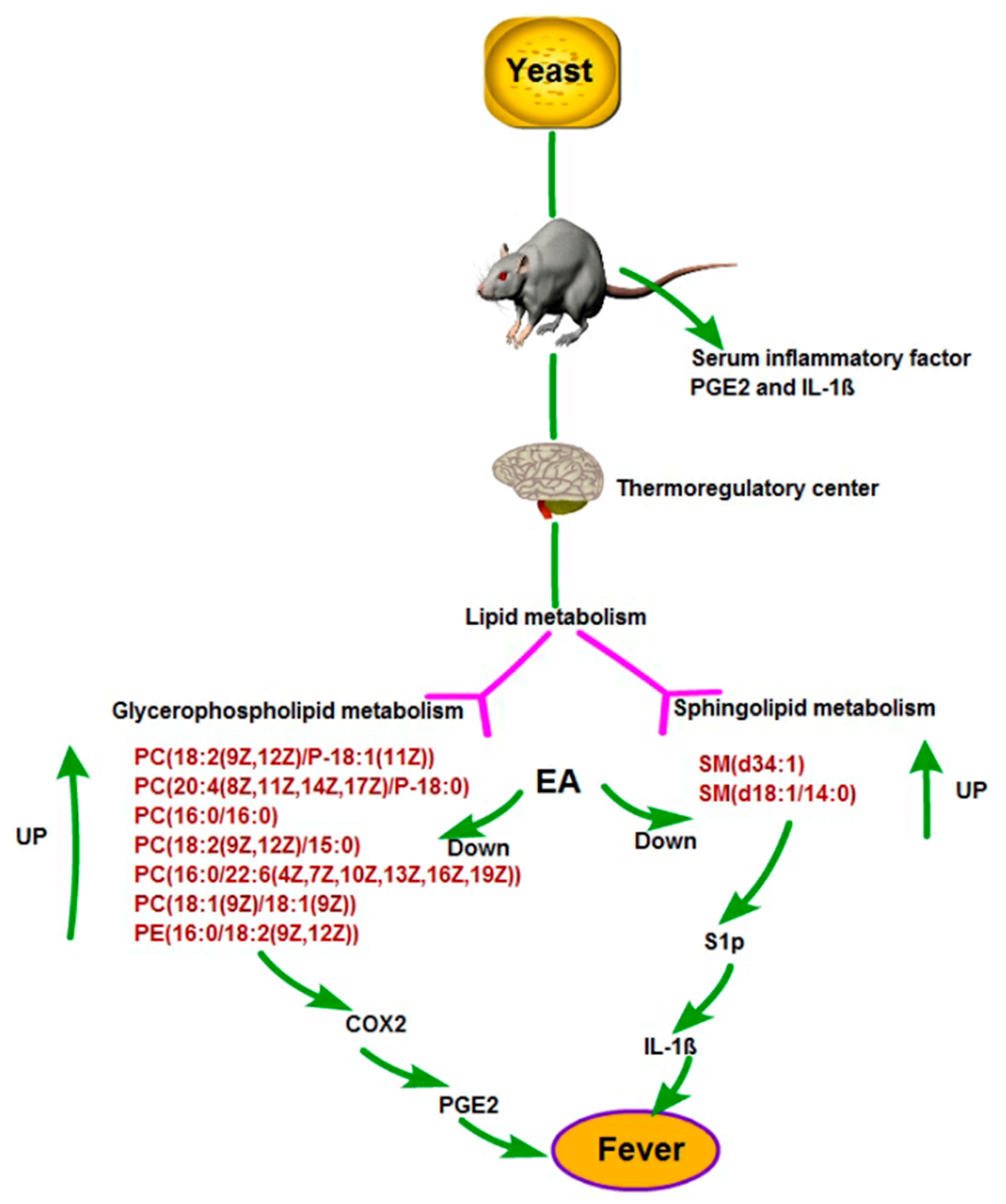
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals and Model Construction
4.3. Sample Collection and Cytokines
4.4. Sample Preparation and UPLC-MS Analysis
4.4.1. Tissue Extraction
4.4.2. UPLC-MS Conditions
5. Data Processing and Statistical Analysis
5.1. Data Preprocessing
5.2. Principal Component Analysis
5.3. Hierarchical Cluster Analysis and Pearson Correlation Coefficients
5.4. Differential Metabolites Selected
5.5. KEGG Annotation and Enrichment Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lammert, E. Metabolism of Human Diseases: Organ Physiology and Pathophysiology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 313–317. [Google Scholar]
- Khalil, S.N.; Hahn, B.J.; Chumpitazi, C.E.; Rock, A.D.; Kaelin, B.A.; Macias, C.G. A multicenter, randomized, open-label, active-comparator trial to determine the efficacy, safety, and pharmacokinetics of intravenous ibuprofen for treatment of fever in hospitalized pediatric patients. BMC Pediatr. 2017, 17, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogoina, D. Fever, fever patterns and diseases called ‘fever’—A review. J. Infect. Public Health 2011, 4, 108–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito, H.O.; Barbosa, F.L.; Reis, R.C.D.; Fraga, D.; Borges, B.S.; Franco, C.R.; Zampronio, A.R. Evidence of substance P autocrine circuitry that involves TNF-α,IL-6,and PGE2 in endogenous pyrogen-induced fever. J. Neuroimmunol. 2016, 293, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Barberà, C.M.; Baroja, M.A.; Gomez, A.I.; Machado, F.; Di Virgilio, F.; Pelegrín, P. P2X7 receptor-stimulation causes fever via PGE2 and IL-1β release. FASEB J. 2012, 26, 2951–2962. [Google Scholar] [CrossRef] [PubMed]
- Marais, M.; Maloney, S.K.; Gray, D.A. Brain IL-6- and PG-dependent actions of IL-1β and lipopolysaccharide in avian fever. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R791–R800. [Google Scholar] [CrossRef] [Green Version]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef] [Green Version]
- Martín Arias, L.H.; Martín González, A.; Sanz Fadrique, R.; Vazquez, E.S. Cardiovascular Risk of Nonsteroidal Anti-inflammatory Drugs and Classical and Selective Cyclooxygenase-2 Inhibitors: A Meta-analysis of Observational Studies. J. Clin. Pharmacol. 2018, 59, 55–73. [Google Scholar] [CrossRef] [Green Version]
- El-Shitany, N.A.; El-Bastawissy, E.A.; El-Desoky, K. Ellagic acid protects against carrageenan-induced acute inflammation through inhibition of nuclear factor kappa B, inducible cyclooxygenase and proinflammatory cytokines and enhancement of interleukin-10 via an antioxidant mechanism. Int. Immunopharmacol. 2014, 19, 290–299. [Google Scholar] [CrossRef]
- Zhao, L.; Mehmood, A.; Soliman, M.M.; Iftikhar, A.; Iftikhar, M.; Aboelenin, S.M.; Wang, C. Protective Effects of Ellagic Acid Against Alcoholic Liver Disease in Mice. Front. Nutr. 2012, 8, 744520. [Google Scholar] [CrossRef]
- Maruthamuthu, V.; Henry, L.J.K.; Ramar, M.K.; Kandasamy, R. Myxopyrum serratulum ameliorates airway inflammation in LPS-stimulated RAW 264.7 macrophages and OVA-induced murine model of allergic asthma. J. Ethnopharmacol. 2020, 255, 112369. [Google Scholar] [CrossRef]
- Preti, G. Metabolomics comes of age? Scientist 2005, 19, 8–9. [Google Scholar]
- Utpott, M.; Rodrigues, E.; Rios, A.; Mercali, G.D.; Flôres, S.H. Metabolomics:an analytical technique for food processing evaluation. Food Chem. 2021, 366, 130685. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Du, Z.; Yuan, S.; Ma, Q.; Han, Z.; Tu, P.; Jiang, Y. Comparison of the preventive effects of Murraya exotica and Murraya paniculata on alcohol-induced gastric lesions by pharmacodynamics and metabolomics. J. Ethnopharmacol. 2021, 281, 114567. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, L. Chemical Isotope Labeling LC-MS for Metabolomics. In Cancer Metabolomics. Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2021; Volume 1280, pp. 1–18. [Google Scholar]
- Behera, S.K.; Kasaragod, S.; Karthikkeyan, G.; Narayana Kotimoole, C.; Raju, R.; Prasad, T.S.K.; Subbannayya, Y. MS2Compound: A User-Friendly Compound Identification Tool for LC-MS/MS-Based Metabolomics Data. OMICS J. Integr. Biol. 2021, 25, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Tobin, N.H.; Murphy, A.; Li, F.; Brummel, S.S.; Taha, T.E.; Saidi, F.; Owor, M.; Violari, A.; Moodley, D.; Chi, B.; et al. Comparison of dried blood spot and plasma sampling for untargeted metabolomics. Metabolomics 2021, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Yu, J.; Wang, N.; Zhao, S.; Han, W.; Chen, D.; Li, L.; Li, L. High-Coverage Metabolome Analysis Reveals Significant Diet Effects of Autoclaved and Irradiated Feed on Mouse Fecal and Urine Metabolomics. Mol. Nutr. Food Res. 2021, 65, e2100110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Li, S.; Dai, Y.; Li, X.; Wang, Q.; Wang, G.; Ma, Y.; Gu, X.; Zhang, C. The Potential Antipyretic Mechanism of Gardeniae Fructus and Its Heat-Processed Products With Plasma Metabolomics Using Rats With Yeast-Induced Fever. Front. Pharmacol. 2019, 10, 491. [Google Scholar] [CrossRef] [Green Version]
- Qian, W.; Shan, J.; Shen, C.; Yang, R.; Xie, T.; Di, L. Brain Metabolomics Reveal the Antipyretic Effects of Jinxin Oral Liquid in Young Rats by Using Gas Chromatography–Mass Spectrometry. Metabolites 2019, 9, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haley, M.J.; Lawrence, C.B. The blood-brain barrier after stroke: Structural studies and the role of transcytotic vesicles. J. Cereb. Blood Flow Metab. 2017, 37, 456–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daviss, B. Growing pains for metabolomics. Scientist 2005, 19, 25–28. [Google Scholar]
- Jordan, K.W.; Nordenstam, J.; Lauwers, G.Y.; Rothenberger, D.A.; Alavi, K.; Garwood, M.; Cheng, L.L. Metabolomic characterization of human rectal adenocarcinoma with intact tissue magnetic resonance spectroscopy. Dis. Colon Rectum 2009, 52, 520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, J.L.; Vidal-Puig, A. Current challenges in metabolomics for diabetes research: A vital functional genomic tool or just a ploy for gaining funding? Physiol. Genom. 2008, 34, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nejad, K.H.; Dianat, M.; Sarkaki, A. Ellagic acid improves electrocardiogram waves and blood pressure against global cerebral ischemia rat experimental models. Electron. Physician 2015, 7, 1153–1162. [Google Scholar]
- Lee, W.J.; Ou, H.C.; Hsu, W.C.; Chou, M.M.; Tseng, J.J.; Hsu, S.L.; Tsai, K.-L.; Sheu, W.H.-H. Ellagic acid inhibits oxidized LDL mediated LOX-1 expression, ROS generation and inflammation in human endothelial cells. J. Vasc. Surg. 2010, 52, 1290–1300. [Google Scholar] [CrossRef] [Green Version]
- Altnda, F.; Rabetli, M.E.; Zdek, U.; Koyun, N.; Alhalboosi, J.K.I.; Elasan, S. Combined treatment of sinapic acid and ellagic acid attenuates hyperglycemia in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2021, 156, 112443. [Google Scholar] [CrossRef]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef] [PubMed]
- Engström, L.; Ruud, J.; Eskilsson, A.; Larsson, A.; Mackerlova, L.; Kugelberg, U.; Qian, H.; Vasilache, A.M.; Larsson, P.; Engblom, D.; et al. Lipopolysaccharide-induced fever depends on prostaglandin E2 production specifically in brain endothelial cells. Endocrinology 2012, 153, 4849–4861. [Google Scholar] [CrossRef] [Green Version]
- Romanovsky, A.; Almeida, M.; Aronoff, D.; Ivanov, A.; Konsman, J.; Steiner, A.; Turek, V. Fever and hypothermia in systemic inflammation: Recent discoveries and revisions. Front. Biosci. 2005, 10, 2193–2216. [Google Scholar] [CrossRef]
- Zasłona, Z.; Pålsson-McDermott, E.M.; Menon, D.; Haneklaus, M.; Flis, E.; Prendeville, H.; Corcoran, S.E.; Peters-Golden, M.; O’Neill, L.A.J. The Induction of Pro-IL-1β by Lipopolysaccharide Requires Endogenous Prostaglandin E2 Production. J. Immunol. 2017, 198, 3558–3564. [Google Scholar] [CrossRef] [Green Version]
- Dennis, E.A. Liberating Chiral Lipid Mediators, Inflammatory Enzymes, and LIPID MAPS from Biological Grease. J. Biol. Chem. 2016, 291, 24431–24448. [Google Scholar] [CrossRef] [Green Version]
- Fahy, E.; Subramaniam, S.; Murphy, R.C.; Nishijima, M.; Raetz, C.R.; Shimizu, T.; Spener, F.; van Meer, G.; Wakelam, M.J.; Dennis, E.A. Update of the LIPIDMAPS comprehensive classification system for lipids. J. Lipid Res. 2009, 50, S9–S14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donnell, V.B.; Dennis, E.A.; Wakelam, M.J.O.; Subramaniam, S. LIPID MAPS: Serving the next generation of lipid researchers with tools, resources, data, and training. Sci. Signal. 2019, 12, 2964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kihara, Y. Introduction: Druggable Lipid Signaling Pathways. In Druggable Lipid Signaling Pathways. Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2020; Volume 1274, pp. 1–4. [Google Scholar]
- Hishikawa, D.; Hashidate, T.; Shimizu, T.; Shindou, H. Diversity and function of membrane glycero phospholipids generated by the remodeling pathway in mammalian cells. J. Lipid Res. 2014, 55, 799–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kita, Y.; Shindou, H.; Shimizu, T. Cytosolic phospholipase A2 and lysophospholipid acyltransferases. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 838–845. [Google Scholar] [CrossRef]
- Shindou, H.; Shimizu, T. Acyl-CoA: Lysophospholipid acyltransferases. J. Biol. Chem. 2009, 284, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef] [Green Version]
- Inceoglu, B.; Schmelzer, K.R.; Morisseau, C.; Jinks, S.L.; Hammock, B.D. Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (EETs). Prostaglandins Other Lipid Mediat. 2007, 82, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Smith, W.L.; Urade, Y.; Jakobsson, P.J. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem. Rev. 2011, 111, 5821–5865. [Google Scholar] [CrossRef] [Green Version]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Trayssac, M.; Hannun, Y.A.; Obeid, L.M. Role of sphingolipids in senescence: Implication in aging and age-related diseases. J. Clin. Investig. 2018, 128, 2702–2712. [Google Scholar] [CrossRef]
- Proia, R.L.; Hla, T. Emerging biology of sphingosine-1-phosphate: Its role in pathogenesis and therapy. J. Clin. Investig. 2015, 125, 1379–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaho, V.A.; Chun, J. ‘Crystal’ clear?Lysophospholipid receptor structure insights and controversies. Trends Pharmacol. Sci. 2018, 39, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.L.; Huang, C.C.; Tseng, T.T.; Liu, S.-C.; Tsai, C.-H.; Fong, Y.-C.; Tang, C.-H. S1P facilitates IL-1β production in osteoblasts via the JAK and STAT3 signaling pathways. Environ. Toxicol. 2020, 35, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zeng, Y.Z.; Ren, K.; Zhu, X.; Tan, Y.; Li, Y.; Li, Q.; Yi, G.-H. S1P promotes inflammation-induced tube formation by HLECs via the S1PR1/NF-κB pathway. Int. Immunopharmacol. 2019, 66, 224–235. [Google Scholar] [CrossRef]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0-The Human Metabolome Database in 2013. Nucleic Acids Res. 2013, 41, 801–807. [Google Scholar] [CrossRef]
- Zhu, Z.J.; Schultz, A.W.; Wang, J.; Johnson, C.; Yannone, S.M.; Patti, G.J.; Siuzdak, G. Liquid chromatography quadrupole time-of-flight mass spectrometry characterization of metabolites guided by the METLIN database. Nat. Protoc. 2013, 3, 451–460. [Google Scholar] [CrossRef]
- Fraga, C.G.; Clowers, B.H.; Moore, R.J.; Zink, E.M. Signature-discovery approach for sample matching of a nerve-agent precursor using liquid chromatography-mass spectrometry, XCMS, and chemometrics. Anal. Chem. 2010, 10, 4165–4173. [Google Scholar] [CrossRef]
- Kanehisa, G. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]



| No. | Index | Formula | Compounds | FC | NG vs. MG | MG vs. APG | MG vs. EAG | |||
|---|---|---|---|---|---|---|---|---|---|---|
| NG vs. MG | p Value | Type | p Value | Type | p Value | Type | ||||
| 1 | MADN0334 | C8H8O3 | 2-Hydroxyphenylacetic acid | 2.1562 | 0.0399 # | ↓ | 0.2466 | - | 0.0148 * | ↑ |
| 2 | MADN0343 | C9H13N2O9P | 3′-UMP | 0.465 | 0.0347 # | ↑ | 0.0151 * | ↓ | 0.1955 | - |
| 3 | MADN0408 | C10H15N2O8P | Thymidine-5′-phosphate (dTMP) | 0.3211 | 0.0128 # | ↑ | 0.0015 ** | ↓ | 0.1721 | ↓ |
| 4 | MADN0469 | C41H74NO8P | PE (16:0/20:4) | 0.3419 | 0.0258 # | ↑ | 0.0865 | - | 0.0905 | ↓ |
| 5 | MADN0495 | C39H79N2O6P | SM (d34:1) | 0.3601 | 0.0223 # | ↑ | 0.0322 * | ↓ | 0.0327 * | ↓ |
| 6 | MADP0192 | C8H17NO2 | Methacholine | 2.4518 | 0.0196 # | ↓ | 0.1032 | - | 0.0175 * | ↓ |
| 7 | MADP0433 | C39H79N2O6P | SM (d18:1/16:0) | 0.3526 | 0.0166 # | ↑ | 0.0500 * | ↓ | 0.0503 | ↓ |
| 8 | MADP0435 | C44H82NO7P | PC (18:2(9Z, 12Z)/P-18:1 (11Z)) | 0.3662 | 0.0458 # | ↑ | 0.0577 | - | 0.0464 * | ↓ |
| 9 | MADP0436 | C46H84NO7P | PC (20:4(8Z, 11Z, 14Z, 17Z)/P-18:0) | 0.4763 | 0.0495 # | ↑ | 0.0437 * | ↓ | 0.0357 * | ↓ |
| 10 | MADP0438 | C44H80NO7P | PC (O-16:0/20:5 (5Z, 8Z, 11Z, 14Z, 17Z)) | 0.4072 | 0.0492 # | ↑ | 0.0488 * | ↓ | 0.0388 * | ↓ |
| 11 | MADP0440 | C38H76NO8P | PC (15:0/15:0) | 0.3294 | 0.0411 # | ↑ | 0.0936 | - | 0.0505 | ↓ |
| 12 | MADP0442 | C40H80NO8P | PC (16:0/16:0) | 0.3509 | 0.0317 # | ↑ | 0.0730 | - | 0.0430 * | ↓ |
| 13 | MADP0444 | C45H90N2O6P+ | SM (d16:1/24:1(15Z)) | 0.3685 | 0.0331 # | ↑ | 0.0368 * | ↓ | 0.0641 | ↓ |
| 14 | MADP0445 | C44H80NO8P | PC (18:1 (9Z)/18:3 (6Z, 9Z, 12Z)) | 0.2118 | 0.0202 # | ↑ | 0.0112 * | ↓ | 0.1082 | ↓ |
| 15 | MADP0450 | C41H78NO8P | PC (18:2 (9Z, 12Z)/15:0) | 0.4158 | 0.0342 # | ↑ | 0.0747 | - | 0.0141 * | ↓ |
| 16 | MADP0452 | C45H91N2O6P | SM (d18:1/22:0) | 0.3602 | 0.0364 # | ↑ | 0.0830 | - | 0.0713 | ↓ |
| 17 | MADP0455 | C37H75N2O6P | SM (d18:1/14:0) | 0.4935 | 0.0149 # | ↑ | 0.0207 * | ↓ | 0.0278 * | ↓ |
| 18 | MADP0459 | C41H81N2O6P | SM (d18:1/18:1(9Z)) | 0.2787 | 0.0245 # | ↑ | 0.0380 * | ↓ | 0.0565 | ↓ |
| 19 | MADP0468 | C46H80NO8P | PC (16:0/22:6 (4Z, 7Z, 10Z, 13Z, 16Z, 19Z)) | 0.4856 | 0.0483 # | ↑ | 0.0754 | - | 0.0328 * | ↓ |
| 20 | MADP0469 | C42H80NO8P | PC (16:0/18:2 (11Z, 13Z)) | 0.3724 | 0.0461 # | ↑ | 0.0569 | - | 0.0600 | ↓ |
| 21 | MADP0470 | C41H83N2O6P | SM (d18:1/18:0) | 0.3249 | 0.0298 # | ↑ | 0.0708 | - | 0.0559 | ↓ |
| 22 | MADP0472 | C39H74NO8P | PE (16:0/18:2(9Z, 12Z)) | 0.3882 | 0.0353 # | ↑ | 0.0994 | - | 0.0355 * | ↓ |
| 23 | MADP0473 | C48H84NO8P | PC (18:0/22:6 (4Z, 7Z, 10Z, 13Z, 16Z, 19Z)) | 0.3796 | 0.0466 # | ↑ | 0.1106 | - | 0.0625 | ↓ |
| 24 | MADP0475 | C44H84NO8P | PC (18:1 (9Z)/18:1 (9Z)) | 0.3025 | 0.0252 # | ↑ | 0.0126 * | ↓ | 0.0334 * | ↓ |
| 25 | MADP0478 | C44H86NO8P | PC (18:0/18:1 (9Z)) | 0.3128 | 0.0439 # | ↑ | 0.0899 | - | 0.1194 | ↓ |
| 26 | MEDP1928 | C10H16N2O2 | Cyclo (Pro-Val) | 2.7492 | 0.0000 ## | ↓ | 0.3157 | - | 0.6655 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, F.; Xu, L.; Zhu, H.; Chen, Y.; Li, Y.; Nong, L.; Zeng, Y.; Cen, S. The Potential Antipyretic Mechanism of Ellagic Acid with Brain Metabolomics Using Rats with Yeast-Induced Fever. Molecules 2022, 27, 2465. https://doi.org/10.3390/molecules27082465
Xie F, Xu L, Zhu H, Chen Y, Li Y, Nong L, Zeng Y, Cen S. The Potential Antipyretic Mechanism of Ellagic Acid with Brain Metabolomics Using Rats with Yeast-Induced Fever. Molecules. 2022; 27(8):2465. https://doi.org/10.3390/molecules27082465
Chicago/Turabian StyleXie, Fengfeng, Liba Xu, Hua Zhu, Yaling Chen, Yinlan Li, Lizhen Nong, Yanfang Zeng, and Sijie Cen. 2022. "The Potential Antipyretic Mechanism of Ellagic Acid with Brain Metabolomics Using Rats with Yeast-Induced Fever" Molecules 27, no. 8: 2465. https://doi.org/10.3390/molecules27082465
APA StyleXie, F., Xu, L., Zhu, H., Chen, Y., Li, Y., Nong, L., Zeng, Y., & Cen, S. (2022). The Potential Antipyretic Mechanism of Ellagic Acid with Brain Metabolomics Using Rats with Yeast-Induced Fever. Molecules, 27(8), 2465. https://doi.org/10.3390/molecules27082465





