Attenuation of Scopolamine-Induced Amnesia via Cholinergic Modulation in Mice by Synthetic Curcumin Analogs
Abstract
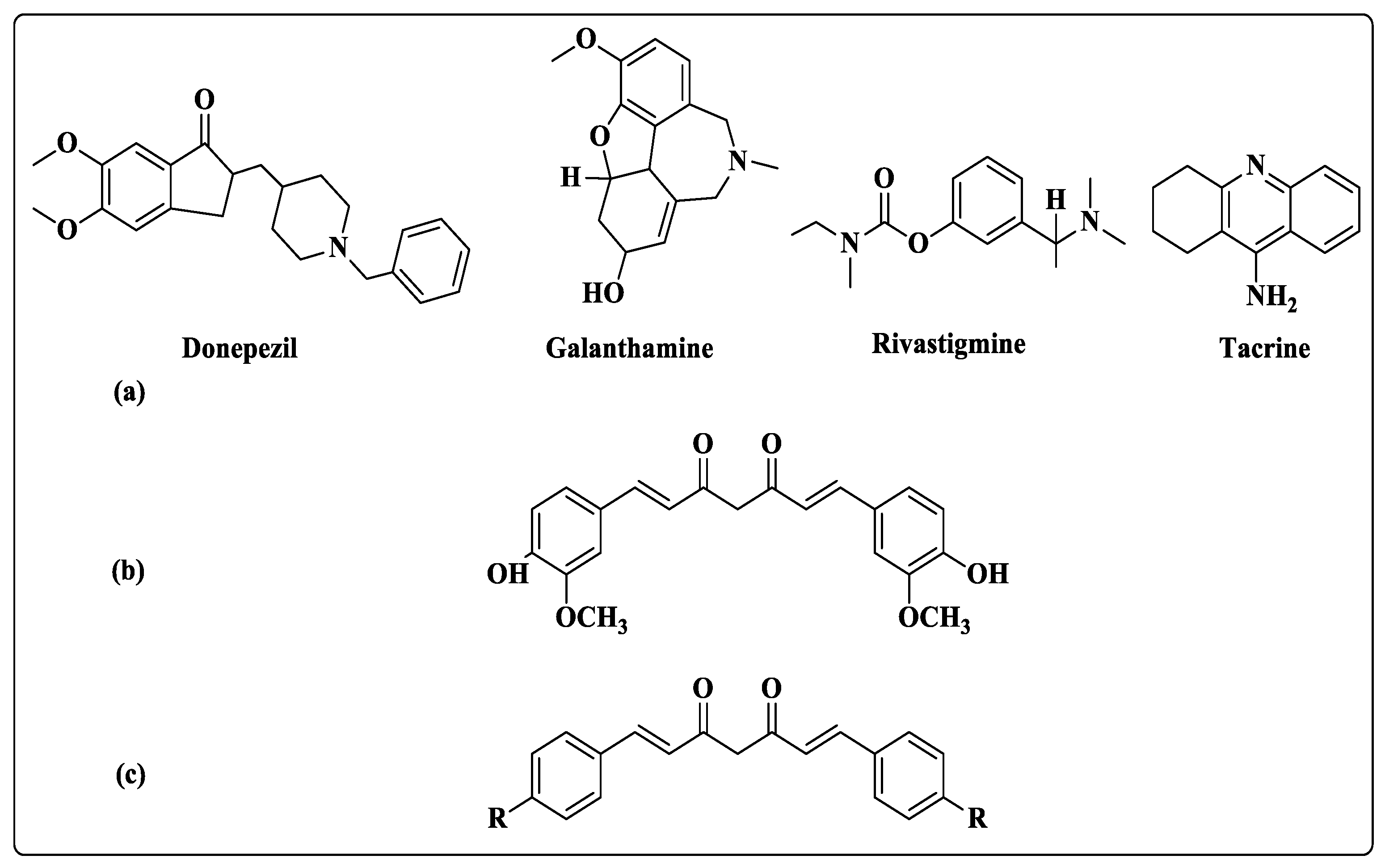
1. Introduction
2. Results
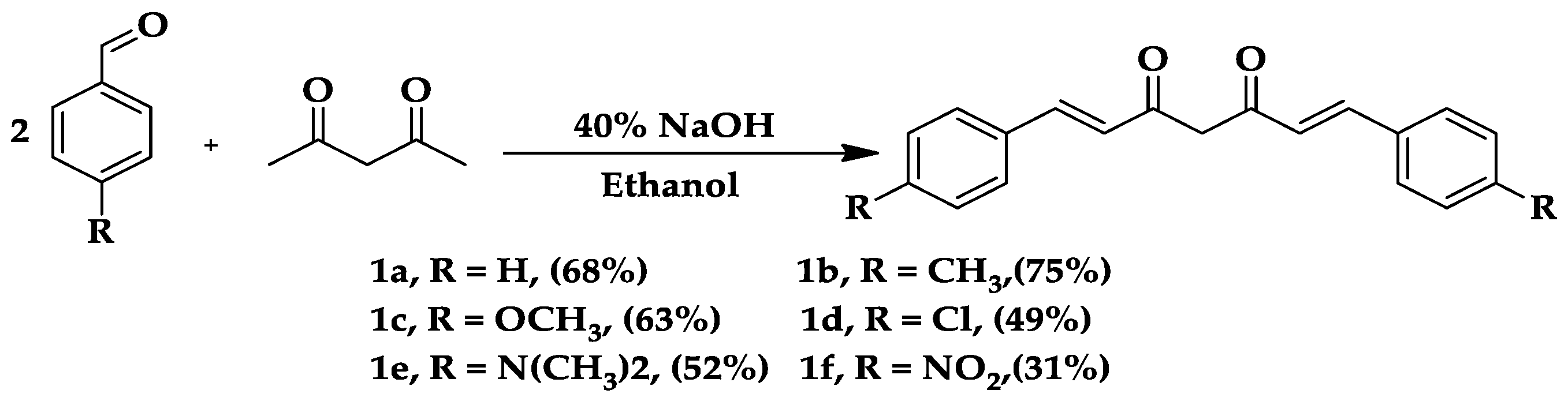
2.1. In Vitro Cholinesterase Activity
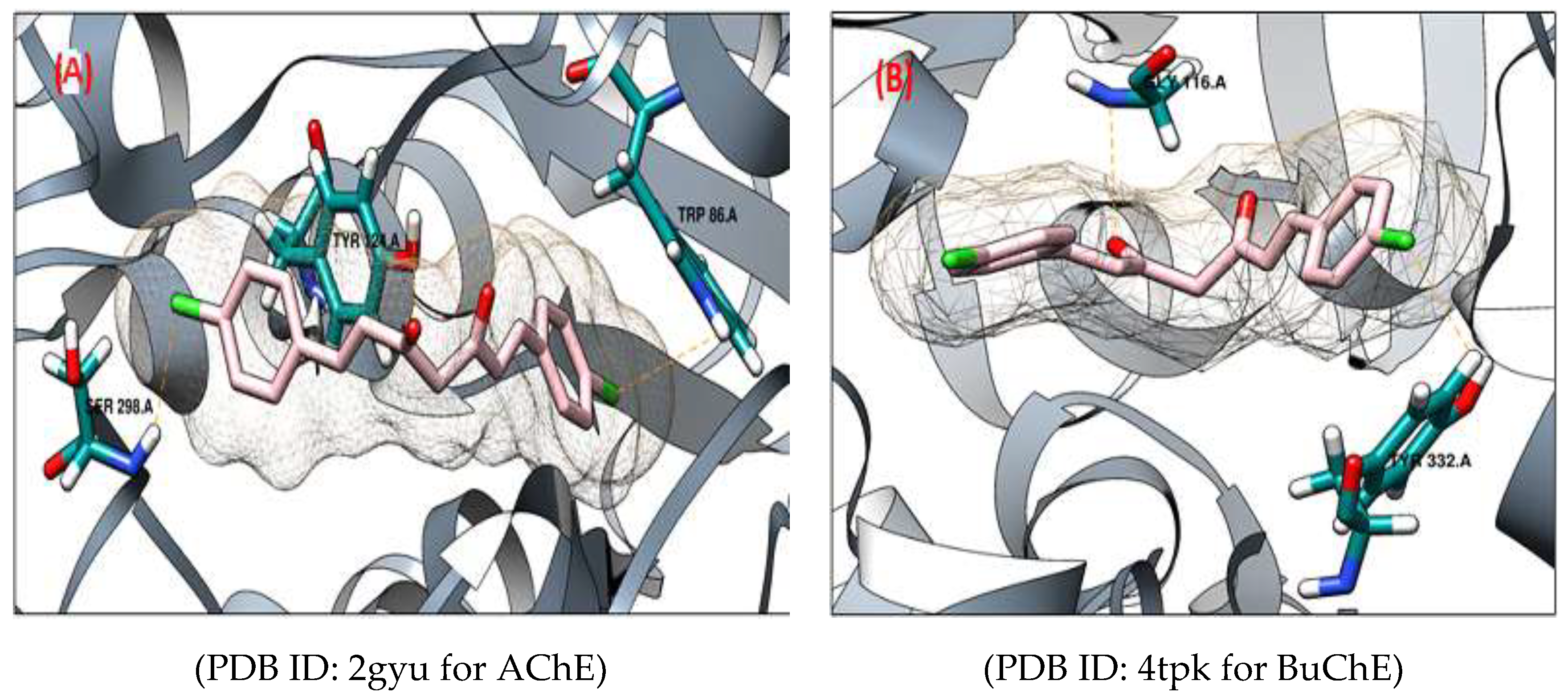
2.2. Molecular Docking
Validation of Docking for Anticholinesterases
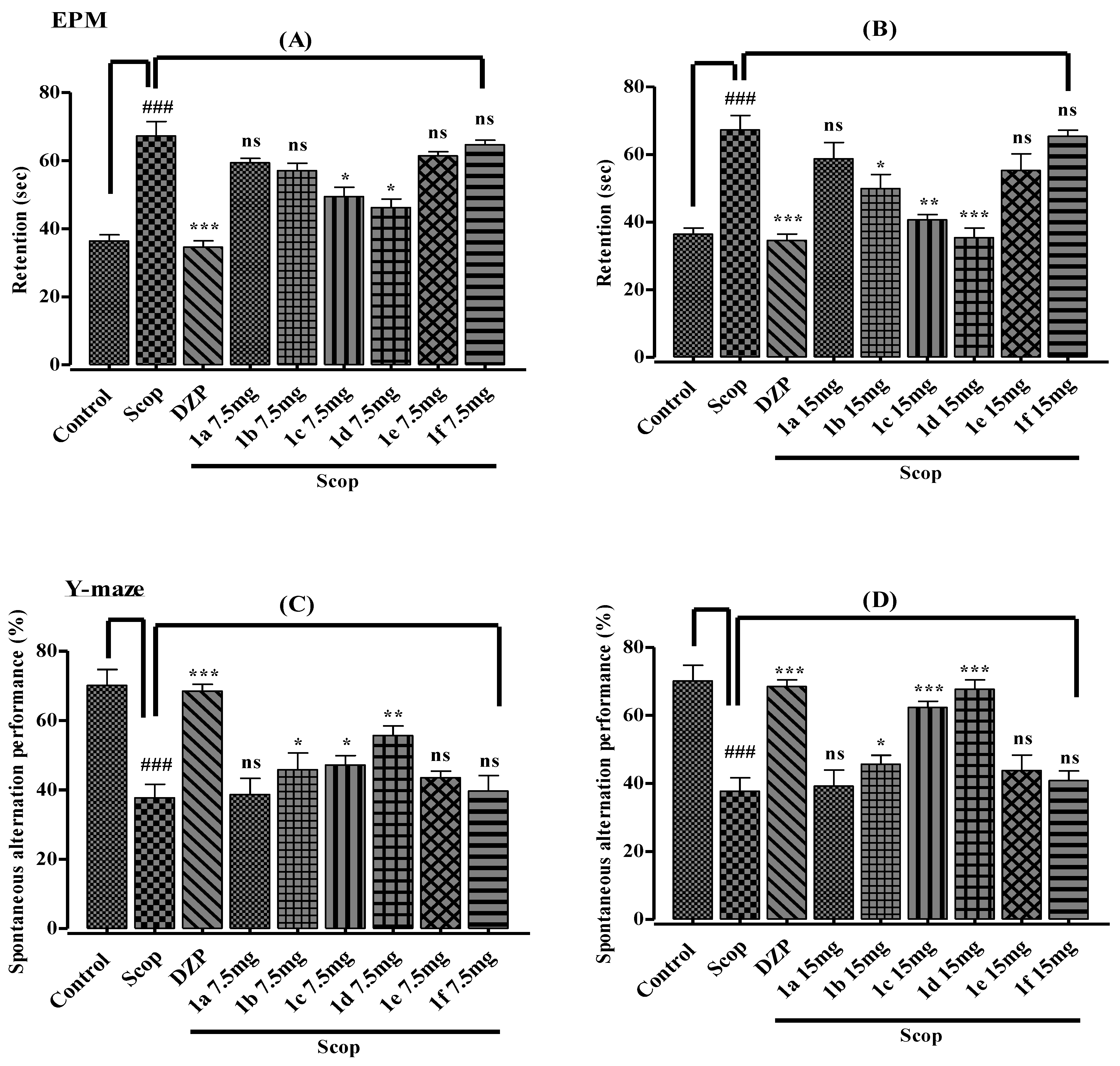
2.3. In Vivo Behavioral Study
2.3.1. Elevated Plus Maze
2.3.2. Y-Maze Test
2.3.3. Novel Object Recognition Test
2.4. Biomarker Study
Effect of Synthesized Curcumin Analogs on AChE and BuChE Activity in the Brain Hippocampus
3. Discussion
4. Materials and Methods
4.1. Methods
4.1.1. General Procedure for the Synthesis of Curcumin Analogs (1a–1f)
Synthesis of (1E,6E)-1,7-diphenylhepta-1,6-diene-3,5-dione (1a)
Synthesis of (1E,6E)-1,7-di-p-tolylhepta-1,6-diene-3,5-dione (1b)
Synthesis of (1E,6E)-1,7-bis(4-methoxyphenyl)hepta-1,6-diene-3,5-dione (1c)
Synthesis of (1E,6E)-1,7-bis(4-chlorophenyl)hepta-1,6-diene-3,5-dione (1d)
Synthesis of (1E,6E)-1,7-bis(4-(dimethylamino)phenyl)hepta-1,6-diene-3,5-dione (1e)
Synthesis of (1E,6E)-1,7-bis(4-nitrophenyl)hepta-1,6-diene-3,5-dione (1f)
4.2. In Vitro Cholinesterase Assay
4.3. Molecular Docking Study
4.3.1. Receptors Preparation
4.3.2. Re-Docking Setup
4.4. Acute Toxicity Study
4.5. In Vivo Analysis
4.5.1. Animals and Dosing
4.5.2. Elevated Plus Maze
4.5.3. Y-Maze Test
4.5.4. Novel Object Recognition Test (NORT)
4.6. Assessment of Biochemical Parameters and Biomarker Study
4.6.1. Cholinesterase Activity
Acetylcholinesterase (AChE) Activity
Butyrylcholinesterase (BuChE) Activity
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Elbini-Dhouib, I.; Doghri, R.; Ellefi, A.; Degrach, I.; Srairi-Abid, N.; Gati, A. Curcumin attenuated neurotoxicity in sporadic animal model of alzheimer’s disease. Molecules 2021, 26, 3011. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Ahmad, S.; Wadood, S.; Shah, A.; Ghias, M.; Ullah, A.; Rahman, S.U.; Kamal, Z.; Khan, F.A.; Khan, N.M.; et al. Neuroprotective Potential of Synthetic Mono-Carbonyl Curcumin Analogs Assessed by Molecular Docking Studies. Molecules 2021, 26, 7168. [Google Scholar] [CrossRef] [PubMed]
- Chainoglou, E.; Hadjipavlou-litina, D. Curcumin in Health and Diseases: Alzheimer’s Disease and Curcumin Analogues, Derivatives, and Hybrids. Int. J. Mol. Sci. 2020, 21, 1975. [Google Scholar] [CrossRef] [PubMed]
- Voulgaropoulou, S.D.; Van Amelsvoort, T.A.M.J.; Prickaerts, J.; Vingerhoets, C. The effect of curcumin on cognition in Alzheimer’s disease and healthy aging: A systematic review of pre-clinical and clinical studies. Brain Res. 2019, 1725, 146476. [Google Scholar] [CrossRef] [PubMed]
- Nazir, N.; Zahoor, M.; Nisar, M.; Karim, N.; Latif, A.; Ahmad, S.; Uddin, Z. Evaluation of neuroprotective and antiamnesic effects of elaeagnus umbellate thunb. On scopolamine-induced memory impairment in mice. BMC Complement. Med. Ther. 2020, 20, 143. [Google Scholar] [CrossRef]
- Ghias, M.; Shoaib, M.; Ali Shah, S.W.; Umar, M.N.; Ullah, S.; Ali, N.; Shah, I.; Ullah, S. Nootropic effects of synthetic flavonoid derivatives on scopolamine induced memory impairment in mice via cholinesterase inhibition and antioxidant system. Pak. J. Pharm. Sci. 2019, 32, 2325–2332. [Google Scholar]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Tipton, K.F. Assessment of Enzyme Inhibition: A Review with Examples from the Development of Monoamine Oxidase and Cholinesterase Inhibitory Drugs. Molecules 2017, 22, 1192. [Google Scholar] [CrossRef]
- Boiangiu, R.S.; Brinza, I.; Hancianu, M.; Orhan, I.E.; Eren, G.; Gündüz, E.; Ertas, H.; Hritcu, L.; Cioanca, O. Cognitive facilitation and antioxidant effects of an essential oil mix on scopolamine-induced amnesia in rats: Molecular modeling of in vitro and in vivo approaches. Molecules 2020, 25, 1519. [Google Scholar] [CrossRef]
- Knox, D. The Role Of Basal Forebrain Cholinergic Neurons In Fear and Extinction Memory. Neurobiol. Learn. Mem. 2016, 133, 39–52. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Lian, W.; Fang, J.; Xu, L.; Zhou, W.; Kang, D.; Xiong, W.; Jia, H.; Liu, A.L.; Du, G.H. DL0410 ameliorates memory and cognitive impairments induced by scopolamine via increasing cholinergic neurotransmission in mice. Molecules 2017, 22, 410. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.; Martinez, A. Targeting Beta-Amyloid Pathogenesis Through Acetylcholinesterase Inhibitors. Curr. Pharm. Des. 2006, 12, 4377–4387. [Google Scholar] [CrossRef] [PubMed]
- Orhan, G.; Orhan, I.; Sener, B. Recent Developments in Natural and Synthetic Drug Research for Alzheimers Disease. Lett. Drug Des. Discov. 2006, 3, 268–274. [Google Scholar] [CrossRef]
- Olsen, C.E.; Poulsen, H.D.; Lublin, H.K.F. Drug therapy of dementia in elderly patients. A review. Nord. J. Psychiatry 2005, 59, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Mbese, Z.; Khwaza, V.; Aderibigbe, B.A. Curcumin and Its Derivatives as Potential Therapeutic Agents in Prostate, Colon and Breast Cancers. Molecules 2019, 24, 4386. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Yang, S.; Jiang, L.; Zhao, Y.; Shao, L.; Xiao, J.; Ye, F.; Li, Y.; Li, X. Synthesis and anti-bacterial properties of mono-carbonyl analogues of curcumin. Chem. Pharm. Bull. 2008, 56, 162–167. [Google Scholar] [CrossRef]
- Ahmed, T.; Gilani, A.H. Inhibitory effect of curcuminoids on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia may explain medicinal use of turmeric in Alzheimer’s disease. Pharmacol. Biochem. Behav. 2009, 91, 554–559. [Google Scholar] [CrossRef]
- Lee, W.; Loo, C.; Bebawy, M.; Luk, F.; Mason, R.S. Curcumin and its Derivatives: Their Application in Neuropharmacology and Neuroscience in the 21 st Century. Curr. Neuropharmacol. 2013, 11, 338–378. [Google Scholar] [CrossRef]
- Naqvi, F.; Haider, S.; Naqvi, F.; Saleem, S.; Perveen, T.; Batool, Z. A comparative study showing greater effects of curcumin compared to donepezil on memory function in rats. Pak. J. Pharm. Sci. 2019, 32, 53–60. [Google Scholar]
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Misra, K.; Priyadarsini, I.K.; Rajasekharan, K.N.; et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem. Pharmacol. 2008, 76, 1590–1611. [Google Scholar] [CrossRef] [PubMed]
- Wadood, A.; Riaz, M.; Jamal, S.B.; Shah, M. Interactions of ketoamide inhibitors on HCV NS3/4A protease target: Molecular docking studies. Mol. Biol. Rep. 2014, 41, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, Y.; Zou, P.; Wang, J.; He, W.; Shi, D.; Li, H.; Liang, G.; Yang, S. Synthesis and biological evaluation of a novel class of curcumin analogs as anti-inflammatory agents for prevention and treatment of sepsis in mouse model. Drug Des. Devel. Ther. 2015, 9, 1663–1678. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Food, J.M.; Kalpana, C.; Menon, V.P. Inhibition of Nicotine-Induced Toxicity by Curcumin and Curcumin Analog: A Comparative Study. J. Med. Food 2004, 7, 467–471. [Google Scholar]
- Chlebek, J.; Korábečný, J.; Doležal, R.; Štěpánková, Š.; Pérez, D.I.; Hošťálková, A.; Opletal, L.; Cahlíková, L.; Macáková, K.; Kučera, T.; et al. In Vitro and In Silico Acetylcholinesterase Inhibitory Activity of Thalictricavine and Canadine and Their Predicted Penetration across the Blood-Brain Barrier. Molecules 2019, 24, 1340. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, Y.; Wu, Y.; Wang, W.; Xiao, L. The Curcumin Derivative, H10, Suppresses Hormone-Dependent Prostate Cancer by Inhibiting 17 b-Hydroxysteroid Dehydrogenase Type 3. Front. Pharmacol. 2020, 11, 637. [Google Scholar] [CrossRef]
- Aman, L.O.; Kartasasmita, R.E.; Tjahjono, D.H. Virtual screening of curcumin analogues as DYRK2 inhibitor: Pharmacophore analysis, molecular docking and dynamics, and ADME prediction. F1000Research 2021, 10, 394. [Google Scholar] [CrossRef]
- Raghu, G.; Karunanithi, A.; Kannan, I.; Preetha, L.K. Molecular docking study on curcumin and its derivatives as inhibitors of BACE1 in the treatment of Alzheimer’s disease. Natl. J. Physiol. Pharm. Pharmacol. 2017, 8, 244–250. [Google Scholar] [CrossRef]
- Pahaye, D.B.; Bum, E.N.; Taïwé, G.S.; Ngoupaye, G.T.; Sidiki, N.; Clarisse, F.; Moto, O.; Kouemou, N.; Jacqueline, S.; Njapdounke, K.; et al. Neuroprotective and Antiamnesic Effects of Mitragyna inermis Willd (Rubiaceae) on Scopolamine-Induced Memory Impairment in Mice. Behav. Neurol. 2017, 2017, 5952897. [Google Scholar] [CrossRef]
- Kumar, S.; Maheshwari, K.K.; Singh, V. Protective effects of Punica granatum seeds extract against aging and scopolamine induced cognitive impairments in mice. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Reeta, K.H.; Mehla, J.; Gupta, Y.K. Curcumin is protective against phenytoin-induced cognitive impairment and oxidative stress in rats. Brain Res. 2009, 1301, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Noorafshan, A.; Asadi-Golshan, R.; Karbalay-Doust, S.; Abdollahifar, M.A.; Rashidiani-Rashidabadi, A. Curcumin, the Main Part of Turmeric, Prevents Learning and Memory Changes Induced by Sodium Metabisulfite, a Preservative Agent, in Rats. Exp. Neurobiol. 2013, 22, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Cleal, M.; Fontana, B.D.; Ranson, D.C.; Redhead, E.S.; Parker, M.O.; Parker, M.O. The Free-movement pattern Y-maze: A cross-species measure of working memory and executive function. Behav. Res. Methods 2021, 53, 536–557. [Google Scholar] [CrossRef] [PubMed]
- Ru, M.; Liu, H. Association between Y-Maze Acquisition Learning and Major Histocompatibility Complex Class II Polymorphisms in Mice. Biomed Res. Int. 2018, 5, 6381932. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.L. VTE on an elevated T-maze. J. Comp. Psychol. 1943, 36, 99–107. [Google Scholar] [CrossRef]
- Postu, P.A.; Sadiki, F.Z.; El Idrissi, M.; Cioanca, O.; Trifan, A.; Hancianu, M.; Hritcu, L. Pinus halepensis essential oil attenuates the toxic Alzheimer’s amyloid beta (1-42)-induced memory impairment and oxidative stress in the rat hippocampus. Biomed. Pharmacother. 2019, 112, 108673. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Lee, C.; Park, G.H.; Jang, J.H. Amelioration of Scopolamine-Induced Learning and Memory Impairment by α-Pinene in C57BL/6 Mice. Evid.-Based Complement. Altern. Med. 2017, 2017, 4926815. [Google Scholar] [CrossRef]
- Baxter, M.G. “I’ve seen it all before” Explaining age-related impairments in object recognition. Theoretical comment on Burke et al. Behav. Neurosci. 2010, 124, 706–709. [Google Scholar] [CrossRef]
- Lueptow, L.M. Novel Object Recognition Test for the Investigation of Learning and Memory in Mice. J. Vis. Exp. 2017, 126, e55718. [Google Scholar] [CrossRef]
- Rabiei, Z.; Setorki, M. Effect of hydroalcoholic echium amoenum extract on scopolamine-induced learning and memory impairment in rats. Pharm. Biol. 2018, 56, 672–677. [Google Scholar] [CrossRef]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Carapina da Silva, C.; Pacheco, B.S.; das Neves, R.N.; Dié Alves, M.S.; Sena-Lopes, Â.; Moura, S.; Borsuk, S.; de Pereira, C.M.P. Antiparasitic activity of synthetic curcumin monocarbonyl analogues against Trichomonas vaginalis. Biomed. Pharmacother. 2019, 111, 367–377. [Google Scholar] [CrossRef]
- Hung, S.; Hong, Y.; Lin, K.; Hua, Y.; Kuo, C.; Hu, A. Efficient Photodynamic Killing of Gram-Positive Bacteria by Synthetic Curcuminoids. Int. J. Mol. Sci. 2020, 21, 9024. [Google Scholar] [CrossRef] [PubMed]
- Natalia Milklasova, R.M. Cytotoxic activity of palladium (II) complexes of (1e, 6E)-1,7-bis(4-dimethylamino)phenyl) hepta-1,6-diene-3,5-dione against human colon carcinoma. Stud. Ubb Chem. 2016, 3, 109–116. [Google Scholar]
- Shoaib, M.; Shah, I.; Adikhari, A.; Ali, N.; Ayub, T.; Ul-Haq, Z.; Shah, S.W.A. Isolation of flavonoides from Artemisia macrocephala anticholinesterase activity: Isolation, characterization and its in vitro anticholinesterse activity supported by molecular docking. Pak. J. Pharm. Sci. 2018, 31, 1347–1354. [Google Scholar]
- Faheem, M.; Jamal, S.B. Identification of Zika Virus NS5 Novel Inhibitors through Virtual Screening and Docking Studies. Life Sci. 2020, 1, 2–6. [Google Scholar] [CrossRef]
- Jamal, S.B.; Hassan, S.S.; Tiwari, S.; Viana, M.V.; Turjanski, G.; Barh, D.; Benevides, D.J.; Ullah, A.; Baumbach, J.; Ghosh, P.; et al. An integrative in-silico approach for therapeutic target identification in the human pathogen Corynebacterium diphtheriae. PLoS ONE 2017, 12, e0186401. [Google Scholar] [CrossRef]
- Wadood, A.; Jamal, S.B.; Riaz, M.; Mir, A. Computational analysis of benzofuran-2-carboxlic acids as potent Pim-1 kinase inhibitors. Pharm. Biol. 2014, 52, 1170–1178. [Google Scholar] [CrossRef]
- Singh, V.; Kahol, A.; Singh, I.P.; Saraf, I.; Shri, R. Evaluation of anti-amnesic effect of extracts of selected Ocimum species using in-vitro and in-vivo models. J. Ethnopharmacol. 2016, 193, 490–499. [Google Scholar] [CrossRef]
- Mushtaq, A.; Anwar, R.; Ahmad, M. Lavandula stoechas (L) a very potent antioxidant attenuates dementia in scopolamine induced memory deficit mice. Front. Pharmacol. 2018, 9, 1375. [Google Scholar] [CrossRef]
- George, L.; Ellman, K. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar]



| Compound | AChEI (IC50 (nM)) | BuChEI (IC50 (nM)) |
|---|---|---|
| 1a | 2615.42 | 5347.16 |
| 1b | 733.84 | 2159.08 |
| 1c | 467.18 | 1356.14 |
| 1d | 112.52 | 378.43 |
| 1e | 3267.95 | 6635.82 |
| 1f | 5839.96 | 9664.71 |
| Donepezil | 9.31 | 33.65 |
| Comp | AChEI (IC50 nM) | Docking Score | Binding Energy (GBVI/WSA) | BuChEI (IC50 nM) | Docking Score | Binding Energy (GBVI/WSA) |
|---|---|---|---|---|---|---|
| 1a | 2615.42 | −7.371 | −13.101 | 5347.16 | −7.871 | −13.207 |
| 1b | 733.84 | −8.236 | −14.082 | 2159.08 | −8.054 | −14.749 |
| 1c | 467.18 | −10.427 | −18.127 | 1356.14 | −8.556 | −15.217 |
| 1d | 112.52 | −12.089 | −21.159 | 378.43 | −10.962 | −19.081 |
| 1e | 3267.95 | −7.391 | −13.330 | 6635.82 | −6.918 | −12.652 |
| 1f | 5839.96 | −6.998 | −12.146 | 9664.71 | −6.202 | −11.023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, H.; Ahmad, S.; Shah, S.W.A.; Ullah, A.; Ali, N.; Almehmadi, M.; Ahmad, M.; Khalil, A.A.K.; Jamal, S.B.; Ahmad, H.; et al. Attenuation of Scopolamine-Induced Amnesia via Cholinergic Modulation in Mice by Synthetic Curcumin Analogs. Molecules 2022, 27, 2468. https://doi.org/10.3390/molecules27082468
Hussain H, Ahmad S, Shah SWA, Ullah A, Ali N, Almehmadi M, Ahmad M, Khalil AAK, Jamal SB, Ahmad H, et al. Attenuation of Scopolamine-Induced Amnesia via Cholinergic Modulation in Mice by Synthetic Curcumin Analogs. Molecules. 2022; 27(8):2468. https://doi.org/10.3390/molecules27082468
Chicago/Turabian StyleHussain, Haya, Shujaat Ahmad, Syed Wadood Ali Shah, Abid Ullah, Niaz Ali, Mazen Almehmadi, Manzoor Ahmad, Atif Ali Khan Khalil, Syed Babar Jamal, Hanif Ahmad, and et al. 2022. "Attenuation of Scopolamine-Induced Amnesia via Cholinergic Modulation in Mice by Synthetic Curcumin Analogs" Molecules 27, no. 8: 2468. https://doi.org/10.3390/molecules27082468
APA StyleHussain, H., Ahmad, S., Shah, S. W. A., Ullah, A., Ali, N., Almehmadi, M., Ahmad, M., Khalil, A. A. K., Jamal, S. B., Ahmad, H., & Halawi, M. (2022). Attenuation of Scopolamine-Induced Amnesia via Cholinergic Modulation in Mice by Synthetic Curcumin Analogs. Molecules, 27(8), 2468. https://doi.org/10.3390/molecules27082468







